Abstract
Objectives: Free radicals play an important role in the onset and progression of many diseases. The aim of this study was to investigate the contribution of oxidative stress in the pathology of aggressive (AgP) and chronic (CP) periodontitis and its relation with the clinical periodontal status.
Methods: Eighty subjects were divided into two groups: 20 patients with AgP and 20 patients with CP with their 20 corresponding matched controls, based on clinical attachment loss (CAL), probing pocket depth (PPD), and bleeding on probing (BOP). Saliva reactive oxygen species (ROS), lipid peroxidation, and non-enzymatic antioxidant defences were measured by luminol-dependent chemiluminescence assay, as thiobarbituric acid-reactive substances (TBARs) and total radical-trapping antioxidant potential (TRAP), respectively. Pearson's correlation and multivariate analysis were used to determine the relationship between ROS and TBARs and the clinical parameters.
Results: ROS and TBARs were increased in AgP while TRAP was decreased, comparing with CP. In AgP, a strong and positive correlation was observed between ROS and TBARs and they were closely associated with CAL and PPD.
Discussion: In AgP, but not in CP, oxidative stress is a high contributor to periodontal pathology and it is closely associated with the clinical periodontal status.
Keywords: Oxidative stress, ROS, TRAP, TBARs, Aggressive periodontitis, Chronic periodontitis
Introduction
Free radicals including reactive oxygen species (ROS) and reactive nitrogen species (RNS) exist in normal cells at low but measurable concentrations.1 Their levels depend on the balance between their rates of production and their rates of clearance by the endogenous antioxidant systems. When ROS/RNS production is increased in such form that the antioxidative response is unable to reset the system, the result is an oxidative stress status. Different mechanisms operate to defend the organism from oxidative insults. Those mechanisms can be grouped into enzymatic and non-enzymatic. Enzymatic defences are mainly comprised by catalase, superoxide dismutase, and glutathione peroxidase activities, while non-enzymatic protectors include a series of organic molecules that can scavenge or inactivate reactive species.2
It is widely accepted that free radicals play an important role in the onset and progression of many diseases including rheumatoid arthritis,3 chronic obstructive pulmonary disease,4 AIDS,5 atherosclerosis,6 and more recently periodontal disease.7,8
Periodontal disease is an inflammatory disorder in which tissue damage occurs through the complex interaction between periodontal pathogens and components of the host defence mechanism. Two principal forms of periodontitis are currently recognized, chronic (CP), and aggressive periodontitis (AgP). AgP is a specific form of periodontal disease with a higher rate of progression and patterns of tissue destruction, mostly affecting younger individuals. Despite differences in their clinical phenotypes,9 no unequivocal pathophysiological foundation that differentiates between CP and AgP has been established. Chronic and aggressive periodontitis lesions cannot be distinguished on the basis of histopathologic features10 or microbial colonization profiles,11 although there is evidence of immunological differences, including the presence of neutrophil abnormalities in AgP.12 Neutrophils play a central role in the initial host inflammatory response to the periodontal pathogens and protect the host tissues by killing various pathogenic bacteria either by non-oxidative or oxidative means, in an intracellular or extracellular environment. Non-oxidative killing is mediated by various lysosomal enzymes, peptides and proteins, including lysozyme, bactericidal/permeability-increasing proteins, cationic proteins, defensins, and lactoferrin. Generation of ROS (superoxide, hydrogen peroxide, hydroxyl radicals, hypochlorous acid, and chloramines) contributes to the oxidative killing of the invading microorganisms.13 Unfortunately, the ROS generated during an oxidative burst response can cause considerable collateral damage and are directly responsible for infection-associated tissues injuries. Neutrophils from patients with AgP are hyperactive and primed and appear to release enhanced levels of oxygen radicals, inflammatory mediators such as cytokines and matrix-degrading enzymes.14,15 This hyperactivity and reactivity of neutrophils destroys the adjacent host tissues and contributes to the destructive changes observed in inflammatory periodontitis.16 An excess of ROS can cause oxidative stress and damage to critical biomolecules resulting in deleterious biological effects.17 Thus, the present study was undertaken to find out the association between ROS and the destruction of periodontal tissue in patients with AgP in comparison with CP. For this purpose ROS, total non-enzymatic antioxidant response and thiobarbituric acid-reactive substances (TBARs), as a signal of lipid peroxidation, were determined in saliva from patients with AgP, CP and their matched controls, and related with clinical periodontal status. Determinations were done in saliva because of its availability of measurable substrates and ease and safety of collection that make it a promising area for biomarker discovery in a wide array of human biomedical conditions. Assays for perturbations of salivary redox homeostasis may be salient for diagnosis, prognosis, and monitoring of treatment in systemic disorders as disparate as diabetes and scleroderma as well as in the management of local pathologies like periodontitis and oral cancers.18
Materials and methods
Study population
Study subjects were recruited from the patient's population of a private dental clinic, from January to August 2014. The protocol was approved by the Ethics Committee of the School of Dentistry, University of Buenos Aires, Argentina (protocol number 11/06/2012-18), and the study was conducted in accordance with the Declaration of Helsinki (version 2008). Completed medical and dental histories were obtained from all subjects. The inclusion criteria for the study group was the presence of established AgP or CP according to World Workshop in Periodontology criteria,19 based on the measurement of clinical attachment loss (CAL), probing pocket depth (PPD), and bleeding on probing (BOP), in subjects who had not had periodontal check-up in the previous 6 months. Therefore, a total of 20 individuals with AgP and 20 with CP were selected. Since there was a significant difference in the age of AgP and CP patients, two control groups were formed with subjects of similar ethnic, income levels, age and gender than patients. All the subjects gave their informed consent and exclusion criteria included: smokers, cardiovascular, or respiratory diseases, systemic inflammatory conditions or non-plaque induced oral inflammatory conditions, immunodeficiency, current pregnancy, or lactation and medicine use.
