ABSTRACT
Objectives: Determine the effects of a 12-month exercise and lifestyle intervention program on changes in plasma biomarkers of oxidative stress in pre-dialysis chronic kidney disease (CKD) patients.
Methods: A total of 136 stage 3–4 CKD patients were randomized to receive standard nephrological care with (N = 72) or without (N = 64) a lifestyle and exercise intervention for 12 months. Plasma total F2-isoprostanes (IsoP), glutathione peroxidase (GPX) activity, total antioxidant capacity (TAC), anthropometric and biochemical data were collected at baseline and at 12 months.
Results: There were no significant differences between groups at baseline. There were no significant differences in changes for standard care and lifestyle intervention, respectively, in IsoP (p = 0.88), GPX (p = 0.87), or TAC (p = 0.56). Patients identified as having high IsoP at baseline (>250 pg/mL) had a greater decrease in IsoP with lifestyle intervention compared to standard care; however, the difference was not statistically significant (p = 0.06). There was no difference in the change in kidney function (eGFR) between standard care and lifestyle intervention (p = 0.33).
Discussion: Exercise and lifestyle modification in stage 3–4 CKD did not produce changes in systemic biomarkers of oxidative stress over a 12-month period, but patients with high IsoP may benefit most from the addition of intervention to standard care.
KEYWORDS: Chronic kidney disease, exercise and lifestyle modification, oxidative stress
Introduction
Chronic kidney disease (CKD) is a common and serious condition caused by a wide range of etiological factors. Systemic biomarkers of oxidative stress have been associated with the progression of CKD [1–3]. Oxidative stress is defined as an imbalance in oxidants and antioxidants causing disruption to redox-sensitive cell processes [4]. Numerous clinical trials, not necessarily in CKD, have demonstrated that reducing oxidative stress through dietary antioxidant compounds yields little benefit to disease progression [5,6]. The complexity and heterogeneity of a typical CKD patient population, with associated comorbidities and poor lifestyle habits, highlights the importance of identifying and characterizing alterations to the systemic redox environment during CKD, to employ appropriate therapies to target oxidative stress in this population.
Cardiovascular disease (CVD) is the leading cause of morbidity and mortality in the CKD population, with patients more likely to die from CVD than progress to end stage kidney disease [7]. Oxidative stress has been implicated as a common mediator linking CKD and CVD [8]. Conventional pharmacological strategies targeting CVD and comorbidities are only partially effective in reducing the progression of CKD [9]. Improvements in physical activity may underlie the benefits on health and the burden of disease in the CKD and CVD populations, but there is, to date, little supporting evidence.
Measuring a dynamic process, such as oxidative stress, is difficult, and relies primarily on the quantification of specific end-products from free radical damage. F2-isoprostanes (IsoP) are considered stable and one of the better available measures [10]. IsoP are end-products of non-enzymatic peroxidation of arachidonyl lipids and have been studied extensively in the relationship between oxidative stress and CKD [1,2]. A change in endogenous antioxidant levels can influence the overall development of oxidative damage and should, therefore, be considered in assessments of systemic oxidative stress. Identifying site-specific changes in reactive oxygen species (ROS) generation in vivo is difficult; however, considering that oxidative stress likely influences overall disease progress, systemic markers need to be considered.
The primary aim of this study was to investigate the effect of exercise training and lifestyle intervention on systemic biomarkers of oxidative stress in stage 3–4 CKD patients otherwise on standard nephrology care, over a 12-month period. The secondary aim was to investigate changes in oxidative stress biomarkers in patients with elevated oxidative stress at baseline.
Methods
Study design
This study was a sub-study of an ongoing open-label randomized controlled trial, known as the LANDMARK-III trial (Longitudinal Assessment of Numerous Discrete Modifications of the Atherosclerotic Risk Factors in Kidney Disease). LANDMARK-III is a 3-year study comparing the effect of a nurse practitioner-led model of care with standard nephrology care on cardiovascular risk factors. Patients attending the Nephrology Outpatients Department at the Princess Alexandra Hospital Australia were screened.
Subjects
Patients were eligible for inclusion if they were aged 18–75 years, had moderate CKD (stages 3 and 4; estimated GFR [eGFR] 25–60 ml/min per 1.73 m2), and had one or more uncontrolled yet modifiable cardiovascular risk factors that were: blood pressure exceeding target (>130/80 mmHg, or >120/75 mmHg for those with diabetes or proteinuria > 1 g/ 24 h); overweight (body mass index [BMI] >25 kg/m2); poor diabetic control (hemoglobin A1c [HbA1c] >7%); or hyperlipidemia (low-density lipoprotein [LDL-C] <2.5 or <2.0 mmol/L in those with diabetes or existing coronary heart disease). Exclusion criteria were: intervention for, or symptomatic, coronary artery disease (within 3 months); current heart failure (according to New York Heart Association class III and IV) or significant valvular heart disease; pregnant or planning to become pregnant; previous kidney transplant; and life expectancy or anticipated time to dialysis or transplant <6 months.
Patient groups
Patients were randomized into two arms: Exercise and lifestyle intervention group, or usual care control group, in a 1:1 ratio using a computer random assignment program, and stratified for renal function, gender and diabetes status following baseline visits.
Control group
The control group received standard nephrology care, which included a review by a nephrologist, recommended lifestyle modification but no specific information or education, and referral to an allied health professional on an ad hoc basis.
Exercise training and lifestyle intervention group
In addition to standard nephrology care, risk factor management was provided by a multidisciplinary clinic, including a CKD nurse practitioner, dietician, exercise physiologist, diabetic educator, psychologist, and social worker to target risk factors to national guidelines [11,12], and has been described previously [13]. Briefly, exercise training incorporated at least 150 min/week of moderate intensity exercise initially supervised by an accredited exercise physiologist for 8 weeks, followed by a home-based program. All prescribed exercise was individualized. A typical gym session consisted of 20–30 min aerobic training on a treadmill or stationary bike, 20 min of whole-body resistance training followed by 10 min of aerobic training on a bike or rowing ergometer. On completion of the gym-based training, participants were provided with a swiss ball, therabands, and an exercise booklet with different exercises, tips, and goals. The intensity and types of exercises were individualized according to each patient’s condition, home equipment, and interests. A typical home-based program comprised of whole-body resistance training exercises utilizing the swiss ball and theraband for 20–30 min, 2–3 days per week, with moderate to high intensity aerobic exercise on most days of the week. Regular follow-up via telephone and/or email was performed to maintain motivation, encourage accountability, address barriers to compliance, and offer gym refresher sessions. Patients also underwent 4 weeks of group behavior and lifestyle modification facilitated by a dietician and psychologist focusing on sustainable diet and behavior change to assist with weight loss.
Oxidative stress biomarkers
Total IsoP, glutathione peroxidase (GPX) activity, and total antioxidant capacity (TAC) were measured in plasma according to previously published protocols. Briefly, total IsoP were determined via negative chemical ionization gas chromatography-tandem mass spectroscopy [14]. GPX activity was measured via a spectrophotometric assay based on the oxidation of NADPH to NADP+ [15,16]. TAC was measured via a spectrophotometric assay based on the inhibition of the absorbance of the radical cation ABTS+ [17]. The coefficient of variance (CV) for the IsoP, GPX, and TAC assays was 6.7%, 2.4%, and 1.9% respectively. Samples were measured in duplicate.
