Abstract
Objective
To estimate oxidative stress and antioxidant components during different stages of autoimmune liver diseases and assess their possible implication on disease progression.
Methods
We determined several markers of oxidative injury (isoprostane, aldehydes, protein carbonyls, 3-nitrotyrosine, and myeloperoxidase) and antioxidant components (glutathione, glutathione peroxidase, glutathione reductase, superoxide dismutase, and catalase) in whole blood, serum, and urine in 49 patients with autoimmune cholestatic liver diseases (AC) and 36 patients with autoimmune hepatitis (AIH) and healthy subjects matched for sex and age.
Results
Both AC and AIH patients had increased levels of all lipid and protein oxidative injury products and significantly decreased whole blood glutathione levels compared to controls. AIH patients had significantly higher levels of aldehydes and glutathione peroxidase activity and significantly lower protein carbonyl levels compared to AC patients. Protein carbonyl and isoprostane levels increased and glutathione levels decreased gradually with progression from mild fibrosis to severe fibrosis and cirrhosis in both AC and AIH patients. In addition, both cirrhotic AC and AIH patients had significantly higher protein carbonyls compared to non-cirrhotics.
Discussion
We provide novel findings in support of a major contribution of oxidant/antioxidant imbalance in the progression of liver injury in AC and AIH.
Keywords: Autoimmune hepatitis, Primary biliary cirrhosis, Lipid peroxidation, Glutathione, Antioxidant enzymes, Protein carbonyls
Introduction
The pathophysiological mechanisms underlying progression from the initial autoimmune attack to the development of fibrosis and ultimately to cirrhosis and liver failure in patients with autoimmune liver diseases, namely, autoimmune hepatitis (AIH), primary biliary cirrhosis (PBC), and primary sclerosing cholangitis (PSC) are poorly understood.1–3 Several reports have suggested the involvement of oxidative injury in animal models of acute and chronic liver disease and particularly in rat model of cholestasis induced by bile duct ligation,4,5 as well as in a variety of human liver disorders.5–7 Data on the presence of components of oxidative stress and antioxidant defence and their potential contribution to progression of AIH and PBC are scarce and limited to evaluation of individual markers in serum or in liver.8,9
The aim of the present study was to determine several components of oxidative injury and antioxidant defence in patients with different stages of AIH and cholestatic liver diseases in order to assess their potential implication in disease progression. We determined lipid peroxidation products like trans-4-hydroxy-2-nonenal (4-HNE), malondialdehyde (MDA), and 8-isoprostane (8-iso), protein modification products such as 3-nitrotyrosine (3-NT) or protein carbonyls (PC) in parallel with enzymatic and non-enzymatic antioxidant activities and subsequently correlated their activity with clinical and laboratory parameters associated with disease severity, as well as administration and response to treatment.
Materials and methods
Subjects
Eighty-five patients with autoimmune liver diseases (14 male, 71 female) followed at the Department of Medicine, Medical School, Thessaly University, were studied prospectively. Patients were divided into two groups: Group 1 consisted of 49 patients with autoimmune cholestatic liver diseases (AC; 41 PBC and 8 PSC).2,3 According to Ludwig's classification 2 patients had stage 1, 26 stage 2, 2 stage 3, and 6 stage 4 PBC.10 All PSC patients (n = 8) had stage I fibrosis and for this reason they were analysed together with stage I PBC patients (stage I AC group). Nine PBC patients were not receiving any treatment, while the remaining (32 PBC, 8 PSC) were under ursodeoxycholic acid (UDCA; 15 mg/kg/day for PBC and 20 mg/kg/day for PSC). Response to treatment was defined as normalization of liver enzymes.2
Group 2 (AIH group) consisted of 36 AIH patients.11 All but three patients (because of the presence of burn-out cirrhosis) were under immunosuppression with prednisolone (median dose: 5 mg/day, range: 0–10 mg/day) and/or mycophenolate mofetil (MMF; median dose: 1 g/day, range: 0–2 g/day) as part of a single-centre uncontrolled trial.12 Response to treatment was defined according to our report12 and the American Association for the Study of Liver Diseases criteria.13 According to Ishak scoring system,14 14 patients had minimal/mild, 6 had moderate, and 10 had severe fibrosis (cirrhosis). Other causes of cholestasis or hepatitis, Wilson's disease, and haemochromatosis were excluded appropriately. Mean follow-up of AC and AIH patients was 59 ± 26 and 68 ± 75 months, respectively.
Voluntary blood donors, age- and sex-matched, for each group of patients were also studied. All were seronegative for hepatitis C virus, hepatitis B virus, and human immunodeficiency virus with no previous history of hepatitis and/or chronic alcohol consumption. None of the patients and controls had received antioxidant supplementation at least 2 months preceding their inclusion in the study. Demographic characteristics and personal habits of patients and controls are presented in Table 1. All patients and controls consented to participate in the study. The study was approved by the Ethical Committee of the University of Thessaly, Medical School.
Table 1.