Clinical examination
All periodontal measurements were performed in four quadrants using a first-generation probe (Hu-Friedy Mfg. Co., Chicago, IL, USA) by a single calibrated investigator (G.A.S.). PPD (measurements were rounded off to the nearest millimetre marking) and clinical attachment level (CAL, measuring the distance from the cemento-enamel junction to the bottom of the probable pocket) were assessed at six sites per tooth (mesiopalatal, palatal, distopalatal, mesiobuccal, buccal, and distobuccal) and BOP (scored as: −, no bleeding or +, bleeding within 30 seconds after probing) at four sites per tooth (mesiopalatal, distopalatal, mesiobuccal, and distobuccal).
Collection of saliva
Unstimulated saliva was collected at 10 am the day after the periodontal diagnosis.20 Subjects were asked to refrain from eating or drinking 2 hours prior to collection. Whole saliva was collected by spitting into an ice-cooled graduated vessel. Subjects spat out every 30 seconds during 5 minutes. The volume of saliva was recorded and expressed as ml/min. The resulting saliva was stored in aliquots at −20°C until determinations were performed.21
Reagents
Luminol (5-amino-2,3-dihydro-1,4-phthalazinedione), 2-thiobarbituric acid (TBA), glycine, and 1,1,3,3-tetraethoxypropane (TTP) were purchased from Sigma Chemical Co. (St Louis, MO, USA). 2,2′-Azo-bis-(2-methylpropionamidine) dihydrochloride (ABAP) and 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox) were from Aldrich. 1-Butanol and acetic acid were from Cicarelli (San Lorenzo, Argentina), and hydrochloric acid and absolute ethanol were purchased from Merck (Darmstadt, Germany). The other reagents were of the highest quality available.
Measurement of ROS
ROS production was determined by a chemiluminescence assay using luminol as described by Kobayashi et al.22 with minor modifications. Briefly, 5 µl of saliva was pippeted into white 96-well microplates (Nunc, Thermoscientific) containing 200 µl of phosphate buffered saline. 5 µl of luminol (5 mM) were added to each well and luminescence was assessed in a Biotek Instruments Synergy HT multiplate reader. Hydrogen peroxide was used as positive control. ROS production was expressed as relative light units (RLU).
Determination of total radical-trapping antioxidant potential (TRAP)
TRAP, representing the non-enzymatic antioxidant defences, was measured in the saliva of patients and control subjects as described by Lissi et al.23 and Vargas et al.24 The reaction medium consisted of 10 mM ABAP and 5 µM luminol. ABAP is a source of free radicals that react with luminol yielding chemiluminescence. The resulting chemiluminescence was measured in a Biotek Instruments Synergy HT multiplate reader. The addition of 2 µl of saliva decreased the chemiluminescence to basal levels, for a period proportional to the amount of antioxidants present in the sample, until luminol radicals were regenerated (induction time). Different concentrations of Trolox (vitamin E water-soluble analogue) were used for calibration. A comparison between the induction time of known concentrations of Trolox and of saliva, allows calculation of TRAP as the equivalent of Trolox concentration which is necessary to produce the same induction time. Results are thus expressed as micromolar Trolox.
