Abstract
Objectives: The multifunctional drug niclosamide (NSD), extensively employed therapeutically, is a frequent pollutant of surface waters. Considering the environmental importance of photodegradative processes for this type of contaminant, the kinetic and mechanistic aspects of the possible visible-light-mediated photooxidation of NSD were studied under naturalistic conditions.
Methods: The visible-light absorber riboflavin (vitamin B2) was employed as a photosensitizer. The vitamin can usually be found in natural waters and is the most common endogenous photosensitizer in mammals.
The interaction of NSD with electronically excited states of Rf and with photogenerated reactive oxygen species (ROS) was evaluated through conventional UV spectroscopy, laser flash photolysis, time-resolved phosphorescence detection of singlet molecular oxygen (O2(1Δg)), and polarographic dosage of dissolved oxygen.
Results: Ground state NSD quenched the long-lived triplet excited state of Rf (3Rf*) and the photogenerated ROS (O2(1Δg)) and superoxide radical anion . As a result, NSD was photooxidized. The rate constants for the interaction NSD–O2(1Δg) are particularly low, in the order of 106/M/s, although the whole interaction is attributable to a pure reactive process. The O2(1Δg) quenching was faster in alkaline medium, favored by the ionization of the NSD phenolic group.
Under Rf-photosensitization, NSD was degraded very much more rapidly than phenol, the latter being considered a paradigmatic water-contaminant model compound.
NSD may behave as an antioxidant in bio-environments, as demonstrated employing the photooxidizable amino acid tryptophan as a relevant biological target.
Discussion: The results indicate that a -mediated process is the main route for the Rf-sensitized photooxidation of NSD. Photodegradation of the biocide in the presence and absence of phenol and tryptophan was quantitatively evaluated, discussed, and interpreted in terms of competitive quenching processes of 3Rf*, O2(1Δg), and by the substrates.
Keywords: Niclosamide, Photodegradation, Photooxidation, Reactive oxygen species, Riboflavin
Introduction
Niclosamide (NSD) is a multifunctional drug normally found as natural surface waters due to its widespread use as a biocide.1 NSD's chemical structure and the respective absorption spectra for the molecular and OH-ionized species are shown in Fig. 1. The drug is incorporated into wastewaters mainly through animal excretion and anthropogenic industrial activities. The toxicity of NSD to several non-target fish species and snails has been demonstrated.2,3 Some years ago, the interaction of NSD with DNA was reported. The study suggested that NSD-toxicity can be caused by this interaction, after reductive activation.4
Figure 1.
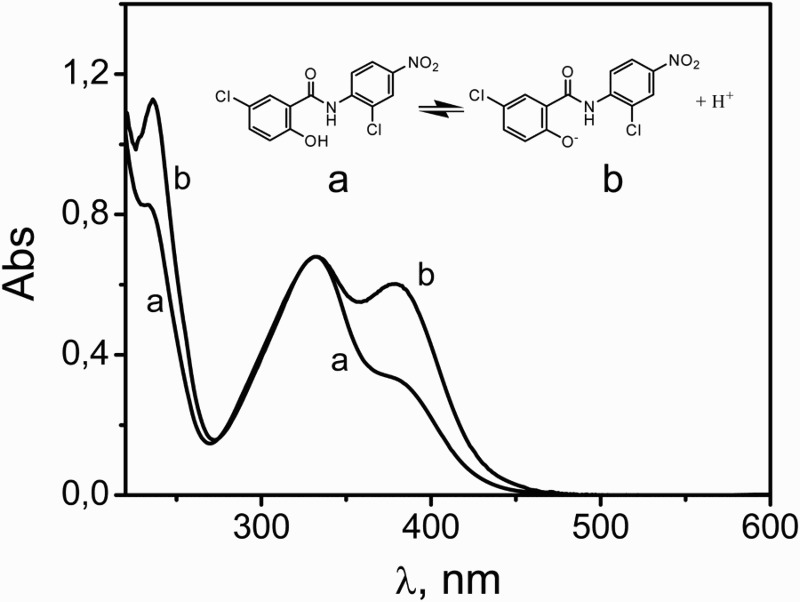
Acid-base equilibrium of NSD species: (a) NSD in MeOH, (b) NSD in MeOH plus 50 mM NaOH. The spectra were normalized at 330 nm.
However, although some research works emphasize the toxicity of NSD, others attribute a very low level of noxious health effects on mammals.5 In parallel, several beneficial biomedical properties have been assigned to the tenicide: a number of recent studies have established anticancer activities of the drug, both in vitro and in vivo models.6 Furthermore, NSD possesses significant activity toward tuberculosis and as a chemotherapeutic drug against trypanosomiasis.7
The variety of properties mentioned makes NSD a particularly interesting target for stability studies. The results of these experiments would be useful not only in the depuration of natural waters contaminated with NSD, but also for the prevention of NSD degradation when used as a health-beneficial drug.
In 2003, Dawson8 reviewed the processes of NSD's fate in environmental conditions. His work points out that NSD breaks down in natural waters through hydrolysis, microbial degradation, and direct photolysis. Mainly, it was established that degradation under laboratory conditions is highly dependent on microbial activity, suggesting that hydrolysis plays a limited role in the degradation of NSD.
A very recent study from Zaazaa et al.9 goes forward in the thermal degradation of NSD through an interesting kinetic and mechanistic study in alkaline solution. A novel double divisor-ratio-spectra method allowed determination of NSD in the presence of its degradation products, the mutagenic metabolites 2-chloro-4-nitro aniline and 5-chloro salicylic acid.10 Under established pseudo-first-order conditions, the degradation half-life of NSD at 80°C was 8.35 hours in 0.8 M NaOH methanolic solution.
