Abstract
Antioxidant activity and hepatoprotective properties of the aqueous extract and tetrahydrofuran-extracted phenolic fractions of Halimeda opuntia (Linnaeus) Lamouroux were investigated in rats with chemically induced liver injury. Total polyphenols were determined by using the Folin–Ciocalteau reagent. Liver damage was induced by CCl4 and assessed by a histological technique. Reverse transcription/polymerase chain reaction (RT/PCR) analysis showed increased superoxide dismutase (SOD) and catalase (CAT) gene expression and activities in the group treated with free phenolic acid (FPA) fractions of H. opuntia, suggesting inducing effects on both enzymes. In addition, rats treated with FPA fractions displayed lower liver thiobarbituric acid reactive substance (TBARS) levels than those observed for rats in the CCl4-treated group. These data suggest that the phenolic fractions from H. opuntia may protect the liver against oxidative stress-inducing effects of chemicals by modulating its antioxidant enzymes and oxidative status.
Keywords: Seaweed, Natural antioxidants, Halimeda opuntia, Hepatoprotection
Introduction
Reactive oxygen species (ROS) are by-products of cellular metabolism. However, overproduction of ROS leads to oxidation of biomolecules and consequent cell damage. Antioxidants can alleviate the noxious effects of in vivo oxidative stress1 through inhibiting the generation of ROS, directly scavenging free radicals or increasing the expression of the genes encoding the antioxidant enzymes involved in the elimination of ROS, such as superoxide dismutase (SOD), catalase, and glutathione peroxidase (GPx).2,3
Seaweeds are commonly consumed as vegetables in Asia and other western countries and have also been used as traditional medicines for the treatment of several conditions.4,5 Indeed, seaweeds are rich in bioactive compounds that exhibit several phytotherapeutic properties and have excellent potential as functional food ingredients for reducing the incidence of chronic diseases.6
In the waters of the Caribbean, seaweeds are exposed to sunlight and oxygen, which leads to the formation of ROS. However, the absence of oxidative damage in their structural and functional components suggests that they have an efficient antioxidant defense system. For this reason, several seaweed extracts have attracted increasing scientific interest.7
Significant antioxidant activity of seaweed extracts has been observed in vitro and in animal models, indicating great potential for phytotherapeutic, nutraceutical, or both applications. Of the numerous compounds exhibiting these properties, polyphenols are particularly interesting as they can display antioxidant activity at low concentrations.8,9
The green seaweeds of the Halimeda opuntia species occur widely, mainly in shallow waters of tropical regions. They are harmless, very easy to collect, and a potential source of phytomedicines. Halimeda spp. have been investigated for different medicinal properties10–16 including antioxidant activity.17,18 Animal studies carried out in our laboratory have shown that Halimeda incrassata effectively attenuates oxidative stress in cells by scavenging free radicals, inhibiting lipoperoxidation and exhibiting neuroprotective and hepatoprotective activities.19–21 The antioxidant activity of Halimeda spp. has been attributed to the presence of polyphenolic compounds such as flavonoids, phenolic, and cinnamic acids.22–24
In view of these considerations, the aim of this paper was to investigate the antioxidant and hepatoprotective effects of polyphenol-rich fractions from seaweed H. opuntia (Linnaeus) Lamouroux on acute liver damage induced by CCl4 in Wistar rats. The activity of hepatic antioxidant enzymes and their gene expression levels were determined and used to assess these effects.
Materials and methods
Seaweed collection and preparation of hydrophilic fractions
H. opuntia (Linnaeus) Lamouroux was collected in December 2010 in Bajo de Santa Ana, Havana City, Cuba. Specimens were identified and a voucher was deposited for future reference at the Seaweeds Laboratory at the Marine Research Center of the University of Havana.
Freshly collected specimens were washed with distilled water and dried at room temperature (26°C) for 7–10 days. After milling and sieving, the dry powder was extracted with distilled water (1:5 w/v) at room temperature (±22°C) and centrifuged at 800 × g and 4°C for 20 minutes. The supernatant was collected, lyophilized, and kept at −20°C until time of analysis. The yield of extraction (% w/w of seaweed on a dry weight basis) was 4.3%. Polyphenols were extracted according Krygier et al.24 Free phenolic acids (FPAs) were extracted by suspending dry seaweed in tetrahydrofuran. Total phenolic content was determined as in Vidal et al.23 and expressed as milligrams of gallic acid equivalents (GAE)/g dry seaweed.
In vitro antioxidant activity
Reducing power
Reducing power was determined by a slightly modified method described by Oyaizu.25 Absorbance was measured against a water blank at 700 nm on a VIS-723 G spectrophotometer (Rayleigh, Beijing, China). Absorbance increments were directly proportional to reducing power increments. Reducing power activity was expressed as ascorbic acid equivalents. One equivalent corresponds to 20 µg of ascorbic acid with an absorbance of 0.139.
