Abstract
Objectives: Liver is considered a target organ affected by lead toxicity. Oxidative stress is among the mechanisms involved in liver damage. Here we investigated the effects of the natural alkaloid berberine on oxidative stress and hepatotoxicity induced by lead in rats.
Methods: Animals received an aqueous solution of lead acetate (500 mg Pb/l in the drinking water) and/or daily oral gavage of berberine (50 mg/kg) for 8 weeks. Rats were then weighed and used for the biochemical, molecular, and histological evaluations.
Results: Lead-induced oxidative stress, shown by increasing lipid peroxidation along with a concomitant decrease in hepatic levels of thiol groups, total antioxidant capacity, the activities of superoxide dismutase, catalase, glutathione peroxidase, and glutathione-S-transferase, and reduced versus oxidized glutathione ratio. Berberine corrected all the disturbances in oxidative stress markers induced by lead administration. Berberine also prevented the elevated levels of enzymes (alanine aminotransferase, aspartate aminotransferase, and alkaline phosphatase) and the decrease in body weight and albumin. The protective effects of berberine were comparable with silymarin. Furthermore, berberine attenuated liver damage, shown by decreased necrosis and inflammatory cell infiltration.
Discussion: Berberine represents a potential therapeutic option against lead-induced hepatotoxicity through inhibiting lipid peroxidation and enhancing antioxidant defenses.
Conclusion: Berberine exerted protective effects on lead-induced oxidative stress and hepatotoxicity in rats.
Keywords: Berberine, Liver, Lead, Hepatotoxicity, Oxidative stress, Rats
Introduction
Lead is one of the most abundant toxic metals and has been detected in all parts of the environment and in biological systems.1 Sources of human exposure to this metal include many foods, drinking water, and dust. Lead exposure or lead poisoning is known to cause a large spectrum of physiological, biochemical, and behavioral disorders in experimental animals and in humans.2–4 Liver is considered a target organ in relation to the toxic effect of lead. Several mechanisms have been proposed to explain lead-induced toxicity.1
Although the exact mechanisms by which lead induces oxidative damage in various organ systems are not completely understood, evidence indicates that multiple complex mechanisms are involved. Some studies have confirmed the possible involvement of reactive oxygen species (ROS) in lead-induced toxicity.3,5–7 Any compound that induces oxidative stress does so by accelerating the generation of pro-oxidants and at the same time may also reduce cellular antioxidant defenses. Studies have reported that exposure to lead will lead to the disruption of the reducing status of a tissue and drive ROS formation, which will damage essential biomolecules such as proteins, lipids, and DNA.8,9 Considering the importance of oxidative stress in lead-induced toxicity, it is reasonable that administration of an antioxidant may be an important therapeutic approach in lead intoxication.1,8–10
Berberine is an isoquinoline alkaloid present in a number of important medicinal plant species, such as Berberis aristata and Berberis aquifolium. Berberine exhibits multiple pharmacological properties, including antibacterial,11 anti-hypertensive,12 anti-inflammatory,13 anti-diabetic,14 and anti-hyperlipidemic activities.15 It has been shown that berberine ameliorates liver fibrosis in mice with CCl4-induced liver injury and inhibits the proliferation of hepatic stellate cells in a dose- and time-dependent manner.16 Berberine also has hepatoprotective effects on liver toxicity induced by doxorubicin17 and cyclophosphamide18 in mice and alleviates the elevation of serum marker enzymes in addition to its antioxidant and anti-inflammatory effects.
Heavy metals including lead are the oldest toxins known to humans. The most commonly affected organ is liver. Furthermore, generation of oxidative stress has been considered as one of the major mechanisms behind heavy metal toxicity. To our knowledge, no study has been carried out concerning the protective effects of berberine on heavy metal-induced oxidative stress and toxicity in liver. Taking the above into account, we aimed to assess the effects of berberine on lipid peroxidation and antioxidant defense systems in a rat model of lead-induced liver injury and to compare its effects with those of the classic antioxidant and hepatoprotector silymarin.
Materials and methods
Drugs and chemicals
Berberine, lead acetate (Pb(CH3COO)2), and silymarin (purity >98%) were obtained from Sigma (St Louis, MO, USA). 2,2′-Dinitro-5,5′-dithiodibenzoic acid (DTNB), 2,4,6-tri(2-pyridyl)-1,3,5-triazine (TPTZ), 2-thiobarbituric acid (TBA), trichloroacetic acid (TCA), n-butanol, Tris–hydrochloric acid, ethylenediaminetetraacetic acid, sodium acetate, glacial acetic acid, phosphoric acid, potassium chloride, tetramethoxypropane (TMP), ferric chloride (FeCl3·6H2O), ferrous sulfate, and hydrochloric acid were obtained from Merck (Darmstadt, Germany). All other chemicals were of analytical grade.
Animals
Locally bred male Wistar rats (250–300 g) were obtained from the breeding colony of the Iran Pasteur Institute, Tehran. Four rats were housed per cage and were kept at a constant temperature of 20 ± 2°C with a 12:12-hour light/dark cycle (lights on at 7:00 AM). Food and water were available ad libitum in the home cages. Rats were divided randomly into four experimental groups. Each experimental group consisted of seven animals that were chosen randomly from different cages and each was used only once. The rats were habituated to the environment for at least 1 week before the start of the experiment. Animals were handled in accordance with the criteria outlined in the Guide for the Care and Use of Laboratory Animals (National Institutes of Health (NIH) publication 86-23; revised 1985; http://www.oacu.od.nih.gov/regs/guide/guidex.htm). The protocols were also approved by the Animal Experimentation Ethics Committee of Bu-Ali Sina University (No. 12A-1325) and all procedures were performed in accordance with the recommendations for the proper care and use of laboratory animals. All procedures and experiments were performed between 10:00 and 14:00.