The photochemical fading of NSD was initially observed by Strufe and Gönnert11 in 1962, upon irradiation with ultraviolet light. Some relevant quantitative data collected in Dawsońs review are half-lives of ca. 42 hours and ca. 7 days for NSD in solution, exposed to direct sunlight and upon photoirradiation in the range 290–405 nm, respectively.12
During manufacturing, storage, or even after therapeutic administration, NSD might, in the presence of native photosensitizers, be able to generate potentially aggressive agents, such as reactive oxygen species (ROS). The same hypothesis is valid for the cases when the drug is a contaminant of surface waters. According to our knowledge, no studies have specifically examined this possibility. In this context, the objective of the present work was to evaluate the stability of NSD under irradiation conditions similar to those frequently found in nature, in the presence of riboflavin (Rf, vitamin B2), a common accompanying substance that could eventually act as a native photogenerator of ROS.13–15 Rf was chosen as a photosensitizer in order to mimic natural scenarios. The vitamin constitutes one of the most important endogenous visible-light-absorbing photosensitizers in mammals. From the environmental point of view, Rf is present as traces in natural watercourses and water bodies.
Experimental
Materials
NSD (5-chloro-N-(2-chloro-4-nitrophenyl)-2-hydroxybenzamide), superoxide dismutase from bovine erythrocytes (SOD), and Rf were from Sigma. Rose Bengal (RB), furfuryl alcohol (FFA), monodeuterated methanol (CH3OD), bromoxynil, and NaOH were from Aldrich (Milwaukee, WI, USA). Sodium azide (NaN3) was from Merck. All these compounds were used as received.
Apparatus and methods
Ground state absorption spectra were registered in a Hewlett Packard 8452A diode array spectrophotometer.
Stationary photolysis in the visible range was carried out with a home-made photolyzer provided with two blue LEDs (467 ± 30 nm) or two green LEDs (510 ± 45 nm) employed for Rf-containing and RB-containing solutions, respectively.
Irradiation at 254 nm of matched absorbances of NSD and bromoxynil (the actinometer) in MeOH and MeOH plus 0.05 M NaOH was achieved with an Osram Germicide lamp (15 W) in a 1 × 1 cm fluorescence quartz cuvette provided with a magnetic stirrer.
Continuous photolysis
The reactive rate constant, kr, for the chemical reaction of O2(1Δg) (see further process (11)) was determined by a known method,16 using the expression slope/slopeR = kr [NSD]/krR [R]. The rate constant for the photooxidative step of a reference compound, R, at similar concentration, must be known. SlopeR and slope are the respective slopes for the first-order plots of oxygen consumption by the reference compound and by the substrate, under sensitized irradiation. The reference R employed was FFA, with a reported krR value in MeOH of 2.1 × 107/M/s.17 Oxygen consumption in MeOH was monitored with a 97-08 Orion electrode, employing RB as sensitizer. It was assumed that the reaction of O2(1Δg) with NSD is the only path for oxygen uptake.
Laser flash photolysis experiments
Argon-saturated aqueous solutions of Rf 0.04 mM were irradiated with a flash photolysis (LFP) apparatus. The disappearance of 3Rf*, generated by a 355 nm laser pulse, was monitored by the first-order decay of the solution absorbance at 670 nm. At this wavelength, interference from other species is negligible.
A ns Nd:YAG laser system (Spectron) at 355 nm was used for excitation, employing a 150-W Xenon lamp as source for the analyzing light. The detection system comprised a PTI (Photon Technology International) monochromator and a red-extended photomultiplier (Hamamatsu R666). Details of acquisition, monitoring, and handling of the decay 3Rf* signal have been described elsewhere.18
NSD presents a relatively strong absorption at 355 nm (ϵ355∼9700/M/cm), which is the excitation wavelength employed to generate triplet excited Rf. This condition enables the determination of the rate constant for the quenching of triplet excited Rf by NSD since a very low concentration of the quencher (Abs355∼0.01 as an upper limit) was enough to obtain a good Stern–Volmer representation. Nevertheless, it was not possible to observe any transient species derived from triplet excited Rf. In this case, a relatively high NSD concentration is required to reach the quenching of a significant fraction of the excited species.
Time-resolved phosphorescence detection of O2(1Δg)
The overall quenching rate constant for the deactivation of O2(1Δg) by NSD (kt, the sum of kq plus kr, processes (10) and (11), respectively, see later) was determined using a previously reported system. MeOD, instead of MeOH, was used as solvent in order to enlarge the lifetime of O2(1Δg). A lifetime of 21 μs has been obtained for O2(1Δg) in MeOD in the absence and in the presence of NaOH 0.05 M.
Results and discussion
Photosensitized irradiation
The spectral changes produced upon photoirradiation of a methanolic solution of Rf 0.035 mM plus NSD 0.45 mM with light of λ > 450 nm are shown in Fig. 2. NSD is transparent at the irradiation wavelength. For comparative purposes, the absorption spectrum of the sensitizer alone also is shown in Fig. 2. The mentioned spectral changes, in both NSD and the sensitizer, could be attributed to phototransformations. The absorbance change in the photolyzed mixture at 378 nm, as a function of irradiation time, is shown in inset A of Fig. 2 (trace b). When only the sensitizer was photoirradiated, under identical experimental conditions, the representation of these absorbance changes shows a totally different time profile (trace a). This observation indicates that both components of the photolyzed mixture are being transformed.
Figure 2.
