Abstract
Background: Diabetic retinopathy (DR) is one of the main complications in patients with diabetes and has been the leading cause of visual loss since 1990. Oxidative stress is a biological process resulting from excessive production of reactive oxygen species (ROS). This process contributes to the development of many diseases and disease complications. ROS interact with various cellular components to induce cell injury. Fortunately, there is an antioxidan t system that protects organisms against ROS. Indeed, when ROS exceed antioxidant capacity, the resulting cell injury can cause diverse physiological and pathological changes that could lead to a disease like DR.
Objective: This paper reviews the possible mechanisms of common and novel biomarkers involved in the development of DR and explores how these biomarkers could be used to monitor the damage induced by oxidative stress in DR, which is a significant complication in people with diabetes.
Conclusion: The poor control of glucemy in pacients with DB has been shown contribute to the development of complications in eyes as DR.
Keywords: Biomarkers, Diabetic retinopathy, Oxidative stress, Retinal damage
Diabetic retinopathy
Diabetes mellitus (DM) is a complex metabolic disease that will affect a projected 380 million patients by the year 2025. Tragically, this disease will lead to blindness in approximately 4 million people around the world due to the development of diabetic retinopathy (DR).1 In 1998, the prevalence and severity of DR was greater in non-Hispanic black and Mexican-American populations than in non-Hispanic whites with type 2 diabetes in the USA.2 In 2008, the prevalence of both DR and vision-threatening DR in adults equal to or greater than 40 years old, especially among non-Hispanic black individuals, was high.3
Diabetic patients tend to have increased levels of reactive oxygen metabolites (ROM) in serum when compared with healthy subjects. A study carried out by Naruse et al.4 demonstrated that ROM increased rapidly in serum as DR progressed. This increase was notoriously high during DR progression in patients with type 2 diabetes whose levels of lipoperoxide, catalase, glutathione peroxidase, and nitric oxide (NO) catabolites increased significantly in erythrocyte.5
Signs of retinal vascular activation and injury induced by diabetes and elevated glucose are associated with increased arginase activity and decreased levels of bioavailable NO,6 which suggests that overactive arginase contributes to DR by reducing NO and increasing oxidative stress the activity of arginase, which competes NO synthase for the common substrate l-arginine. Inducible nitric oxide synthase is one of the three NOS isoforms that generate NO by conversion of l-arginine to l-citrulline.7 NO generated from S-nitrosoglutation acts as a second messenger, which has been shown to control important cellular processes through S-nitrosylation by regulating of the expression or activity of certain proteins. Moreover, NO is necessary for physiological functions such as host defense, neurotransmission, and vasodilation, whereas the generation of reactive nitrogen species is implicated in the pathophysiology of degenerative diseases.
Mechanisms involved in DR
Diabetes results in an increased flux through the hexosamine biosynthetic pathway that increases posttranslational modifications of Ser/Thr residues on proteins by O-linked β-N-acetylglucosamine. O-GlcN-acylation is involved in the regulation of many nuclear and cytoplasmic proteins in a manner similar to protein phosphorylation.8 Diabetes is accompanied by an increased risk of developing chronic macro- and microvascular complications including DR.9
DR is an eye disorder affecting the human retina and is believed to be caused by an elevated amount of insulin in the blood10 Intensive insulin therapy has been found to delay the progression of this clinical disorder, and there is level 1 evidence for intensive glycemic control to reduce the progression of DR in persons with type 2 diabetes.
DR is a complex condition; inflammation and oxidative stress are involved in crucial pathways that affect the pathogenesis of DR.11 DR is characterized by pericyte and neuronal cell loss, formation of acellular-occluded capillaries, micro aneurysms, increased leukostasis, and thickening of the vascular basement membrane. Pericyte death is initiated when hyperglycemia persistently activates protein kinase C-δ (PKC-δ, encoded by Prkcd) and the p38 mitogen-activated protein kinase as the expression of a previously unknown target of PKC-δ signaling increases. This signaling cascade leads to PDGF receptor dephosphorylation and a reduction in downstream signaling by this receptor, resulting in pericyte apoptosis.12 Thioredoxin interacting protein represents a novel gene and drug target to prevent pericyte loss and the progression of DR.13 These alterations progressively affect the integrity of retinal microvessels, leading to the breakdown of the blood–retinal barrier and widespread hemorrhage and neovascularization, which appear on the surface of the retina along with microaneurysms, hemorrhages, and exudates.14 Growing evidence suggests that local inflammation and oxidative stress are critical factors in the pathogenesis of DR.15 Proinflammatory cytokines (TNF-alpha and IL-1), secretory phospholipase A2 IIA, and lipoprotein-PLA2 are implicated in vascular inflammation,16 suggest that understanding cytokine-induced changes in lipid metabolism will promote the development of novel concepts and steer bench-to-bedside therapeutic developments.