Laboratory assessment
Venous blood and spot urine samples were collected from all patients following an overnight fast. For oxidative stress assays, plasma samples were stored at −80°C with butyl hydoxytoluene (10 µL of 100 mM to each 1.5 mL Eppendorf tube) to prevent artifactual oxidation. Kidney function was determined as the eGFR using the Modified Diet in Renal Disease formula based on the isotype dilution mass spectroscopy standardized creatinine assay (MDRD175) [18]. Blood urea, hemoglobin (Hb), HbA1c, high density lipoprotein (HDL), low-density lipoprotein (LDL), cholesterol, and triglycerides were measured using standard automated laboratory techniques by Queensland Health Pathology. Demographic data relating to comorbidities and medication use were collected from patient histories.
Statistical analysis
Full statistical methods are outlined in Supplementary Material S1. Results are expressed as mean ± SD for normally distributed data, median ± interquartile range (IQR) for non-normally distributed data, or total number (n) percentage (%) for categorical data. Baseline characteristics and change scores over 12 months (Δ 12-mo) were compared between groups using independent t-tests for normally distributed data, and Mann–Whitney U-tests for non-normally distributed data. A linear-mixed model was employed to determine the effect of exercise and lifestyle intervention for changes in IsoP, GPX, or TAC over 12 months. Pearson’s product-moment correlations (r) and Spearman’s correlations (rs) were performed between changes in oxidative stress measures and other secondary measures. Data were analyzed using the statistical software SPSS version 22 (Chicago, IL). Statistical significance was defined as P≤0.05.
Results
Patient characteristics
The baseline characteristics of this patient group are summarized in Table 1. There were no significant group differences at baseline. Men comprised the majority of each group and mean BMI of each patient group was within the obese range. The prevalence of comorbidities was high with 96% and 69% of patients having hypertension and hyperlipidemia, respectively. All patients were taking at least one medication. There was no statistically significant difference (p > 0.05; independent samples t-test and Pearson’s Chi-Square tests) between patient groups at baseline for each variable.
Table 1. Baseline demographics.
| Standard care (N = 64) | Lifestyle intervention (N = 72) | |
|---|---|---|
| Women | 29 (41) | 30 (40) |
| Age (yr) | 63.5 ± 9.7 | 60.5 ± 14.2 |
| Height (cm) | 168.6 ± 8.9 | 169.0 ± 15.5 |
| Weight (kg) | 91.3 ± 29.2 | 90.2 ± 22.2 |
| BMI (kg/m2) | 31.2 ± 8.1 | 33.0 ± 6.0 |
| Clinical markers | ||
| eGFR (ml/min per 1.73 m2) | 40.0 ± 16.0 | 37.0 ± 12.0 |
| Urea (mmol/L) | 9.6 ± 4.5 | 10.4 ± 5.4 |
| Hb (g/L) | 132.1 ± 13.8 | 131.7 ± 16.4 |
| HbA1c (%) | 6.2 ± 1.4 | 6.1 ± 1.5 |
| HDL (mmol/L) | 1.0 ± 0.4 | 1.1 ± 0.6 |
| LDL (mmol/L) | 2.3 ± 1.0 | 2.5 ± 1.0 |
| Cholesterol (mmol/L) | 4.3 ± 1.6 | 4.4 ± 0.9 |
| Triglycerides (mmol/L) | 1.6 ± 1.5 | 1.5 ± 1.0 |
| Risk factors | ||
| Diabetes | 28 (40) | 32 (43) |
| Hyperlipidemia | 47 (67) | 47 (63) |
| Myocardial infarction | 14 (20) | 11 (15) |
| Heart failure | 2 (3) | 3 (4) |
| Peripheral vascular disease | 8 (11) | 17 (23) |
| Hypertension | 61 (87) | 70 (95) |
| Stent | 8 (11) | 9 (12) |
| Coronary artery bypass graft | 5 (7) | 7 (9) |
| Medications | ||
| ACEi | 35 (50) | 34 (45) |
| β-Blocker | 31 (44) | 23 (31) |
| Thiazide | 16 (23) | 13 (17) |
| Statin | 42 (60) | 47 (63) |
| Insulin | 16 (23) | 15 (20) |
| Allopurinol | 13 (19) | 9 (12) |
| Smoking history | ||
| Never | 25 (39) | 21 (29) |
| Former | 31 (48) | 39 (54) |
| Current | 8 (12) | 11 (15) |
| Unknown | 0 (0) | 1 (1) |
| Primary renal disease | ||
| Diabetes type 1 | 0 (0) | 1 (1) |
| Diabetes type 2 | 14 (22) | 10 (14) |
| Glomerulonephritis | 2 (3) | 7 (10) |
| Renal vascular disease | 5 (8) | 6 (8) |
| FSGS | 3 (5) | 2 (3) |
| IgA nephropathy | 4 (6) | 4 (6) |
| PKD | 0 (0) | 4 (6) |
| Analgesic nephropathy | 0 (0) | 2 (3) |
| Reflux nephropathy | 0 (0) | 1 (1) |
| Calculi | 0 (0) | 1 (1) |
| Pyelonephritis | 1 (1) | 0 (0) |
| Other | 24 (37) | 22 (31) |
| Unknown | 12 (19) | 11 (15) |
Values are mean ± SD (normal distribution), median ± IQR (non-normal distribution), or number (%) for categorical variables. Abbreviations: BMI, body mass index; eGFR, estimated glomerular filtration rate; FSGS, focal segmental glomerulosclerosis; HbA1c, Hemoglobin A1c; Hb, Hemoglobin; HDL, high density lipoprotein; LDL, low-density lipoprotein; PKD, polycystic kidney disease.
Changes in oxidative stress biomarkers and kidney function
There were no statistically significant differences in mean IsoP, GPX, or TAC, between patients undergoing standard care and patients undergoing the lifestyle intervention over 12 months (Figure 1a, p = 0.880; Figure 1c, p = 0.787; Figure 1d, p = 0.560, respectively). Patients identified as having high IsoP at baseline (defined as > 250 pg/mL at baseline) had a mean decrease in IsoP following standard care (−38.1 ± 81.6 pg/mL; 12% decrease from baseline) that was more pronounced in patients undergoing lifestyle intervention (−106.2 ± 157.8 pg/mL; 28% decrease from baseline); however, differences in mean Δ 12-mo IsoP between groups, in patients with high IsoP were not statistically significant (p = 0.064) (Figure 1b). Owing to the large heterogeneity of the change values, the observed power of this relationship was only 16%, suggesting a type 2 statistical error. A linear regression of the mean may also exist in this patient sub-set since these values are high at baseline [19]. A linear-mixed model performed on GPX measurements demonstrated the main effect of time (12 months) has a significant effect on GPX activity in patients, regardless of whether they were receiving standard care or lifestyle intervention (Figure 1c; 95% CI, −0.3 to −0.1, t[−6.1] = −0.2, p < 0.0001). Full linear-mixed model results are provided in Supplementary Material S2–S4.
