Abstract
Objectives
Oxidative stress and inflammation have been reported to be higher in subjects with depression, but it is unclear whether this is due to inadequate dietary antioxidant intake or the pathophysiology of depression. The aim of this study was to assess the association between dietary and serum antioxidant status with depression scales in young male university students.
Methods
This research was a case–control study carried out on 60 male university students (30 students diagnosed with depression and 30 matched healthy controls). Beck Depression Inventory-II was used to assess the major depressive disorder (MDD) scales. A semi-quantitative food frequency questionnaire and 2-day 24-h recalls were used for dietary assessment. Dietary and serum total antioxidant capacity (TAC) and high-sensitive C-reactive protein (hs-CRP) concentrations were also measured.
Results
MDD subjects consumed less fruits (P < 0.05), legumes (P < 0.001), nuts and seeds (P = 0.003), vitamin C (P = 0.005), beta carotene (P < 0.001), lutein, and zeaxanthin (P = 0.006) than the controls. Moreover, the depressed group had lower serum TAC levels than their controls (P < 0.05). There were no significant differences in serum hs-CRP concentrations and dietary TAC levels between the study groups.
Discussion
Students with depression had significantly lower intake of dietary antioxidants. However, dietary TAC and serum hs-CRP levels were not significantly different between depressed and normal university male students. Intake of foods rich in antioxidants is encouraged in male students.
Keywords: Antioxidants, CRP, Depression, Oxidative stress, Students
Introduction
Depression is a debilitating mental disorder that affects 350 million people worldwide. It is estimated that depression is responsible for 50–70% of suicides.1 The World Health Organization (WHO) predicts that depression will become the second most prevalent disorder (after ischemic heart disease) by the year 2020.2
Oxidative stress, the imbalance between the oxidant and antioxidant systems, induces damage to DNA, proteins, and fatty acids.3 The brain is very sensitive to oxidative stress due to its modest antioxidant defense mechanisms, high oxygen consumption, high content of polyunsaturated fatty acids which are highly vulnerable to oxidative damage, the reducing potential of certain neurotransmitters, and the presence of redox-catalytic metals such as iron and copper.4 The presence of oxidative stress in depression has already been established and more recent studies show that depression is accompanied by increased reactive oxygen species and significantly decreased antioxidant status.3 However, it is not known whether this is due to inadequate dietary antioxidant intake or the pathophysiology of depression per se.
Recently, more papers have been published indicating the important role of inflammation in the pathophysiology of depression. Depression is accompanied by increased plasma levels of pro-inflammatory cytokines and altered levels of acute phase reactants such as hs-CRP.3
The total antioxidant capacity (TAC) is the capacity of enzymatic and non-enzymatic antioxidants required to reduce oxidants and its measurement provides useful information about the overall antioxidant status including those antioxidants not well measured or not well recognized.5
Recently, there has been increasing interest in the key role of diet in depression. More studies regarding the association between dietary antioxidants and depression have focused on individual antioxidants. For example, intakes of vitamin C and beta carotene have been shown to be lower in college students with depression compared with controls.6 Another study showed lower intakes of vitamin C, beta cryptoxanthin, and lutein in older adults with depression compared with non-depressed subjects.7
However, studying these antioxidants may provide an incomplete picture of the relationship between dietary antioxidants and mental health. Measuring the TAC of plant foods rich in antioxidants such as fruits, vegetables, nuts, and seeds is a more practical approach because there are numerous antioxidants (certain vitamins and phytochemicals with antioxidant characteristics) in these foods need to be measured.8
This study was aimed to assess associations between depression scales and serum TAC, hs-CRP, and intake of dietary antioxidants in university male students. We hypothesized that a lower dietary intake of antioxidants would be found among subjects with depression than their healthy counterparts.
Methods
Subjects
In the Fall and Winter 2012–2013, subjects were recruited from students at the international division of the Ahvaz Jundishapur University of Medical Sciences and Azad University, City of Abadan, Iran, both located by the Persian Gulf. Inclusion criteria were being male students aged 18–25 years with no medical or psychiatric disorders except depression, non-smoking, and taking no antioxidant supplements. Participants were divided into two groups: the major depressive disorder (MDD) group including 30 male students who had Beck Depression Inventory-II (BDI-II) scores higher than 20 and the control group including 30 age-matched male students with BDI-II scores lower than 14 and no current or past history of depression symptoms. All subjects were selected in the same catchment area.
Anthropometric measurements and body composition
Subject's body height, weight, and waist and hip circumferences were recorded according to WHO standard procedures with the least amount of clothing. The body fat percent was measured using a Bodystat Quadscan 4000 (Bodystat Ltd, Douglas, UK).