Screening and gene expression
England is a world leader in DR screening, having offered 85.7% of eligible diabetic patients a screening program. The British Diabetic Association (Diabetes UK) has established standard values for any DR screening program of at least 80% sensitivity and 95% specificity. Papavasileiou et al.17 suggested that a well-implemented program would provide timely treatment, reduce the need for vitrectomy and blind registration, and serve as a benchmark in planning the delivery of services in a similar population.
People with Down's syndrome, who have three copies of chromosome 21, almost never acquire DR. This protection appears to be due to the elevated levels in tumor cells of endostatin,18 an anti-angiogenic protein, derived from collagen XVIII. Its gene is located on chromosome 21.
Uncoupling protein 1 (UCP1) reduces mitochondrial production of reactive oxygen species (ROS), and deleterious polymorphisms in the UCP1 gene are candidate risk factors for the development of DR. This protein is expressed in the retinas of people with type 1 DM. Brondani et al.19 suggest that the 3826A/G polymorphism influences UCP1 expression and reduces mitochondrial production of ROS.
On the other hand, Payne et al.20 suggested that the molecular pathways contributing to signal transduction in the retina exhibit a high energy demand with functional and structural consequences. Subsequent vascularization and elevated metabolic rates contribute to oxidative stress and influence age-related processes that increase oxidative load, resulting in chronically elevated levels of oxidative stress and ROS.
Ocular tissues are prone to damage from ROS due to constant exposure of the eye to sunlight, atmospheric oxygen, and environmental chemicals. Furthermore, free radical (FR)-catalyzed peroxidation of long-chain polyunsaturated acids (LCPUFAs) such as arachidonic acid and docosahexaenoic acid leads to generation of LCPUFA metabolites including isoprostanes and neuroprostanes that may further exert pharmacological and toxicological actions in ocular tissues.21
Reactive radical species
An FR can be generated through different pathways, but the most frequent mechanism in living organisms is the addition of an electron to a stable molecule (Fig. 1). Most of the molecules of an organism are non-radicals, which mean that they have only an even number of electrons in their atomic orbits.
Figure 1.
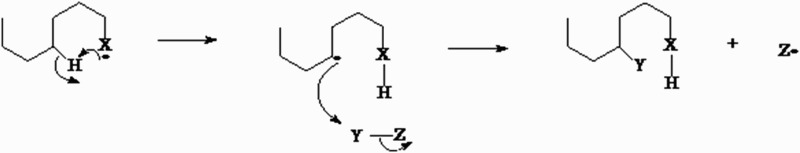
Pathway of FR generation through the addition of an electron to a stable molecule. The movement of electrons is accomplished by hydrogen attack on carbon atoms that substitute a released substrate.
Once generated, FRs interact with other molecules through redox reactions to reach a stable electronic configuration. In a redox reaction, electron transfer between the participating chemical species will take place. One chemical species loses free electrons (oxidation process) and the other gains electrons (reduction process). The oxidation of one chemical species implies the reduction of another (Fig. 2).
Figure 2.
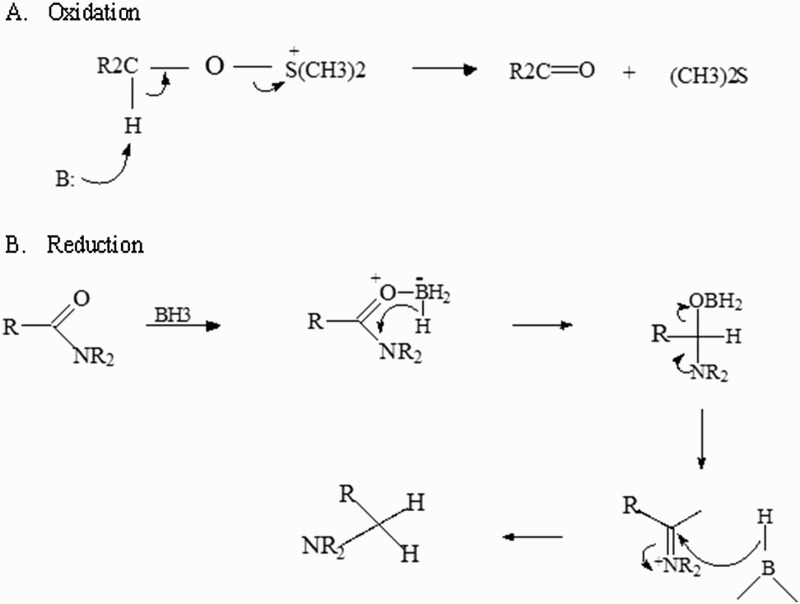
FRs interact with other molecules through redox reactions to obtain a stable electron configuration. In a redox reaction, electron transfer among the participating chemical species will occur. One molecule loses free electrons (A): oxidation process and the other gains electrons (B): reduction process. This balance is necessary in reactions or interactions between proteins that participate in any cellular biochemical pathways.