Determination of thiobarbituric acid-reactive substances (TBARs)
TBARs were determined fluorometrically in the saliva of patients and control subjects as described by Wasowicz et al.21 Briefly, 10 µl of saliva or an adequate volume of malondialdehyde (MDA) working standard solution was pippeted into 96-well microplates (Nunc, Thermoscientific) containing distilled water to a final volume of 200 µl. After addition of 100 µl of 29 mM TBA in 8.75 M acetic acid, the samples were placed in a water bath and heated for 1 hour at 95–100°C. After cooling the samples, 2.5 µl of 5 mM HCl was added, and the reaction mixture was extracted by a brief vortexing with 350 µl of 1-butanol. The butanolic phase was then separated by centrifugation at 1500 × g for 10 minutes, and its fluorescence (485/20 and 528/20 nm excitation and emission, respectively) assessed in a Biotek Instruments Synergy HT multiplate reader. The calibration curve was prepared with MDA generated by hydrolysis of TTP. The stock standard solution of MDA was prepared by dissolving TTP in ethanol. Just before use, the solution was diluted in distilled water to yield a 2 µM MDA working standard.
Statistical analysis
Statistical significance of differences was determined by one-way ANOVA followed by Neuman–Keuls multiple comparison test. Student t test was used for comparing two groups. Pearson correlation analysis was done using GRAPHPAD Prism version 5.03 for Windows (GraphPad Software, San Diego, CA, USA). Categorical multivariate analysis was used to establish the relation between ROS, TBARs, and the clinical parameters. The level of statistical significance is set to P < 0.05.
Results
Clinical findings
Demographic and clinical data from patients with AgP and CP are presented in Table 1. The criteria used to select the study participants, in particular the absence of periodontal treatment during the previous 6 months, led to a small sample size. Due to the difference in age of the two disease groups, each of them was matched with subjects representing two control groups. As can be seen in the table, both disease groups showed significant higher CAL and PPD than control groups, tested by one-way ANOVA followed by Neuman–Keuls multiple comparison test. Patients with AgP had more periodontal destruction than patients with CP, as disclosed from the higher CAL and PPD observed.
Table 1. The table shows the demographic and clinical data from patients with aggressive (AgP) and chronic (CP) periodontitis and their matched controls.
| Parameter/group | Age (years) mean (range) |
Gender | Number of teeth (range) | Number of sites checked up | CAL (mm) | Number of sites CAL > 4 mm, median (range) | PPD (mm) | Number of sites PPD > 5 mm, median (range) |
|---|---|---|---|---|---|---|---|---|
| Control AgP | 19.5 (17–23) |
10 F 10 M |
28–30 | 168–180 | 0.23 ± 0.06 | 0 | 2.4 ± 0.06 | 0 |
| Patients AgP | 19.5 (17–23) |
10 F 10 M |
28–30 | 168–180 | a5.9 ± 0.17*** | 5 (4–6) | b5.5 ± 0.07*** | 3 (2–5) |
| Control CP | 37.4 (32–40) |
10 F 10 M |
28–30 | 168–180 | 0.25 ± 0.06 | 0 | 2.5 ± 0.06 | 0 |
| Patients CP | 37.4 (32–40) |
10 F 10 M |
26–28 | 168–180 | 4.2 ± 0.25*** | 3 (0–5) | 5.1 ± 0.2*** | 3 (0–5) |
Data are the mean ± SEM. CAL: clinical attachment level; PPD: probing pocket depth. F: female, M: male.
***Significantly different from controls, P < 0.001.
aSignificantly different from CP, P < 0.001.
bSignificantly different from CP, P < 0.01.
Laboratory findings
The levels of ROS in unstimulated saliva differed widely in the AgP group while no difference was observed between the two control groups (Fig. 1A). When comparing ROS generation in each group with its respective control, a significant difference was found in both AgP and CP (controls AgP: 1082 ± 64; AgP: 7032 ± 400, P < 0.001; controls CP: 1085 ± 79; CP: 1522 ± 61.7, P < 0.001).
Figure 1.
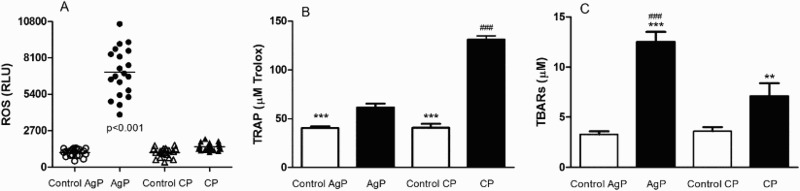
(A) Generation of ROS expressed as RLU, in saliva from patients with aggressive (AgP) and chronic periodontal (CP) disease and their matched controls. (B) TRAP expressed as µM Trolox in saliva from patients with aggressive (AgP) and chronic (CP) periodontal disease and their matched controls. ***Significantly different from controls (P < 0.001); ###significantly different from AgP (P < 0.001). (C) Lipid peroxidation measured as TBARs (µM) in saliva from patients with aggressive (AgP) and chronic (CP) periodontal disease and their matched controls. ***Significantly different from controls (P < 0.001); **significantly different from controls (P < 0.01); ###significantly different from CP (P < 0.001).