Psychiatry assessment
The full 21-item version of BDI-II was used to assess symptoms of depression and the total scores were then calculated.9 Validity and reliability of the Persian version of the BDI-II in non-clinical samples had been assessed before.10 The following BDI-II cutoff values were applied for classifying the subjects: scores below 14, 14 to 19, 20 to 28, and 29 to 63 were regarded as normal, mild, moderate, and severe depression, respectively. MDD was defined as scores higher than 20 (moderate-to-severe depression), and the controls were defined as scores lower than 14 (normal). Subjects with mild depression (BDI-II scores of 14–19) were excluded from the study.
Dietary assessment
The semi-quantitative food frequency questionnaire (semi-FFQ) was used to assess the dietary intake during the preceding year. Validity and reproducibility of the semi-FFQ had previously been assessed.11,12 The questionnaire included a list of foods with standard serving sizes typically consumed by Iranians. Participants reported their frequency of consumption of each food item on a daily, weekly, or monthly basis. The amount reported for each food item was converted to a daily intake value. The size of consumed foods was expressed in grams using household measures.13 Each food item was then coded and analyzed for nutrients using Nutritionist IV software (version 3.5; First Databank Division, The Hearst Corporation, San Bruno, CA, USA), which has been corrected for Iranian foods. Participants also completed a 24-hour food recall questionnaire for 2 days to ensure the amount of nutrients consumed.
To assess dietary TAC, ferric reducing-antioxidant power (FRAP) and trolox equivalent antioxidant capacity (TEAC) values were calculated for each participant. To determine the amounts of each food FRAP and TEAC, we used the tables published in previous papers.14–16 For analyzing similar food items (e.g. several types of oranges), we used the overall mean value. For each participant, the frequencies of consumption of each food were multiplied by their related FRAP and TEAC values and then summed to obtain dietary TAC. The FRAP assay measures the ability of antioxidants to reduce ferric ions to ferrous ions and the TEAC assay measures the ability of antioxidants to quench a radical cation in both lipophilic and hydrophilic environments.16
Assessment of serum TAC and hs-CRP
Five milliliters of fasting venous blood samples were collected from each participant to measure serum TAC and hs-CRP levels. The samples were centrifuged within 1 hour, stored at −20°C, and analyzed within a week. The TAC of serum was measured using a Biorex kit (Biorex Diagnostics Limited, Antrim, UK). The TAC test was performed in accordance with the supplier's instruction. In brief, ABTS (2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonate)) is incubated with a peroxidase (metmyoglobin) and H2O2 to produce the radical cation ABTS+. This has a relatively stable blue-green colour, which is measured at 660 nm. Antioxidants in the serum cause suppression of this colour production to a degree, which is proportional to their concentrations. The assay results are expressed in mmol trolox equiv/l. Serum hs-CRP levels were measured using a fluorescence immunoassay i-CHROMATM kit (BoditechMed, Gangwondo, Korea) with the limit of detection of 0.10 mg/l.
Statistical analyses
The Kolmogorov–Smirnov test and independent sample t test were performed to show normal distribution of variables and to compare variable means between the two groups, respectively. Simple correlation analysis (Pearson's correlation coefficient) was applied to illustrate the linear correlation between continuous quantitative variables. Hierarchical multiple regression was used to investigate the influence of demographic, dietary, inflammatory, and antioxidant factors on depression. Independent variables were entered in three blocks. The first block contained anthropometric variables (height, weight, body mass index (BMI), waist-to-hip ratio, body fat percent, and also age), the second block contained dietary variables (energy intake, daily servings of antioxidant-rich food groups, antioxidant vitamins, and dietary TAC), and the third block contained inflammatory and antioxidant variables (serum hs-CRP and TAC levels). All statistical analyses were conducted using the Statistical Package for the Social Sciences (SPSS Inc., version 16, Chicago, IL, USA). A P value of less than 0.05 was considered significant.
Medical ethics
The study was approved by the Ahvaz Jundishapur University of Medical Sciences' Research Ethics Committee (no. B-91/013). All subjects gave their written informed consent.
Results
General characteristics of the participants are shown in Table 1. In both depressed and control groups, the average age, calorie intake, height, weight, BMI, waist-to-hip ratio, and body fat percent were not significantly different.
Table 1.