The molecule losing electrons is known as a reducing agent, and the molecule gaining electrons is known as an oxidant agent. When an FR reacts with a non-radical molecule, it can lose or gain electrons, or simply join the molecule. In any case, the non-radical molecule turns into an FR and a chain reaction is triggered: one FR generates another FR. The reaction will stop only when two FRs meet.22
Several authors have classified FRs according to their functional groups.23 The most common is an oxygen FR, in which oxygen is the functional center (Fig. 3).
Figure 3.
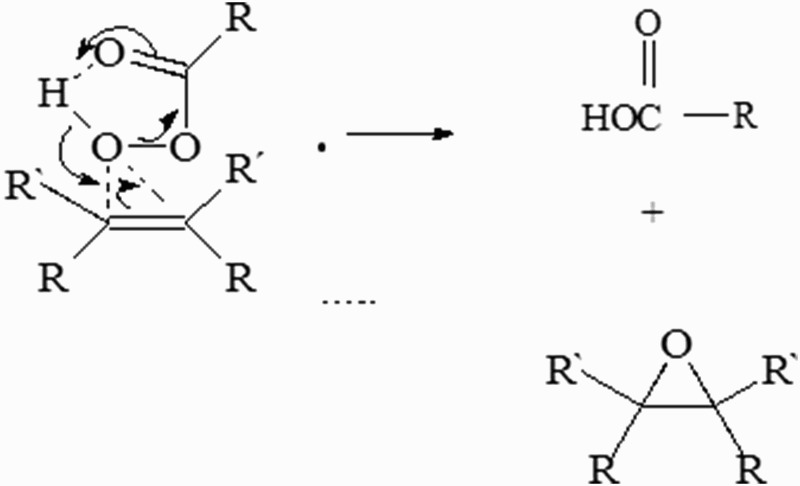
The most frequent FR produced is the oxygen radical, in which oxygen is the functional center. The movement of electrons is through atoms or molecules with electrophilic properties, and this substrate is critical because it participates in all cellular processes of inflammation.
Thiol radical groups contain sulfur (S), and other radicals contain carbon (C), phosphorous (P), or nitrogen (N) in their reactive groups. FRs are generated from normal metabolic reactions, and exogenous factors can increase their production.24 This group of FR is formed by one superoxide anion, one hydroxyl radical, and FRs that come from organic compounds: alcoxyl, peroxyl, hydrogen peroxide, and singlet oxygen.25
Therefore, the general term ROS is used to indicate chemical species that act like oxidants but are not FRs such as hydrogen peroxide, hypochlorous acid, hydroperoxides, and epoxide metabolites.26
The major site of superoxide production is considered to be the respiratory chain in mitochondria, but the exact mechanism and the precise location of the physiologically relevant ROS generation within the respiratory chain have not yet been determined. The mechanism of ROS generation could be relevant because evidence indicates that oxidative stress is a crucial factor in the pathogenesis of clinical disorders.27 However, recent studies suggest that the ubiquitination of the mitochondrial transcription factor A impedes its transport to the mitochondria, resulting in suboptimal mtDNA transcription and mitochondria dysfunction. The inhibition of ubiquitination restores mitochondrial homeostasis and inhibits the development and progression of DR.28 Oxygen-derived FRs such as hydroxyl and hydroperoxyl species have been shown to oxidize phospholipids and other membrane lipid components leading to lipid peroxidation. Lipid peroxidation has been reported to play a significant role in the progression of DR.21
Prevention and treatment
Recent studies describe three treatments for DR that are very effective in reducing vision loss from this disease. Even people with advanced DR have a 90% chance of maintaining their vision when they are treated before the retina is severely damaged. These three treatments are laser surgery, corticosteroid or anti-vascular endothelial growth factor (VEGF) injection into the eye, and vitrectomy. These treatment options do not cure DR. New insights into the pathophysiology of DR are needed to develop new methods to improve its detection, treatment, and prevention and to understand the underlying molecular mechanisms (see Table 1) that control the incidence and progression of the disease.
Table 1. Clinical disorders and biomarkers related to DR.