Experimental design
After acclimatization to the laboratory conditions, the animals were randomly divided into five groups (n = 7): (1) control group, where the rats received lead-free redistilled water and daily oral gavage of physiological saline during the whole course of the experiment; (2) lead-treated group, animals received an aqueous solution of lead acetate (500 mg Pb/l of drinking water); (3) lead + berberine-treated group, animals received an aqueous solution of lead acetate (500 mg Pb/l in the drinking water) and daily oral gavage of berberine (50 mg/kg) dissolved in physiological saline; (4) berberine-treated group, animals received a daily oral gavage administration of berberine (50 mg/kg) and (5) lead + silymarin-treated group, where rats received an aqueous solution of lead acetate (500 mg Pb/l in the drinking water) and daily oral gavage of silymarin (200 mg/kg). The treatments in all groups lasted for 8 weeks. The treatment protocols and the doses used were based on previously published studies.1,17,19,20 At the end of treatments, rats in each group were weighed and then used for biochemical analysis and histological evaluation. Rats were anesthetized with ketamine/xylazine mixture (ketamine 67 mg/kg, xylazine 6 mg/kg, i.p.) and about 5 ml of blood was drawn by cardiac puncture into heparin tubes. The plasma was collected after centrifugation at 1800g for 10 minutes and stored in a −70°C freezer until further analysis of biochemical parameters. Immediately after blood collection, animals were sacrificed by an overdose of sodium pentobarbital (200 mg/kg, i.p.). The liver of each rat was removed, washed with ice-cold saline at −80°C, and homogenized in cold potassium phosphate buffer (0.05 M, pH 7.4). Liver homogenates were centrifuged at 2800g for 10 minutes at 4°C. The resulting supernatant was used for determination of oxidant/antioxidant markers.
Parts of liver obtained from each rat were fixed in 10% formalin solution for 24 hours, dehydrated in ascending grades of mixtures of ethyl alcohol–water, cleaned in xylene, and embedded in paraffin. Sections of the tissue were prepared by using a rotary microtome and stained with hematoxylin and eosin (H&E) dye, which was mounted in a neutral deparaffinated xylene medium for microscopic observations. Sections were examined under light microscope by a pathologist unaware of the treatment protocol.
Analysis of serum biochemical parameters
The concentrations of albumin (g/dl) as well as the activities of alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase (ALP) were measured using standard techniques and commercial kits (Pars Azmoon Co., Iran) with an auto analyzer (Gcsan Chem 2000, Spain).
Assay of malondialdehyde level
Malondialdehyde (MDA) content, a measure of lipid peroxidation, was assayed in the form of TBA-reacting substances. Briefly, the samples were diluted by1.5 ml TCA (20% w/v) and centrifuged at 3000g for 10 minutes. The precipitate was dissolved in sulfuric acid and 1.5 ml of the mixture was added to 1.5 ml of TBA (0.2% w/v). The mixture was then incubated for 1 hour in a boiling water bath. Following incubation, 2 ml of n-butanol was added, the solution centrifuged, cooled, and the absorption of the supernatant recorded in 532 nm. A calibration curve of tetraethoxypropane standard solutions was used to determine the concentrations of TBA + MDA adducts in samples.21
Assay of total sulfhydryl groups
Total thiol content was estimated based on the Ellman22 method. In this method, thiol groups interact with DTNB to form a yellow complex dianion (5-thio-2-nitrobenzoic acid), which shows maximum absorbance in 412 nm.
Assay of total antioxidant capacity level
The ferric reducing/antioxidant power (FRAP) method was used to determine total antioxidant capacity (TAC) levels, which depend upon the reduction of ferric tripyridyltriazine complex to the ferrous tripyridyltriazine by a reductant at low pH. This ferrous tripyridyltriazine complex has an intensive blue color and can be monitored at 593 nm.23 The FRAP reagent consists of 300 mM acetate buffer (pH = 3.6), 10 mM TPTZ in 40 mM HCl, and 20 mM FeCl3·6H2O in the ratio of 10:1:1. Briefly, 50 μl of homogenate was added to 1.5 ml freshly prepared and pre-warmed (37°C) FRAP reagent in a test tube and incubated at 37°C for 10 minutes. The absorbance of the blue-colored complex was read against reagent blank (1.5 ml FRAP reagent + 50 μl distilled water) at 593 nm. Standard solutions of FeII in the range of 100–1000 mM were prepared from ferrous sulfate (FeSO4·7H2O) in distilled water. FRAP values were expressed as micromole ferric ions reduced to ferrous form/G-protein.23
Assay of superoxide dismutase activity
The supernatant of liver homogenate was obtained as described above. Superoxide dismutase (SOD) activity was measured as previously reported.24 Briefly, supernatant was incubated with xanthine and xanthine oxidase in potassium phosphate buffer (pH 7.8, 37°C) for 40 minutes, and then nitrobluetetrazolium (NBT) was added. Thereafter, blue formazan was monitored spectrophotometrically at 550 nm. The amount of protein that inhibited NBT reduction by 50% was defined as 1 nitrite unit of SOD activity.
Assay of catalase activity
The Claiborne25 method was used. Briefly, H2O2 was added to a mixture of 50 mM potassium phosphate buffer (pH 7.0) and supernatant and the rate of H2O2 decomposition was assessed by measuring the absorbance changes at 240 nm for 2 minutes. One unit of catalase (CAT) activity is defined as 1 μM of H2O2 that is decomposed in 1 minute.
Assay of glutathione peroxidase and glutathione-S-transferase (GST)
Glutathione peroxidase (GPx) and glutathione-S-transferase (GST) were assayed using ELISA kits (Diagnostics Biochem Canada Inc., Ontario, Canada).
Assay of reduced versus oxidized glutathione ratio (GSH/GSSG)
Total glutathione (GSH and GSSG) was measured using a colorimetric assay kit. The kit uses DTNB and glutathione reductase. It uses 1-methyl-2-vinylpyridinium trifluoromethanesulfonate as scavenger instead of N-ethylmaleimide. Liver harvested at 24 hours of reperfusion was minced and homogenized (g/10 ml) in 5% metaphosphoric acid. The procedure was followed according to manufacturer's instruction and the levels were quantified as micromolar GSH or GSSG based on standards supplied with the kit.
Statistical analysis
All data are expressed as mean ± SEM. The analysis was performed using the SPSS statistical software package (version 21.5; SPSS, Chicago, IL, USA). Differences among groups were tested by one-way analysis of variance (ANOVA) with Tukey as post hoc test. Probability values less than 0.05 were considered significant.
Results
Effects of berberine on the body weight and serum biochemical parameters
The effects of different treatments on the body weight and serum biochemical parameters at the end of treatments are depicted in Table 1. Eight weeks of lead treatment caused a significant reduction in body weight (P < 0.001). Berberine prevented the decrease in body weight of lead-treated rats so that there was no significant difference between the berberine-treated lead group and the untreated control animals (P > 0.05). Furthermore, berberine did not change the body weight of control rats compared to the untreated group at the end of the experiment (P > 0.05).