Age, gender, BMI, dietary and lifestyle habits of patients and controls
| AC controls | AC patients | AIH controls | AIH patients | |
|---|---|---|---|---|
| Gender (male/female) | (8/42) | (6/43) | (10/31) | (8/28) |
| Age (years) | 54 ± 15 | 57 ± 14 | 49 ± 17 | 50 ± 15 |
| BMI (kg/(h)2) | 26 ± 4 | 26 ± 4 | 26 ± 3 | 26 ± 6 |
| Smokers | 33% | 16% | 18% | 16% |
| Smoking (pack-years) | 29 ± 24 | 29 ± 27 | 26 ± 50 | 27 ± 59 |
| Alcohol consumption (1–7 drinks/week) | 36% | 24% | 15% | 16% |
| Meat consumption >4 times/week | 35% | 20% | 30% | 26% |
| Fruit consumption >7 times/week | 55% | 52% | 65% | 60% |
| Vegetables consumption >7 times/week | 80% | 53% | 75% | 80% |
Values shown as percentages % or as mean ± SD.
BMI, body mass index.
Specimen processing
Plasma, serum, and urine were collected after overnight fasting. Plasma and urine were protected from oxidation during storage by addition of the antioxidant butylated hydroxytoluene (200 µg/ml). For myeloperoxidase (MPO) measurement, leucocytes were isolated from the blood according to Cooray et al.15
Antioxidants
Copper, zinc superoxide dismutase (CuZn-SOD), catalase (CAT), glutathione peroxidase (GSH-Px), and GSH-reductase activities were measured in the erythrocyte lysates on a UV–VIS Recording Spectrophotometer (UV-2100S, Shimadzu, Japan). Erythrocytes were lysed with cold distilled water (1:4).
Measurement of CuZn-SOD activity was performed using Ransod reagents (Randox Laboratories, London, UK) according to McCord and Fridovich.16 GSH-reductase activity was determined according to Goldberg and Spooner,17 GSH-Px according to Paglia and Valentine,18 and CAT determination according to Aeby19 whereas determination of reduced glutathione (GSH) and oxidized glutathione (GSSG) was carried out according to Tietze.20
Oxidative injury markers
15-F2t-isoprostane in urine and serum 3-NT levels were determined using commercially available enzyme-linked immunosorbent assay kit ((Oxford Biomedical Research, Oxford, UK), (HyCult Biotechnology, Uden, The Netherlands)). PC in serum were determined according to Levine et al.21 while MDA and 4-HNE according to Gérard-Monnier et al.22 Leucocyte MPO activity was measured according to the method of Marquez and Dunford.23
Statistical analysis
The results were expressed as mean ± standard deviation (SD) or median (range) as appropriate. Data were analysed by unpaired t-test, Mann–Whitney U test, analysis of variance, and the Fisher's post hoc least-significant difference as the post hoc test corrected for multiple comparisons, as well as the non-parametric test Kruskal–Wallis, where applicable. A two sided P < 0.05 was considered as statistically significant.
Results
Oxidative stress markers in patients with AC, AIH, and their respective controls are shown in Tables 2 and 3. AIH patients had significantly higher levels of aldehydes (MDA + 4-HNE) (P = 0.02) and GSH-Px activity (P = 0.02) and significantly lower PC levels (P = 0.001) compared to AC patients.
Table 2.
Markers of lipid peroxidation, protein oxidation, activities of oxidant enzymes and antioxidant defence in controls and AC group in relation to disease stage and treatment response
| Parameters | Controls (n = 50) | AC patients (n = 49) | Cirrhotics with AC (n = 6) | Non-cirrhotics with AC (n = 43) | Naïve patients with AC (n = 9) | Treated patients with AC (n = 40) | Non-responders to treatment with AC (n = 9) (3 patients with cirrhosis) | Responders to treatment with AC (n = 31) (3 patients with cirrhosis) |