The total radical-trapping antioxidant potential, evaluated as TRAP, revealed a significantly higher activity in both patient groups as compared with controls (Fig. 1B), but patients within the CP group exhibited significantly higher TRAP values than those of the AgP group.
Lipid peroxidation measured as TBARs was significantly higher in AgP patients as compared with controls and CP patients group (Fig. 1C). On the other hand, CP patients showed increased TBARs when compared with control groups (Fig. 1C).
Correlations
In order to establish the relation between ROS and TBARs and protein salivary levels in both groups, Pearson's correlation analyses were carried out (Figs. 2 and 3). As can be seen in Fig. 2A, the significant and high r2 found in the AgP group suggests that lipid peroxidation is closely related to ROS. However, no significant relation was found in CP group (Fig. 2B). On the other hand, salivary protein concentration showed only a positive and significant but weak correlation with ROS in AgP group (Fig. 3A).
Figure 2.
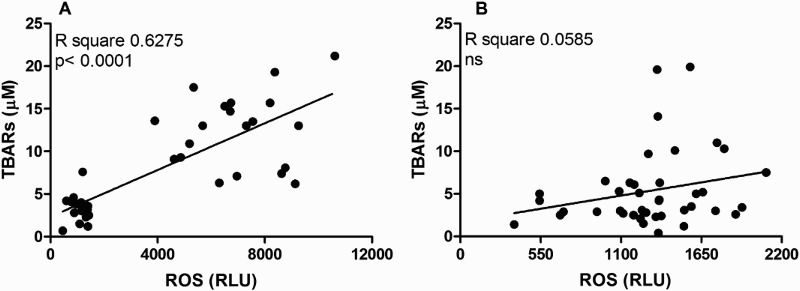
Pearson correlation analysis between salivary levels of ROS and lipid peroxidation measured as salivary TBARs in AgP (A) and CP (B) groups.
Figure 3.
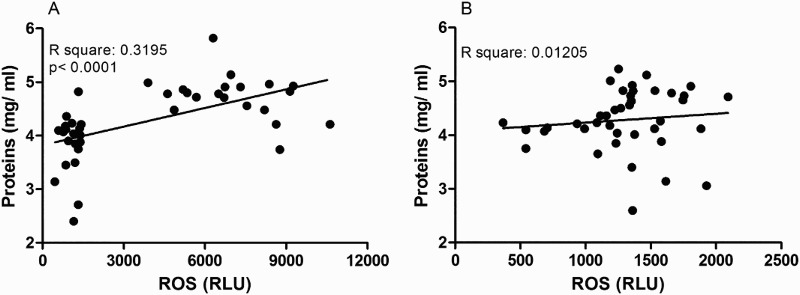
Pearson correlation analysis between salivary levels of ROS and salivary protein concentration in AgP (A) and CP (B) groups.
Correlations between clinical parameters and ROS and TBARs levels in saliva were investigated in both groups (Figs. 4 and 5A and B). During the analysis, while statistically significant strong and positive correlations were observed between clinical parameters and ROS and TBARs levels in saliva from AgP group, statistically significant, yet weak correlations, were observed between clinical parameters and ROS values in CP group. Only a very weak significant relation was observed with the saliva TBARs and PPD (Figs. 4 and 5A and B). To further analyse the relationship between ROS and TBARs and the periodontal status, a multivariate regression analysis was performed. As can be seen in Table 2, in AgP group the salivary levels of ROS may be closely associated with CAL and PPD being weaker the association with TBARs. On the other hand, in CP group only ROS may be associated with PPD and CAL, although the latter association was weak (Table 2). Conversely, as disclosed by the multivariate regression analysis, in CP group no association was observed between TBARs and the clinical parameters.
Figure 4.
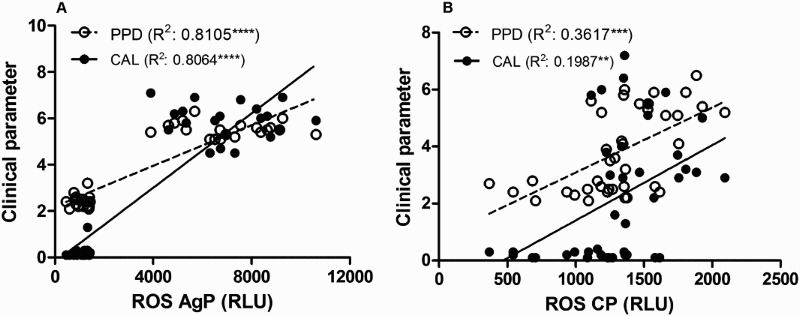
Pearson correlation analysis between ROS and the clinical parameters PPD and the CAL in AgP (A) and CP (B) groups. ****Significant correlation (P < 0.0001). **Significant correlation (P < 0.01).