| Disorders | Tissue | Effect or biomarkers | Reference |
|---|---|---|---|
| DR | Retina | Two single-nucleotide polymorphisms (rs1073203 and rs4838605) were found to be significantly associated with DR | McAuley et al.29 |
| DR | Retinal capillary cell | Retinal capillary cell apoptosis and the number of degenerative capillaries were increased by three- to fourfold. Gene expression of mtDNA-encoded proteins was decreased, and VEGF, interleukin-1β, and NF-κB levels were elevated | Kowluru et al.30 |
| DR | Retinal pigment epithelium (RPE) | Glycogen storage is increased in the RPE of diabetic patients and demonstrates the role of glycogen deposits in the pathogenesis of DR | Hernández et al.31 |
| Retinal inflammation | Neural retina | Cells derived from the stromal fraction of adipose tissue are able to rescue the neural retina from hyperglycemia-induced degeneration | Rajashekhar et al.32 |
| Retinal inflammation | Retina | Upregulation of oxidative/nitrosative stress, A2AAR, ENT1, Iba1, TNF-α, ICAM1, retinal cell death, and downregulation of AK | Elsherbiny et al.33 |
| Diabetic retina | Retina | Brain-derived neurotrophic factor (BDNF) is reduced by high-mobility group box-1, thiobarbituric acid-reactive substances↑ | Abu El-Asrar et al.34 |
| Retinopathy induced by hyperoxia (80% O2) | Retinal microglia | Activation of microglia and induction of microvascular injury through the release of Sema3A from adjacent neurons | Rivera et al. 35 |
| Experimental DR | Retina | SS31, a mitochondria-targeted antioxidant peptide in the retina of diabetic patients, could be a potential new treatment for DR | Huang et al.36 |
| DR | Retina | Adiponectin on the retinal vasculature may help improve potential therapies for retinal vascular disorders | Omae et al.37 |
| DR | Retina | Increased binding of Nrf2 to Keap1; its translocation to the nucleus is compromised, contributing to decreased GSH levels; regulation of Nrf2–Keap1 by pharmacological or molecular means could serve as a potential adjunct therapy to combat oxidative stress and inhibit the development of DR | Zhong et al.38 |
| Diabetic retina | Cultured retina | The expression patterns of HO-1, Nox2, Nox4 in db/db mouse retinas, and the suppressive effects of NADPH oxidase inhibitors on the expression of HO-1, which is at least partially mediated by NADPH oxidase | He et al.39 |
| Retinopathy animal model | Retina | Lack of glutathione peroxidase-1 was associated with increased oxidative stress, an increase in the retinal avascular area, and upregulation of retinal VEGF | Tan et al.40 |
| Diabetic retina | Retina | Sigma receptor 1 is a non-opioid transmembrane protein that plays a key role in modulating retinal stress. It may be an important target in retinal disease | Ha et al.41 |
| DR | Plasma | Decreased plasma purpose Dickkopf-1 levels, which may contribute to Wnt/β-catenin pathway activation, are associated with the presence and progression of DR | Qiu et al.42 |
| DR | Retina | Strategies targeting T-cell lymphoma invasion and metastasis–Ras-related C3 botulinum toxin substrate 1 (TIAM1–RAC1) signaling could have the potential to halt the progression of DR in the early stages of the disease | Kowluru et al.43 |
| DR | Retina | Hypoxia might be involved in DR development through the stimulation of two key events of RD, such as neoangiogenesis and apoptosis | Cervellati et al.44 |
| Diabetic retina | Retina | Levels of circulating oxidized LDL immune complexes predict the development of DR | Fu et al.45 |
| DR | Retina | Therapies targeting the retinal dopaminergic system may be beneficial in early-stage DR | Aung et al.46 |
Up (↑), down (↓).
Many treatment options have recently been developed for the clinical management of DR complications. Table 2 shows experimental data and some relevant substances thought to ameliorate oxidative stress and prevent or retard the development of DR in animal models. However, clinical observations also suggest that reducing oxidative stress may help reverse pathological disturbances in DR. Early protection of retinal neurons in DR cases can protect against damage of retinal vessels, thereby helping ameliorate the progression of DR.
Table 2. Substances with antioxidant activity used in DR.
| Substance | Effects | Reference |
|---|---|---|
| Alpha-lipoic acid | ALA treatment has been shown to suppress expression of vascular endothelial growth factor, angiopoietin 2, and erythropoietin via blockade of superoxide formation | Nebbioso et al.47 |
| Telmisartan | BDNF and glutathione are increased in the sera and the retina of a DR animal model, decreasing signs of apoptosis | Ola et al.48 |
| Phlorizin | Significantly reduced fasting blood glucose and levels of advanced glycation end products and remarkably inhibited retina cell apoptosis and the expression of glial fibrillary acidic protein in the retinas | Zhang et al.49 |
| Photobio-modulation | Daily 670-nm PBM treatment (6 J/cm2) resulted in significant reduction in diabetes-induced death of retinal ganglion cells | Tang et al.50 |
| Astaxanthin | Reduced apoptosis of retinal ganglion cells and improved the levels of superoxide anion, malondialdehyde (a marker of lipid peroxidation), 8-hydroxy-2-deoxyguanosine (indicator of oxidative DNA damage), and manganese superoxide dismutase activity in the retinal tissue | Dong et al.51 |
| Hydrogen sulfide | Abated oxidative stress, alleviated mitochondrial dysfunction, suppressed NF-κB activation and attenuated inflammation in DR animal models | Si et al.52 |
| Resveratrol | Alleviated hyperglycemia, induced weight loss, enhanced lipid peroxidation index, and oxidization to reduce the glutathione ratio and superoxide dismutase activity in the retina | Soufi et al.53 |
| Hesperetin (flavonoids) | Showed inhibitory effects on caspase-3, which could be effective for the prevention of DR | Kumar et al.54 |
| Green tea, rich source of epigallocatechin gallate | Protects the retina in DR due to an increase in the expression of glial fibrillary acidic protein, oxidative retinal markers, and glutamine synthetase levels | Silva et al.55 |
| Methylene blue or apocynin | Pharmaceuticals targeting photoreceptor oxidative stress could offer a unique therapy for DR | Du et al.56 |
| Tauroursodeoxycholic acid | Decreased protein carbonyl groups and ROS production | Gaspar et al.57 |
Over half of the people with young-onset diabetes, regardless of type, exhibit retinopathy within 10–12 years of disease duration, which emphasizes the need for regular eye screening and aggressive control of glucose and blood pressure to prevent ocular damage.58
Conclusions
Poor glycemic control and oxidative stress have been shown to contribute to the development of complications in the eye such as DR. Diabetic patients should be educated on eye complications that may arise from their condition. Regular eye screening with a fundus camera should be part of the routine management of diabetes.