|---|---|---|---|---|---|---|---|---|
| 8-Iso ng/mg creatinine (U) | 1 (0.01–2.7) | 1.8*** (0.2–3.7) | 2.6*** (1.7–3.2) | 1.8**** (0.2–3.7) | 2.5** (2.2–3.4) | 1.8**** (0.2–3.7) | 2.1*** (0.6–3.2) | 1.8**** (0.2–3.7) |
| Aldehydes μM (P) | 0.3 (0.05–4.7) | 1.2*** (0.1–3.8) | 0.4 (0.1–1) | 1.3*** (0.1–3.7) | 2.1* (0.3–3.8) | 0.9** (0.1–3.8) | 0.3 (0.1–2.1) | 1.7**** (0.2–3.8) |
| 3-NT nM (P) | 11 (1.5–20) | 14** (1.3–70) | 22 (1.8–50) | 14 (1.3–70) | 6.9 (2.5–20) | 14 (1.3–70) | 14 (1.9–40) | 13 (1.3–70) |
| PC mg/mg protein (S) | 1.6 (0.1–10) | 4.1**** (0.01–21) | 8.1**** (5.9–21) | 3.7****/+ (0.02–19) | 5.4 (0.02–4.6) | 4*** (0.02–21) | 3.6*** (0.02–21) | 4.2*** (0.14–15) |
| MPO U/106cells (L) | 12 (1–142) | 19 (1.3–511) | 13 (2–39) | 20 (1.3–511) | 7 (1.3–65) | 25** (1.3–511) | 10 (1.3–511) | 26 (2–245) |
| GSSG (GSH + GSSG) (WB) | 5.1 (0.1–36) | 14*** (0.2–92) | 27** (1.5–75) | 12 (0.2–92) | 12 (0.2–71) | 10** (0.2–92) | 13 (0.2–92) | 14 (0.4–71) |
| GSH μM (WB) | 1101 (276–5409) | 475**** (4–2743) | 209**** (89–659) | 495*** (4–2743) | 258** (18–2743) | 500**** (18–1872) | 426**** (4–1400) | 494** (18–1872) |
| Catalase kU/gHb (RC) | 142 (50–449) | 148 (11–504) | 144 (79–265) | 153 (11–504) | 144 (28–430) | 130 (11–504) | 124** (14–294) | 162 (11–504) |
| SOD kU/gHb (RC) | 43 (8–68) | 51*** (14–78) | 52 (25–60) | 50** (14–78) | 46 (24–78) | 51** (14–60) | 52** (29–78) | 50** (14–60) |
| GSH-Px U/gHb (RC) | 3.6 (0.3–25) | 4.9 (0.1–63) | 7.8 (0.8–16) | 3.4 (0.1–63) | 3 (0.8–63) | 3.5 (0.1–63) | 3.4 (0.4–25) | 3.7 (0.1–63) |
| Glutathione reductase U/gHb (RC) | 1.6 (0.08–10) | 2.6 (0.1–36) | 2.9 (0.5–16) | 2.6 (0.1–36) | 3.3 (0.83–36) | 2.6 (0.1–37) | 2.7 (0.22–37) | 2.6 (0.1–35) |
| AST U/l (S) | 20 (12–44) | 23 (12–100) | 37* (19–100) | 22 (12–48) | 18 (15–30) | 23 (12–100) | 25 (15–100) | 21 (12–39) |
| ALT U/l (S) | 20 (11–57) | 26** (6–86) | 44 (12–58) | 25** (6–86) | 18 (6–31) | 26** (6–86) | 31** (12–86) | 21 (6–50) |
| ALP U/l (S) | 61 (32–96) | 81**** (28–279) | 130* (41–191) | 79**** (28–279) | 67 (41–120) | 120**** (28–279) | 127**** (46–279) | 73 (28–117) |
| γ-GT U/l (S) | 14 (5–44) | 28**** (10–213) | 71* (11–196) | 27**** (10–213) | 25 (12–75) | 30**** (10–213) | 52**** (13–213) | 21 (10–37) |
| Bilirubin mg/dl (S) | 0.5 (0.1–1) | 0.6*** (0.23–1.7) | 1.1** (0.53–1.7) | 0.6** (0.23–1.6) | 0.45 (0.26–1.6) | 0.6**** (0.23–1.7) | 0.7**** (0.23–1.7) | 0.6 (0.29–1.1) |
| UDCA (duration of intake in months) | 29 (1–168) | 50 (12–168) | 29 (1–91) | 29 (1–168) | 25 (2–91) | 38 (1–168) |
Values shown as medians with range. P values (<0.05) derived by the Mann–Whitney U test (*vs. control group, +vs. cirrhotic group).
*0.1 > P > 0.05, **/+P ≤ 0.05, ***P ≤ 0.01, ****P ≤ 0.001. GSSG/(GSH + GSSG) was expressed as %.
U, urine; P, plasma; S, serum; WB, whole blood; L, leucocytes; RC, red blood cells; UDCA, ursodeoxycholic acid.
Table 3.
Markers of lipid peroxidation, protein oxidation, activities of oxidant enzymes and antioxidant defence in controls and AIH group in relation to disease stage and treatment response
| Parameters | Controls (n = 41) | AIH patients (n = 36) | Cirrhosis (n = 10) | No cirrhosis (n = 26) | Non-responders to treatment (n = 10) (4 patients with cirrhosis) | Responders to treatment (n = 23) (6 patients with cirrhosis) |
|---|---|---|---|---|---|---|
| 8-Iso ng/mg creatinine (U) | 1 (0.02–3) | 1.3*** (0.01–8.5) | 2.6** (0.4–8.5) | 0.9 (0.01–5) | 0.9 (0.01–8.5) | 1.2 (0.4–4.2) |
| Aldehydes μM (P) | 0.3 (0.05–4.7) | 1.7**** (0.2–7.9) | 3**** (0.18–7.8) | 1.7**** (0.17–6.6) | 1.7*** (0.2–7.9) | 0.8**** (0.2–7) |