Figure 5.
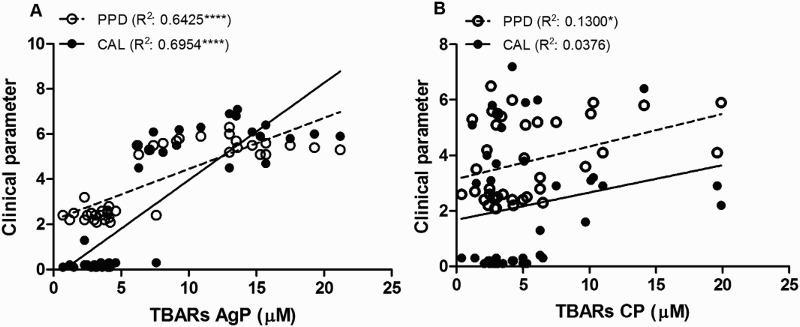
Pearson correlation analysis between lipid peroxidation measured as TBARs and the clinical parameters PPD and the CAL in AgP (A) and CP (B) groups. ****Significant correlation (P < 0.0001). *Significant correlation (P < 0.05).
Table 2. Values of η2 (effect size) obtained by multivariate analysis between the values of ROS and TBARs in saliva, with CAL and PPD in aggressive (AgP) and chronic (CP) periodontitis groups.
| Dependent variables | Independent variables | |||
|---|---|---|---|---|
| ROS (RLU) | TBARs (µM) | |||
| AgP | CP | AgP | CP | |
| CAL (mm) | 0.49 (P < 0.0001) |
0.17 (P = 0.008) |
0.21 (P = 0.004) |
0.01 (P = 0.54) |
| PPD (mm) | 0.52 (P < 0.0001) |
0.32 (P < 0.0001) |
0.11 (P = 0.039) |
0.07 (P = 0.087) |
Discussion
Increasing evidence over the last years confirms that the oxidative stress-dependent tissue injury may be involved in many diseases in humans.1,25 The pathogenesis of these diseases, including periodontitis, could be clarified with the use of biomarkers of oxidative stress in different body fluids. Saliva constantly bathes the teeth and oral mucosa acting as an antibacterial solution, an ion reservoir, a lubricant, and a buffer. In addition to these host protective properties, saliva constitutes a first line of defence against free radical mediated oxidative stress.26,27 It has been claimed that imbalances in levels of free reactive oxygen radicals with antioxidants, play a major role in development of oral diseases and inflammation.28,29
Given the growing importance of the ROS measurement in saliva, as a help tool in the initial evaluation and follow-up of periodontal disease, and the clinical usefulness of the luminol-dependent chemiluminescence assay, that can quantify minor changes in PMN oxidative metabolism,30 the levels of ROS were quantified in saliva from patients with AgP and CP and their matched controls. Thus, ROS formation was detected in saliva not only from patients with AgP or CP but in control subjects. ROS play important roles in physiological and immune-inflammatory reactions7 and spontaneous release of by crevicular PMN occurs in healthy individuals.29 Supragingival plaque induced emission of chemiluminescence by normal peripheral blood neutrophils,31 and at least and H2O2 are produced by dental-plaque-stimulated neutrophils. Bacterial products may lead to altered PMN function, modifying their response to a second stimulus. This action of preparing PMN for stimulation is referred to as ‘priming’.29 In this study, it was observed that saliva of patients with AgP released larger amounts of ROS than patients with CP. Neutrophils in AgP are in a hyperactive, primed state.16 This hyperactivity may be attributed to circulating factors15 or genetic make-up of individual or environmental effects.32 These hyper responsive neutrophils may explain the difference in ROS production between patients with AgP or CP, observed in this study. Gingival epithelial cells are highly susceptible to oxidative stress elicited by PMN-derived oxidants33 and ROS production is considered as one of the major reasons for the periodontal tissue destruction seen in periodontitis.13,16 In the human body, there is an antioxidant mechanism to maintain the balance of oxidation–reduction.2 The breakdown of this balance could lead to an oxidative stress. There are two possible causes for the excessive oxidative stress: increased ROS with no concomitant or less increased levels of antioxidants, or substantially decreased levels of antioxidants with no marked change of ROS. The oxidative stress index based on the measurement of oxidant/antioxidant imbalances has been a useful and practical marker of the oxidative damage that occurs in periodontitis.8 Here, patients with AgP showed high levels of ROS but very low antioxidant activity. However, patients within CP showed lesser production of ROS and higher antioxidant activity. Thus, the non-enzymatic antioxidant defences, measured as TRAP, were incremented in both, AgP and CP as compared