Figure 1.
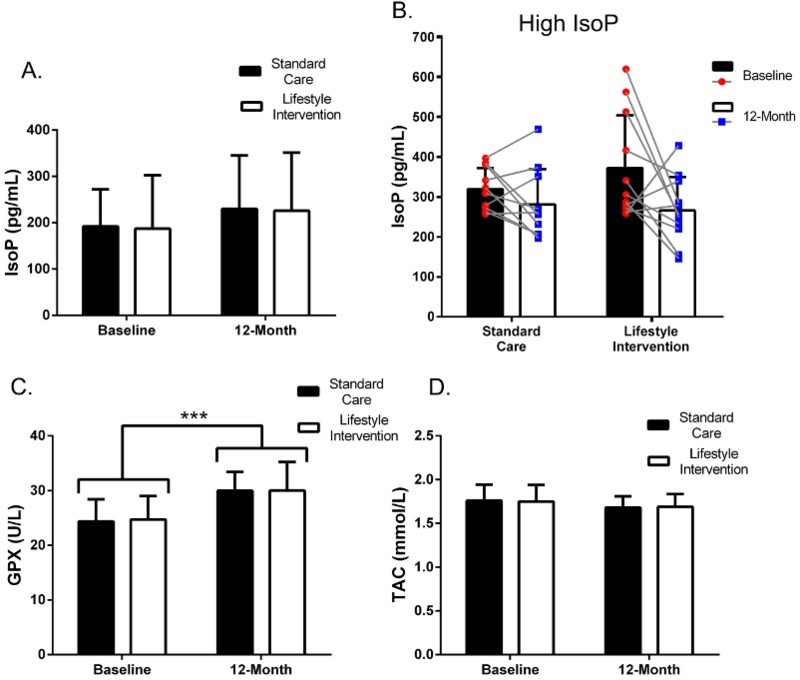
Changes in oxidative stress biomarkers following 12-month exercise and lifestyle intervention. (a) Plasma IsoP over 12 months in standard care and lifestyle intervention treatment groups. (b) A CKD patient subgroup that had high (>250 pg/mL) IsoP at baseline and repeated measures comparison show individual subject changes over 12 months in patients undergoing exercise and lifestyle intervention compared to those receiving standard care. (c) Plasma GPX and (d) plasma TAC over 12 months in patients undergoing exercise and lifestyle intervention compared to those receiving standard care. Values are presented as mean ± SEM. Δ 12-mo log transformed values underwent either an unpaired t-test (IsoP and TAC) or a Mann–Whitney U-test (GPX) for parametric and non-parametric data, respectively. A linear-mixed model incorporated the time-point, patient group, and the interaction between time-point and patient group as the main effects on plasma IsoP, GPX, or TAC. *** indicates p < 0.0001.
There were no significant differences between kidney function (eGFR) for standard care (−0.2 ± 7.8 mL/min/1.73m2) and lifestyle intervention (−1.6 ± 6.7 mL/min/1.73m2) over 12 months (p = 0.33).
Clinical correlations to changes in oxidative stress biomarkers
Biomarkers of oxidative stress correlated to various clinical parameters at baseline regardless of patient treatment allocation (Table 2), and when separated into standard and lifestyle intervention groups (Table 3). Changes in IsoP, GPX, and TAC correlated to various clinical changes over 12 months in standard care and intervention patient groups (Table 4). Selected patient characteristics, comorbidities, and medications were associated with changes in oxidative stress biomarkers in standard care and lifestyle intervention groups (Table 5). Smoking history and primary renal disease did not influence changes in oxidative stress biomarkers over 12 months (Supplementary Material S5).
Table 2. Correlation analysis between clinical parameters and oxidative stress biomarkers at baseline in all patients.
| Baseline | ||||||
|---|---|---|---|---|---|---|
| IsoP (pg/mL) | GPX (U/L) | TAC (mmol/L) | ||||
| Baseline | rs | p | rs | p | rs | p |
| Kidney function | ||||||
| eGFR (ml/min/1.73m2) | −0.007 | 0.942 | 0.235** | 0.008 | −0.192* | 0.030 |
| Urea (mmol/L) | 0.046 | 0.611 | 0.065 | 0.467 | 0.060 | 0.503 |
| Body parameters | ||||||
| BMI (kg/m2) | 0.209* | 0.020 | −0.147 | 0.106 | 0.064 | 0.479 |
| Weight (kg) | 0.095 | 0.295 | −0.135 | 0.136 | 0.101 | 0.260 |
| Glycemic control | ||||||
| HbA1c (%) | 0.034 | 0.707 | 0.190* | 0.035 | −0.049 | 0.589 |
| Blood profile | ||||||
| Hb (g/L) | −0.176* | 0.048 | −0.043 | 0.636 | 0.220* | 0.012 |
| HDL (mmol/L) | 0.274** | 0.002 | 0.193* | 0.033 | 0.067 | 0.456 |
| LDL (mmol/L) | 0.129 | 0.168 | 0.094 | 0.318 | 0.054 | 0.564 |
| Cholesterol (mmol/L) | 0.333** | <0.001 | 0.092 | 0.311 | −0.026 | 0.775 |
| Triglycerides (mmol/L) | 0.272** | 0.002 | −0.148 | 0.101 | 0.053 | 0.556 |
Spearman’s correlation was performed on continuous clinical parameters and oxidative stress biomarkers to obtain a coefficient (rs) and p value of the relationship. *p < 0.05; **p < 0.01. Shaded boxes indicate Pearson’s correlation that was performed to confirm significant relationship on normally distributed data. All analyses were performed on log transformation data. Abbreviations: IsoP, isoprostanes; GPX, glutathione peroxidase; TAC, total antioxidant capacity; eGFR, estimated glomerular filtration rate; BMI, body mass index; HbA1c, Hemoglobin A1c; Hb, Hemoglobin; HDL, high density lipoprotein; LDL, low-density lipoprotein.