| 3-NT nM (P) | 9 (1.5–20) | 12*** (1.6–100) | 12 (2.3–48) | 15 (1.64–100) | 6.5 (2.1–100) | 12 (1.6–48) |
| PC mg/mg protein (S) | 1.8 (0.1–9.9) | 1.8* (0.1–14) | 3.8*** (0.6–15) | 1.5+ (0.15–6.8) | 6.7** (0.14–14) | 1.9 (0.5–6.3) |
| MPO U/106cells (l) | 12 (1–141) | 10.5 (1.2–463) | 11.5 (1.5–87) | 10.5 (1.2–462) | 9.7 (1.2–463) | 12 (1.5–160) |
| GSSG/(GSH + GSSG) (WB) | 4.3 (0.2–15) | 9.5** (0.1–93) | 8.6 (1.4–85) | 12.4 (0.1–93) | 6.7** (0.3–93) | 7.9** (0.1–85) |
| GSH μM (WB) | 1135 (293–5409) | 512*** (51–5541) | 293**** (51–5541) | 511***/++ (88–2977) | 519**** (88–5541) | 800** (51–1033) |
| Catalase kU/gHb (RC) | 146 (40–449) | 173 (5–772) | 147 (5–320) | 215** (22–772) | 236*** (5–772) | 148 (17–409) |
| SOD kU/gHb (RC) | 44 (8–68) | 48 (13–59) | 37 (16–59) | 47 (13–59) | 24 (13–59) | 45 (13–59) |
| GSH-Px U/gHb (RC) | 3.6 (0.3–25) | 8.6** (0.6–36) | 7 (2.2–32) | 8.8** (0.6–35) | 8.9** (1.6–36) | 8.9*** (0.6–33) |
| Glutathione reductase U/gHb (RC) | 2.4 (0.1–10) | 2.1 (0.08–36) | 2.2 (0.08–36) | 2.3 (0.08–8.9) | 2.6 (0.1–36) | 2.3 (0.2–8) |
| AST U/l (S) | 20 (12–30) | 25*** (10–117) | 29* (10–65) | 25** (10–117) | 52*** (18–117) | 23 (10–49) |
| ALT U/l (S) | 18 (9–29) | 25*** (10–160) | 23** (10–81) | 26*** (12–160) | 56 **** (20–160) | 20 (10–43) |
| ALP U/l (S) | 61 (32–91) | 61 (26–320)* | 64 (49–136)* | 60 (26–320)* | 106*** (30–300) | 61 (26–116) |
| γ-GT U/l (S) | 13 (5.2–27) | 22.5**** (8–277) | 21** (8–170) | 23**** (8–277) | 35**** (12–240) | 15 (8–69) |
| Bilirubin mg/dl (S) | 0.5 (0.1–1) | 0.7*** (0.3–1.96) | 1*** (0.3–1.96) | 0.6** (0.2–1.32) | 0.68*** (0.3–1.96) | 0.5 (0.3–1) |
| Prednisolone (duration of intake in months) | 30 (1–114) | 54 (11–101) | 13 (1–114) | 16 (1–58) | 36 (3–114) | |
| MMF (duration of intake in months) | 26 (1–94) | 41 (11–84) | 16 (1–94) | 16 (1–60) | 30 (3–94) |
Values shown as medians with range. P values (<0.05) derived by the Mann–Whitney U test (*vs. control group, +vs. cirrhotic group).
*0.1 > P > 0.05, **P ≤ 0.05, ***/+P ≤ 0.01, ****/++P ≤ 0.001. GSSG/(GSH + GSSG) was expressed as %.
U, urine; P, plasma; S, serum; WB, whole blood; L, leucocytes; RC, red blood cells; MMF, mycophenolate mofetil.
Oxidative stress in the AC group
Patients with AC had significantly increased levels of 8-iso (P < 0.0001), aldehydes (P = 0.005), 3-NT (P = 0.05), PC (P = 0.0009), GSSG/total GSH ratio (P = 0.002), and superoxide dismutase (SOD) activity (P = 0.01) compared to controls while whole blood GSH was significantly reduced (P ≤ 0.0001) (Table 2).
Oxidative stress in relation to cirrhosis in AC patients
8-Iso and PC levels were significantly increased in both cirrhotic (n = 6, P = 0.01 and P < 0.0001, respectively) and non-cirrhotic patients (n = 43, P < 0.001 and P < 0.0001, respectively) compared to controls (Table 2). GSSG/total GSH ratio was also significantly higher in cirrhotics (P = 0.05), while there was no difference between non-cirrhotics and controls (Table 2). In contrast, GSH levels were significantly decreased both in patients with cirrhosis and non-cirrhosis compared to controls (P = 0.001 and P = 0.01, respectively; Table 2).
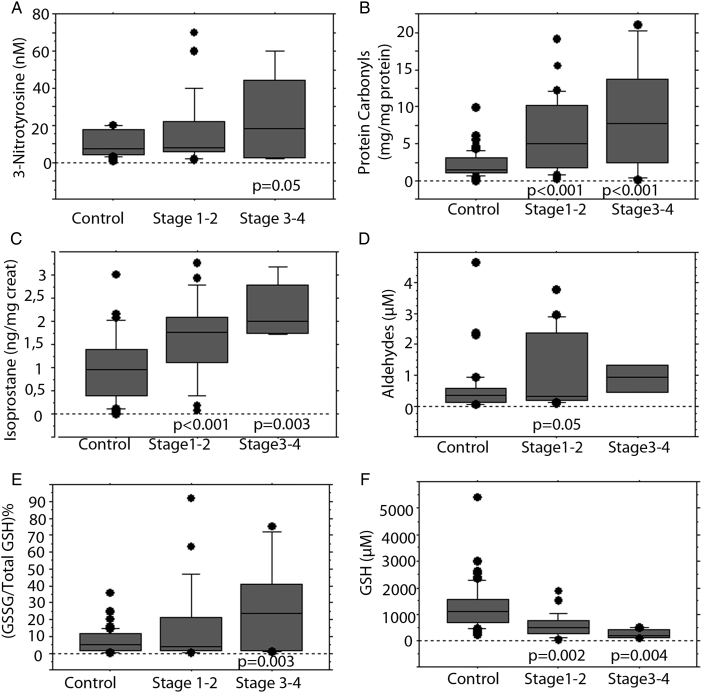
AC patients had a gradual increase of 3-NT, PC, 8-iso, aldehydes, and the GSSG/total GSH ratio from minimal–mild fibrosis (stages 1–2, n = 36) to moderate–severe fibrosis (stages 3–4, n = 8) (Fig. 1A–E). 8-Iso and PC levels were significantly increased in all stages compared to controls (Fig. 1B and C).
Figure 1.