2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging assay
The free radical scavenging activity of the H. opuntia extract was determined using the DPPH assay.26 Aliquots of the extract (10–40 µg of GAE) were suspended in a methanolic solution of DPPH (60 µM). After 30 minutes, the absorbance was measured against a blank solution at 517 nm. Radical scavenging activity was calculated as follows:
where Asample is the absorbance of the sample containing the extract and Ablank is the absorbance of the blank solution.
Animals
Male Wistar rats, weighing between 120 and 150 g, were provided by the University of São Paulo Vivarium, and were housed in boxes (six rats each) in a room with controlled lighting (12-hour light/dark cycle) at 25°C and 60% humidity. The rats had free access to water and to a standard food diet according to the Guidelines for Laboratory Animal Care and Use. Animal studies were approved by the Animal Experimentation Ethics Committee of the Faculty of Pharmaceutical Sciences of the University of São Paulo, Brazil.
Animal treatment schedule
Hepatic injury was induced in rats by intraperitoneal administration of a single dose of 3 ml of CCl4 (1:1 in olive oil). Gallic acid (GA), a known antioxidant compound, was used as reference.
The animals were grouped as follows:
Control: treated daily with vehicle (1.0 ml, per oral (p.o.)) for 20 days.
CCl4: treated daily with vehicle (1.0 ml, p.o.) for 20 days, followed by treatment with CCl4 on day 21.
GA: treated daily with GA (100 mg/kg, p.o.) for 20 days, followed by treatment with CCl4 on day 21.
Ho 20: treated daily with H. opuntia-FPA (20 mg/kg, p.o.) for 20 days, followed by treatment with CCl4 on day 21.
Ho 80: treated daily with H. opuntia-FPA (80 mg/kg, p.o.) for 20 days, followed by treatment with CCl4 on day 21.
At the end of the treatment, blood and liver samples of each animal were collected. Serum was separated and assayed for thiobarbituric acid reactive substance (TBARS) levels. Liver homogenates were prepared and assayed for TBARS and antioxidant enzyme activity.
TBARS assay
TBARS levels, as a marker of lipid peroxidation, were measured in liver homogenates and serum using the method of Ohkawa et al.27 The results were expressed as nanomoles per milligram of protein.
SOD determination
The cytoplasmic SOD activity was determined according to McCord and Fridovich28 using a reaction mixture containing cytochrome C (100 mM), xanthine (500 mM), ethylenediaminetetraacetic acid (1 mM), and KCN (200 mM) and potassium phosphate buffer (0.05 M – pH 7.8). The results were expressed as units per milligram of protein. One unit of SOD activity was defined as the amount of enzyme required to inhibit the reaction rate by 50% at 25°C and pH 7.8.
Catalase activity (CAT) determination
The CAT activity was determined as described by Beutler.29 The method is based on the decrease in optical density at 230 nm (molar extinction coefficient – 0.071/mM/ cm) as a result of the decomposition of hydrogen peroxide by catalase at 37°C. The results were expressed as units per milligram of protein. One unit of CAT activity was defined as the amount of enzyme required to hydrolyze 1 mol of hydrogen peroxide per minute at 37°C and pH 8.0.
GPx determination
The GPx activity in the cytosolic fraction was determined by Sies.30 One unit of enzyme activity was defined as the amount of enzyme required to oxidize 1 µmol of NADPH per minute at 30°C at pH 7.0.
Reverse transcription/polymerase chain reaction (RT/PCR)
RNA extraction: CAT and SOD gene expression evaluation
RNA was extracted by mixing 100 mg of rat liver and 1000 µl of trizol (Invitrogen, New York City, New York). After the addition of 200 µl of chloroform (Merck, Darmstadt, Hessen, Germany), vortex mixing for 15 seconds, incubation at room temprature for 5 minutes, and centrifugation at 12 000 × g and 4°C for 15 minutes, the supernatant (400 µl) was collected, avoiding the interphase, and mixed with 500 µl of isopropanol by vortexing for 5 seconds. It was then centrifuged at 12 000 × g and 4°C for 5 minutes and the supernatant was discarded. The resulting pellet was washed with 1 ml of ethanol (75%), gently vortex-mixed and centrifuged at 7500 × g and 4°C for 10 minutes. The supernatant was discarded again. The pellet was resuspended in 20 µl of RNAse-free distilled water, incubated at 50°C for 10 minutes, and stored at −70°C.
Reverse transcription
Five micrograms of RNA was added to 1.0 µl of primer (Cu/Zn SOD or CAT), 1.0 µl of dNTP (10 mM), and 4.0 µl of sterile distilled water. The reaction was started by a heating step at 65°C for 5 minutes and then it was quickly chilled on ice. After adding 4.0 µl of 5X First-Strand Buffer (Invitrogen), 2.0 µl of DTT (0.1 M, Invitrogen), and 1.0 µl of RNAseOUTribonuclease inhibitor (Invitrogen), the mixture was incubated at 37°C for 2 minutes. After that 1.0 µl of M-MLV reverse transcriptase (200 U/μl, Invitrogen) was added and the mixture was incubated at 37°C for 50 minutes. The reaction was stopped by a heating step at 70°C for 15 minutes. The PCR product (cDNA) was stored at −70°C.