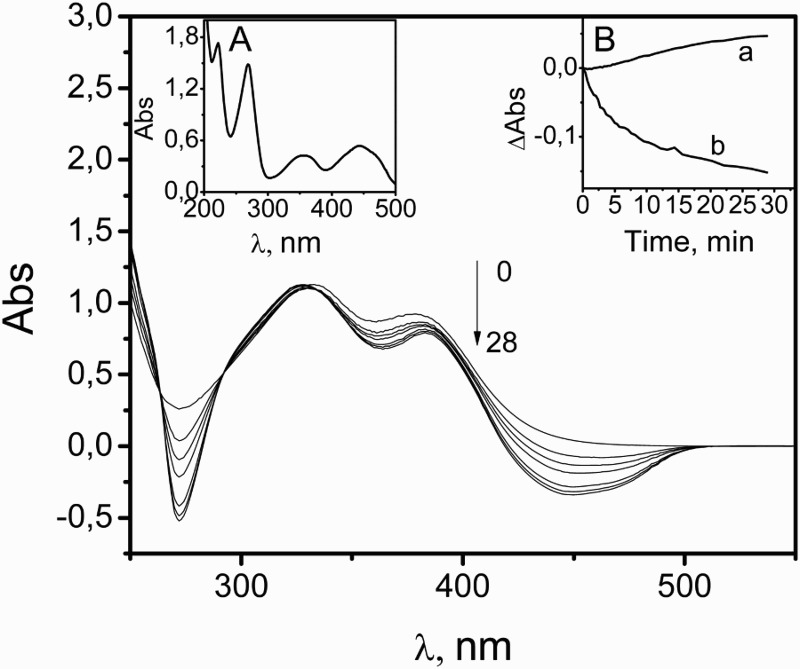
Changes in the UV-vis absorption spectrum of a methanolic solution of 0.05 mM Rf plus 0.03 mM NSD upon photoirradiation, taken vs. 0.05 mM Rf in the same solvent. Inset (A): absorption spectrum of 0.05 mM Rf in MeOH shown for comparative purposes. Inset (B): monitoring of the absorption changes at 378 nm upon photoirradiation of: Rf 0.05 mM in MeOH (a); the main figure (b).
Photoirradiation of the Rf-NSD methanolic mixtures, under the described experimental conditions, gave rise to oxygen uptake. A slight decrease in the rate of oxygen consumption (ROC) could be observed in the presence of 2 mM sodium azide, whereas in the presence of 1 μg/ml SOD, the ROC was greatly reduced. The salt and the protein are frequently employed to confirm/discard the respective participation of the ROS singlet molecular oxygen (O2(1Δg)) and superoxide anion radical in a given process.19 Results are shown in the bar diagram of Fig. 3, where there is also evident a small ROC when the sensitizer alone is photolyzed.
Figure 3.
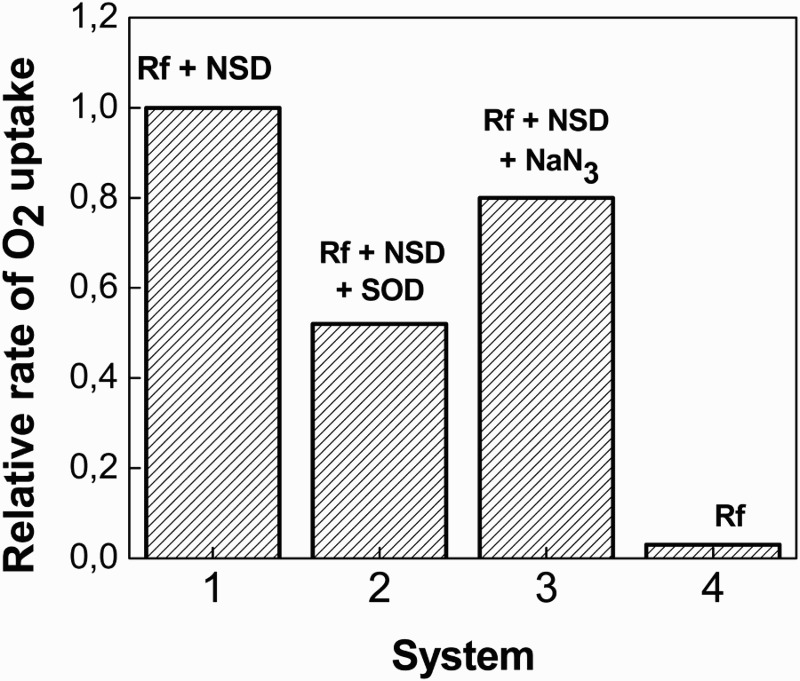
Bar diagram for the relative rates of oxygen uptake for 0.5 mM NSD + Riboflavin (A445 = 0.4) as a function of photoirradiation time in MeOH, in the absence (1) and in the presence of 1 μg/ml SOD (2); and 1 mM NaN3 (3). Bar (4) corresponds to the relative rate for riboflavin (A445 = 0.4) in MeOH.
It is known that NaN3 deactivates O2(1Δg) with a rate constant value kt = 2.2 × 108/M/s in MeOH (symbols of the rate constants are defined below). From a simple calculation through the Stern–Volmer equation, it arises that a decrease of ca. 85% must be expected for the lifetime of O2(1Δg) in a solution of the mixture NSD–NaN3 as compared to the O2(1Δg) lifetime in the presence of NSD alone. On this basis, the slight decrease in the ROC observed in Fig. 3 due to the presence of 1 mM NaN3 strongly suggests the existence of at least a second efficient pathway for oxygen consumption, generated upon Rf-photoirradiation and possibly represented by the reaction NSD–O2•−.
The interaction of NSD with electronically excited states of Rf
Experimental results indicate a possible interaction of NSD with electronically excited states of Rf and/or with photogenerated ROS. The quenching of singlet excited Rf (1Rf*) by NSD can be disregarded. Since a lifetime of ca. 5 ns has been reported for 1Rf*, a NSD concentration in the sub-mM range, similar to those employed in the present work, is not enough to significantly intercept the excited singlet species of the vitamin.
It is known that, under visible-light irradiation, the anaerobic photodegradation of Rf predominantly proceeds through its electronically excited triplet state (reaction (1)).20 The rate of the process can be estimated by monitoring the absorbance-decay of the absorption band centered at 445 nm. Comparative irradiations of Ar-saturated aqueous solutions of Rf in the presence and in the absence of 0.5 mM NSD indicated that this rate is increased in the presence of NSD by ca. 18% (data not shown). This finding strongly suggests a quenching process of the electronic excited triplet state of Rf (3Rf*).
In aerated solutions, reactions (4) and (5) may occur, leading to generation of O2•− (reaction 6).