Markers of oxidative stress in controls and AC patients according to Ludwig's stage. Levels of 3-NT (A), PC (B), isoprostane (C), aldehydes (D), oxidized/total GSH ratio (E), and reduced glutathione (F). The results are expressed as median, interquartile range, and range. P values refer to the comparison between each stage to controls (Mann–Whitney U test).
In contrast, AC patients showed a gradual decrease of GSH from stage 1–2 to stage 3–4 compared to controls (P = 0.002 and P = 0.004, respectively; Fig. 1F). Apart from PC levels (P = 0.03), no other statistical significant difference was observed between cirrhotic and non-cirrhotic AC patients (Table 2).
Oxidative stress in AC patients according to response to treatment
There were no significant differences regarding oxidative stress markers and antioxidant activities between treatment-naïve patients and those receiving UDCA at the time of investigation, as well as between AC patients with response to UDCA treatment (n = 31), compared to those not responding to UDCA (n = 9), although treatment-naïve and treated patients, as well as responders and non-responders, had statistically significant differences compared to controls (Table 2).
Oxidative stress in the AIH group
Patients with AIH had significantly increased levels of 8-iso (P = 0.009), aldehydes (P ≤ 0.0001), 3-NT (P = 0.004), GSSG/total GSH ratio (P = 0.03), and GSH-Px activity (P = 0.02) compared to controls while whole blood GSH was significantly reduced (P = 0.01) (Table 3).
Oxidative stress in relation to cirrhosis in AIH patients
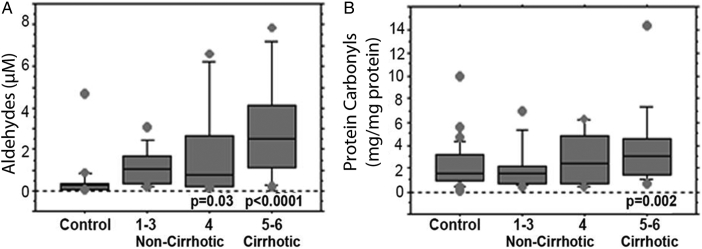
Aldehyde levels were significantly increased in both cirrhotic (n = 10, P < 0.0001) and non-cirrhotic patients (n = 26, P < 0.0001) compared to controls, whereas 8-iso and PC levels were significantly elevated only in cirrhotics compared to controls (P = 0.05 and P = 0.002) (Table 3). In addition, PC levels were significantly higher in cirrhotics compared to non-cirrhotic patients (P = 0.01, Table 3). Αnalysing cirrhotic patients according to the presence of biochemical activity, only patients with biochemical activity showed significant elevation of 8-iso compared to controls (2.8 ng/mg creatinine (1.5–5.8) vs. 1 ng/mg creatinine (0.02–3), P = 0.03). GSH levels were significantly reduced in cirrhotic compared to non-cirrhotic patients (P = 0.0003) but were abnormal in both cirrhotic and non-cirrhotic patients (P < 0.0001 and P = 0.01 respectively; Table 3).
AIH patients had a gradual increase of aldehyde and PC levels from mild to severe fibrosis (Fig. 2A and B). Actually, aldehyde levels were significantly elevated in patients with moderate (0.8 µM (0.2–6.6)) and severe fibrosis (3 µΜ (0.2–7.8)) compared to controls (P = 0.03 and P < 0.0001, respectively; Fig. 2A), whereas PC levels were significantly higher only between AIH patients with severe fibrosis and controls (3.8 mg/mg protein (0.6–15) vs. 1.8 mg/mg protein (0.1–9.9); P = 0.002) (Fig. 2B). Furthermore, GSH-Px and CAT activities were significantly increased in non-cirrhotic patients with AIH compared to controls (P = 0.02 and P = 0.04, respectively; Table 3).
Figure 2.
Products of oxidative stress injury products in controls and AIH patients according to the stage of fibrosis. Products of lipid (A) and protein (B) oxidation. The results are expressed as median, interquartile range, and range. P values refer to the comparison between each stage to controls (Mann–Whitney U test).
Oxidative stress in AIH patients in relation to response to treatment
AIH patients not responding to immunosuppression (n = 10) had significantly increased aldehyde (P = 0.006), PC levels (P = 0.05), GSSG/total GSH ratio (P = 0.05), CAT (P = 0.008), and GSH-Px activities (P = 0.05) and lower GSH levels (P = 0.0006) compared to controls (Table 3). AIH patients responding to immunosuppressive treatment (n = 23) had significantly higher levels of aldehyde (P ≤ 0.001), GSH-Px (P = 0.003), and GSSG/total GSH ratio (P ≤ 0.05) and decreased GSH levels (P ≤ 0.05) compared to controls (Table 3). Differences in oxidative stress products and markers of antioxidant defence were evident between responders and non-responder AIH patients, though did not reach statistical significance (Table 3).
Discussion
Three major findings emerge from the present study. First, oxidative stress is a significant feature of autoimmune liver diseases and is evident from early stages of both diseases. Second, disturbances in antioxidant defence are involved in the progress of both diseases contributing probably to progression towards cirrhosis. Third, immunosuppression can ameliorate protein oxidation, by reducing PC levels.
To our knowledge, this is the first study to evaluate lipid peroxidation products, protein oxidation markers, and MPO concurrent with enzymatic and non-enzymatic antioxidant activities in patients with autoimmune liver diseases. Previous work in patients with autoimmune liver diseases has dealt with the determination of individual parameters of oxidative stress, including GSH status, lipid peroxidation products, or intrahepatic 3-NT accumulation.8,24–26 PC levels are reported for the first time in the literature.