Table 3. Correlation analysis between clinical parameters and oxidative stress biomarkers at baseline in patients randomized to standard care or lifestyle intervention.
| Standard care (Baseline) | Lifestyle intervention (Baseline) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IsoP (pg/mL) | GPX (U/L) | TAC (mmol/L) | IsoP (pg/mL) | GPX (U/L) | TAC (mmol/L) | |||||||
| Baseline | rs | p | rs | p | rs | p | rs | p | rs | p | rs | p |
| Kidney function | ||||||||||||
| eGFR (ml/min/1.73m2) | 0.002 | 0.985 | 0.242 | 0.061 | −0.178 | 0.166 | −0.027 | 0.832 | 0.242 | 0.052 | −0.202 | 0.104 |
| Urea (mmol/L) | 0.100 | 0.444 | 0.112 | 0.391 | 0.119 | 0.357 | 0.040 | 0.751 | 0.024 | 0.847 | −0.014 | 0.913 |
| Body parameters | ||||||||||||
| BMI (kg/m2) | 0.195 | 0.139 | −0.136 | 0.303 | −0.141 | 0.284 | 0.241 | 0.055 | −0.149 | 0.241 | 0.253 | 0.042* |
| Weight (kg) | 0.123 | 0.353 | −0.149 | 0.261 | −0.064 | 0.627 | 0.066 | 0.606 | −0.125 | 0.325 | 0.261 | 0.036* |
| Glycemic control | ||||||||||||
| HbA1c (%) | 0.056 | 0.671 | 0.292 | 0.023* | −0.050 | 0.702 | 0.006 | 0.964 | 0.091 | 0.478 | −0.054 | 0.669 |
| Blood profile | ||||||||||||
| Hb (g/L) | −0.180 | 0.166 | −0.195 | 0.133 | 0.211 | 0.100 | −0.180 | 0.150 | 0.095 | 0.478 | 0.242 | 0.051 |
| HDL (mmol/L) | 0.179 | 0.184 | 0.239 | 0.070 | 0.006 | 0.926 | 0.373 | 0.003** | 0.139 | 0.274 | 0.107 | 0.395 |
| LDL (mmol/L) | 0.214 | 0.132 | 0.160 | 0.263 | −0.099 | 0.490 | 0.068 | 0.590 | 0.067 | 0.596 | 0.183 | 0.144 |
| Cholesterol (mmol/L) | 0.434 | 0.001** | 0.014 | 0.915 | −0.155 | 0.238 | 0.236 | 0.058 | 0.160 | 0.202 | 0.101 | 0.421 |
| Triglycerides (mmol/L) | 0.344 | 0.008** | −0.156 | 0.239 | −0.119 | 0.364 | 0.207 | 0.097 | −0.152 | 0.227 | 0.236 | 0.056 |
Spearman’s correlation was performed on continuous clinical parameters and oxidative stress biomarkers to obtain a coefficient (rs) and p value of the relationship. *p < 0.05; **p < 0.01. Shaded boxes indicate Pearson’s correlation that was performed to confirm significant relationship on normally distributed data. All analyses were performed on log transformation data. Abbreviations: IsoP, isoprostanes; GPX, glutathione peroxidase; TAC, total antioxidant capacity; eGFR, estimated glomerular filtration rate; BMI, body mass index; HbA1c, Hemoglobin A1c; Hb, Hemoglobin; HDL, high density lipoprotein; LDL, low-density lipoprotein.
Table 4. Correlation analysis between clinical parameters and oxidative stress biomarkers over 12months in patients undergoing standard care or lifestyle intervention.
| Standard care (Δ 12-Months) | Lifestyle intervention (Δ 12-Months) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IsoP (pg/mL) | GPX (U/L) | TAC (mmol/L) | IsoP (pg/mL) | GPX (U/L) | TAC (mmol/L) | |||||||
| Δ 12-months | rs | p | rs | p | rs | p | rs | p | rs | p | rs | p |
| Kidney function | ||||||||||||
| eGFR (ml/min/1.73m2) | 0.075 | 0.637 | 0.104 | 0.492 | −0.001 | 0.994 | 0.257 | 0.088 | 0.081 | 0.586 | −0.291 | 0.042* |
| Urea (mmol/L) | 0.006 | 0.969 | −0.074 | 0.623 | 0.133 | 0.374 | −0.015 | 0.923 | −0.104 | 0.481 | 0.275 | 0.055 |
| Body parameters | ||||||||||||
| BMI (kg/m2) | 0.110 | 0.489 | 0.076 | 0.613 | −0.191 | 0.197 | −0.046 | 0.764 | −0.003 | 0.983 | 0.095 | 0.515 |
| Weight (kg) | 0.107 | 0.498 | 0.083 | 0.582 | −0.184 | 0.217 | −0.014 | 0.927 | 0.015 | 0.922 | 0.108 | 0.460 |
| Glycemic control | ||||||||||||
| HbA1c (%) | 0.192 | 0.229 | 0.192 | 0.205 | 0.003 | 0.983 | 0.071 | 0.651 | 0.029 | 0.847 | −0.135 | 0.366 |
| Blood profile | ||||||||||||
| Hb (g/L) | 0.092 | 0.560 | −0.116 | 0.444 | 0.170 | 0.253 | 0.083 | 0.586 | −0.008 | 0.956 | −0.105 | 0.478 |
| HDL (mmol/L) | 0.317 | 0.044* | 0.269 | 0.074 | −0.016 | 0.915 | 0.087 | 0.569 | 0.121 | 0.412 | 0.010 | 0.948 |
| LDL (mmol/L) | −0.040 | 0.814 | 0.137 | 0.393 | −0.046 | 0.774 | 0.082 | 0.591 | 0.111 | 0.459 | −0.181 | 0.217 |
| Cholesterol (mmol/L) | 0.139 | 0.387 | 0.152 | 0.320 | −0.122 | 0.420 | −0.139 | 0.361 | −0.146 | 0.323 | 0.147 | 0.312 |
| Triglycerides(mmol/L) | 0.293 | 0.063 | 0.002 | 0.991 | −0.305 | 0.039* | 0.077 | 0.616 | −0.195 | 0.185 | 0.080 | 0.584 |
Spearman’s correlation was performed on continuous clinical parameters and oxidative stress biomarkers to obtain a coefficient (rs) and p value of the relationship. * denotes p < 0.05. Shaded boxes indicate Pearson’s correlation that was performed to confirm significant relationship on normally distributed data. All analyses were performed on log transformation data. Abbreviations: IsoP, isoprostanes; GPX, glutathione peroxidase; TAC, total antioxidant capacity; eGFR, estimated glomerular filtration rate; BMI, body mass index; HbA1c, Hemoglobin A1c; Hb, Hemoglobin; HDL, high density lipoprotein; LDL, low-density lipoprotein.
Table 5. Influence of categorical patient characteristics on changes in oxidative stress biomarkers in patients undergoing standard care or lifestyle intervention.