PCR amplification
Five microliters of cDNA was amplified in a 50.0-μl reaction mixture containing 5.0 µl of Tris (hydroxymethyl) aminomethane–hydrochloride buffer (20 mM; pH 8.4), KCl (500 mM), 1.5 µl of MgCl2 (50 mM), 1.0 µl of dNTP (10 mM), 35.1 µl of diethyl pyrocarbonate, 1.0 µl of primer (SOD or CAT), and 0.4 µl of Taq polymerase (5 U/μl). After an initial denaturation at 94°C for 3 minutes in a thermal cycler (Bio-Rad, Hercules, California, USA), 35 cycles (at 94°C for 45 seconds, at 55°C for 30 seconds, at 72°C for 1.3 minutes, and 72°C for 10 minutes) were carried out. Finally, the mixture was chilled at 4°C. The PCR amplification products were analyzed by electrophoresis on a 2.0% agarose gel (Sigma, St. Louis, Missouri, USA) at 60 V. The gel was stained with 0.5 µg/ml ethidium bromide, visualized on a fluorescence table (Vilber-Lourmat, Marne-la-Vallée, France), and photographed with a digital camera. CAT-262 bp (C to T) and SOD-242 bp (C to T) were genotyped using the following primers (Promega, Madison, AL, USA):
CAT 1 – 5′-GCG AAT GGA GAG GCA GTG TAC-3′
CAT 2 – 5′-GAG TGA CGT TGT CTT CAT TAG CAC TG-3′
Cu/Zn SOD 1 – 5′-TCT AAG AAA CAT GGC GGT CC-3′
Cu/Zn SOD 2 – 5′-CAG TTAGCA GGC CAGCAG AT-3′
Statistical analysis
All experiments were carried out in triplicate and results were expressed as mean values ± standard deviations and the significance level was set at P < 0.05. In vivo antioxidant activity measurements were compared in terms of mean values using a one-way analysis of variance and the Tukey post-test.
Results and discussion
Antioxidant activity assays
In view of the potential health applications of seaweeds, the genus Halimeda has been investigated for numerous health-beneficial properties, including antioxidant activity.10–16
The total polyphenolic content of the aqueous extract of H. opuntia was 97.2 ± 7.3 µg of GAE/g dry seaweed. Vidal et al.22 reported similar polyphenolic content (74.3 mg of polyphenols/g dry seaweed) in a study on the antioxidant activity of a water-soluble extract of H. opuntia using gas–liquid chromatography. Their research suggests that the antioxidant properties of the seaweed extract are at least partly related to its content of phenolic acids. Yoshie et al.21 have also observed high levels of polyphenols in two Halimeda species.
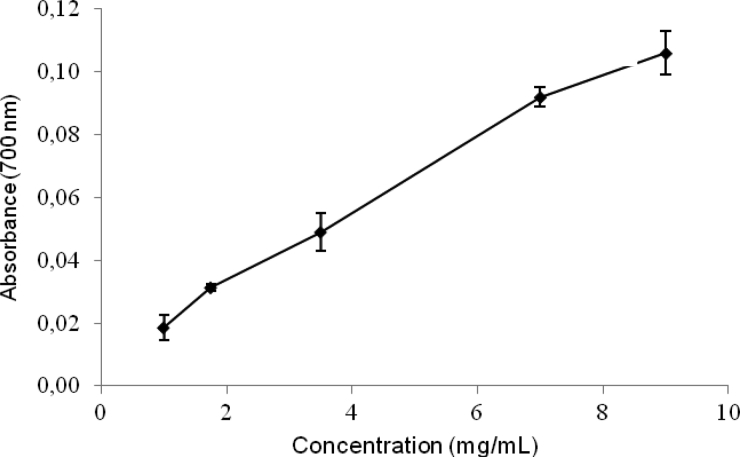
The DPPH scavenging and the reducing power assays are valuable tools for the determination of the antioxidant activity of natural products. Fig. 1 and Table 1 show the reducing power and DPPH results for the aqueous extract of H. opuntia, respectively. Both methods delivered similarly high antioxidant activity results, which are in agreement with those observed for aqueous extracts of other Halimeda species in studies using different methods.18–20 As shown in Fig. 1, the aqueous extract at 1 mg of polyphenols/ml showed a significantly lower absorbance value (0.0185) in comparison with that obtained for the positive control (Optical density (O.D.) 0.139 for 20 µg), suggesting that a pure extract would have displayed a potent antioxidant activity. Our results are consistent with the results of Kuda and Ikemori,31 who observed similar reducing power results for several seaweeds found in Japan, as well as a significant correlation between antioxidant capacity and polyphenolic content.
Figure 1.
Reducing power versus concentration of polyphenols in the aqueous extract of Halimeda opuntia. Results expressed as mean ± standard deviation.
Table 1.