Our results demonstrate a marked increase of lipid peroxidation (8-iso and aldehydes) and protein oxidation (3-NT and PC) products in AIH and AC patients compared to healthy. In our AC patients, we observed a significant augmentation of 8-iso with progression of fibrosis, as assessed by Ludwig's score. Our data complement previous work26,27 and demonstrate increased lipid peroxidation to be an early event in the disease progress and probably not just a consequence of the disease. This hypothesis is further supported by Paradis et al.,27 who provided immunohistochemical evidence of 4-HNE adducts in normal bile ducts with no detectable features of cell death. Reactive oxygen species (ROS) generation by inflammatory cells, accumulation of cytotoxic bile acids and intrahepatic copper are some of the mechanisms that contribute to lipid peroxidation induction in PBC.28
Similarly, PC levels increased with progression of liver disease. PC was the only marker of oxidative stress that differed significantly between cirrhotic and non-cirrhotic AC patients, being higher in the former group. The use of PC is regarded as more advantageous oxidative stress marker compared to lipid peroxidation products, taking into account that they are more stable compared to other oxidative stress biomarkers and are an index of severe oxidative protein damage.29
Moreover, similar to others24 we observed a significant decrease in total GSH levels in AC patients compared to controls. Reduced intracellular glutathione levels were linked to increased perinuclear expression of 4-HNE in the damaged bile ducts of PBC patients.30 Of note, we showed a gradual decrease of total GSH levels and a gradual increase of 8-iso levels with deterioration of fibrosis stage in AC, suggesting that reduced intrahepatic glutathione reserves might contribute to increased lipid peroxidation which stimulates collagen synthesis.31 GSSG/total GSH ratio was also significantly increased only in cirrhotics with AC compared to controls, indicating a defective glutathione redox state.
Of interest, among enzymatic antioxidants, only SOD was increased in AC patients and particularly in early disease stage compared to controls. This may indicate overexpression of enzymatic activity at early stages to counteract for the increased burden of ROS production. Decreased or unaltered levels of antioxidants at advanced stages of disease could be explained by consumption of antioxidant elements necessary for neutralizing the oxidative damage. Changes in enzymatic antioxidants develop in situ and this could probably explain the fact that no significant changes were noted in other antioxidant enzymes.
So far, UDCA is the only approved treatment for PBC.2 UDCA has been shown to have a hepatoprotective effect mainly via up-regulation of γ-glutamyl-cysteine-synthetase expression, which leads to increased rate of GSH synthesis.32 In our study, UDCA treatment was associated with a relative increase of GSH levels compared to non-treated patients, even though the difference was not significant. UDCA failed to improve any parameter of oxidative stress, as demonstrated also by others.33
Data on parameters of oxidative stress in AIH are scarce. We have found increased levels of lipid peroxidation products and PC in AIH patients compared to controls. Similar to AC patients, there was a significant correlation between both lipid peroxidation products and PC levels with fibrosis stage, suggesting that increased levels of oxidant stress may contribute to perpetuation of liver injury in AIH. These data are in agreement with Pemberton et al.8 reporting increased levels of lipid peroxidation that correlated significantly with the necroinflammatory activity and fibrosis score. In a previous study, Sanz-Cameno et al.25 revealed enhanced NT accumulation in AIH patients and provided evidence of significant correlation with severity of liver damage. Even though 3-NT was increased in all AIH and AC patients, we could not find significant changes in the blood between different stages of liver disease probably due to small number of patients in each stage of liver injury. Similar to patients with AC, PC was the only marker that was significantly elevated in cirrhotics compared to non-cirrhotic AIH patients. This association is of immense importance since published data have pointed towards a link between oxidative and nitrosative stress and induction/exaggeration of autoimmunity, as covalent binding of lipid peroxidation products with proteins can lead to formation of neoantigens.34,35 In parallel, AIH patients with cirrhosis had significantly lower GSH levels compared to non-cirrhotics, suggesting a major contribution of depletion of antioxidant reserves in disease progression.
Of note, comparison between AC and AIH groups with chronic hepatitis B patients revealed significantly higher NT levels in autoimmune liver disease groups (Supplementary Material 1). Whether changes in individual parameters of oxidative stress are disease specific needs further investigation in larger studies and it is not within the aim of the present study.
Systemic use of corticosteroids alone or in conjunction with azathioprine or MMF is considered the mainstay of treatment for patients with AIH.12,13 The anti-inflammatory action of corticosteroids depends partially on interference with ROS formation.36 We have demonstrated lower PC and CAT levels, higher median GSH levels in AIH patients responding to immunosuppressive treatment compared to non-responders. Similar data have been reported in patients with infiltrative Grave's ophthalmopathy.37 Our data suggest that corticosteroids were more effective than UDCA in ameliorating protein oxidation products.
Our data suggest that increased levels of oxidant stress in AC and AIH patients might correspond to liver inflammatory activity and fibrosis in both diseases. In this context, though speculative at present, our results may be of value in order to design future antioxidant therapies that can be used as complementary treatments, mainly for non-responder patients to immunosuppressive regimens. In addition, studies in larger cohort of patients may help to identify individual parameters of oxidative stress or antioxidant protection mechanisms that could serve as markers of treatment response but this needs external validation.