| Standard care (Δ 12-month) | Lifestyle intervention (Δ 12-month) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IsoP (pg/mL) | GPX (U/L) | TAC (mmol/L) | IsoP (pg/mL) | GPX (U/L) | TAC (mmol/L) | |||||||
| Mean | p | Mean | p | Mean | p | Mean | p | Mean | p | Mean | p | |
| Gender | ||||||||||||
| Male | 13.7 ± 71.7 | 0.233 | 4.8 ± 3.5 | 0.881 | −0.02 ± 0.1 | 0.506 | 9.0 ± 72.4 | 0.196^ | 4.7 ± 5.6 | 0.029*^ | −0.02 ± 0.2 | 0.128 |
| Female | 64.7 ± 158.7 | 5.1 ± 4.8 | −0.05 ± 0.2 | 69.8 ± 199.5 | 7.5 ± 6.0 | −0.10 ± 0.2 | ||||||
| Diabetes | ||||||||||||
| Yes | −14.6 ± 146.6 | 0.215^ | 6.4 ± 3.2 | 0.044 | −0.03 ± 0.2 | 0.321^ | −9.33 ± 143.5 | 0.059 | 5.2 ± 6.3 | 0.511^ | −0.09 ± 0.2 | 0.236 |
| No | 36.4 ± 71.1 | 3.7 ± 4.4 | −0.04 ± 0.1 | 55.6 ± 145.0 | 5.8 ± 8.3 | −0.02 ± 0.2 | ||||||
| Hypertension | ||||||||||||
| Yes | 34.0 ± 116.3 | 0.482 | 5.3 ± 3.8 | 0.011* | −0.04 ± 0.1 | 0.051 | 20.1 ± 149.5 | 0.141 | 5.2 ± 6.3 | 0.032*^ | −0.07 ± 0.2 | 0.092 |
| No | 47.6 ± 151.7 | 0.8 ± 5.4 | 0.1 ± 0.01 | 108.2 ± 74.2 | 9.9 ± 9.6 | 0.1 ± 0.3 | ||||||
| PVD | ||||||||||||
| Yes | −29.0 ± 57.6 | 0.069 | 3.9 ± 3.0 | 0.471 | −0.1 ± 0.3 | 0.264 | 20.4 ± 113.2 | 0.922 | 3.8 ± 4.3 | 0.061^ | −0.1 ± 0.2 | 0.378 |
| No | 45.1 ± 121.9 | 5.1 ± 4.3 | −0.02 ± 0.1 | 29.0 ± 156.5 | 6.4 ± 5.7 | −0.04 ± 0.21 | ||||||
| Allopurinol | ||||||||||||
| Yes | 84.9 ± 144.7 | 0.055 | 5.7 ± 4.5 | 0.622^ | −0.03 ± 0.2 | 0.940 | 63.3 ± 50.8 | 0.091^ | 5.9 ± 9.3 | 0.872^ | −0.1 ± 0.2 | 0.194 |
| No | 21.1 ± 107.1 | 5.5 ± 4.0 | −0.03 ± 0.1 | 14.5 ± 142.2 | 5.5 ± 5.8 | −0.03 ± 0.2 | ||||||
| ACEi | ||||||||||||
| Yes | 20.4 ± 125.8 | 0.085 | 5.05 ± 3.4 | 0.774 | −0.03 ± 0.2 | 0.972 | 12.3 ± 105.9 | 0.263^ | 5.3 ± 4.6 | 0.487 | −0.04 ± 0.2 | 0.715 |
| No | 53.7 ± 107.1 | 4.8 ± 5.1 | −0.03 ± 0.1 | 56.7 ± 164.1 | 5.4 ± 7.3 | −0.06 ± 0.2 | ||||||
| Thiazide | ||||||||||||
| Yes | 16.9 ± 105.4 | 0.809 | 7.1 ± 4.9 | 0.025*^ | −0.04 ± 0.1 | 0.776 | 30.6 ± 99.8 | 0.583 | 6.7 ± 4.9 | 0.039*^ | −0.1 ± 0.2 | 0.345 |
| No | 42.7 ± 123.3 | 5.1 ± 4.7 | −0.03 ± 0.1 | 25.7 ± 163.1 | 4.8 ± 6.4 | −0.03 ± 0.2 | ||||||
| Insulin | ||||||||||||
| Yes | 57.4 ± 143.3 | 0.655 | 7.2 ± 3.9 | 0.102 | −0.1 ± 0.2 | 0.261 | −17.7 ± 150.4 | 0.487 | 5.6 ± 7.9 | 0.895 | −0.2 ± 0.2 | 0.018* |
| No | 28.9 ± 111.1 | 4.2 ± 4.0 | −0.01 ± 0.1 | 39. 4 ± 148.6 | 5.3 ± 5.5 | −0.01 ± 0.2 | ||||||
Mean ± SD (normal distribution) or median ± IQR (non-normal distribution) oxidative stress Δ 12-month values between each patient characteristic (categorical) were compared by a unpaired t-test for normally distributed data or a Mann–Whitney U-test for non-normally distributed data denoted by (^). * denotes p < 0.05. All tests were performed on log transformed data. Abbreviations: IsoP, isoprostanes; GPX, glutathione peroxidase; TAC, total antioxidant capacity; PVD, peripheral vascular disease; ACEi, angiotensin converting enzyme inhibitor.
Discussion
The influence of metabolism and systemic redox homeostasis may underlie the proven benefits of physical exercise and lifestyle modification in general health and the burden of disease. This study sought to investigate the role of exercise and lifestyle modification on systemic biomarkers of oxidative stress in the progression of moderate (pre-dialysis) CKD. The effects of the exercise and lifestyle intervention on health and fitness outcomes have been previously demonstrated within a smaller cohort of these patients, with an 11% improvement in maximal oxygen consumption (VO2 max), 20% improvement in diastolic tissue velocity (e’), and decreased body mass index (BMI) [13]. We have demonstrated here that changes in systemic biomarkers of oxidative stress over 12 months in stage 3–4 CKD patients undergoing an exercise and lifestyle intervention plus standard nephrology care were not different from patients receiving standard nephrology care alone. However, although patients with high IsoP levels at baseline (>250 pg/mL IsoP) benefited from both standard care and intervention, there was an almost 3-fold difference in the decrease in IsoP in patients on standard care plus intervention (−106.2 ± 157.8 pg/mL) versus standard care alone (−38.1 ± 81.6 pg/mL). The highly variable nature of systemic biomarkers of oxidative stress appears to have contributed to a lack of significance.
Previous studies have demonstrated changes in systemic oxidative stress biomarkers in response to exercise and lifestyle intervention despite the presence of disease [20,21]. However, the majority of studies investigating exercise and lifestyle interventions within chronic disease populations commonly report improvements in functional parameters of disease, yet rarely report changes to oxidative stress biomarkers or mechanisms underlying the benefit [22]. In the hemodialysis CKD population, 4 months of intradialytic exercise training reduced plasma lipid peroxidation that was associated with a reduction in epicardial fat thickness [23]. Chronic heart failure patients undergoing 6-month exercise therapy have been shown to increase skeletal muscle GPX and catalase while reducing lipid peroxidation [24], whereas coronary artery disease patients, who demonstrate improved endothelial dysfunction, do so independent of changes in oxidative stress markers [25]. This variability of oxidative stress responses to different types of exercise, for different periods of time, and within different disease populations, highlights the complexities of adaptations to exercise. We expected to observe a significant improvement in oxidative stress biomarkers in the patient group randomized to the lifestyle intervention compared with the group with standard nephrology care. Failure to do so could indicate a resistance to exercise-induced redox adaptations within a uremic CKD state, or alternately, the beneficial physical adaptations to exercise do not involve changes in oxidative stress in the CKD population. The latter is unlikely considering those patients, identified as having high systemic oxidative stress at baseline, had a trend of reduced IsoP following 12 months of exercise and lifestyle intervention compared to patients receiving standard care. This finding suggests patients with high IsoPs may benefit from therapies aimed at reducing oxidative stress, but further study is needed with a larger patient cohort.