Percentage inhibition of DPPH versus concentration of the aqueous extract of Halimeda opuntia. Results expressed as mean ± standard deviation.
| Concentration (mg/ml) | % Inhibition DPPH |
|---|---|
| 3.0 | 21 ± 2 |
| 4.0 | 27 ± 2 |
| 5.0 | 32 ± 1 |
| 5.5 | 35 ± 1 |
| 6.0 | 39 ± 2 |
| 6.5 | 44 ± 3 |
| 7.0 | 48 ± 1 |
Great antioxidant potential has also been reported in studies using polyphenol-rich fractions of H. incrassata and Halimeda monile.23,32 Zubia et al.17 reported relatively high antioxidant activity of H. monile and Halimeda tuna and found a direct relationship between the content of polyphenols and antioxidant capacity in a study of numerous seaweeds including 17 species of Chlorophyta. In addition, Senevirathne et al.33 investigated polar fractions of Ecklonia cava and observed great reducing ability and significantly high polyphenol content, which are comparable to the results obtained in this study.
Effect of H. opuntia extract on CCl4-induced liver damage in rats
To investigate the hepatoprotective properties of H. opuntia, Wistar rats with CCl4-induced liver injury were treated with a FPA fraction with a total polyphenol content of 5.92 ± 0.85 µg of GAE/g dry seaweed.
We observed that the rats treated with FPA fraction from H. monile or GA proved to be capable of attenuating the changes induced by CCl4.34
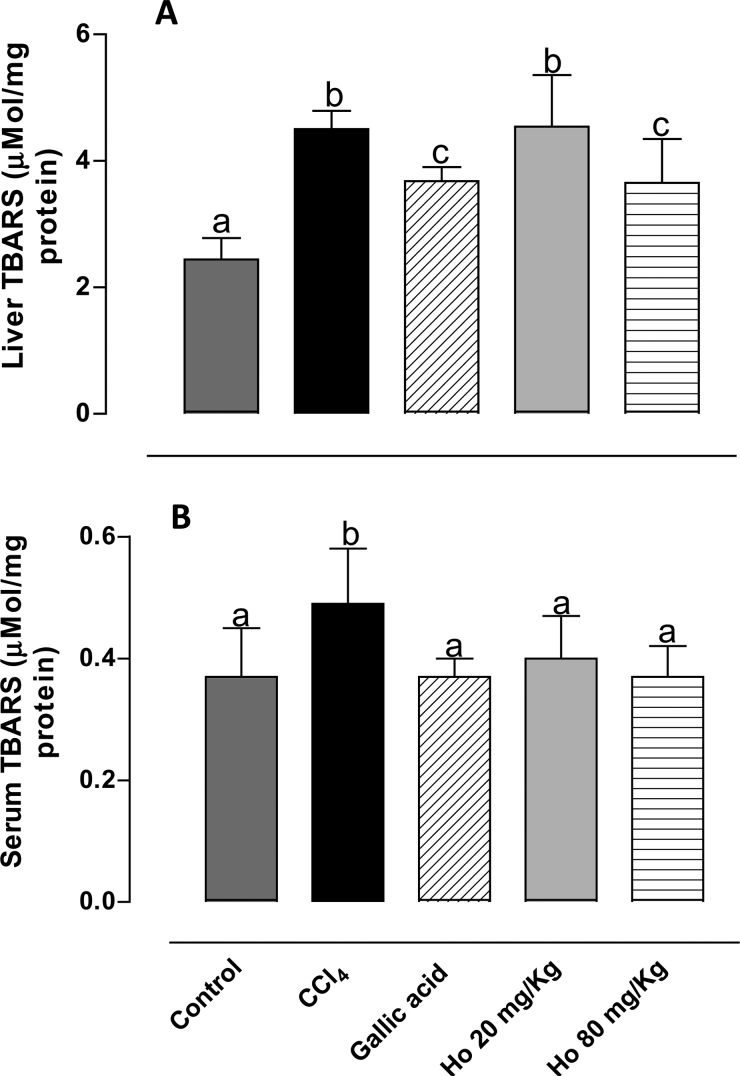
TBARS are produced as a result of lipidic peroxidation. As shown in Fig. 2, TBARS levels in serum and liver tissues in CCl4-treated rats increased, confirming successful induction of oxidative damage. Pre-treatment with H. opuntia (80 mg/kg) led to 20 and 25% reductions in serum and liver TBARS levels, respectively. These results are in agreement with Kim et al.,35 who observed a comparable reduction in hydroperoxide levels in the liver and plasma (30 and 15%, respectively, relative to CCl4-treated group) in a study of rats fed on Saengshik, a non-cooked food containing vegetables and seaweeds. Similarly, a previous study from our laboratory showed that an H. incrassata aqueous extract was effective in reducing TBARS levels by 55% in rats with oxidative stress induced by methylmercury.20
Figure 2.
(A) Liver and (B) serum TBARS levels from control, CCl4-treated, GA-treated, and Halimeda opuntia-treated rats. Different letters indicate statistically significant differences, P < 0.05.