Table 1. Effects of long-term berberine administration on serum ALT, AST, ALP, albumin, and body weight (BW) in different animal groups at the end of the treatment period (n = 7).
| Parameters | Control | Lead | Lead + berberine | Lead + silymarin | Control + berberine |
|---|---|---|---|---|---|
| ALT (U/l) | 74.28 ± 1.51 | 132 ± 7.02*** | 89.71 ± 0.35 | 81.71 ± 3.14 | 70.7 ± 3.48 |
| AST (U/l) | 155.71 ± 3.19 | 197.42 ± 6.04*** | 165.28 ± 8.71 | 159 ± 7.33 | 160.14 ± 5.24 |
| ALP (U/l) | 129.85 ± 3.23 | 191.14 ± 2.39*** | 141.85 ± 1.83 | 135.42 ± 2.54 | 132.71 ± 7.41 |
| Albumin (g/dl) | 3.04 ± 0.14 | 2.34 ± 0.09*** | 2.87 ± 0.04 | 2.91 ± 0.03 | 3.12 ± 0.10 |
| BW (g) | 311 ± 5.7 | 213 ± 9.1*** | 286 ± 2.5 | 292 ± 5 | 331 ± 10 |
The data are represented as mean ± SEM.
Asterisk indicates significant difference compared to control group (***P < 0.001, ANOVA, Tukey's test for post hoc comparisons).
Chronic administration of lead alone to rats caused severe liver damage with significant increases in levels of ALT, AST, and ALP as well as a decrease in the level of albumin (P < 0.001 in each case). However, berberine co-treatment significantly prevented the lead-induced elevation in ALT (P < 0.001), AST (P < 0.01), and ALP (P < 0.001) of treated rats. Furthermore, the concentration of serum albumin was significantly elevated in the berberine-treated lead group compared to rats treated with lead alone (P < 0.01). The protective activity of berberine on serum hepatic biomarkers was comparable with that of the positive control silymarin. There were no significant changes in all the biochemical markers tested between animals treated with berberine alone and the control group (Ps > 0.05).
Effect of berberine on liver content of MDA
Figure 1 shows liver MDA levels in different animal groups. The concentration of lipid peroxidation product MDA was significantly enhanced in the lead group (8.27 ± 0.16 nmol/mg protein) compared to the control group (3.3 ± 0.08 nmol/mg protein) (P < 0.001). However, this increase in MDA was attenuated in lead-treated rats by berberine, as well as by silymarin (P < 0.001 in both cases). There were no significant changes in MDA between the berberine-treated lead group and the control group (P > 0.05). Furthermore, berberine decreased MDA in control rats (2.58 ± 0.27 nmol/mg protein) compared to untreated control animals (3.3 ± 0.08 nmol/mg protein) (P < 0.05).
Figure 1.
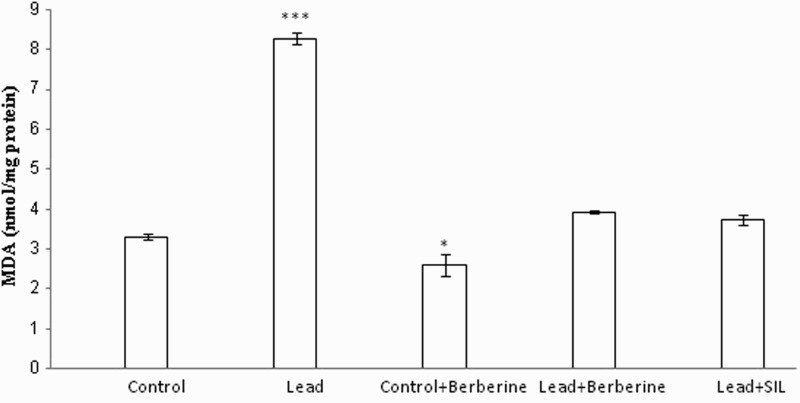
Effects of long-term berberine administration on lipid peroxidation measured as hepatic MDA content in control, lead, berberine (50 mg/kg) treated control (Control + Berberine), berberine (50 mg/kg) treated lead (Lead + Berberine), and silymarin (200 mg/kg) treated lead (Lead+ SIL) groups (n = 7) at 8 weeks after treatments. The data are represented as mean ± SEM. *P < 0.05 and ***P < 0.001 (as compared to control group).
Effect of berberine on hepatic total thiol concentration
There was a significant decrease in total sulfhydryl (SH) groups of the lead-treated group (1.9 ± 0.22 µmol/g protein) compared to the control group (3.45 ± 0.12 µmol/g protein) (P < 0.001) (Fig. 2). Both berberine and silymarin treatment in lead-treated animals significantly attenuated the increase in total thiol concentration of liver samples (P < 0.01 for both). Interestingly, berberine also elevated the total thiol concentration of control rat liver (4.47 ± 0.19 µmol/g protein) compared to untreated control animals (3.45 ± 0.12 µmol/g protein) (P < 0.05) (Fig. 2).
Figure 2.
Effects of long-term berberine administration on total thiol concentration in liver homogenates of control, lead, berberine (50 mg/kg) treated control (Control + Berberine), berberine (50 mg/kg) treated lead (Lead + Berberine), and silymarin (200 mg/kg) treated lead (Lead+ SIL) groups (n = 7) at 8 weeks after treatments. The data are represented as mean ± SEM. *P < 0.05 and ***P < 0.001 (as compared to control group).
Effect of berberine on hepatic TAC
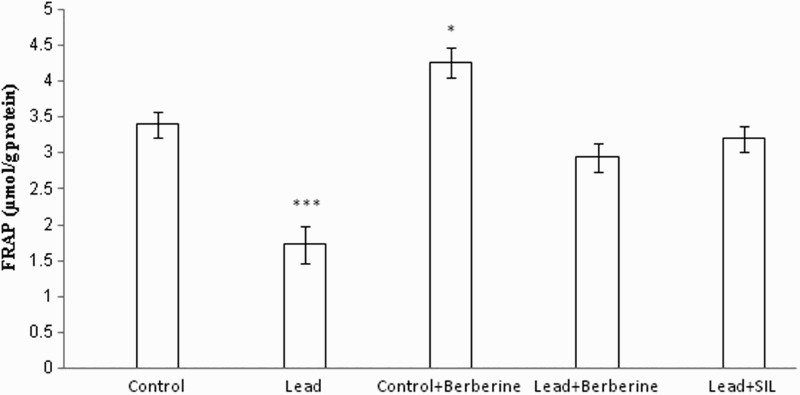
Chronic lead administration caused a significant reduction in TAC (FRAP value) of liver homogenate (1.72 ± 0.25 µmol/g protein) compared to controls (3.4 ± 0.18 µmol/g protein) (P < 0.001) (Fig. 3). Berberine increased FRAP values in the lead-treated (2.94 ± 0.19 µmol/g protein) and control (4.25 ± 0.2 µmol/g protein) groups compared to the respective untreated control groups (1.72 ± 0.25, 3.4 ± 0.18 µmol/g protein, respectively) (P < 0.01, P < 0.05, respectively) (Fig. 3). There was also a significant difference in FRAP value between the lead only and silymarin- and lead-treated groups (P < 0.001).