GPX activity over 12 months significantly increased in CKD patients, regardless of the intervention therapy, as well as being associated with various comorbidities and medication use in this population. GPX is an intracellular endogenous antioxidant responsible for the scavenging of ROS, as well as a crucial regulator of intracellular glutathione homeostasis, which in itself is vital for maintaining the cellular redox environment [26]. Previous studies have demonstrated dampened GPX activity with decreased kidney function (eGFR) in mild–moderate CKD patients [27], which is consistent with the baseline data we report. Increased GPX activity over 12 months may indicate a greater ability to counter ROS and ensuing oxidative stress. However, this effect is not due to exercise and lifestyle modification in the current study. Increased GPX may also indicate an increase in ROS and, therefore, increased adaptive ability to reduce ROS through GPX activity. This is interesting considering that proximal tubular epithelial cells are the main source of plasma GPX activity in the kidney [28].
A high degree of variability in systemic oxidative stress biomarkers was evident over 12 months, highlighting the difficulty in measuring systemic oxidative stress via available biomarkers. IsoPs are generated from arachidonic acid through cyclooxygenase-independent pathways following free radical damage, and are considered a reliable marker primarily because they are stable [29]. Despite IsoP being chemically stable and reliably assayed, the biological variation of IsoP remains uncharacterized and appears to be large, as our results suggest. There have been claims that IsoP levels vary with time of day, and from day to day, within samples taken from the same subjects at different times [30,31]. This is relevant considering that oxidative stress is a highly dynamic process that can change significantly as a result of such influences. Therefore, it is essential that systemic biomarkers of oxidative stress take biological factors of variation into account [32]. Within the current CKD cohort, this was difficult given the complexity of comorbid disorders, many of which have previously shown a relationship to oxidative stress, including peripheral vascular disease [33], hypertension [34], obesity [35], as well as medication use, such as allopurinol [36], and ACEi [37]. There is a notable increase in IsoPs levels in some patients in both groups which indicate that individual patient circumstances can influence the biological reactivity of oxidative stress biomarkers. However, a definitive explanation of individual variation is difficult and circumstantial in this heterogeneous cohort of patients. Plasma IsoPs in both patient groups correlated significantly with changes in blood lipid profiles, probably because IsoP is a primary measure of lipid peroxidation. Specifically, HDL is the major lipoprotein carrier for IsoP [38], and is more susceptible to oxidation than LDL [39,40], which is consistent with the observed positive correlation with IsoP in the standard care patient group over 12 months. Blood lipids may, therefore, need to be considered for the interpretation of oxidative stress.
Kidney function, measured by eGFR, did not change significantly over time or with respect to the intervention, as would be expected in a cohort of mild–moderate CKD patients with existing kidney damage. The use of eGFR equations incorporating serum creatinine levels as an assessment tool for kidney function has limitations and should be interpreted with caution. Improvements in eGFR, within the exercise and lifestyle intervention groups, may be offset by increases in serum creatinine due to increased physical activity or lean body mass. Creatinine is a breakdown product of creatinine phosphate in muscle and is produced at a constant rate that is freely excreted by the kidneys during resting conditions. Healthy individuals undergoing moderate/intense physical activity have higher serum and urinary creatinine compared to sedentary individuals [41]. Furthermore, creatinine clearance can return to resting levels 1 h after stopping physical exercise; however, serum creatinine remains elevated [42]. Whether these changes apply to patients with established CKD remains unknown; however, the biological possibility does exist. A recent in-depth review of GFR estimation noted increased accuracy of eGFR formulas that incorporate serum cystatin C [43], namely, the 2012 CKD-EPI creatinine-cystatin C equation [44], and may offer greater insight into kidney function changes in exercising CKD populations.
In summary, this study has demonstrated that exercise training and lifestyle intervention in patients with stage 3–4 CKD on standard nephrology care does not produce significant changes in systemic biomarkers of oxidative stress. Limitations to the present study include the open label design increasing the chance of observer and performance biases, the relatively short duration, and small sample size increasing the likelihood of type 2 statistical errors. The observed results may not be generalizable to other centers considering that this is a single-center study design. GPX activity was responsive to the progression of CKD independent of exercise and lifestyle changes. CKD patients identified with high systemic levels of IsoP were generally more responsive to exercise and lifestyle interventions, and may present an ideal population to treat with oxidative stress-targeted therapies. One positive outcome of the study is that patients, randomized to undergo exercise and lifestyle intervention, now possess additional physical fitness, support, and knowledge, to improve general health and potentially reduce their disease burden, regardless of a known underlying mechanism.
Supplementary Material
Notes on contributors
David M. Small is currently a postdoctoral researcher at Cornell University, Department of Biomedical Engineering. His PhD investigated the role of oxidative stress in acute and chronic kidney pathologies and how alterations in mitochondrial dynamics influence nephron function. His main research interests are in understanding cellular adaptations to injury and stress in kidney and cardiovascular diseases. These research interests involve the application of multiphoton microscopy techniques to in vivo models of disease.
Dr. Kassia S. Beetham has a PhD in Clinical Exercise Physiology. Specifically, her research investigates the impact of exercise training on cardiovascular risk factors in chronic kidney disease. Dr Beetham also has an interest in high-intensity interval training compared to moderate intensity continuous training in patients with chronic diseases.
Dr. Erin J. Howden is an integrative physiologist with an interest in understanding the adaptive capability and regulation of the circulation in order to promote healthy ageing. While human lifespan is increasing, healthy lifespan has reduced, meaning although humans are living longer they are living with significant disease burden. Thus, Erin's research seeks to determine whether exercise training can improve physiological function in various diseases, which represent an advanced ageing phenotype.
Dr. David R. Briskey’s primary research expertise is in biochemistry and biomedical research, with a focus on the role of microbiota on inflammation, oxidative stress and intestinal permeability. He is currently focused on linking the microbiota with chronic diseases. By combining knowledge of the microbiota with the pathogenesis of CKD and CVD he has hypothesized that the microbiota has the capability to affect CVD and CKD through regulation of NO and inflammation. He has demonstrated that probiotics supplementation in an animal model of non-alcoholic fatty liver disease can alter intestinal epithelial permeability and reducing the severity of a chronic disease.
Professor David W. Johnson is currently Director of the Metro South and Ipswich Nephrology and Transplant Service (MINTS) and Medical Director of the Queensland Renal Transplant Service at Princess Alexandra Hospital, Brisbane, Australia. He is President-elect of the International Society for Peritoneal Dialysis (ISPD) and is an International Society of Nephrology (ISN) councillor. He has published over 700 original manuscripts in peer-reviewed journals, presented over 400 abstracts at national and international scientific meetings, led numerous large multi-centre randomised trials (including IDEAL, HONEYPOT and balANZ), and received numerous national and international awards for his contributions to nephrology.
Associate Professor Nicole M. Isbel is currently full time Nephrologist, Princess Alexandra Hospital and Queensland Renal Transplant Service, Brisbane. Assoc Prof School of Medicine, University of QLD; Group leader for the Renal Research Group, Diamentina Health Partners, TRI. aHUS registry National Co-Ordinator Australia, Australian TMA Registry Steering Committee Member. Her research interests include cardiovascular complications in CKD and the use of exercise and lifestyle modification in improving cardiovascular health. Complications post kidney transplantation, complement dysregulation, better use of immunosuppressant medications post-transplant using pharmacokinetic and pharmacogenetic monitoring. She has 159 publications, 3 book chapters and 3 guideline groups.