Disclaimer statements
Contributors GND and ANM had the original idea for the study, designed the study protocol and wrote the paper. ETK in collaboration with ANM did the whole laboratory work and along with EIR collected all the demographic and clinical data and performed the statistical analysis and wrote the first draft of the manuscript. GKK did the interpretation of the histological data of the patients. EIR and GND treated the patients and performed the follow-up assessment while contributed to the final version of the paper. GKK and GND wrote the final version of the paper. All authors have seen and approved the final draft of the paper.
Funding Parts of this work has been supported by the Hellenic Ministry of Education and Religious Affairs (Code: Archimidis) and the Research Committee of the University of Thessaly (Code No: 2466).
Conflict of interest None.
Ethics approval The study was approved by the Ethical Committee of the University of Thessaly, Medical School.
Acknowledgements
We thank Kostas Galanis for assistance in blood collection.
References
- 1.Zachou K, Muratori P, Koukoulis GK, Granito A, Gatselis N, Fabbri A,. et al. Review article: autoimmune hepatitis – its aetiopathogenesis, clinical features, diagnosis and management. Aliment Pharmacol Ther 2013;38:887–913. [DOI] [PubMed] [Google Scholar]
- 2.Lindor KD, Gershwin ME, Poupon R, Kaplan M, Bergasa NV, Heathcote EJ; American Association for Study of Liver Diseases . Primary biliary cirrhosis. Hepatology 2009;50:291–308. [DOI] [PubMed] [Google Scholar]
- 3.Chapman R, Fevery J, Kalloo A, Nagorney DM, Boberg KM, Shneider B,. et al.; American Association for the Study of Liver Diseases Diagnosis and management of primary sclerosing cholangitis. Hepatology 2010;51:660–78. [DOI] [PubMed] [Google Scholar]
- 4.Ljubuncic P, Tann Z, Bomzon A. Evidence of a systemic phenomenon for oxidative stress in cholestatic liver disease. Gut 2000;47:710–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jain SK, Pemberton PW, Smith A, McMahon RF, Burrows PC, Aboutwerat A,. et al. Oxidative stress in chronic hepatitis C: not just a feature of late stage disease. J Hepatol 2002;36:805–11. [DOI] [PubMed] [Google Scholar]
- 6.Albano E. Oxidative mechanisms in the pathogenesis of alcoholic liver disease. Mol Aspects Med 2008;29:9–16. [DOI] [PubMed] [Google Scholar]
- 7.Chalasani N, Deeg MA, Crabb DW. Systemic levels of lipid peroxidation and its metabolic and dietary correlates in patients with nonalcoholic steatohepatitis. Am J Gastroenterol 2004;99:1497–502. [DOI] [PubMed] [Google Scholar]
- 8.Pemberton PW, Aboutwerat A, Smith A, Burrows PC, McMahon RF, Warnes TW. Oxidant stress in type I autoimmune hepatitis: the link between necroinflammation and fibrogenesis? Biochim Biophys Acta 2004;1689:182–9. [DOI] [PubMed] [Google Scholar]
- 9.Wu CT, Eiserich JP, Ansari AA, Coppel RL, Balasubramanian S, Bowlus CL,. et al. Myeloperoxidase-positive inflammatory cells participate in bile duct damage in primary biliary cirrhosis through nitric oxide-mediated reactions. Hepatology 2003;38:1018–25. [DOI] [PubMed] [Google Scholar]
- 10.Ludwig J, Dickson ER, McDonald GS. Staging of chronic nonsuppurative destructive cholangitis (syndrome of primary biliary cirrhosis). Virchows Arch 1978;379:103–12. [DOI] [PubMed] [Google Scholar]
- 11.Hennes EM, Zeniya M, Czaja AJ, Pares A, Dalekos GN, Krawitt EL,. et al. Simplified criteria for the diagnosis of autoimmune hepatitis. Hepatology 2008;48:169–76. [DOI] [PubMed] [Google Scholar]
- 12.Zachou K, Gatselis N, Papadamou G, Rigopoulou EI, Dalekos GN. Mycophenolate for the treatment of autoimmune hepatitis: prospective assessment of its efficacy and safety for induction and maintenance of remission in a large cohort of treatment-naïve patients. J Hepatol 2011;55:636–46. [DOI] [PubMed] [Google Scholar]
- 13.Manns MP, Czaja AJ, Gorham JD, Krawitt EL, Mieli-Vergani G, Vergani D,. et al. Diagnosis and management of autoimmune hepatitis. Hepatology 2010;51:1–31. [DOI] [PubMed] [Google Scholar]
- 14.Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F,. et al. Histological grading and staging of chronic hepatitis. J Hepatol 1995;22:696–9. [DOI] [PubMed] [Google Scholar]
- 15.Cooray R, Petersson CG, Holmberg O. Isolation and purification of bovine myeloperoxidase from neutrophil granules. Vet Immunol Immunopathol 1993;38:261–72. [DOI] [PubMed] [Google Scholar]
- 16.McCord JM, Fridovich I. Superoxide dismutase. An enzymatic function for erythrocuprein (hemocuprein). J Biol Chem 1969;244:6049–55. [PubMed] [Google Scholar]