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
| (6) |
Subsequently, the species may react with NSD (step (7)) as suggested by both the SOD-dependent oxygen consumption shown in Fig. 3 and the spectral changes observed in Fig. 2.
| (7) |
The energy transfer step (8) is known to proceed in aerated solutions, with a reported quantum yield of 0.47 in MeOH. This reaction could be competitive with the reaction pathway (3),
| (8) |
The oxidative species can decay via the solvent-dependent process (9) or interact with NSD through physical and/or the reactive processes depicted by steps (10) and (11), respectively.
| (9) |
| (10) |
| (11) |
The presence of NSD in the sub-mM concentration range produces a decrease in the 3Rf* lifetime (process (3)). A rate constant value 3 kq = 9.3 × 109/M/s was obtained, by LFP, for the quenching of 3Rf* by NSD through the Stern–Volmer treatment shown in Fig. 4. It has been reported by other authors and by ourselves that 3Rf* is quenched by substituted phenols through an electron transfer process, represented by reaction (3), with rate constant values 3 kq in the order of 109/M/s.21,22 In these cases, a positive identification of the protonated species RfH• (reaction (4)) was made through LFP experiments, strongly suggesting the occurrence of reaction (3). This might be the case in the quenching of 3Rf* by NSD. Nevertheless, we were not able to confirm the participation of the radical species involved in step (3) due to experimental limitations, as stated above.
Figure 4.
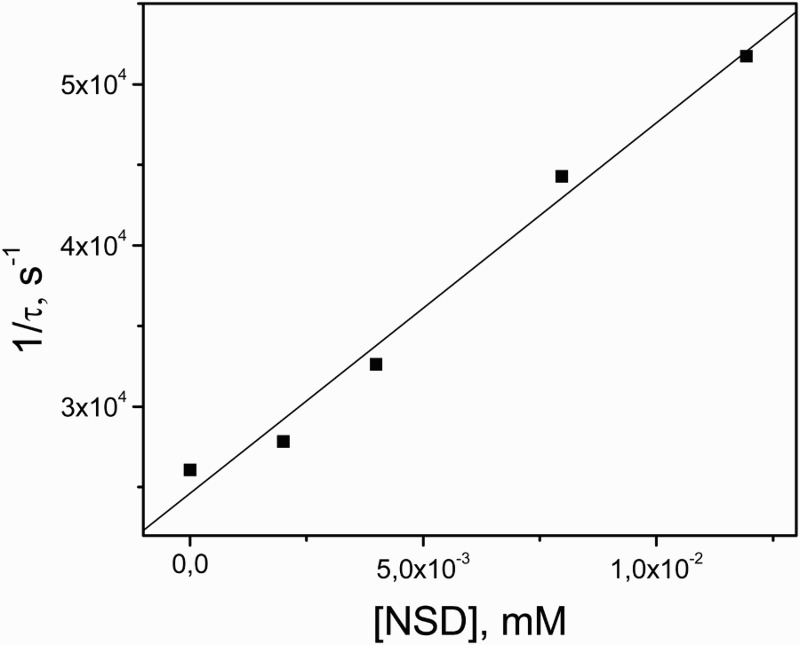
Stern–Volmer plot for the quenching of 3Rf* by NSD in MeOH. The ordinate axis represents the inverse of 3Rf* lifetime in the presence of different NSD concentrations.
The interaction of NSD with O2(1Δg)
The rate constants for the quenching of O2(1Δg) by NSD were evaluated. In order to avoid possible interactions of the substrate with 3Rf* or with ROS aside from O2(1Δg) photogenerated upon Rf photoexcitation, instead of the vitamin the well known O2(1Δg)-sensitizer RB was employed. The xanthene dye is one of the most used photosensitizers in O2(1Δg) reactions. It produces the oxidative species, in MeOH, with a quantum yield of 0.81.23 Visible-light irradiation of RB (Abs549 = 0.5) plus 0.025 mM NSD in methanolic solution produces absorbance changes in the wavelength range of NSD absorption (Fig. 5, inset). The presence of NSD in the sub-mM concentration range quenches the O2(1Δg) phosphorescence emission, as detected by TRPD experiments. This experiment unambiguously demonstrates the existence of the interaction NSD–O2(1Δg), which may be physical in nature (process (10)) and/or chemical (reactive, process (11)). It has been reported often that the interaction O2(1Δg) with different phenols occurs through processes highly dependent on the ionization degree of the phenolic OH groups.24 Hence, the rate constant kt, accounting for the overall O2(1Δg)-deactivation by NSD, was determined in pure MeOD and in MeOD/0.05 M NaOH.
Figure 5.
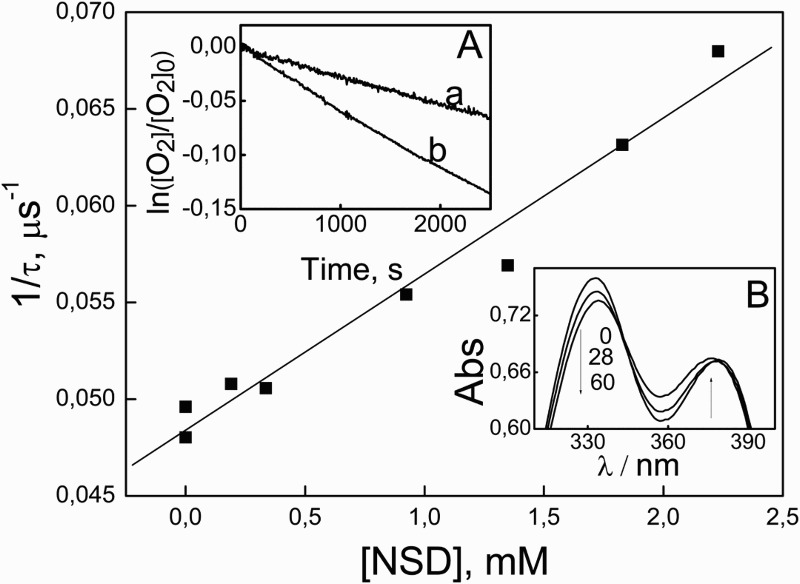
Stern–Volmer plot for the quenching of O2(1Δg) by NSD in MeOD plus 50 mM NaOH. Inset (A): first-order plot for oxygen uptake upon visible-light irradiation by solutions containing RB (A549 = 0.32) plus: 0.5 mM FFAc (a) and 0.5 mM NSD (b), in MeOH plus 50 mM NaOH. Inset (B): spectral evolution of 0.04 mM NSD plus RB A557 = 0.54 vs. RB A557 = 0.54 upon photoirradiation (cutoff 400 nm) in MeOH plus 50 mM NaOH.