A/Prof Glenda C. Gobe heads the Translational Research Laboratories of the Centre for Kidney Disease Research, University of Queensland Diamantina Institute (UQDI), located in the Translational Research Institute at Princess Alexandra Hospital, Brisbane, Australia. She is known for her research into molecular pathways controlling apoptosis particularly in the kidney, and her experimental and translational research into molecular strategies is aimed at improving patient outcome in acute and chronic kidney disease. Dr Gobe is also Director of Research Training at the UQDI, and has Australian and overseas postgraduate and undergraduate students taking part in her research training programs.
Jeff S. Coombes is a Professor of Human Movement and Nutrition Sciences at the University of Queensland. His research interests focus on determining the optimal exercise prescription for improving health. With theoretical backgrounds in biochemistry and physiology he conducts human studies and basic science projects. His findings have emphasized the importance of cardiorespiratory fitness for health benefits and many of his current projects are using high-intensity interval to improve fitness and investigate outcomes.
Funding Statement
The work was supported by the National Health and Medical Research Council Australia, through Australian Fellowship award [# 511081] to Prof. Wendy Hoy, and the Centre for Research Excellence (Chronic Kidney Disease in Queensland, CKD.QLD) and the Centre for Chronic Disease at the University of Queensland.
Acknowledgments
The authors sincerely thank the renal research and data management team at the Nephrology Department, Princess Alexandra Hospital, Brisbane Australia, as well as all nurses, dieticians, psychologists, diabetic educators, social workers, and exercise physiologists that provided the exercise and lifestyle intervention. Dr Anne Bernard from QFAB provided statistical support. Dr Christudas Morais provided advice on data analysis and presentation.
Disclaimer statements
Ethics approvals: The study received approval from the Princess Alexandra Human Research Ethics Committee (HREC 2007/190) and University of Queensland Medical Research Ethics Committee (MREC 2008000184), and was registered at www.anzctr.org.au (Registration Number ANZCTR 12608000337370). Participants provided written informed consent and the study complied with the Declaration of Helsinki.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
David W. Johnson http://orcid.org/0000-0001-5491-3460
References
- [1].Dounousi E, Papavasiliou E, Makedou A, et al. Oxidative stress is progressively enhanced with advancing stages of chronic kidney disease. Am J Kidney Dis. 2006;48:752–760. doi: 10.1053/j.ajkd.2006.08.015 [DOI] [PubMed] [Google Scholar]
- [2].Karamouzis I, Sarafidis PA, Karamouzis M, et al. Increase in oxidative stress but not in antioxidant capacity with advancing stages of chronic kidney disease. Am J Nephrol. 2008;28(3):397–404. doi: 10.1159/000112413 [DOI] [PubMed] [Google Scholar]
- [3].Kuchta A, Pacanis A, Kortas-Stempak B, et al. Estimation of oxidative stress markers in chronic kidney disease. Kidney Blood Press Res. 2011;34(1):12–19. doi: 10.1159/000321508 [DOI] [PubMed] [Google Scholar]
- [4].Small DM, Coombes JS, Bennett N, et al. Oxidative stress, anti-oxidant therapies and chronic kidney disease. Nephrology. 2012;17(4):311–321. doi: 10.1111/j.1440-1797.2012.01572.x [DOI] [PubMed] [Google Scholar]
- [5].Bjelakovic G, Nikolova D, Gluud LL, et al. Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. Cochrane Db Syst Rev. 2012;3:CD007176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Jun M, Venkataraman V, Razavian M, et al. Antioxidants for chronic kidney disease. Cochrane Db Syst Rev. 2012;10:CD008176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. New Engl J Med. 2004;351(13):1296–1305. doi: 10.1056/NEJMoa041031 [DOI] [PubMed] [Google Scholar]
- [8].Cachofeiro V, Goicochea M, de VSG, et al. Oxidative stress and inflammation, a link between chronic kidney disease and cardiovascular disease. Kidney Int. 2008;74(111):S4–S9. doi: 10.1038/ki.2008.516 [DOI] [PubMed] [Google Scholar]
- [9].Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. New Engl J Med. 2001;345(12):861–869. doi: 10.1056/NEJMoa011161 [DOI] [PubMed] [Google Scholar]
- [10].Tucker PS, Dalbo VJ, Han T, et al. Clinical and research markers of oxidative stress in chronic kidney disease. Biomarkers. 2013;18(2):103–115. doi: 10.3109/1354750X.2012.749302 [DOI] [PubMed] [Google Scholar]
- [11].Johnson DW. Prevention of progression of kidney disease – CARI guidelines. Aust Fam Phys. 2007;36(5):353. [PubMed] [Google Scholar]
- [12].NHF Guide to management of hypertension: national heart foundation 2008. Sydney, Australia; 2010. [Google Scholar]
- [13].Howden EJ, Leano R, Petchey W, et al. Effects of exercise and lifestyle intervention on cardiovascular function in CKD. Clin J Am Soc Nephro. 2013;8(9):1494–1501. doi: 10.2215/CJN.10141012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Briskey DR, Wilson GR, Fassett RG, et al. Optimized method for quantification of total F(2)-isoprostanes using gas chromatography-tandem mass spectrometry. J Pharm Biomed Anal. 2014;90:161–166. doi: 10.1016/j.jpba.2013.11.028 [DOI] [PubMed] [Google Scholar]
- [15].Wheeler CR, Salzman JA, Elsayed NM, et al. Automated assays for superoxide dismutase, catalase, glutathione peroxidase, and glutathione reductase activity. Anal Biochem. 1990;184(2):193–199. doi: 10.1016/0003-2697(90)90668-Y [DOI] [PubMed] [Google Scholar]
- [16].Andersen HR, Nielsen JB, Nielsen F, et al. Antioxidative enzyme activities in human erythrocytes. Clin Chem. 1997;43(4):562–568. [PubMed] [Google Scholar]
- [17].Rice-Evans C and Miller NJ. Total antioxidant status in plasma and body fluids. Method Enzymol. 1994;234:279–293. doi: 10.1016/0076-6879(94)34095-1 [DOI] [PubMed] [Google Scholar]
- [18].Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999;130(6):461–470. doi: 10.7326/0003-4819-130-6-199903160-00002 [DOI] [PubMed] [Google Scholar]
- [19].Margaritelis NV, Theodorou AA, Paschalis V, et al. Experimental verification of regression to the mean in redox biology: differential responses to exercise. Free Radic Res. 2016;50(11):1237–1244. doi: 10.1080/10715762.2016.1233330 [DOI] [PubMed] [Google Scholar]