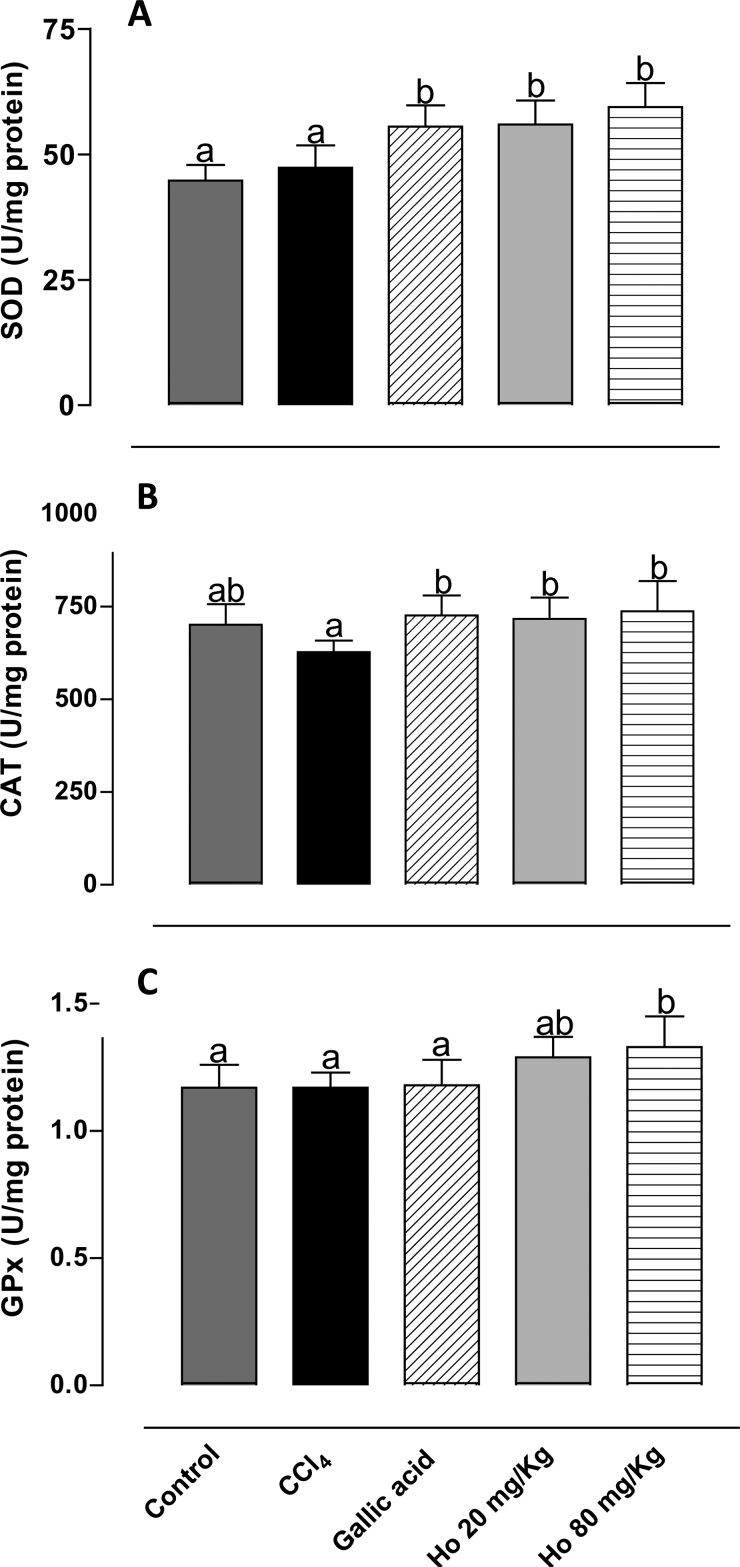
Of the numerous compounds of the antioxidant defense system in mammals, including low-molecular-weight compounds and enzymes, three stand out as most important: CAT, SOD, and GPx. The activity of these enzymes may be altered by CCl4 treatment. Punitha and Rajasekaran36 demonstrated that CCl4 treatment significantly reduced the activity of the antioxidant enzymes. However, Ozturk et al.37 observed in the CCl4-treated group significant increases in kidney SOD and CAT activities. In this study, we also investigated the ability of the H. opuntia extract to induce antioxidant enzyme activity. Fig. 3 shows the effects of different treatments on the activities of CAT, SOD, and GPx. Treatment with the seaweed led to a significant increase in the activity of all enzymes, which in turn resulted in enhanced antioxidant defense.38 These results suggest potent hepatoprotective activity of the phenolic fraction of H. opuntia. Kim et al.35 observed a comparable rise in SOD activity in rats fed on Saengshik for 4 weeks. Mancini-Filho et al.34 reported considerable increase in the activities of SOD and CAT in rats treated with polyphenol-rich fractions (80 mg/kg) of H. monile. Batista-Gonzalez et al.32 reported similar results using four-fold lower doses of E. cava fractions. High antioxidant enzyme activity has been reported through repeated administration of Sargassum extracts.39,40 Treatment with Caulerpa prolifera, Laurencia obtusata, and Porphyra haitanensis extracts also led to a rise in enzyme activity.41,42
Figure 3.