General characteristics of the subjects (mean ± SD)
| Variables | Control group (n = 30) | Depression group (n = 30) | P value |
|---|---|---|---|
| Beck scores | 5.7 ± 2.2 | 27.8 ± 5.3 | <0.001* |
| Age (years) | 21.3 ± 1.6 | 20.6 ± 1.4 | 0.071 |
| Energy intake in MJ (kcal) | 10.5 ± 1.1 | 10.9 ± 1.3 | 0.151 |
| (2504.8 ± 266.4) | (2615.0 ± 317.2) | ||
| Height (cm) | 174.9 ± 6.2 | 175.2 ± 6.7 | 0.858 |
| Weight (kg) | 67.3 ± 10.7 | 71.5 ± 11.7 | 0.154 |
| BMI (kg m−2) | 22.1 ± 3.3 | 23.3 ± 3.4 | 0.159 |
| WHR | 0.84 ± 0.1 | 0.86 ± 0.1 | 0.198 |
| %BF | 12.8 ± 3.9 | 13.7 ± 4.4 | 0.406 |
| Body fat weight (kg) | 8.9 ± 4.4 | 10.1 ± 4.6 | 0.307 |
*Significant at the 0.001 level. ;
BMI, body mass index; WHR, waist-to-hip ratio; %BF, body fat percent.
Dietary analysis revealed that the depressed group consumed less fruits (P < 0.05), legumes (P < 0.001), nuts and seeds (P = 0.003), vitamin C (P = 0.005), beta carotene (P = 0.001), lutein, and zeaxanthin (P = 0.006) than the control group (Table 2). No statistically significant difference was observed between the two groups in terms of dietary TAC.
Table 2.
Intake of antioxidant-rich food groups and nutrients in the two study groups (mean ± SD)
| Variables | Control group (n = 30) | Depression group (n = 30) | P value |
|---|---|---|---|
| Fruits (servings/day) | 2.8 ± 0.7 | 2.4 ± 0.7 | 0.039* |
| Vegetables (servings/day) | 3.2 ± 0.7 | 3.0 ± 0.6 | 0.408 |
| Whole grains (servings/day) | 1.2 ± 0.5 | 1.1 ± 0.5 | 0.848 |
| Legumes (servings/day) | 1.25 ± 0.3 | 0.96 ± 0.3 | <0.001*** |
| Nuts and seeds (servings/day) | 0.87 ± 0.2 | 0.70 ± 0.1 | 0.003** |
| Tea and coffee (cups/day) | 1.9 ± 1.3 | 2.2 ± 0.8 | 0.437 |
| Chocolates (servings/day) | 0.53 ± 0.3 | 0.52 ± 0.2 | 0.836 |
| Seasoning (teaspoons/day) | 0.74 ± 0.4 | 0.55 ± 0.4 | 0.095 |
| Vegetable oils (servings/day) | 1.6 ± 0.6 | 1.7 ± 0.7 | 0.377 |
| Vitamin C (mg/day) | 106.9 ± 15.3 | 94.9 ± 16.7 | 0.005** |
| Vitamin E (mg/day) | 11.3 ± 3.6 | 11.6 ± 4.6 | 0.841 |
| Alpha carotene (μg/day) | 426.7 ± 96.3 | 394.8 ± 58.7 | 0.128 |
| Beta carotene (μg/day) | 2890.6 ± 475.5 | 2425.1 ± 539.0 | 0.001** |
| Beta cryptoxanthin (μg/day) | 131.1 ± 38.9 | 129.0 ± 44.8 | 0.850 |
| Lycopene (μg/day) | 2913.6 ± 805.3 | 2761.0 ± 759.0 | 0.453 |
| Lutein and zeaxanthin (μg/day) | 1732.0 ± 451.6 | 1353.7 ± 572.9 | 0.006** |
| Total carotenoids (μg/day) | 8094.0 ± 1444.8 | 7063.6 ± 1486.6 | 0.009** |
| Dietary FRAP (mmol Fe(II)/day) | 10.07 ± 2.41 | 9.68 ± 2.48 | 0.536 |
| Dietary TEAC (mmol trolox equiv./day) | 5.34 ± 1.79 | 5.70 ± 2.40 | 0.513 |
* Significant at the 0.05 level.
** Significant at the 0.01 level.
*** Significant at the 0.001 level.
FRAP, ferric reducing-antioxidant power; TEAC, trolox equivalent antioxidant capacity.
Biochemical analysis showed that the depressed group had lower serum TAC than the controls (P < 0.05) (Table 3). No significant differences in serum hs-CRP concentrations were observed between the groups.
Table 3.