- [20].Alessio HM and Goldfarb AH. Lipid peroxidation and scavenger enzymes during exercise: adaptive response to training. J Appl Physiol. 1988;64(4):1333–1336. [DOI] [PubMed] [Google Scholar]
- [21].Radak Z, Taylor AW, Ohno H, et al. Adaptation to exercise-induced oxidative stress: from muscle to brain. Exerc Immunol Rev. 2001;7:90–107. [PubMed] [Google Scholar]
- [22].Wang YL, Shu KH, Yang MF, et al. The impact of body weight management in chronic kidney disease patients with obesity. J Renal Nutr. 2013;23(5):372–379. doi: 10.1053/j.jrn.2013.04.004 [DOI] [PubMed] [Google Scholar]
- [23].Wilund KR, Tomayko EJ, Wu PT, et al. Intradialytic exercise training reduces oxidative stress and epicardial fat: a pilot study. Nephrol Dial Transpl. 2010;25(8):2695–2701. doi: 10.1093/ndt/gfq106 [DOI] [PubMed] [Google Scholar]
- [24].Linke A, Adams V, Schulze PC, et al. Antioxidative effects of exercise training in patients with chronic heart failure: increase in radical scavenger enzyme activity in skeletal muscle. Circulation. 2005;111(14):1763–1770. doi: 10.1161/01.CIR.0000165503.08661.E5 [DOI] [PubMed] [Google Scholar]
- [25].Luk TH, Dai YL, Siu CW, et al. Effect of exercise training on vascular endothelial function in patients with stable coronary artery disease: a randomized controlled trial. Eur J Prev Cardiol. 2012;19(4):830–839. doi: 10.1177/1741826711415679 [DOI] [PubMed] [Google Scholar]
- [26].Godoy JR, Oesteritz S, Hanschmann EM, et al. Segment-specific overexpression of redoxins after renal ischemia and reperfusion: protective roles of glutaredoxin 2, peroxiredoxin 3, and peroxiredoxin 6. Free Radical Bio Med. 2011;51(2):552–561. doi: 10.1016/j.freeradbiomed.2011.04.036 [DOI] [PubMed] [Google Scholar]
- [27].Crawford A, Fassett RG, Coombes JS, et al. Glutathione peroxidase, superoxide dismutase and catalase genotypes and activities and the progression of chronic kidney disease. Nephrol Dial Transpl. 2011;26(9):2806–2813. doi: 10.1093/ndt/gfq828 [DOI] [PubMed] [Google Scholar]
- [28].Avissar N, Ornt DB, Yagil Y, et al. Human kidney proximal tubules are the main source of plasma glutathione peroxidase. Am J Physiol. 1994;266(2 Pt 1):C367–C375. [DOI] [PubMed] [Google Scholar]
- [29].Roberts LJ, Morrow JD. Products of the isoprostane pathway: unique bioactive compounds and markers of lipid peroxidation. Cell Mol Life Sci. 2002;59(5):808–820. doi: 10.1007/s00018-002-8469-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Helmersson J, Basu S. F2-isoprostane and prostaglandin F2αmetabolite excretion rate and day to day variation in healthy humans. Prostag Leukotr Ess Fatty Acids. 2001;65(2):99–102. doi: 10.1054/plef.2001.0295 [DOI] [PubMed] [Google Scholar]
- [31].Kanabrocki EL, Murray D, Hermida RC, et al. Circadian variation in oxidative stress markers in healthy and type II diabetic men. Chronobiol Int. 2002;19(2):423–439. doi: 10.1081/CBI-120002914 [DOI] [PubMed] [Google Scholar]
- [32].Strobel NA, Fassett RG, Marsh SA, et al. Importance of understanding pre-analytical variability in biomarker development. Int J Cardiol. 2011;150(2):223–224. doi: 10.1016/j.ijcard.2011.05.014 [DOI] [PubMed] [Google Scholar]
- [33].Roller RE, Renner W, Dorr A, et al. Oxidative stress and increase of vascular endothelial growth factor in plasma of patients with peripheral arterial occlusive disease. Thromb Haemost. 2001;85(2):368. [PubMed] [Google Scholar]
- [34].Velayutham PK, Adhikary SD, Babu SK, et al. Oxidative stress-associated hypertension in surgically induced brain injury patients: effects of beta-blocker and angiotensin-converting enzyme inhibitor. J Surg Res. 2013;179(1):125–131. doi: 10.1016/j.jss.2012.09.005 [DOI] [PubMed] [Google Scholar]
- [35].Matsuda M, Shimomura I. Increased oxidative stress in obesity: implications for metabolic syndrome, diabetes, hypertension, dyslipidemia, atherosclerosis, and cancer. Obes Res Clin Pract. 2013;7(5):e330–e341. doi: 10.1016/j.orcp.2013.05.004 [DOI] [PubMed] [Google Scholar]
- [36].Vina J, Gomez-Cabrera MC, Lloret A, et al. Free radicals in exhaustive physical exercise: mechanism of production, and protection by antioxidants. IUBMB Life. 2000;50(4–5):271–277. doi: 10.1080/15216540051080994 [DOI] [PubMed] [Google Scholar]
- [37].Nakamura A, Shikata K, Nakatou T, et al. Combination therapy with an angiotensin-converting-enzyme inhibitor and an angiotensin II receptor antagonist ameliorates microinflammation and oxidative stress in patients with diabetic nephropathy. J Diabetes Invest. 2013;4(2):195–201. doi: 10.1111/jdi.12004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Proudfoot JM, Barden AE, Loke WM, et al. HDL is the major lipoprotein carrier of plasma F2-isoprostanes. The J Lipid Res. 2009;50(4):716–722. doi: 10.1194/jlr.M800607-JLR200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Raveh O, Pinchuk I, Schnitzer E, et al. Kinetic analysis of copper-induced peroxidation of HDL, autoaccelerated and tocopherol-mediated peroxidation. Free Radical Bio Med. 2000;29(2):131–146. doi: 10.1016/S0891-5849(00)00332-4 [DOI] [PubMed] [Google Scholar]
- [40].Nagyova A, Krajcovicova-Kudlackova M, Klvanova J. LDL and HDL oxidation and fatty acid composition in vegetarians. Ann Nutr Metab. 2001;45(4):148–151. doi: 10.1159/000046722 [DOI] [PubMed] [Google Scholar]
- [41].Baxmann AC, Ahmed MS, Marques NC, et al. Influence of muscle mass and physical activity on serum and urinary creatinine and serum cystatin C. Clin J Am Soc Nephrol. 2008;3(2):348–354. doi: 10.2215/CJN.02870707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Poortmans JR, Gulbis B, De Bruyn E, et al. Limitations of serum values to estimate glomerular filtration rate during exercise. Brit J Sport Med. 2013;47(18):1166–1170. doi: 10.1136/bjsports-2012-090976 [DOI] [PubMed] [Google Scholar]
- [43].Levey AS, Fan L, Eckfeldt JH, et al. Cystatin C for glomerular filtration rate estimation: coming of age. Clin Chem. 2014;60(7):916–919. doi: 10.1373/clinchem.2014.225383 [DOI] [PubMed] [Google Scholar]
- [44].Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. New Engl J Med. 2012;367(1):20–29. doi: 10.1056/NEJMoa1114248 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