Oxidative stress reduction and the restoration of the retinal antioxidant system using exogenous antioxidants or anti-inflammatory drugs are promising issues for further research.
Acknowledgments
We thank Dr Cyril Ndidi Nwoye, a native English speaker and language professor, for the critical review and translation of this manuscript. Besides, manuscript was edited by Taylor & Francis Service.
Disclaimer statements
Contributors All authors contributed equally.
Funding None.
Conflict of interest The authors declare that there is no conflicts of interest regarding the publication of this paper.
Ethics approval Yes.
References
- 1.Tarr JM, Kaul K, Chopra M, Kohner EM, Chibber R. Pathophysiology of diabetic retinopathy. ISRN Ophthalmology 34356, eCollection 2013. [DOI] [PMC free article] [PubMed]
- 2.Harris MI, Klein R, Cowie CC, Rowland M, Byrd-Holt DD. Is the risk of diabetic retinopathy greater in non-Hispanic blacks and Mexican Americans than in non-Hispanic whites with type 2 diabetes? A U.S. population study. Diabetes Care 1998;21:1230–5. doi: 10.2337/diacare.21.8.1230 [DOI] [PubMed] [Google Scholar]
- 3.Zhang X, Saaddine JB, Chou CF, Cotch MF, Cheng YJ, Geiss LS, et al. Prevalence of diabetic retinopathy in the United States, 2005–2008. JAMA 2010;304:649–56. doi: 10.1001/jama.2010.1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Naruse R, Suetsugu M, Terasawa T, Ito K, Hara K, Takebayashi K, et al. Oxidative stress and antioxidative potency are closely associated with diabetic retinopathy and nephropathy in patients with type 2 diabetes. Saudi Medi J 2013;34:135–141. [PubMed] [Google Scholar]
- 5.Rodríguez-Carrizalez AD, Castellanos-González JA, Martínez-Romero EC, Miller-Arrevillaga G, Villa-Hernández D, Hernández-Godínez PP, et al. Oxidants, antioxidants and mitochondrial function in non-proliferative diabetic retinopathy. J Diabetes 2014;6:167–75. doi: 10.1111/1753-0407.12076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel C, Rojas M, Narayanan SP, Zhang W, Xu Z, Lemtalsi T, et al. Arginase as a mediator of diabetic retinopathy. Front Immunol 2013;4:173. doi: 10.3389/fimmu.2013.00173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pannu R, Singh I. Pharmacological strategies for the regulation of inducible nitric oxide synthase: neurodegenerative versus neuroprotective mechanisms. Neurochem Int 2006;49:170–80. doi: 10.1016/j.neuint.2006.04.010 [DOI] [PubMed] [Google Scholar]
- 8.Semba RD, Huang H, Lutty GA, Van Eyk JE, Hart GW. The role of O-GlcNAc signaling in the pathogenesis of diabetic retinopathy. Proteom Clin Appl 2014;8:218–31. Doi: 10.1002/prca.201300076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ezquer F, Ezquer M, Arango-Rodriguez M, Conget P. Could donor multipotent mesenchymal stromal cells prevent or delay the onset of diabetic retinopathy? Acta Ophthalmol 2014;92:e86–95. doi: 10.1111/aos.12113 [DOI] [PubMed] [Google Scholar]
- 10.Kuo JZ, Guo X, Klein R, Klein BE, Genter P, Roll K, et al. Adiponectin, insulin sensitivity and diabetic retinopathy in Latinos with type 2 diabetes. J Clin Endocrinol Metab 2015;10:3348–55. Doi: 10.1210/jc.2015-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bucolo C, Marrazzo G, Platania CB, Drago F, Leggio GM, Salomone S. Fortified extract of red berry, Ginkgo biloba, and white willow bark in experimental early diabetic retinopathy. J Diabetes Res 2013;2013:1–6, article no. 432695. Doi: 10.1155/2013/432695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geraldes P, Hiraoka-Yamamoto J, Matsumoto M, Clermont A, Leitges M, Marette A, et al. Activation of PKC-δ and SHP-1 by hyperglycemia causes vascular cell apoptosis and diabetic retinopathy. Nat Med 2009;15:1298–306. doi: 10.1038/nm.2052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Devi TS, Hosoya K, Terasaki T, Singh LP. Critical role of TXNIP in oxidative stress, DNA damage and retinal pericyte apoptosis under high glucose: implications for diabetic retinopathy. Exp Cell Res 2013;319:1001–12. doi: 10.1016/j.yexcr.2013.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chiu CJ, Taylor A. Dietary hyperglycemia, glycemic index and metabolic retinal diseases. Prog Retin Eye Res 2011;30:18–53. doi: 10.1016/j.preteyeres.2010.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams M, Hogg RE, Chakravarthy U. Antioxidants and diabetic retinopathy. Curr Diabetes Rep 2013;13:481–7. doi: 10.1007/s11892-013-0384-x [DOI] [PubMed] [Google Scholar]