Activity of (A) SOD, (B) CAT, and (C) GPx in liver tissues from control, CCl4-treated, GA-treated, and Halimeda opuntia-treated rats. Different letters indicate statistically significant differences, P < 0.05.
Expression levels of antioxidant enzymes by PCR
The levels of CAT and SOD in liver tissues increased after repeated administration of either the H. opuntia aqueous extract or GA, whereas they decreased after CCl4 induction as can be seen in Fig. 4, which shows alterations in catalase gene expression assessed by the RT/PCR technique. Treatment with the H. opuntia FPA (band 2) resulted in higher catalase gene expression compared with that observed in the CCl4-treated group (band 4). A review by Stevenson and Hurst43 discusses recent evidence that polyphenols also have indirect antioxidant effects through induction of endogenous protective enzymes and these inductive or signalling effects may occur at concentrations much lower than those required for effective radical scavenging.
Figure 4.
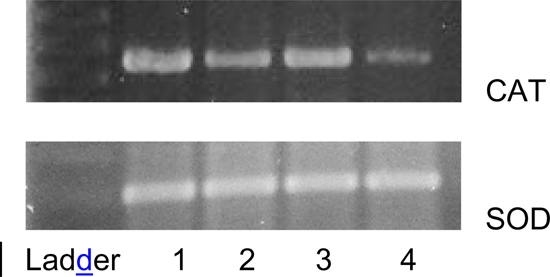
RT-PCR analysis of CAT and SOD expression in liver tissues on agarose gel: (1) control; (2) Halimeda opuntia 80 mg/kg, (3) GA, and (4) CCl4.
Nine phenolic acids including ferulic acid, GA, and p-coumaric acid have been reported to be present in H. opuntia. Yeh44 suggested that these three phenolic acids modulate phase II antioxidant enzymes and phase II sulphate conjugative enzymes, and seem to selectively induce hepatic mRNA transcripts for Cu, Zn-SOD, GPx, and CAT, probably through up-regulation for gene transcription as well as the Nrf2 transcription factor.
Reduced serum and liver TBARS levels after treatment with H. opuntia indicate great antioxidant capacity of the seaweed, which is consistent with observations made in a study of rats treated with natural (phenolic acids from H. monile) and synthetic (GA) antioxidants.34
Conclusion
Treatment with FPA fractions of H. opuntia led to attenuation of liver damage induced by CCl4 in Wistar rats. Reduced serum and liver TBARS levels suggest hepatoprotective activity of the seaweed. Treatment with synthetic GA led to similar results. Whereas CAT and SOD levels in rat liver tissues decreased as a result of oxidative damage induced by CCl4, they increased after treatment with either the H. opuntia FPA fraction or GA, suggesting great antioxidant capacity of H. opuntia and its potential use as a phytodrug, nutraceutical, or both.
Acknowledgement
The authors wish to thank Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for the financial support provided (project No. 471344/06-5).
References
- 1.Aruoma OI. Free radical, oxidative stress, and antioxidants in human health and disease. JAOCS 1998;75(2):199–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Charles AL, Chang C-K, Wu M-L, Huang T-C. Studies on the expression of liver detoxifying enzymes in rats fed seaweed (Monostroma nitidum). Food Chem Toxicol 2007;45:2390–6. [DOI] [PubMed] [Google Scholar]
- 3.Finley JW, Kong A-N, Hintze KJ, Jeffrey EH, Ji LL, Lei XG. Antioxidants in foods: state of the science important to the food industry. J Agric Food Chem 2011;59:6837–46. [DOI] [PubMed] [Google Scholar]
- 4.MacArtain P, Gill CIR, Brooks M, Campbell R, Rowland IR. Nutritional value of edible seaweeds. Nutr Rev 2007;65(12):535–43. [DOI] [PubMed] [Google Scholar]
- 5.Cornish ML, Garbary DJ. Antioxidant from macroalgae: potential application in human health and nutrition. Algae 2010;25(4):155–71. [Google Scholar]
- 6.Lordan S, Ross RP, Stanton C. Marine bioactives as functional food ingredients: potential to reduce the incidence of chronic diseases. Mar Drugs 2011;9:1056–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sampath-Wiley P, Neefus CD, Jahnke LS. Seasonal effects of sun exposure and emersion on intertidal seaweed physiology: fluctuations in antioxidant contents, photosynthetic pigments and photosynthetic efficiency in the red alga Porphyra umbilicalis Kützing (Rhodophyta, Bangiales). J Exp Mar Biol Ecol 2008;36:83–91. [Google Scholar]