Serum TAC and hs-CRP of the two study groups (mean ± SD)
| Variables | Control group (n = 30) | Depression group (n = 30) | P value |
|---|---|---|---|
| Serum TAC (mmol trolox equiv./l) | 1.92 ± 0.34 | 1.69 ± 0.33 | 0.012* |
| Serum hs-CRP (mg/l) | 2.05 ± 1.57 | 2.70 ± 2.04 | 0.175 |
*Significant at the 0.05 level.
TAC, total antioxidant capacity; hs-CRP = high-sensitive C-reactive protein.
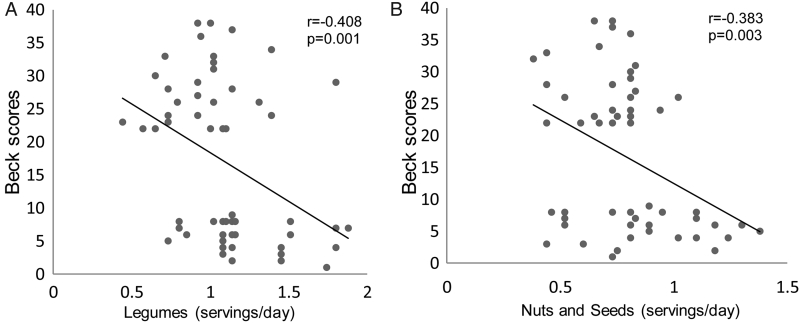
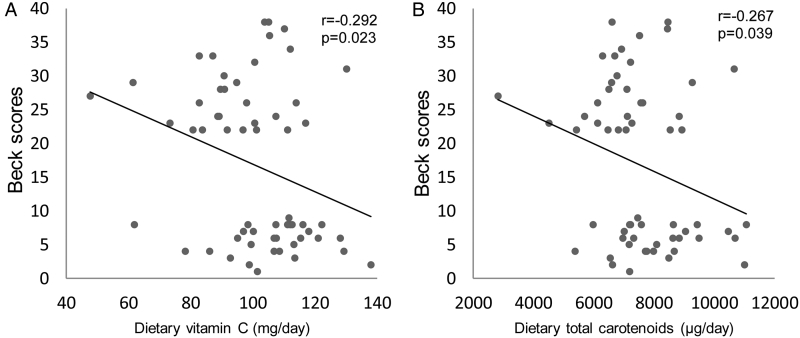
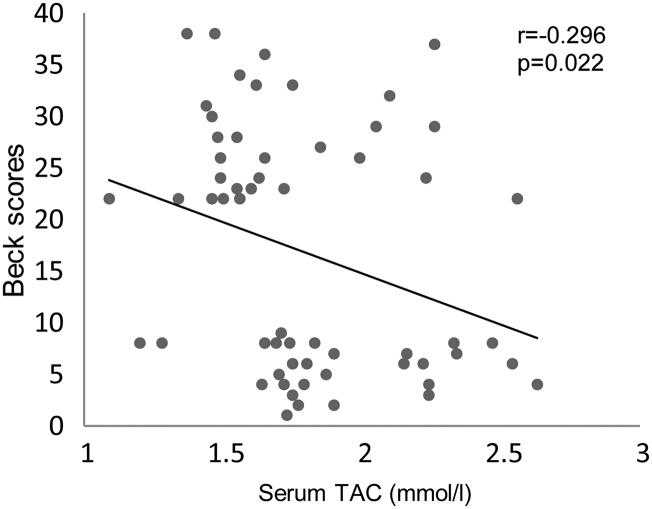
Pearson's correlation analysis showed negative association between Beck scores (severity of depression) and daily servings of legumes (r = −0.408, P = 0.001), nuts and seeds (r = −0.383, P = 0.003) (Fig. 1), dietary vitamin C (r = −0.292, P < 0.05), dietary total carotenoids (r = −0.267, P < 0.05) (Fig. 2), and serum TAC (r = −0.296, P < 0.05) (Fig. 3). However, these variables showed no significant correlations with depression scores in MDD subjects (figures not shown). There was no significant correlation between serum TAC, hs-CRP levels, and the intake of dietary antioxidant vitamins or TAC scores.
Figure 1.
Correlation between Beck scores (severity of depression) and (A) daily servings of legumes, (B) daily servings of nuts and seeds in all subjects studied. The significant negative correlations suggest that subjects with depression are more likely to follow unhealthy diet compared with subjects without depression.
Figure 2.
Correlation between Beck scores (severity of depression) and (A) dietary vitamin C intake (milligrams per day), (B) dietary total carotenoids intake (micrograms per day) in all subjects studied. The significant negative correlations between dietary antioxidant vitamin intake and depression scores suggest that subjects with depression had significantly lower intake of some foods that are high in antioxidants.