Figure 3.
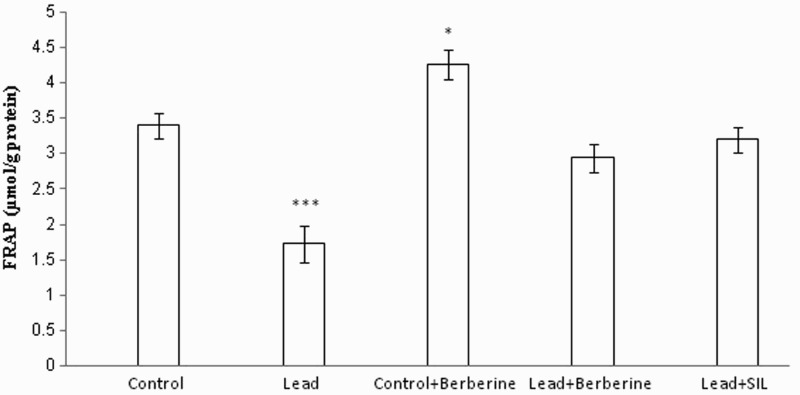
Effects of long-term berberine administration on antioxidant power (FRAP value) in liver homogenates of control, lead, berberine (50 mg/kg) treated control (Control + Berberine), berberine (50 mg/kg) treated lead (Lead + Berberine), and silymarin (200 mg/kg) treated lead (Lead+ SIL) groups (n = 7) at 8 weeks after treatments. The data are represented as mean ± SEM. *P < 0.05 and ***P < 0.001 (as compared to control group).
Effect of berberine on hepatic SOD activity
Figure 4 shows levels of SOD activity in different animal groups. Chronic lead treatment caused a significant reduction in SOD activity (6.64 ± 0.74 unit/mg protein) in liver tissue compared to the control group (15 ± 0.3 unit/mg protein) (P < 0.001). Berberine prevented the decrease in SOD activity induced by lead (P < 0.05). Furthermore, there was no significant difference in SOD activity between the berberine-treated lead group and the untreated control group (P > 0.05). Berberine treatment also elevated liver SOD activity in control rats (20 ± 1.78 unit/mg protein vs. 15 ± 0.3 unit/mg protein) (P < 0.05).
Figure 4.
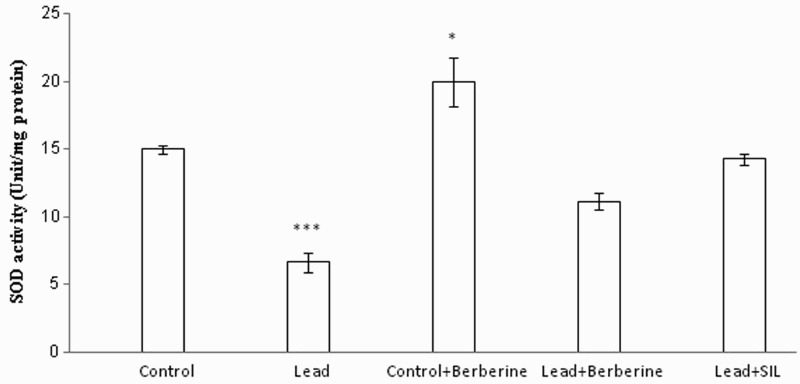
Effects of long-term berberine administration on SOD activity in liver homogenates of control, lead, berberine (50 mg/kg) treated control (Control + Berberine), berberine (50 mg/kg) treated lead (Lead + Berberine), and silymarin (200 mg/kg) treated lead (Lead + SIL) groups (n = 7) at 8 weeks after treatments. The data are represented as mean ± SEM. *P < 0.05 and ***P < 0.001 (as compared to control group).
Effect of berberine on hepatic CAT activity
Rats chronically treated with lead showed a significant reduction in activity of the defensive enzyme CAT in liver homogenates samples (3 ± 0.34 unit/mg protein) compared to the control group (6 ± 0.27 unit/mg protein) (P < 0.001) (Fig. 5). Berberine treatment elevated CAT activity in the lead group (5.28 ± 0.35 unit/mg protein) and also the control group (8.21 ± 0.34 unit/mg protein) compared to the respective untreated lead (3 ± 0.34 unit/mg protein) and control rats (6 ± 0.27 unit/mg protein) (P < 0.001, P < 0.01, respectively) (Fig. 5). Similarly, silymarin prevented the alterations in the activity of SOD and CAT induced by lead administration (P < 0.001 in each case) (Figs. 5 and 6).
Figure 5.
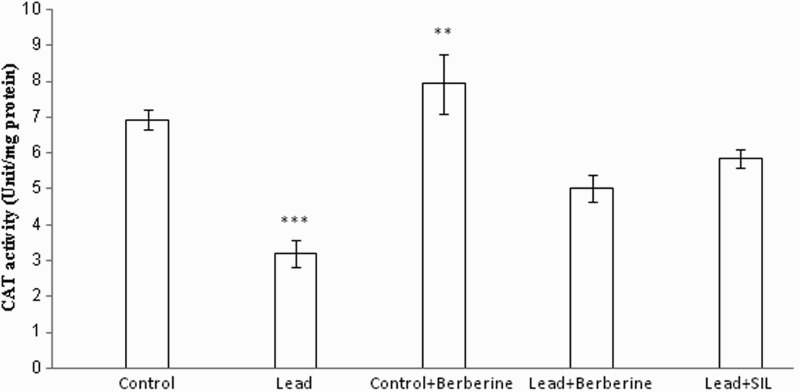
Effects of long-term berberine administration on CAT activity in liver homogenates samples of control, lead, berberine (50 mg/kg) treated control (Control + Berberine), berberine (50 mg/kg) treated lead (Lead + Berberine), and silymarin (200 mg/kg) treated lead (Lead + SIL) groups (n = 7) at 8 weeks after treatments. The data are represented as mean ± SEM. **P < 0.01 and ***P < 0.001 (as compared to control group).