The rate constant kr was determined monitoring oxygen consumption upon photoirradiation of RB-NSD mixtures, by means of an already described actinometric method. Results indicate kt = 5.1 ± 0.8 × 105/M/s in MeOD; kr = 5.0 ± 0.4 × 105/M/s in MeOH; kt = 8.7 ± 0.1 × 106/M/s in MeOD plus 0.05 M NaOH and kr = 8.6 ± 0.1 × 106/M/s in MeOH plus 0.05 M NaOH. Experimental data for the determination of kt and kr values are shown in Fig. 5.
It is known that kr/kt values represent a measure for the effectiveness of the degradation pathway in a O2(1Δg)-mediated process. At both pH values tested in these experiments, and within the estimated error, the calculated kr/kt ∼ 1 indicates a high efficiency in the O2(1Δg)-mediated degradation by the tenicide, in spite of the low values obtained for the absolute rate constants.
To our knowledge, the only report on NSD oxidation was published in 2003 by Alemu et al.25 The oxidation proceeds in a similar way to a series of Schiff bases, which present structures analogous to NSD structure. The substrate initially gives a radical cation, which is deprotonated and further oxidized to form a di-radical cation that, after losing another proton, gives a cyclic product.
Kinetic-mechanistic evaluation of NSD photooxidation
The feasibility of the electron transfer process between NSD and Rf (reaction (3)) can be evaluated in thermodynamic terms through the Gibbs free energy for electron transfer, ΔET G0 = E0(NSD/NSD+) – E0(Rf/Rf−) – ERf* + C. A value of 0.75 V was reported for E0(NSD/NSD+), the electrode potential of the donor, in aqueous solution. E0(Rf/Rf−) is the standard electrode potential of Rf, the acceptor (−0.80 V), ERf* is the 3Rf* energy (2.17 eV), and C is the coulombic energy term (−0.06 V).26 The obtained ΔET G0 value of −0.68 V constitutes evidence for the thermodynamic feasibility of process (7). As a consequence, the species could be formed through processes (4)–(6), provided that process (7) is kinetically competitive with O2(1Δg) generation, step (5). Since 3 kq = 9.3 × 109/M/s for the NSD and kET = 1.2 × 109/M e in MeOH (i.e., 1/9 of the diffusional value),27 for the same concentrations of NSD and dissolved O2(3Σg−) it can be inferred that the rate value for the generation of both RfH• − the initial precursor − is ca. eight times faster than the corresponding one for O2(1Δg), and will be the prevailing oxidation pathway. In addition to the relatively low kr value exhibited by NSD and the limited inhibitory effect of NaN3 on the ROC (Fig. 3), this finding implies a less important role for the O2(1Δg)-oxidative pathway as compared to the -mediated oxidation, under Rf photosensitization.
Comparative experiments on the photooxidation of NSD in the presence of phenol and tryptophan
In parallel experiments and for comparative purposes, the ROC by a photoirradiated solution of 0.035 mM Rf plus 0.45 mM phenol (PHE) or plus 0.45 mM of tryptophan (Trp) were evaluated. The latter was performed in the absence and in the presence of 0.45 mM NSD. The ROC by the different mixtures can be taken as a measure of their respective photodegradabilities under established experimental conditions.
PHE is considered as a paradigmatic pollutant model in the context of surface waters contamination, and the amino acid (AA) participate in photooxidative damages of proteins through photodynamic oxidation.28 In both cases, Rf plays the role of a naturally occurring ROS-photogenerator. Results are shown in Fig. 6.
Figure 6.
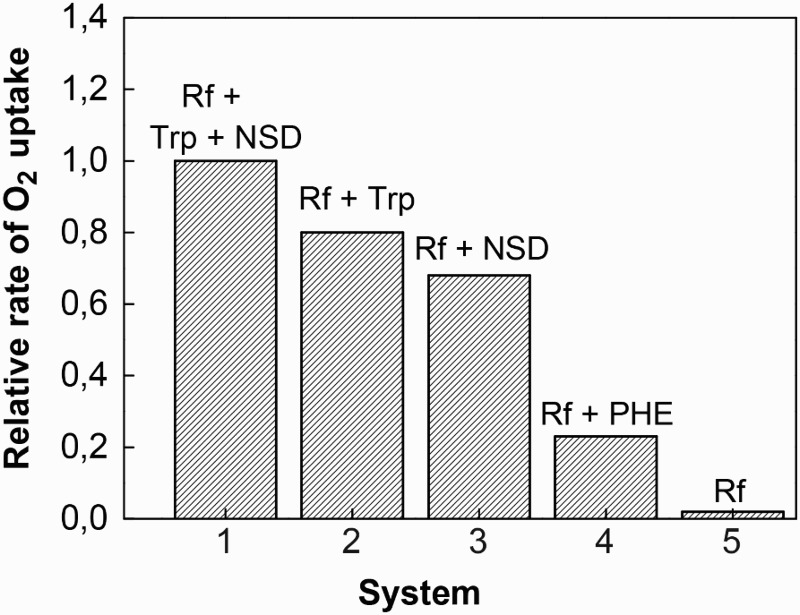
Bar diagram for the relative rates of oxygen uptake as a function of photoirradiation time (cut-off 400 nm) for 0.035 mM Riboflavin + 0.45 mM NSD + 0.45 mM Trp (1); 0.035 mM Riboflavin + 0.45 mM Trp (2); 0.035 mM Riboflavin + 0.45 mM NSD (3); 0.035 mM Riboflavin + 0.45 mM PHE (4). Bar (5) corresponds to the relative rate for 0.035 mM Riboflavin in MeOH.