- 16.Chen H, Wen F, Zhang X, Su SB. Expression of T-helper-associated cytokines in patients with type 2 diabetes mellitus with retinopathy. Mol Vis 2012;18:219–26. [PMC free article] [PubMed] [Google Scholar]
- 17.Papavasileiou E, Dereklis D, Oikonomidis P, Grixti A, Vineeth Kumar B, Prasad S, et al. An effective programme to systematic diabetic retinopathy screening in order to reduce diabetic retinopathy blindness. Hellenic J Nucl Med 2014;17(Suppl 1):30–34. [PubMed] [Google Scholar]
- 18.Ryeom S, Folkman J. Role of endogenous angiogenesis inhibitors in Down syndrome. J Craniofacial Surg 2009;20(Suppl 1):595–6. doi: 10.1097/SCS.0b013e3181927f47 [DOI] [PubMed] [Google Scholar]
- 19.Brondani LA, de Souza BM, Duarte GC, Kliemann LM, Esteves JF, Marcon AS, et al. The UCP1−3826A/G polymorphism is associated with diabetic retinopathy and increased UCP1 and MnSOD2 gene expression in human retina. Invest Ophthalmol Vis Sci 2012;53:7449–57. doi: 10.1167/iovs.12-10660 [DOI] [PubMed] [Google Scholar]
- 20.Payne AJ, Kaja S, Naumchuk Y, Kunjukunju N, Koulen P. Antioxidant drug therapy approaches for neuroprotection in chronic diseases of the retina. Int J Mol Sci 2014;15:1865–86. doi: 10.3390/ijms15021865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Njie-Mbye YF, Kulkarni-Chitnis M, Opere CA, Barrett A, Ohia SE. Lipid peroxidation: pathophysiological and pharmacological implications in the eye. Front Physiol 2013;4:366, eCollection. doi: 10.3389/fphys.2013.00366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Halliwell B, Gutteridge JMC, Cross CE. Free radicals, antioxidants, and human disease: where are we now? J Lab Clin Med 1992;119:598–620. [PubMed] [Google Scholar]
- 23.Gaál T, Speake BK, Mezes M, Noble RC, Surai PF, Vajdovich P. Antioxidant parameters and ageing in some animal species. Comp Haematol Int 1996;6:208–13. doi: 10.1007/BF00378112 [DOI] [Google Scholar]
- 24.Miller JK, Brzezinska-Slebodzinska E, Madsen FC. Oxidative stress, antioxidants, and animal function. J Dairy Sci 1993;76:2812–23. doi: 10.3168/jds.S0022-0302(93)77620-1 [DOI] [PubMed] [Google Scholar]
- 25.Halliwell B, Gutteridge JMC. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J 1984;219:1–14. doi: 10.1042/bj2190001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Juarez Olguín H, Calderon Guzman D. Free radicals: formation, types and effects in Central Nervous System. In: Dimitri K, Vasily S, (eds.) Handbook of free radicals: formation, types and effects. New York: Nova Science Publishers; 2009. p. 539–56. [Google Scholar]
- 27.Adam-Vizi V. Production of reactive oxygen species in brain mitochondria: contribution by electron transport chain and non-electron transport chain sources. Antioxid Redox Signal 2005;8:1140–9. doi: 10.1089/ars.2005.7.1140 [DOI] [PubMed] [Google Scholar]
- 28.Santos JM, Mishra M, Kowluru RA. Posttranslational modification of mitochondrial transcription factor A in impaired mitochondria biogenesis: implications in diabetic retinopathy and metabolic memory phenomenon. Exp Eye Res 2014;121C:168–77. doi: 10.1016/j.exer.2014.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McAuley AK, Wang JJ, Dirani M, Connell PP, Lamoureux E, Hewitt AW. Replication of genetic loci implicated in diabetic retinopathy. Invest Ophthalmol Vis Sci 2014; pii: iovs.13-13559v1 Doi: 10.1167/iovs.13-13559. [DOI] [PubMed] [Google Scholar]