Figure 6.
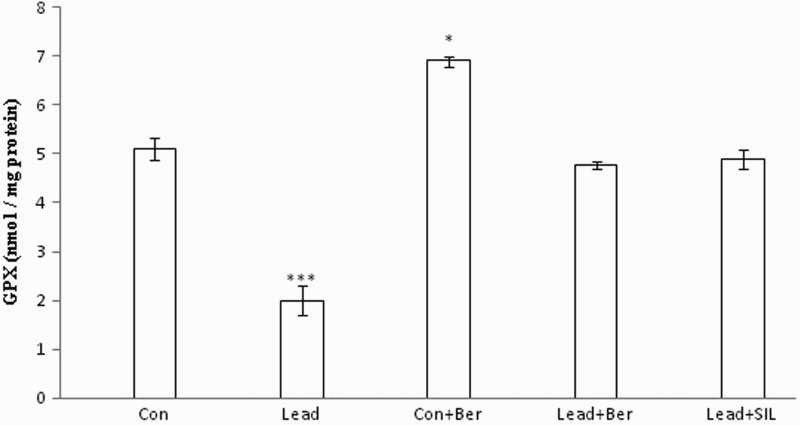
Effects of long-term berberine administration on GPx activity in liver homogenates of control, lead, berberine (50 mg/kg) treated control (Control + Berberine), berberine (50 mg/kg) treated lead (Lead + Berberine), and silymarin (200 mg/kg) treated lead (Lead + SIL) groups (n = 7) at 8 weeks after treatments. The data are represented as mean ± SEM. *P < 0.05 and ***P < 0.001 (as compared to control group).
Effect of berberine on hepatic GPx and GST activity
Figures 6 and 7 show hepatic levels of these enzymes in different animal groups. The levels of GPx and GST in liver homogenates of the lead-treated group (2 ± 0.3 nmol/mg protein, 2.5 ± 0.09 µmol/mg protein, respectively) were decreased compared to the control group (5.1 ± 0.22 nmol/mg protein, 5 ± 0.17 µmol/mg protein, respectively) (P < 0.001 in both cases). Treatment with berberine resulted in significant attenuation of the lead-induced alterations of these enzymes (P < 0.05 in all cases). Furthermore, berberine also increased hepatic levels of GPx and GST in the control group (P < 0.05 in both instances). There were no significant differences in the activity of these two enzymes between the berberine- and silymarin-treated lead groups (P > 0.05 in both cases).
Figure 7.
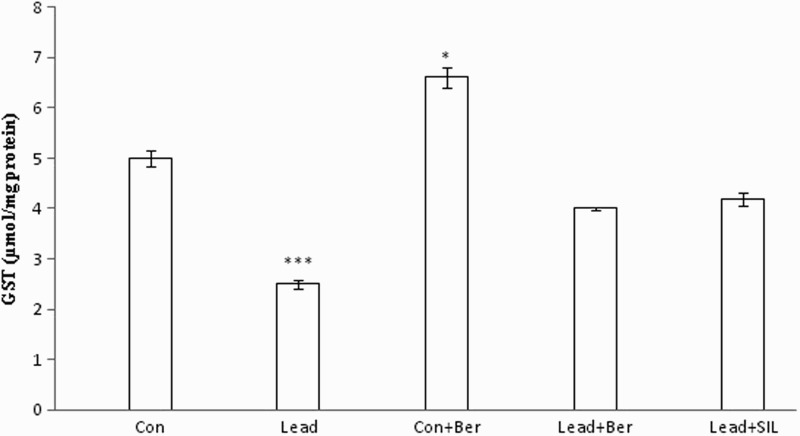
Effects of long-term berberine administration on GST activity in liver homogenates of control, lead, berberine (50 mg/kg) treated control (Control + Berberine), berberine (50 mg/kg) treated lead (Lead + Berberine), and silymarin (200 mg/kg) treated lead (Lead + SIL) groups (n = 7) at 8 weeks after treatments. The data are represented as mean ± SEM. *P < 0.05 and ***P < 0.001 (as compared to control group).
Effect of berberine on hepatic ratio of GSH/GSSG
The hepatic ratio of GSH/GSSG was decreased in the lead-treated group (0.29 ± 0.02) in comparison with the control group (0.7 ± 0.01) (P < 0.001) (Fig. 8). Berberine administration increased the ratio in both the lead-treated (0.5 ± 0.09) and control (1.1 ± 0.04) groups (P < 0.01 in each case). There was no significant difference in GSH/GSSG ratio between the berberine and silymarin-treated lead groups (Ps > 0.05) (Fig. 8).
Figure 8.
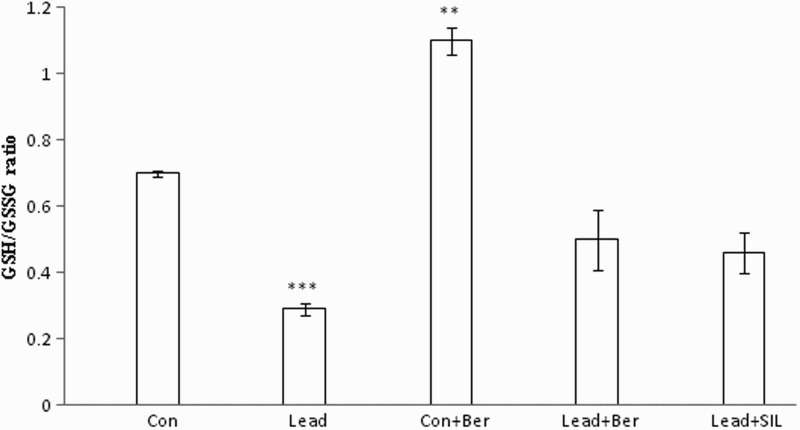
Effects of long-term berberine administration on GSH/GSSG in liver homogenates of control, lead, berberine (50 mg/kg) treated control (Control + Berberine), berberine (50 mg/kg) treated lead (Lead + Berberine), and silymarin (200 mg/kg) treated lead (Lead + SIL) groups (n = 7) at 8 weeks after treatments. The data are represented as mean ± SEM. **P < 0.01 and ***P < 0.001 (as compared to control group).