with healthy controls indicating an antioxidant response to the oxidative insult. But the low increment in AgP group could not maintain the balance of oxidation-reduction and ROS production outweighs salivary antioxidant capacity. When antioxidant systems are unable to counteract their action efficiently, tissue damage can result. In other words, the balance between oxidative stress and antioxidant level was perturbed mainly in inflammatory AgP, and the enhanced production of ROS led to cellular damage. Low ROS often behaves as an inductor stimulus, whereas higher levels may result in injury.34 The ROS-related tissue destruction could be measured by the final product, MDA which is a stable end product of peroxidation of lipids by ROS.35 Although the origin of MDA detected in saliva from patients with periodontitis is unknown, it seems probable that it is produced locally in the oral cavity due to ROS generated during inflammation.36 The group of compounds called TBARs is heterogeneous, but it is still used as a marker of oxidative stress, as the vast majority of TBARs are products of oxidative damage of lipids.36 In the present study, salivary TBARs levels were significantly higher in AgP patients than in CP patients or healthy controls, and their salivary levels were shown to be closely related to ROS production. However, in CP group no relation was found between ROS and TBARs. In addition, in AgP group but not in CP group, ROS levels correlated with salivary proteins. It is known that salivary proteins derive from salivary glands but in periodontal disease they may have another origin, such as the inflammatory process or tissue damage. This difference may indicate that in AgP, which is a specific form of periodontal disease with a higher rate of progression, oxidative stress is one of the factors involved in tissue damage. Conversely, in CP which is a chronic inflammatory reaction where there was not a high production of ROS and they could be partially neutralized by the salivary antioxidant system, it is probable that other components of the host defence mechanisms or periodontal pathogens were implicated in tissue destruction. Furthermore, a strong and positive correlation was observed between salivary levels of ROS and TBARs and the clinical parameters, CAL and PPD in AgP group. However, weak correlations were found in CP group. Different mechanisms, including DNA damage, lipid peroxidation, protein damage, oxidation of important enzymes, and stimulation of proinflammatory cytokine release, have been implicated for the cause of tissue damage by ROS.37,38 Thus, the difference in the course of oxidative stress in AgP and CP could provide a different perspective as to why periodontal infections follow a chronic or aggressive course. It is important to note that the targeted variables were detected in saliva which has become a widely recognized source of information, both in clinical studies and basic research. The diagnostic potential of saliva is attributed to its molecular profile, which reflects an individual's physiological status at the moment of collection. The sampling is really non-invasive making it especially useful in studies involving repeated sampling. It represents the major intra-oral condition regarding the saliva state and composition.18
In conclusion, based on the presented data it is possible to hypothesize that, in AgP patients, hyper responsive PMN produced excessive ROS leading to an oxidative stress which may be an important contributor to the higher rate of progression of the disease.
Funding Statement
This work was supported by Universidad de Buenos Aires (grant UBACYT N° 20020110100135).
Disclaimer statements
Contributors All authors contributed equally.
Conflict of interest The authors have no conflict of interest to disclose.
Ethics approval The protocol of this investigation was approved by the Ethics Committee of the School of Dentistry, University of Buenos Aires, Argentina, and the study was conducted in accordance with the Declaration of Helsinki (version 2008).