The rate of oxygen uptake by the system Rf + NSD is practically three-fold higher than that observed for the mixture Rf + PHE (Fig. 6). This result suggests a higher photodegradability for NSD in a hypothetical contaminated medium. It has been previously established that under Rf-photosensitization the molecular form of PHE is degraded mainly through a -mediated mechanism. The reported 3 kq = 4.8 × 108/M/s for reaction (3) in MeOH,29 with PHE instead of NSD, indicates that the rate for the initial step for generation is ca. 20 times slower than the corresponding one for NSD. It is also known that in methanolic solution PHE quenches O2(1Δg) by an exclusive physical interaction with an extremely poor rate constant value kt = 2.5 × 104/M/s.30 In synthesis, all the experimental data related to potential interaction of PHE with Rf-photogenerated ROS justify the observed lower reactivity of PHE as compared to the tenicide reactivity.
The observed ROC for Trp is a little higher than the respective one exhibited by NSD under identical conditions (Fig. 6). Besides, the photooxidation rate of the AA plus NSD is very much lower than the simple addition of the respective ROCs by the individual substrates in the presence of Rf. This can be due to a differential contribution of the individual mixture components to the whole oxidative process. The overall mechanism may be affected by the interaction of either the initial by-products generated upon photoirradiation or by interactions of the substrates with the electronically excited states of the sensitizer that generate ROS.
The photodynamic action on aromatic AAs is well known.31 Mainly Type II (O2(1Δg)-mediated) and Type-I (radical-mediated) reactions are the reported mechanisms responsible for degradation of Trp under Rf-photosensitization.32 A rate constant value kt = 6 × 106/M/s in MeOH has been reported for the interaction O2(1Δg)–Trp.33 It is also known that Trp quenches 3Rf* (reaction (3) with Trp instead of NSD) with 3 kq values in the range of the diffusion limit.34 For Trp, the primary products are the reduced flavin radicals (RfH•) and oxidized radicals of the AA. The overall reaction observed was conversion of dissolved O2(3Σg−) to . On this basis, the apparently complex photoprocesses in the system NSD + Trp + Rf could be simplified by considering a scheme that includes quenching of 3Rf* by Trp and NSD, production of O2(1Δg) and and interaction of the generated ROS with the oxidizable substrates. The ROC from NSD remains as a low fraction of the overall contribution by the mixture, but the rate for isolated Trp and for the mixture are not significantly different. This effect could be attributed to the quenching of 3Rf* by NSD and Trp. The process would start a Type I mechanism. As Type II is the dominant route in the Rf-sensitized photooxidation of Trp,35 the quenching of the excited triplet flavin by NSD would decrease the stationary concentration of O2(1Δg) with the concomitant reduction of the overall ROC by the mixture. In a biological medium, the role exerted by NSD can be interpreted as a sort of photoprotection against vitamin B2-mediated oxidation of a Trp-containing proteinaceous environment.
Direct photolysis of NSD
To our knowledge, no quantitative information on the evaluation of direct UV photolysis of NSD has been reported. Hence, considering the direct photoirradiation as a possible alternative for NSD degradation, the quantum yield for this process, upon 254 nm-irradiation (phir(254)), was determined in MeOH and MeOH plus 0.05 M NaOH. The phenolic derivative bromoxynil was used as an actinometer, for which a φr(254) = 0.18 has been reported, in neutral water. The φr(254) values of 0.0010 ± 0.0004 and 0.0013 ± 0.0004 were obtained for NSD in MeOH in the absence and in the presence of 0.05 M NaOH, respectively. Typical results are shown in Fig. 7.
Figure 7.
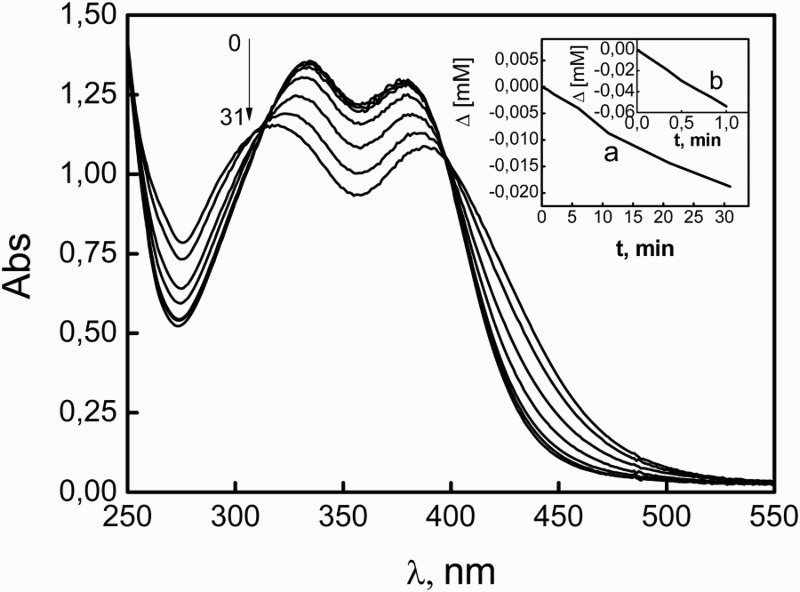
Evolution of the absorption spectrum of 0.08 mM NSD in MeOH plus 50 mM NaOH upon photolysis at 254 nm. Numbers on the spectra represent irradiation time, in minutes. Inset (a): molar consumption as a function of irradiation time for 0.08 mM NSD in MeOH plus 50 mM NaOH monitored at 380 nm. Inset (b): molar consumption as a function of irradiation time for 0.16 mM BXN in H2O (the actinometer) monitored at 285 nm.