Effect of berberine on liver histopathology
There were no abnormalities or histological changes observed in the livers of normal rats, as illustrated in Fig. 9A. The liver of the berberine-treated group also showed normal histological structures (Fig. 9B). Lead exposure caused severe hepatic necrosis and disappearance and disarrangement of hepatic lobular structures. Degenerated hepatocytes with pyknotic nuclei, inflammatory cell infiltrations, and distended portal veins were also observed (Fig. 9C). Figure 9D illustrates that animals given lead and berberine administration showed some regions of recovery with restoration of the normal architecture of the hepatic lobules and the hepatocytes in a manner that was comparable with the reference silymarin-treated group (Fig. 9E).
Figure 9.
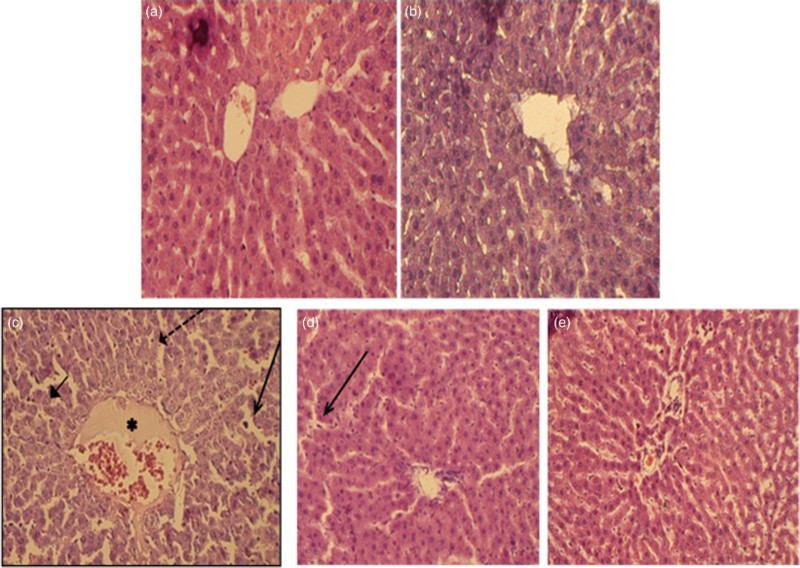
Paraffin sections stained by H&E for histopathological examination of liver tissues of rats as follows: (A) control rats; (B) rats fed with berberine (50 mg/kg, i.g.); (C) rats treated with lead (500 mg Pb/l in the drinking water); (D) rats treated with lead (500 mg Pb/l in the drinking water) and fed with berberine (50 mg/kg, i.g.) and (E) rats treated with lead (500 mg Pb/l in the drinking water) and fed with silymarin (200 mg/kg, i.g.). The long arrow indicates leukocyte infiltration. The short arrow indicates pyknotic nucleus. The dashed arrow indicates hepatic cell necrosis. The asterisk indicates the distended portal vein.
Discussion
The liver is a major organ in detoxification and is one of the principal organs affected by lead toxicity.19 In the present study, chronic lead administration was found to cause significant increases in serum ALT, AST, and ALP. Damage to hepatocyte membranes will cause release of these enzymes into the circulation.26 Furthermore, the decreased concentration of serum albumin observed here could be attributed to the interference of lead with protein synthesis or by the binding of lead to some metal-binding proteins and their removal through detoxification processes.27 Generally, these results may indicate degenerative changes and hypofunction of liver.28 These data confirm prior reports that lead has a harmful and stressful influence on hepatic tissue.29
Histological examination of the liver tissue of the animals treated with lead also supported the occurrence of damage to hepatic structural integrity by revealing severe destruction of hepatic architecture, necrosis, and infiltration of inflammatory cells. Similar observations have been reported previously.30,31
Accumulating evidence shows that liver injury following lead exposure is associated with oxidative damage. Lead is not able to induce production of free radicals directly, but it indirectly influences the processes of lipid peroxidation, leading to loss of membrane integrity and tissue damage.32 In this study, chronic administration of lead resulted in an increase in lipid peroxidation, as indicated by the significant increase in liver MDA. There was also a significant decrease in SH group levels in the liver of lead-treated rats that may be due to the ability of lead to bind to SH groups. TAC levels, which serve as a useful marker for demonstrating total changes in antioxidant status, were decreased in the lead-treated group. SOD and CAT, as antioxidant enzymes that scavenge harmful ROS, are also potential targets for lead toxicity.1 SOD provides the first line of defense against free radicals by making toxic superoxide into less toxic H2O2.33 The activities of the antioxidant defense enzymes SOD, CAT, GPx, GST, and the GSH/GSSG ratio were decreased in liver of lead-treated animals.
The decrease in GPx activity in the liver of lead-exposed rats could be due to lead's displacement of the selenocysteine group from the active site. The selenium-containing functional group at the active site of GPx is responsible for its catalytic functions.34 Furthermore, the significant reduction in GST levels could be due to lead acetate effects on sulfhydryl groups as lead is known to be able to bind irreversibly with –SH groups on proteins, rendering them useless.19
Berberine is an alkaloid extracted from coptis, cork, and other traditional Chinese medicines that possesses a broad spectrum of pharmacological and therapeutic activities such as anti-diabetic, anti-inflammatory, and anti-hyperlipidemic effects.14,15 In the present study, chronic treatment with berberine protected against liver damage induced by lead as indicated by the significant improvement in the biochemical parameters of liver function and the body weight. The beneficial effects of berberine in biochemical examinations were comparable with the reference hepatoprotective drug silymarin. The protective effect of berberine was further confirmed in the liver by histopathological examination. Administration of berberine significantly attenuated hepatocyte necrosis and inflammatory cell infiltration into lead-injured liver.
There is ongoing debate about the effects of berberine in different experimental models of hepatotoxicity. For example, Janbaz and Gilani35 reported that berberine administration (4 mg/kg) after CCl4-induced hepatotoxicity exhibited no effect in reducing hepatic damage. Sun et al.,36 however, reported that berberine protected against liver injury as evidenced by decreased ALT and AST activities in the CCl4 model of hepatotoxicity. The apparent discrepancy between the two studies may be due to the dosages, animal species, and animal models used. The present study suggests that the dosages and duration of berberine treatment may be important factors. Berberine (4 mg/kg) administered for 2 days in the study of Janbaz and Gilani was far below the dosage (50 mg/kg) and the duration of treatment (8 weeks) in our work. The protective effects of berberine against lead-induced hepatotoxicity in the present experiments are in accordance with the reported effects of berberine in experimental models of liver fibrosis16 and doxorubicin-induced hepatotxicity.17
Berberine has diverse biological effects such as free radical scavenging and anti-apoptotic and anti-carcinogenic actions.19 The effects on anti-apoptotic and free radical regulatory genes such as Bcl-2, Bax, c-myc, and p53 may be responsible for the protective properties exhibited by berberine.37 Interestingly, we found that berberine markedly decreased the content of MDA and increased SH group level in the liver of lead-treated animals. This suggests that berberine could, at least partly, attenuate oxidative stress by increasing SH group levels. The measurement of TAC is another useful tool for detecting the prevention of chemical-induced oxidative stress.33,38 In this study, treatment with berberine resulted in a significant improvement in TAC. Berberine also increased the activity of antioxidant enzymes in liver samples.