References
- [1].Halliwell B, Gutteridge JM. Role of free radicals and catalytic metal ions in human disease: an overview. Meth Enzymol 1990;186:1–85. doi: 10.1016/0076-6879(90)86093-B [DOI] [PubMed] [Google Scholar]
- [2].Scandalios J. Oxidative stress: molecular perception and transduction of signals triggering antioxidant gene defenses. Braz J Med Biol Res 2005;38:995–1014. doi: 10.1590/S0100-879X2005000700003 [DOI] [PubMed] [Google Scholar]
- [3].Mapp PI, Grootveld MC, Blake DR. Hypoxia, oxidative stress and rheumatoid arthritis. Br Med Bull 1995;51:419–36. doi: 10.1093/oxfordjournals.bmb.a072970 [DOI] [PubMed] [Google Scholar]
- [4].Macnee W, Rahman I. Oxidants and antioxidants as therapeutic targets in chronic obstructive pulmonary disease. Am J Resp Crit Care Med 1999;160:S58–65. doi: 10.1164/ajrccm.160.supplement_1.15 [DOI] [PubMed] [Google Scholar]
- [5].Elbim C, Pillet S, Prevost MH, Preira A, Girard PM, Rogine N, et al. Redox and activation status of monocytes from human immunodeficiency virus-infected patients: relationship with viral load. J Virol 1999;73:4561–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Halliwell B. The role of oxygen radicals in human disease, with particular reference to the vascular system. Haemostasis 1993;1(suppl.):118–26. [DOI] [PubMed] [Google Scholar]
- [7].Waddington RJ, Moseley R, Embery G. Periodontal Disease Mechanisms: Reactive oxygen species: a potential role in the pathogenesis of periodontal diseases. Oral Dis 2000;6:138–51. doi: 10.1111/j.1601-0825.2000.tb00325.x [DOI] [PubMed] [Google Scholar]
- [8].Baltacıoğlu E, Yuva P, Aydın G, Alver A, Kahraman C, Karabulut E, et al. Lipid peroxidation levels and total oxidant/antioxidant status in serum and saliva from patients with chronic and aggressive periodontitis. Oxidative stress index: a new biomarker for periodontal disease? J Periodontol 2014;85:1432–41. doi: 10.1902/jop.2014.130654 [DOI] [PubMed] [Google Scholar]
- [9].Armitage GC, Cullinan MP. Comparison of the clinical features of chronic and aggressive periodontitis. Periodontology 2000 2010;53:12–27. doi: 10.1111/j.1600-0757.2010.00353.x [DOI] [PubMed] [Google Scholar]
- [10].Smith M, Seymour GJ, Cullinan MP. Histopathological features of chronic and aggressive periodontitis. Periodontology 2000 2010;53:45–54. doi: 10.1111/j.1600-0757.2010.00354.x [DOI] [PubMed] [Google Scholar]
- [11].Armitage GC. Comparison of the microbiological features of chronic and aggressive periodontitis. Periodontology 2000 2010;53:70–88. doi: 10.1111/j.1600-0757.2010.00357.x [DOI] [PubMed] [Google Scholar]
- [12].Ryder MI. Comparison of neutrophil functions in aggressive and chronic periodontitis. Periodontology 2000 2010;53:124–37. doi: 10.1111/j.1600-0757.2009.00327.x [DOI] [PubMed] [Google Scholar]
- [13].Nussbaum G, Shapira L. How has neutrophil research improved our understanding of periodontal pathogenesis? J Clin Periodontol 2011;38(Suppl. 11):49–59. doi: 10.1111/j.1600-051X.2010.01678.x [DOI] [PubMed] [Google Scholar]
- [14].Kantarci A, Oyaizu K, Van Dyke TE. Neutrophil-mediated tissue injury in periodontal disease pathogenesis: findings from localized aggressive periodontitis. J Periodontol 2003;74:66–75. doi: 10.1902/jop.2003.74.1.66 [DOI] [PubMed] [Google Scholar]
- [15].Dias IH, Matthews JB, Chapple IL, Wright HJ, Dunston CR, Griffiths HR. Activation of the neutrophil respiratory burst by plasma from periodontitis patients is mediated by pro-inflammatory cytokines. J Clin Periodontol 2011;38:1–7. doi: 10.1111/j.1600-051X.2010.01628.x [DOI] [PubMed] [Google Scholar]
- [16].Matthews JB, Wright HJ, Roberts A, Ling-Mountford N, Cooper PR, Chapple IL. Neutrophil hyper-responsiveness in periodontitis. J Dent Res 2007;86:718–22. doi: 10.1177/154405910708600806 [DOI] [PubMed] [Google Scholar]
- [17].Luqman S, Rizvi SI. Protection of lipid peroxidation and carbonyl formation in proteins by capsaicin in human erythrocytes subjected to oxidative stress. Phytother Res 2006;20:303–6. doi: 10.1002/ptr.1861 [DOI] [PubMed] [Google Scholar]
- [18].Wang J, Schipper HM, Velly AM, Mohit S, Gornitsky M. Salivary biomarkers of oxidative stress: a critical review. Free Radic Biol Med 2015;85:95–104. doi: 10.1016/j.freeradbiomed.2015.04.005 [DOI] [PubMed] [Google Scholar]
- [19].Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol 1999;4:1–6. doi: 10.1902/annals.1999.4.1.1 [DOI] [PubMed] [Google Scholar]