- 17.Goldberg DM, Spooner RJ. Assay of glutathione reductase. In: Bergmeyen HV. (ed.) Methods of enzymatic analysis. 3rd ed; 1983. Verlag Chemie, Deerfield Beach, FL, USA, p. 258–65. [Google Scholar]
- 18.Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 1967;70:158–69. [PubMed] [Google Scholar]
- 19.Aeby H. Catalase in vitro. Methods Enzymol 1984;105:121–6. [DOI] [PubMed] [Google Scholar]
- 20.Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem 1969;27:502–22. [DOI] [PubMed] [Google Scholar]
- 21.Levine RL, Williams J, Stadtman ER, Shacter E. Carbonyl assays for determination of oxidatively modified proteins. Methods Enzymol 1994;233:346–57. [DOI] [PubMed] [Google Scholar]
- 22.Gérard-Monnier D, Erdelmeier I, Régnard K, Moze-Henry N, Yadan JC, Chaudière J. Reactions of 1-methyl-2-phenylindole with malondialdehyde and 4-hydroxyalkenals. Analytical applications to a colorimetric assay of lipid peroxidation. Chem Res Toxicol 1998;11:1176–83. [DOI] [PubMed] [Google Scholar]
- 23.Marquez LA, Dunford HB. Mechanism of the oxidation of 3,5,3′,5′-tetramethylbenzidine by myeloperoxidase determined by transient- and steady-state kinetics. Biochemistry 1997;36:9349–55. [DOI] [PubMed] [Google Scholar]
- 24.Aboutwerat A, Pemberton PW, Smith A, Burrows PC, McMahon RF, Jain SK,. et al. Oxidant stress is a significant feature of primary biliary cirrhosis. Biochim Biophys Acta 2003;1637:142–50. [DOI] [PubMed] [Google Scholar]
- 25.Sanz-Cameno P, Medina J, García-Buey L, García-Sánchez A, Borque MJ, Martín-Vílchez S,. et al. Enhanced intrahepatic inducible nitric oxide synthase expression and nitrotyrosine accumulation in primary biliary cirrhosis and autoimmune hepatitis. J Hepatol 2002;37:723–9. [DOI] [PubMed] [Google Scholar]
- 26.Kawamura K, Kobayashi Y, Kageyama F, Kawasaki T, Nagasawa M, Toyokuni S,. et al. Enhanced hepatic lipid peroxidation in patients with primary biliary cirrhosis. Am J Gastroenterol 2000;95:3596–601. [DOI] [PubMed] [Google Scholar]
- 27.Paradis V, Kollinger M, Fabre M, Holstege A, Poynard T, Bedossa P. In situ detection of lipid peroxidation by-products in chronic liver diseases. Hepatology 1997;26:135–42. [DOI] [PubMed] [Google Scholar]
- 28.Sokol RJ, Winklhofer-Roob BM, Devereaux MW, McKim JM Jr. Generation of hydroperoxides in isolated rat hepatocytes and hepatic mitochondria exposed to hydrophobic bile acids. Gastroenterology 1995;109:1249–56. [DOI] [PubMed] [Google Scholar]
- 29.Dalle-Donne I, Rossi R, Giustarini D, Milzani A, Colombo R. Protein carbonyl groups as biomarkers of oxidative stress. Clin Chim Acta 2003;329:23–38. [DOI] [PubMed] [Google Scholar]
- 30.Tsuneyama K, Harada K, Kono N, Sasaki M, Saito T, Gershwin ME,. et al. Damaged interlobular bile ducts in primary biliary cirrhosis show reduced expression of glutathione-S-transferase-pi and aberrant expression of 4-hydroxynonenal. J Hepatol 2002;37:176–83. [DOI] [PubMed] [Google Scholar]
- 31.Comporti M, Signorini C, Arezzini B, Vecchio D, Monaco B, Gardi C. F2-Isoprostanes are not just markers of oxidative stress. Free Radic Biol Med 2008;44:247–56. [DOI] [PubMed] [Google Scholar]
- 32.Serviddio G, Pereda J, Pallardó FV, Carretero J, Borras C, Cutrin J,. et al. Ursodeoxycholic acid protects against secondary biliary cirrhosis in rats by preventing mitochondrial oxidative stress. Hepatology 2004;39:711–20. [DOI] [PubMed] [Google Scholar]
- 33.Pemberton PW, Aboutwerat A, Smith A, Warnes TW. Ursodeoxycholic acid in primary biliary cirrhosis improves glutathione status but fails to reduce lipid peroxidation. Redox Rep 2006;11:117–23. [DOI] [PubMed] [Google Scholar]
- 34.Wang G, Cai P, Ansari GA, Khan MF. Oxidative and nitrosative stress in trichloroethene-mediated autoimmune response. Toxicology 2007;229:186–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang G, Wang J, Ma H, Khan MF. Increased nitration and carbonylation of proteins in MRL +/+ mice exposed to trichloroethene: potential role of protein oxidation in autoimmunity. Toxicol Appl Pharmacol 2009;237:188–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Das UN, Mohan IK, Raju TR. Effect of corticosteroids and eicosapentaenoic acid/docosahexaenoic acid on pro-oxidant and anti-oxidant status and metabolism of essential fatty acids in patients with glomerular disorders. Prostaglandins Leukot Essent Fatty Acids 2001;65:197–203. [DOI] [PubMed] [Google Scholar]
- 37.Bednarek J, Wysocki H, Sowiński J. Peripheral parameters of oxidative stress in patients with infiltrative Graves' ophthalmopathy treated with corticosteroids. Immunol Lett 2004;93:227–32. [DOI] [PubMed] [Google Scholar]