Conclusion
We conclude that berberine significantly prevented lead-induced hepatotoxicity, as indicated by serum hepatic biomarkers and histopathological analysis. The present study suggests that berberine can be considered a potential candidate to protect the liver against the deleterious effect of chronic lead intoxication. The antioxidant properties of berberine treatment appeared to underlie the mechanism of protective effects against liver injury in the present study.
Disclaimer statements
Contributors All authors contributed equally.
Funding None.
Conflict of interest There are no conflicts of interest.
Ethics approval Our paper has received ethical approval of Bu-Ali Sina University and all procedures were performed in accordance with the recommendations for the proper care and use of laboratory animals.
Acknowledgment
The authors wish to thank Professor Nigel Calcutt for his valuable suggestions in editing the manuscript.
References
- 1.Wang J, Yang Z, Lin L, Zhao Z, Liu Z, Liu X. Prospective effect of naringenin against lead-induced oxidative stress in rats. Biol Trace Elem Res 2012;146:354–59. doi: 10.1007/s12011-011-9268-6 [DOI] [PubMed] [Google Scholar]
- 2.Ibrahim NM, Eweis EA, el-Beltagi HS, Abdel-Mobdy YE. The effect of lead acetate toxicity on experimental male albino rat. Biol Trace Elem Res 2011;144:1120–32 (retracted article). doi: 10.1007/s12011-011-9149-z [DOI] [PubMed] [Google Scholar]
- 3.Abdou HM, Hassan MA. Protective role of omega-3 polyunsaturated fatty acid against lead acetate-induced toxicity in liver and kidney of female rats. Biomed Res Int 2014;2014:435857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xia D, Yu X, Liao S, Shao Q, Mou H, Ma W. Protective effect of Smilax glabra extract against lead-induced oxidative stress in rats. J Ethnopharmacol 2010;130:414–20. doi: 10.1016/j.jep.2010.05.025 [DOI] [PubMed] [Google Scholar]
- 5.Jia Q, Ha X, Yang Z, Hui L, Yang X. Oxidative stress: a possible mechanism for lead-induced apoptosis and nephrotoxicity. Toxicol Mech Methods 2012;22:705–10. doi: 10.3109/15376516.2012.718811 [DOI] [PubMed] [Google Scholar]
- 6.Li L, Zhang Y, Ma J, Dong W, Song Q, Zhang J, et al. . Salvia miltiorrhiza injection ameliorates renal damage induced by lead exposure in mice. Scientific World Journal 2014;2014:572697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abdel Moneim AE, Dkhil MA, Al-Quraishy S. The protective effect of flaxseed oil on lead acetate-induced renal toxicity in rats. J Hazard Mater 2011;194:250–55. doi: 10.1016/j.jhazmat.2011.07.097 [DOI] [PubMed] [Google Scholar]
- 8.Caylak E, Aytekin M, Halifeoglu I. Antioxidant effects of methionine, α-lipoic acid, N-acetylcysteine and homocysteine on lead-induced oxidative stress to erythrocytes in rats. Exp Toxicol Pathol 2008;60:289–94. doi: 10.1016/j.etp.2007.11.004 [DOI] [PubMed] [Google Scholar]
- 9.Flora SJS, Pande M, Mehta A. Beneficial effect of combined administration of some naturally occurring antioxidants (vitamins) and thiol chelators in the treatment of chronic lead intoxication. Chem Biol Interact 2003;145:267–80. doi: 10.1016/S0009-2797(03)00025-5 [DOI] [PubMed] [Google Scholar]
- 10.Liu CM, Ma JQ, Sun YZ. Protective role of puerarin on lead-induced alterations of the hepatic glutathione antioxidant system and hyperlipidemia in rats. Food Chem Toxicol 2012;49:3119–27. doi: 10.1016/j.fct.2011.09.007 [DOI] [PubMed] [Google Scholar]
- 11.Amin AH, Subbaiah TV, Abbasi KM. Berberine sulfate: antimicrobial activity, bioassay, and mode of action. Can J Microbiol 1969;15:1067–76. doi: 10.1139/m69-190 [DOI] [PubMed] [Google Scholar]
- 12.Bova S, Padrini R, Goldman WF, Berman DM, Cargnelli G. On the mechanism of vasodilating action of berberine: Possible role of inositol lipid signaling system. J Pharmacol Exp Ther 1992;261:318–32. [PubMed] [Google Scholar]
- 13.Akhter MH, Sabir M, Bhide NK. Anti-inflammatory effect of berberine in rats injected locally with cholera toxin. Indian J Med Res 1997;65:133–41. [PubMed] [Google Scholar]
- 14.Lee YS, Kim WS, Kim KH, Yoon MJ, Cho HJ, Shen Y. Berberine, a natural plant product, activates AMP-activated protein kinase with beneficial metabolic effects in diabetic and insulin-resistant states. Diabetes 2006;55:2256–64. doi: 10.2337/db06-0006 [DOI] [PubMed] [Google Scholar]
- 15.Kong W, Wei J, Abidi P, Lin M, Inaba S, Li C. Berberine is a novel cholesterol-lowering drug working through a unique mechanism distinct from statins. Nat Med 2004;10:1344–51. doi: 10.1038/nm1135 [DOI] [PubMed] [Google Scholar]
- 16.Li J, Pan Y, Kan M, Xiao X, Wang Y, Guan F, et al. . Hepatoprotective effects of berberine on liver fibrosis via activation of AMP-activated protein kinase. Life Sci 2014;98:24–30. doi: 10.1016/j.lfs.2013.12.211 [DOI] [PubMed] [Google Scholar]
- 17.Zhao X, Zhang J, Tong N, Chen Y, Luo Y. Protective effects of berberine on doxorubicin-induced hepatotoxicity in mice. Biol Pharm Bull 2012;35:796–800. doi: 10.1248/bpb.35.796 [DOI] [PubMed] [Google Scholar]