This kind of energetically expensive treatment, with an input of ca. 470 kJ/Einstein, could be of interest in case of deposits of residues of the tenicide for which a rapid degradation is required. Nevertheless, results indicate a low photodegradation efficiency for NSD, independent of the different ionization degree of the phenolic OH group.
Final remarks
NSD is photooxidized in methanolic solution by visible-light irradiation in the presence of Rf as sensitizer. Experimental evidence strongly suggests the participation of the ROS and O2(1Δg). Alkaline medium highly favors the O2(1Δg)-mediated oxidative pathway, due to the ionization of the phenolic group of NSD.
In comparative terms and under Rf photosensitization, NSD is degraded very much faster than phenol, the latter being considered a paradigmatic water-contaminant model.
In the context of a possible role of NSD in biological environments, mutual interactions between NSD, Trp, and Rf-electronically excited states, under Rf-photosensitized irradiation, induce photoprotection of the AA against photodynamic degradation.
Highly energetic direct photoirradiation produces NSD degradation with a quantum yield of 0.001.
Acknowledgment
The authors acknowledge M S Mabel Bregliani for helpful assistance in improving the use of English in the manuscript.
Disclaimer statements
Contributors None.
Funding Financial support from Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT), Ministerio de Ciencia y Tecnología de Córdoba, Secretarías de Ciencia y Técnica of the Universidad Nacional de Río Cuarto (SECyT UNRC), of the Universidad Nacional de la Patagonia SJB (SECyT UNP-SJB), and of the Universidad Nacional de la Patagonia Austral (SECyT UNPA), all from Argentina, is gratefully acknowledged.
Conflict of interest None.
Ethics approval Ethics approval is not applicable in this case.
References
- 1.Tomlin C. The pesticide manual. London, UK: British Crop Protection Council and The Royal Society of Chemistry; 1994. [Google Scholar]
- 2.Boogaard MA, Bills TD, Johnson DA. Acute toxicity of TFM and a TFM/niclosamide mixture to selected species of fish, including Lake Sturgeon (Acipenser fulvescens) and Mudpuppies (Necturus maculosus), in laboratory and field exposures. J Great Lakes Res 2003;29:529–41. doi: 10.1016/S0380-1330(03)70514-0 [DOI] [Google Scholar]
- 3.Dai J, Coles GC, Wang J, Liang Y. Toxicity of a novel suspension concentrate of niclosamide against Biomphalaria glabrata. Trans R Soc Trop Med Hyg 2010;104:304–6. doi: 10.1016/j.trstmh.2009.07.015 [DOI] [PubMed] [Google Scholar]
- 4.Abreu FC, Goulart MOF, Oliveira Brett AM. Detection of the damage caused to DNA by niclosamide using an electrochemical DNA-biosensor. Biosens Bioelectron 2002;17:913–19. doi: 10.1016/S0956-5663(02)00082-9 [DOI] [PubMed] [Google Scholar]
- 5.Merschjohann K, Steverding D. In vitro trypanocidal activity of the anti-helminthic drug niclosamide. Exp Parasitol 2008;118:637–40. doi: 10.1016/j.exppara.2007.12.001 [DOI] [PubMed] [Google Scholar]
- 6.Li Y, Li P, Roberts MJ, Arend RC, Samant RS, Buchsbaum DJ. Multi-targeted therapy of cancer by niclosamide: a new application for an old drug. Cancer Lett 2014;349:8–14. doi: 10.1016/j.canlet.2014.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun Z, Zhang Y. Antituberculosis activity of certain antifungal and antihelmintic drugs. Tuber Lung Dis 1999;79:319–20. doi: 10.1054/tuld.1999.0212 [DOI] [PubMed] [Google Scholar]
- 8.Dawson VKJ. Environmental fate and effects of the lampricide bayluscide: a review. Int Assoc Great Lakes Res 2003;29:475–92. doi: 10.1016/S0380-1330(03)70509-7 [DOI] [Google Scholar]
- 9.Zaazaa HA, Abdelrahman MM, Ali NW, Magdy MA, Abdelkawy M. Kinetic study and mechanism of niclosamide degradation. Spectrochim Acta Part A Mol Biomol Spectrosc 2014;132:655–62. doi: 10.1016/j.saa.2014.04.050 [DOI] [PubMed] [Google Scholar]
- 10.Espinosa-Aguirre JJ, Reyes RE, Cortinas de Nava C. Mutagenic activity of 2-chloro-4-nitroaniline and 5-chlorosalicylic acid in Salmonella typhimurium: two possible metabolites of niclosamide. Mutat Res Lett 1991;264:139–45. doi: 10.1016/0165-7992(91)90131-M [DOI] [PubMed] [Google Scholar]
- 11.Strufe R, Gönnert R. Comparative studies on the influence of environmental factors on the efficiency of Bayluscide. Pflanzenschutz-Nach 1962;15:50–70. [Google Scholar]
- 12.Meyling AH, Schutte CHJ, Pitchford RJ. Some laboratory investigations on Bayer 73 and ICI 4223 as molluscicides. Bull Wld Health Organ 1962;27:95–8. [PMC free article] [PubMed] [Google Scholar]
- 13.Heelis PF. The photophysical and photochemical properties of flavins (isoalloxazines). Chem Soc Rev 1982;11:15–39. doi: 10.1039/cs9821100015 [DOI] [Google Scholar]
- 14.Momzikoff A, Santus R, Giraud M. A study of the photosensitizing properties of seawater. Mar Chem 1983;12:1–14. [Google Scholar]