- [20].Sánchez GA, Miozza VA, Delgado A, Busch L. Salivary IL-1β and PGE2 as biomarkers of periodontal status, before and after periodontal treatment. J Clin Periodontol 2013;40:1112–7. doi: 10.1111/jcpe.12164 [DOI] [PubMed] [Google Scholar]
- [21].Wasowicz W, Neve J, Peretz A. Optimized steps in fluorometric determination of thiobarbituric acid-reactive substances in serum: importance of extraction pH and influence of sample preservation and storage. Clin Chem 1993;39:2522–6. [PubMed] [Google Scholar]
- [22].Kobayashi H, Gil-Guzman E, Mahran AM, Sharma RK, Nelson DR, Thomas AJ Jr, et al. Quality control of reactive oxygen species measurement by luminol-dependent chemiluminescence assay. J Androl 2001;22:568–74. [PubMed] [Google Scholar]
- [23].Lissi E, Salim-Hanna M, Pascual C, del Castillo MD. Evaluation of total antioxidant potential (TRAP) and total antioxidant reactivity from luminol-enhanced chemiluminescence measurements. Free Radic Biol Med 1995;18:153–8. doi: 10.1016/0891-5849(94)00117-3 [DOI] [PubMed] [Google Scholar]
- [24].Vargas C, Wajner M, Sirtori L, Goulart L, Chiochetta M, Coelho D, et al. Evidence that oxidative stress is increased in patients with X-linked adrenoleukodystrophy. Biochim Biophys Acta 2004;1688:26–32. doi: 10.1016/j.bbadis.2003.10.004 [DOI] [PubMed] [Google Scholar]
- [25].Dröge W. Free radicals in the physiological control of cell function. Physiol Rev 2002;82:47–95. doi: 10.1152/physrev.00018.2001 [DOI] [PubMed] [Google Scholar]
- [26].Tenovuo J, Lehtonen OP, Aaltonen AS, Vilja P, Tuohimaa P. Antimicrobial factors in whole saliva of human infants. Infect Immun 1986;51:49–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Edgar WM. Saliva: its secretion, composition and functions. Br Dent J 1992;172:305–12. doi: 10.1038/sj.bdj.4807861 [DOI] [PubMed] [Google Scholar]
- [28].Chapple IL. Reactive oxygen species and antioxidants in inflammatory diseases. J Clin Periodontol 1997;24:287–96. doi: 10.1111/j.1600-051X.1997.tb00760.x [DOI] [PubMed] [Google Scholar]
- [29].Battino M, Bullon P, Wilson M, Newman H. Oxidative injury and inflammatory periodontal diseases: the challenge of anti-oxidants to free radicals and reactive oxygen species. Crit Rev Oral Biol Med 1999;10:458–76. doi: 10.1177/10454411990100040301 [DOI] [PubMed] [Google Scholar]
- [30].Battino M, Ferri E, Girotti S, Lenaz G. Free radical scavenging activity of coenzyme Q measured by a chemiluminescent assay. Anal Chim Acta 1991;255:367–71. doi: 10.1016/0003-2670(91)80070-A [DOI] [Google Scholar]
- [31].Charon JA, Joachim F, Champagne C, Torpier G, Capron A. Effect of dental plaque on the oxidative metabolism of normal neutrophils. Oral Microbiol Immunol 1987;2:92–6. doi: 10.1111/j.1399-302X.1987.tb00297.x [DOI] [PubMed] [Google Scholar]
- [32].Stabholz A, Soskolne WA, Shapira L. Genetic and environmental risk factors for chronic periodontitis and aggressive periodontitis. Periodontology 2000 2010;53:138–53. doi: 10.1111/j.1600-0757.2010.00340.x [DOI] [PubMed] [Google Scholar]
- [33].Altman LC, Baker C, Fleckman P, Luchtel D, Oda D. Neutrophil-mediated damage to human gingival epithelial cells. J Periodont Res 1992;27:70–9. doi: 10.1111/j.1600-0765.1992.tb02088.x [DOI] [PubMed] [Google Scholar]
- [34].Battino M, Gorini A, Villa RF, Genova ML, Bovina C, Sassi S, et al. Coenzyme Q content in synaptic and nonsynaptic mitochondria from different brain regions in the ageing rat. Mech Ageing Dev 1995;78:173–87. doi: 10.1016/0047-6374(94)01535-T [DOI] [PubMed] [Google Scholar]
- [35].Nielsen F, Mikkelsen BB, Nielsen JB, Andersen HR, Grandjean P. Plasma malondialdehyde as biomarker for oxidative stress: reference interval and effects of life-style factors. Clin Chem 1997;43:1209–14. [PubMed] [Google Scholar]
- [36].Vlková B, Celec P. Does Enterococcus faecalis contribute to salivary thiobarbituric acid-reacting substances? In Vivo 2009;23:343–5. [PubMed] [Google Scholar]
- [37].Bartold PM, Wiebkin OW, Thonard JC. The effect of oxygen-derived free radicals on gingival proteoglycans and hyaluronic acid. J Periodont Res 1984;19:390–400. doi: 10.1111/j.1600-0765.1984.tb01012.x [DOI] [PubMed] [Google Scholar]
- [38].Finkel T. Reactive oxygen species and signal transduction. IUBMB Life 2001;52:3–6. doi: 10.1080/15216540252774694 [DOI] [PubMed] [Google Scholar]