- 18.Germoush MO, Mahmoud AM. Berberine mitigates cyclophosphamide-induced hepatotoxicity by modulating antioxidant status and inflammatory cytokines. J Cancer Clin Oncol 2014;140:1103–9. doi: 10.1007/s00432-014-1665-8 [DOI] [PubMed] [Google Scholar]
- 19.Haleagrahara N, Jackie T, Chakravarthi S, Rao M, Kulur A. Protective effect of Etlingera elatior (torch ginger) extract on lead acetate-induced hepatotoxicity in rats. J Toxicol Sci 2010;35:663–71. doi: 10.2131/jts.35.663 [DOI] [PubMed] [Google Scholar]
- 20.Zhang BJ, Xu D, Guo Y, Ping J, Chen LB, Wang H. Protection by and anti-oxidant mechanism of berberine against rat liver fibrosis induced by multiple hepatotoxic factors. Clin Exp Pharmacol Physiol 2008;35:303–9. doi: 10.1111/j.1440-1681.2007.04819.x [DOI] [PubMed] [Google Scholar]
- 21.Moore K, Roberts LJ. Measurement of lipid peroxidation. Free Radic Res 1998;28:659–71. doi: 10.3109/10715769809065821 [DOI] [PubMed] [Google Scholar]
- 22.Ellman G. Tissue sulfhydryl groups. Arch Biochem Biophys 1959;82:70–7. doi: 10.1016/0003-9861(59)90090-6 [DOI] [PubMed] [Google Scholar]
- 23.Benzie IFF, Strain J. The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: The FRAP assay. Anal Biochem 1996;239:70–6. doi: 10.1006/abio.1996.0292 [DOI] [PubMed] [Google Scholar]
- 24.Roghani M, Baluchnejadmojarad T. Chronic epigallocatechin-gallate improves aortic reactivity of diabetic rats: Underlying mechanisms. Vascul Pharmacol 2009;51:84–9. doi: 10.1016/j.vph.2009.04.003 [DOI] [PubMed] [Google Scholar]
- 25.Claiborne A. Catalase activity. In: Greenwald RA, (ed.) CRC handbook of methods for oxygen radical research. Boca Raton: CRC; 1985. p. 283–84. [Google Scholar]
- 26.El-Agamy DS, Makled MN, Gamil NM. Protective effects of BML-111 against acetaminophen-induced acute liver injury in mice. J Physiol Biochem 2014;70:141–9. doi: 10.1007/s13105-013-0288-x [DOI] [PubMed] [Google Scholar]
- 27.Yousef MI. Aluminium-induced changes in hemato-biochemical parameters, lipid peroxidation and enzyme activities of male rabbits: protective role of ascorbic acid. Toxicology 2004;199:47–57. doi: 10.1016/j.tox.2004.02.014 [DOI] [PubMed] [Google Scholar]
- 28.Ramadan KS, Khalil OA, Danial EN, Alnahdi HS, Ayaz NO. Hypoglycemic and hepatoprotective activity of Rosmarinus officinalis extract in diabetic rats. J Physiol Biochem 2013;69:779–83. doi: 10.1007/s13105-013-0253-8 [DOI] [PubMed] [Google Scholar]
- 29.El-Nekeety AA, El-Kady AA, Soliman MS, Hassan NS, Abdel-Wahhab MA. Protective effect of Aquilegia vulgaris (L.) against lead acetate-induced oxidative stress in rats. Food Chem Toxicol 2009;47:2209–15. doi: 10.1016/j.fct.2009.06.019 [DOI] [PubMed] [Google Scholar]
- 30.Kubo Y, Yasunaga M, Masuhara M, Terai S, Nakamura T, Okita K. Hepatocyte proliferation induced in rats by lead nitrate is suppressed by several tumor necrosis factor alpha inhibitors. Hepatology 1996;23:104–14. [DOI] [PubMed] [Google Scholar]
- 31.Rijhsinghani K, Choi HS, Burton LA, Paronetto F, Tavoloni N. Immunoelectron microscopy identification of early proliferating cells in rat liver tissue during hyperplasia induced by lead nitrate. Hepatology 1993;17:685–92. doi: 10.1002/hep.1840170424 [DOI] [PubMed] [Google Scholar]
- 32.Sivaprasad R, Nagaraj M, Varalakshmi P. Combined efficacies of lipoic acid and 2,3-dimercaptosuccinic acid against lead-induced lipid peroxidation in rat liver. J Nutr Biochem 2004;15:18–23. doi: 10.1016/j.jnutbio.2003.09.001 [DOI] [PubMed] [Google Scholar]
- 33.Sakeran MI, Zidan N, Rehman H, Aziz AT, Saggu S. Abrogation by Trifolium alexandrinum root extract on hepatotoxicity induced by acetaminophen in rats. Redox Rep 2014;19:26–33. doi: 10.1179/1351000213Y.0000000068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Forstrom JW, Zakowski JJ, Tappel AL. Identification of the catalytic site of rat liver glutathione peroxidase as selenocysteine. Biochemistry 1978;17:2639–44. doi: 10.1021/bi00606a028 [DOI] [PubMed] [Google Scholar]
- 35.Janbaz KH, Gilani AH. Studies on preventive and curative effects of berberine on chemical-induced hepatotoxicity in rodents. Fitoterpia 2000;71:25–33. doi: 10.1016/S0367-326X(99)00098-2 [DOI] [PubMed] [Google Scholar]
- 36.Sun X, Zhang X, Hu H, Lu Y, Chen J, Yasuda K, et al. . Berberine inhibits hepatic stellate cell proliferation and prevents experimental liver fibrosis. Biol Pharm Bull 2009;32:1533–37. doi: 10.1248/bpb.32.1533 [DOI] [PubMed] [Google Scholar]
- 37.Patil JB, Kim J, Jayaprakasha GK. Berberine induces apoptosis in breast cancer cells (MCF-7) through mitochondrial-dependent pathway. Eur J Pharmacol 2010;645:70–8. doi: 10.1016/j.ejphar.2010.07.037 [DOI] [PubMed] [Google Scholar]
- 38.Simeonova R, Bratkov VM, Kondeva-Burdina M, Vitcheva V, Manov V, Krasteva I. Experimental liver protection of n-butanolic extract of Astragalus monspessulanus L. on carbon tetrachloride model of toxicity in rat. Redox Rep 2015;20:145–53. doi: 10.1179/1351000214Y.0000000115 [DOI] [PMC free article] [PubMed] [Google Scholar]