- 15.Chacón JN, McLearie J, Sinclair RS. Singlet oxygen yields and radical contributions in the dye-sensitized photo-oxidation in methanol of esters of polyunsaturated fatty acids (oleic, linoleic, linolenic and arachidonic). Photochem Photobiol 1998;47:647–56. doi: 10.1111/j.1751-1097.1988.tb02760.x [DOI] [PubMed] [Google Scholar]
- 16.Scully FE, Hoingé J. Rate constants for the reaction of singlet oxygen with phenols and other compounds in water. Chemosphere 1987;16:694–99. doi: 10.1016/0045-6535(87)90004-X [DOI] [Google Scholar]
- 17.Wilkinson F, Helman WP, Ross A. Rate constants for the decay and reactions of the lowest electronically excited state of molecular oxygen in solution. An extended and revised compilation. J Phys Chem Ref Data 1995;24:663–1021. doi: 10.1063/1.555965 [DOI] [Google Scholar]
- 18.Massad W, Bertolotti S, Romero M, García NA. A kinetic study on the inhibitory action of sympathomimetic drugs towards photogenerated oxygen active species. The case of phenylephrine. J Photochem Photobiol B Biol 2005;80:130–8. doi: 10.1016/j.jphotobiol.2005.03.010 [DOI] [PubMed] [Google Scholar]
- 19.Afanas'ev IB. Superoxide ion: chemistry and biological implications. Boca Ratón, FL: CRC Press; 1989. [Google Scholar]
- 20.Fritz BJ, Matsui K, Kasai S, Yoshimura A. Triplet lifetime of some flavins. Photochem Photobiol 1987;45:539–41. doi: 10.1111/j.1751-1097.1987.tb05415.x [DOI] [Google Scholar]
- 21.Görner HJ. Oxygen uptake after electron transfer from amines, amino acids and ascorbic acid to triplet flavins in air-saturated aqueous solution. Photochem Photobiol B Biol 2007;87:73–80. doi: 10.1016/j.jphotobiol.2007.02.003 [DOI] [PubMed] [Google Scholar]
- 22.Escalada JP, Pajares A, Gianotti J, Biasutti A, Criado S, Molina P, et al. Photosensitized degradation in water of the phenolic pesticides bromoxynil and dichlorophen in the presence of riboflavin, as a model of their natural photodecomposition in the environment. J Hazard Mater 2011;186:466–72. doi: 10.1016/j.jhazmat.2010.11.026 [DOI] [PubMed] [Google Scholar]
- 23.Amat-Guerri F, López-González MMC, Martínez-Utrilla R, Sastre R. Singlet oxygen photogeneration by ionized and un-ionized derivatives of Rose Bengal and Eosin Y in diluted solutions. J Photochem Photobiol A Chem 1990;53:199–210. doi: 10.1016/1010-6030(90)87124-T [DOI] [Google Scholar]
- 24.García NA. Singlet molecular oxygen-mediated photodegradation of aquatic phenolic pollutants. A kinetic and mechanistic overview. J Photochem Photobiol B Biol 1994;22:185–96. doi: 10.1016/1011-1344(93)06932-S [DOI] [Google Scholar]
- 25.Hailemichael A, Khoabane NM, Tseki PF. Electrochemical oxidation of niclosamide at a glassy carbon electrode and its determination by voltammetry. Bull Chem Soc Ethiop 2003;17:95–106. [Google Scholar]
- 26.Porcal G, Bertolotti SG, Previtali CM, Encinas MV. Electron transfer quenching of singlet and triplet excited states of flavins and lumichrome by aromatic and aliphatic electron donors. Phys Chem Chem Phys 2003;5:4123–8. doi: 10.1039/b306945a [DOI] [Google Scholar]
- 27.Koizumi M, Kato S, Mataga N, Matsuura T, Isui I. Photosensitized reactions. Kyoto, Japan: Kagakudogin Publishing Co; 1978. [Google Scholar]
- 28.Straight RC, Spikes JD. Photosensitized oxidation of biomolecules. In: Frimer AA. (ed.) Singlet O2. Boca Raton, FL: CRC Press; 1985. 4 p. 91–143. [Google Scholar]
- 29.Haggi E, Bertolotti S, García NA. Modeling the environmental degradation of water contaminants. Kinetics and mechanism of the riboflavin-sensitised-photooxidation of phenolic compounds. Chemosphere 2004;55:1501–7. doi: 10.1016/j.chemosphere.2004.01.016 [DOI] [PubMed] [Google Scholar]
- 30.Scurlok R, Rougee M, Bensasson RV. Redox properties of phenols their relationships to singlet oxygen quenching and to their inhibitory effects on benzo(a)pyrene-induced neoplasia. Free Rad Res Commun 1990;8:251–8. doi: 10.3109/10715769009053358 [DOI] [PubMed] [Google Scholar]
- 31.Bertolotti SG, Argüello GA, García NA. Effect of the peptide bond on the singlet molecular oxygen mediated photooxidation of tyrosine and tryptophan dipeptides. A kinetic study. J Photochem Photobiol B Biol 1991;10:57–70. doi: 10.1016/1011-1344(91)80212-Z [DOI] [PubMed] [Google Scholar]
- 32.Yettella RR, Min DB. Effects of Trolox and ascorbic acid on the riboflavin photosensitised oxidation of aromatic amino acids. Food Chem 2010;118:35–41. doi: 10.1016/j.foodchem.2009.04.022 [DOI] [PubMed] [Google Scholar]
- 33.Smith GJ. Photo-oxidation of tryptophan sensitizer by methylene blue. J Chem Soc Faraday Trans 1978;274:1350–4. doi: 10.1039/f29787401350 [DOI] [Google Scholar]
- 34.Heelis PF. The photochemistry of flavins. In: Müller F. (ed.). Chemistry and biochemistry of flavoenzymes. Vol. 1, Boca Ratón, FL: CRC Press; 1991. [Google Scholar]
- 35.De La Rochette A, Silva E, Birlouez-Aragon I, Mancini M, Edwards AM, Moliere P. Riboflavin photodegradation and photosensitizing effects are highly dependent on oxygen and ascorbate concentrations. Photochem Photobiol 2000;72:815–20. [DOI] [PubMed] [Google Scholar]