- 30.Kowluru RA, Zhong Q, Santos JM, Thandampallayam M, Putt D, Gierhart DL. Beneficial effects of the nutritional supplements on the development of diabetic retinopathy. Nutr Metabol 2014;11:8. doi: 10.1186/1743-7075-11-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hernández M, Garcia-Ramírez M, García-Rocha C, Saez-López C, Valverde AM, Guinovart JJ, et al. Glycogen storage in the human retinal pigment epithelium: a comparative study of diabetic and non-diabetic donors. Acta Diabetol 2014;51:543–52. doi: 10.1007/s00592-013-0549-8 [DOI] [PubMed] [Google Scholar]
- 32.Rajashekhar G, Ramadan A, Abburi C, Callaghan B, Traktuev DO, Evans-Molina C, et al. Regenerative therapeutic potential of adipose stromal cells in early stage diabetic retinopathy. PLoS One 2014;9:e84671. doi: 10.1371/journal.pone.0084671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elsherbiny NM, Ahmad S, Naime M, Elsherbini AM, Fulzele S, Al-Gayyar MM, et al. ABT-702, an adenosine kinase inhibitor, attenuates inflammation in diabetic retinopathy. Life Sci 2013;93:78–88. doi: 10.1016/j.lfs.2013.05.024 [DOI] [PubMed] [Google Scholar]
- 34.Abu El-Asrar AM, Nawaz MI, Siddiquei MM, Siddiquei MM. High-mobility group box-1 induces decreased brain-derived neurotrophic factor-mediated neuroprotection in the diabetic retina. Mediators Inflamm 2013;863036 Doi: 10.1155/2013/863036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rivera JC, Sitaras N, Noueihed B, Hamel D, Madaan A, Zhou T, et al. Microglia and interleukin-1β in ischemic retinopathy elicit microvascular degeneration through neuronal semaphorin-3A. Arterioscler Thromb Vasc Biol 2013;33:1881–91. doi: 10.1161/ATVBAHA.113.301331 [DOI] [PubMed] [Google Scholar]
- 36.Huang J, Li X, Li M, Li J, Xiao W, Ma W, et al. Mitochondria-targeted antioxidant peptide SS31 protects the retinas of diabetic rats. Curr Mol Med 2013;13:935–45. doi: 10.2174/15665240113139990049 [DOI] [PubMed] [Google Scholar]
- 37.Omae T, Nagaoka T, Tanano I, Yoshida A. Adiponectin-induced dilation of isolated porcine retinal arterioles via production of nitric oxide from endothelial cells. Invest Ophthalmol Vis Sci 2013;54:4586–94. doi: 10.1167/iovs.13-11756 [DOI] [PubMed] [Google Scholar]
- 38.Zhong Q, Mishra M, Kowluru RA. Transcription factor Nrf2-mediated antioxidant defense system in the development of diabetic retinopathy. Invest Ophthalmol Vis Sci 2013;54:3841–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.He M, Pan H, Xiao C, Pu M. Roles for redox signaling by NADPH oxidase in hyperglycemia-induced heme oxygenase-1 expression in the diabetic retina. Invest Ophthalmol Vis Sci 2013;54:4092–101. doi: 10.1167/iovs.13-12004 [DOI] [PubMed] [Google Scholar]
- 40.Tan SM, Stefanovic N, Tan G, Wilkinson-Berka JL, de Haan JB. Lack of the antioxidant glutathione peroxidase-1 (GPx1) exacerbates retinopathy of prematurity in mice. Invest Ophthalmol Vis Sci 2013;54:555–62. doi: 10.1167/iovs.12-10685 [DOI] [PubMed] [Google Scholar]
- 41.Ha Y, Saul A, Tawfik A, Zorrilla EP, Ganapathy V, Smith SB. Diabetes accelerates retinal ganglion cell dysfunction in mice lacking sigma receptor 1. Mol Vis 2012;18:2860–70. [PMC free article] [PubMed] [Google Scholar]
- 42.Qiu F, He J, Zhou Y, Bai X, Wu G, Wang X, et al. Plasma and vitreous fluid levels of Dickkopf-1 in patients with diabetic retinopathy. Eye (Lond) 2014. Doi: 10.1038/eye.2013.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kowluru RA, Kowluru A, Veluthakal R, Mohammad G, Syed I, Santos JM, et al. TIAM1-RAC1 signalling axis-mediated activation of NADPH oxidase-2 initiates mitochondrial damage in the development of diabetic retinopathy. Diabetologia 2014;57:1047–56. doi: 10.1007/s00125-014-3194-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cervellati F, Cervellati C, Romani A, Cremonini E, Sticozzi C, Belmonte G, et al. Hypoxia induces cell damage via oxidative stress in retinal epithelial cells. Free Rad Res 2014;48:303–12. doi: 10.3109/10715762.2013.867484 [DOI] [PubMed] [Google Scholar]