- 8.Dutra-Rocha F, Crespo-Pereira R, Coelho-Kaplan MA, Laneuville-Teixeira V. Produtos naturais de algas marinhas e seu potencial antioxidante. Braz J Pharmacogn 2007;17(4):631–9. [Google Scholar]
- 9.Athiperumalsami T, Rajeswari VD, Poorna SH, Kumar V, Jesudass LL. Antioxidant activity of seagrasses and seaweeds. Botanica Marina 2010;53:251–7. [Google Scholar]
- 10.Gupta MP, Gomez N, Santana AI, Solis PN, Palacios G. Actividad antimicrobiana de algunas algas de la costa atlántica panameña. Rev Med Panama 1991;16:64–8. [PubMed] [Google Scholar]
- 11.Ballesteros E, Martin D, Uriz MJ. Biological activity of extracts from some Mediterranean macrophytes. Botanica Marina 1995;35:481–5. [Google Scholar]
- 12.Dzeha T, Jaspars M, Tabudravu J. Clionasterol, a triterpenoid from the Kenyan marine green macroalga Halimeda macroloba. West Indian Ocean J Marine Sci 2003;2:157–61. [Google Scholar]
- 13.Huang H-L, Wu S-L, Liao H-F, Jiang C-M, Huang R-L, Chen Y-Y, et al.. Induction of apoptosis by three marine algae through generation of reactive oxygen species in human leukemic cell lines. J Agric Food Chem 2005;53:1776–81. [DOI] [PubMed] [Google Scholar]
- 14.Moo-Puc R, Robledo D, Freile-Pelegrin Y. Evaluation of selected tropical seaweeds for in vitro anti-trichomonal activity. J Ethnopharmacol 2008;120:92–7. [DOI] [PubMed] [Google Scholar]
- 15.Nor Afifah S, Darah I, Shaida Far S, Jain Nordin MK , Mohd Nurul Aili Z. Antimicrobial activity of various extracts of a tropical Chlorophyta macroalgae Halimeda discoidea. J Appl Sci 2010;10(23):3007–13. [Google Scholar]
- 16.Boonchum W, Peerapornpisal Y, Kanjanapothi D, Pekkoh J, Mornlerdpison D, Pumas C, et al.. Antimicrobial and anti-inflammatory properties of various seaweeds from the Gulf of Thailand. Int J Agric Biol 2011;13(1):100–4. [Google Scholar]
- 17.Zubia M, Robledo D, Freile-Pelegrin Y. Antioxidant activities in tropical marine macroalgae from the Yucatan Peninsula, Mexico. J Appl Phycol 2007;19:449–58. [Google Scholar]
- 18.Fallarero A, Loikkanen JJ, Mannisto PT, Castañeda O, Vidal A. Effects of aqueous extracts of Halimeda incrassata (Ellis) Lamouroux and Bryothamnion triquetrum (S.G.Gmelim). How on hydrogen peroxide and methyl mercury-induced oxidative stress in GT1-7 mouse hypothalamic immortalized cells. Phytomedicine 2003;10(1):39–47. [DOI] [PubMed] [Google Scholar]
- 19.Rivero F, Fallarero A, Castañeda O, Dajas F, Manta E, Areces A. Antioxidant activity in vivo and in vitro of Halimeda incrassata aqueous extracts. Cienc Tecnol Aliment Campinas 2003;23:256–63. [Google Scholar]
- 20.Linares AF, Loikkanen J, Jorge MF, Soria RB, Novoa AV. Antioxidant and neuroprotective activity of the extract from the seaweed, Halimeda incrassata (Ellis) Lamouroux, against in vitro and in vivo toxicity induced by methyl-mercury. Vet Humn Toxicol 2004;46(1):1–5. [PubMed] [Google Scholar]
- 21.Yoshie Y, Wang W, Hsieh YP, Suzuki T. Compositional difference of phenolic compounds between two seaweeds, Halimeda spp. J Tokyo Univ Fisheries 2002;88:21–4. [Google Scholar]
- 22.Vidal A, Silva de Andrade-Wartha ER, de Oliveira e Silva AM, Pavan R, Lima A, Fallarero A, et al.. Actividad antioxidante y polifenoles de algas marinas verdes Halimeda opuntia y Halimeda monile. Ars Pharm 2009;50(1):24–31. [Google Scholar]
- 23.Vidal A, Silva de Andrade-Wartha ER, Fallarero A, de Oliveira e Silva AM, Vuorela P, Mancini-Filho J. Antioxidant activity and bioactive components from the seaweed Halimeda incrassata (Ellis) Lamouroux. Braz J Pharmacogn 2011;21(1):53–7. [Google Scholar]
- 24.Krygier K, Sosulski F, Hogge L. Free, esterified, and insoluble-bound phenolic acids. 1. Extraction and purification procedure. J Agric Food Chem 1982;30:330–4. [Google Scholar]
- 25.Oyaizu M. Studies on products of browning reaction: antioxidative activities of products of browning reaction prepared form glucosamine. Jpn J Nutr 1986;44:307–15. [Google Scholar]
- 26.Goupy P, Hugues M, Boivin P, Amiot MJ. Antioxidant composition and activity of barley (Hordeum vulgare) and malt extracts, and of isolated phenolic compounds. J Sci Food Agric 1999;79:1625–34. [Google Scholar]
- 27.Ohkawa H, Ohishi H, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 1979;95:351–8. [DOI] [PubMed] [Google Scholar]
- 28.McCord JM, Fridovich I. Superoxide dismutase, an enzyme function for erythrocuprein (hemocuprein). J Biol Chem 1969;244:6049–55. [PubMed] [Google Scholar]
- 29.Beutler E. Red cell metabolism: manual of biochemical methods. New York: Grune & Stratton; 1975. p. 89–90. [Google Scholar]
- 30.Sies H, Koch OR, Martino E, Boveris A. Increased biliary glutathione disulfide release in chronically ethanol treated rats. FEBS Lett 1979;103:287–90. [DOI] [PubMed] [Google Scholar]
- 31.Kuda T, Ikemori T. Minerals, polysaccharides and antioxidant properties of aqueous solutions obtained from macro algal beach-casts in the Noto Peninsula, Ishikawa, Japan. Food Chem 2009;112:575–81. [Google Scholar]
- 32.Batista-Gonzalez AE, de Oliveira e Silva AM, Vidal-Novoa A, Pinto JR, Portari Mancini DA, Mancini-Filho J. Analysis of antioxidant properties of hydrophilic fractions from seaweed Halimeda monile L. and its function in vivo. J Food Biochem in press; DOI: 10.1111/j.1745-4514.2010.00525.x. [Google Scholar]
- 33.Senevirathne M, Kim S, Siriwardhana N, Ha J, Lee K, Jeon Y. Antioxidant potential of Ecklonia cava on reactive oxygen species scavenging, metal chelating, reducing power and lipid peroxidation inhibition. Food Sci Tech Int 2006;12(1):27–38. [Google Scholar]
- 34.Mancini-Filho J, Vidal A, Batista AE, de Andrade-Wartha ERS, de O e Silva AM, Pinto JR, et al.. Free phenolic acids from the seaweed Halimeda monile with antioxidant effect protecting against liver injury. Z Naturforsch 2009;64c:657–63. [DOI] [PubMed] [Google Scholar]
- 35.Kim H-Y, Kim J-H, Lee S-A, Chang H-E, Park M-H, Hwang S-J, et al.. Saengshik, a formulated health food, prevents liver damage in CCl4-induced mice and increases antioxidant activity in elderly women. J Med Food 2008;11(2):323–330. [DOI] [PubMed] [Google Scholar]
- 36.Punitha SC, Rajasekaran M. antioxidant mediated defense role of Wedelia calendulacea herbal extract against CCl4 induced toxic hepatitis. J Appl Pharmac Sci 2011;01(09):111–5. [Google Scholar]
- 37.Ozturk F, Ucar M, Ozturk IC, Vardi N, Batcioglu K. Carbon tetrachloride-induced nephrotoxicity and protective effect of betaine in Sprague-Dawley rats. Urology 2003;62:353–6. [DOI] [PubMed] [Google Scholar]
- 38.Halliwell B, Aeschbach R, Loliger J, Aruoma OI. The characterization of antioxidants. Food Chem Toxicol 1995;33(7):601–17. [DOI] [PubMed] [Google Scholar]
- 39.Raghavendran HR, Sathivel A, Devaki T. Effect of Sargassum polycystum (Phaeophyceae)-sulphated polysaccharide extract against acetaminophen-induced hyperlipidemia during toxic hepatitis in experimental rats. Mol Cell Biochem 2005;276:89–96. [DOI] [PubMed] [Google Scholar]
- 40.Josephine A, Nithya K, Amudha G, Veena CK, Preetha SP, Varalakshmi P. Role of sulphated polysaccharides from Sargassum wightii in Cyclosporine A-induced oxidative liver injury in rats. BMC Pharmacology 2008;8:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang Q, Li N, Liu X, Zhao Z, Li Z, Xu Z. The structure of a sulphated galactan from Porphyra haitanensis and its in vivo antioxidant activity. Carbohydr Res 2004;339:105–11. [DOI] [PubMed] [Google Scholar]
- 42.Abdel-Wahhab MA, Ahmed HH, Hagazi MM. Prevention of aflatoxin B1-initiated hepatotoxicity in rat by marine algae extracts. J Appl Toxicol 2006;26(3):229–38. [DOI] [PubMed] [Google Scholar]
- 43.Stevenson DE, Hurst RD. Polyphenolic phytochemicals – just antioxidants or much more? Cell Mol Life Sci 2007;64:2900–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yeh C-T, Yen G-C. Induction of hepatic antioxidant enzymes by phenolic acids in rats is accompanied by increased levels of multidrug resistance – associated protein 3 mRNA expression. J Nutr 2006;136:11–5. [DOI] [PubMed] [Google Scholar]