- 45.Fu D, Yu JY, Wu M, Du M, Chen Y, Abdelsamie SA, et al. Immune complex formation in human diabetic retina enhances toxicity of oxidized LDL towards retinal capillary pericytes. J Lipid Res 2014;55:860–69. doi: 10.1194/jlr.M045401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aung MH, Park HN, Han MK, Obertone TS, Abey J, Aseem F, et al. Dopamine deficiency contributes to early visual dysfunction in a rodent model of type 1 diabetes. J Neurosci 2014;34:726–36. doi: 10.1523/JNEUROSCI.3483-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nebbioso M, Pranno F, Pescosolido N. Lipoic acid in animal models and clinical use in diabetic retinopathy. Expert Opin Pharmacother 2013;14:1829–38. doi: 10.1517/14656566.2013.813483 [DOI] [PubMed] [Google Scholar]
- 48.Ola MS, Ahmed MM, Abuohashish HM, Al-Rejaie SS, Alhomida AS. Telmisartan ameliorates neurotrophic support and oxidative stress in the retina of streptozotocin-induced diabetic rats. Neurochem Res 2013;38:1572–9. doi: 10.1007/s11064-013-1058-4 [DOI] [PubMed] [Google Scholar]
- 49.Zhang SY, Li BY, Li XL, Cheng M, Cai Q, Yu F, et al. Effects of phlorizin on diabetic retinopathy according to isobaric tags for relative and absolute quantification-based proteomics in db/db mice. Mol Vis 2013;19:812–21. [PMC free article] [PubMed] [Google Scholar]
- 50.Tang J, Du Y, Lee CA, Talahalli R, Eells JT, Kern TS. Low-intensity far-red light inhibits early lesions that contribute to diabetic retinopathy: in vivo and in vitro. Invest Ophthalmol Vis Sci 2013;54:3681–90. doi: 10.1167/iovs.12-11018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dong LY, Jin J, Lu G, Kang XL. Astaxanthin attenuates the apoptosis of retinal ganglion cells in db/db mice by inhibition of oxidative stress. Mar Drugs 2013;11:960–74. doi: 10.3390/md11030960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Si YF, Wang J, Guan J, Zhou L, Sheng Y, Zhao J. Treatment with hydrogen sulfide alleviates streptozotocin-induced diabetic retinopathy in rats. Brit J Pharmacol 2013;169:619–31. doi: 10.1111/bph.12163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Soufi FG, Mohammad-Nejad D, Ahmadieh H. Resveratrol improves diabetic retinopathy possibly through oxidative stress-nuclear factor κB-apoptosis pathway. Pharmacol Rep 2012;64:1505–14. doi: 10.1016/S1734-1140(12)70948-9 [DOI] [PubMed] [Google Scholar]
- 54.Kumar B, Gupta SK, Srinivasan BP, Nag TC, Srivastava S, Saxena R, et al. Hesperetin rescues retinal oxidative stress, neuroinflammation and apoptosis in diabetic rats. Microvasc Res 2013;87:65–74. doi: 10.1016/j.mvr.2013.01.002 [DOI] [PubMed] [Google Scholar]
- 55.Silva KC, Rosales MA, Hamassaki DE, Saito KC, Faria AM, Ribeiro PA, et al. Green tea is neuroprotective in diabetic retinopathy. Invest Ophthalmol Vis Sci 2013;54:1325–36. doi: 10.1167/iovs.12-10647 [DOI] [PubMed] [Google Scholar]
- 56.Du Y, Veenstra A, Palczewski K, Kern TS. Photoreceptor cells are major contributors to diabetes-induced oxidative stress and local inflammation in the retina. Proc Nat Acad Sci USA 2013;110:15586–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gaspar JM, Martins A, Cruz R, Rodrigues CM, Ambrósio AF, Santiago AR. Tauroursodeoxycholic acid protects retinal neural cells from cell death induced by prolonged exposure to elevated glucose. Neuroscience 2013;253:380–8. doi: 10.1016/j.neuroscience.2013.08.053 [DOI] [PubMed] [Google Scholar]
- 58.Rajalakshmi R, Amutha A, Ranjani H, Ali MK, Unnikrishnan R, Anjana RM, et al. Prevalence and risk factors for diabetic retinopathy in Asian Indians with young onset type 1 and type 2 diabetes. J Diabetes Complications 2014;28:291–7. pii: S1056-8727(13)00334-6 Doi: 10.1016/j.jdiacomp.2013.12.008. [DOI] [PubMed] [Google Scholar]