Figure 3.
Correlation between Beck scores (severity of depression) and serum TAC (millimoles per liter) in all subjects studied. The significant negative correlation suggests that serum antioxidant defense is lower in subjects with depression.
In hierarchical multiple regression, demographic variables were entered at step one, explaining 10.6% of the variance in depression. Dietary variables entered in step two, explained an additional 46.8% of variance. After the entry of the inflammatory and antioxidant variables in step three, the total variance explained by the model was 59.3%. In this model, daily servings of legumes (beta = −0.34, P < 0.05) and serum TAC (beta = −0.29, P < 0.05) were shown as risk factors for depression.
Discussion
In this study, male university students with depression consumed less fruits, legumes, nuts, and seeds than their normal counterparts. These results are consistent with the findings of previous studies showing that depressed people are more likely to follow unhealthy diet compared with people without depression.17,18 In accordance with this finding, several studies have shown that traditional dietary patterns characterized by high intake of fruits, vegetables, whole grains, and fish were associated with lower risk of depression.19–22 Conversely, Western dietary patterns rich in refined grains, sugary products, processed or fried foods, and beer were associated with higher risk of depression.19,22 Furthermore, results from a meta-analysis suggest that antioxidant supplements have less beneficial effects, and even in some cases may be harmful.23 These findings emphasize on the important role of antioxidant phytochemicals in plant foods.
In terms of antioxidant vitamins, the intake of vitamin C and carotenoids such as beta carotene, lutein, and zeaxanthin was lower in students with depression. Furthermore, there was a significant negative correlation between Beck scores (severity of depression) and dietary vitamin C or total carotenoids. Merrill et al.24 demonstrated that dietary changes, which lead to increased vitamin C intake, may reduce depression symptoms. Khanzode et al.25 and Beydoun et al.26 showed that depressed people have lower serum levels of vitamin C and carotenoids, respectively. Our findings are consistent with Park et al.6 and Payne et al.7 as well. Park et al. have shown that depressed Korean female students consumed less vitamin C and beta carotene than controls. Payne et al. have also demonstrated that vitamin C, lutein, and beta cryptoxanthin intakes were lower in depressed older adults. In multivariable models controlled for sex, age, race, education, BMI, vascular comorbidity score, total dietary fat, and alcohol, vitamin C and beta cryptoxanthin remained significant. It is noteworthy that the intake of vitamins C and E was lower than the dietary reference intake in 23.3% and 70% of our subjects, respectively.
Regarding beta carotene, lutein, and zeaxanthin, our findings are in accordance with the findings on carotenoid profiles of the brain. Craft et al.27 demonstrated that xanthophylls accounted for 66–77% of total carotenoids in the human brain. Studies have shown that total xanthophyll and total carotenoid concentrations in the frontal lobes decrease with age and may play an important role in the aetiology of depression.7,27
Regarding serum TAC levels, our findings are in agreement with Cumurcu et al.,5 Galecki et al.,28 and Sarandol et al.29 who have suggested that serum antioxidant defense is lower in the depressed subjects. Furthermore, our findings showed a significant negative correlation between Beck scores and serum TAC levels. Lower serum TAC in the depressed group could be due to lower intake of some food groups such as fruits, legumes, nuts and seeds, or dietary antioxidant vitamins such as vitamin C or total carotenoids. However, there were no significant differences in dietary TAC scores between the groups. In addition, no significant correlation between dietary and serum TAC was observed. This finding is consistent with some studies suggesting that dietary TAC may not be related to serum TAC.30,31
Our findings are also in consistent with Steptoe et al.32 showing no significant association between depressive symptoms and serum hs-CRP. In contrast to these findings, Ezat et al.33 and Miller et al.34 showed that serum hs-CRP levels in the depressed group were significantly higher than the controls. This discrepancy may, in part, be due to differences in the sample size, the inflammatory and immune markers used and also the measures of depressive symptoms.32
To the best of our knowledge, this is the first study that examined the association between dietary TAC and depression in young university students. FRAP and TEAC scores represent a wide range of antioxidant nutrients in plant foods, including those antioxidants that were not well measured or characterized. Despite the fact that the depressed group consumed less fruits, legumes, and nuts and had lower intake of vitamin C and total carotenoids, dietary TAC showed no significant difference between depressed and apparently healthy controls. This could, partially, be due to higher intake of tea, coffee, and plant oils in the depressed group, although the differences did not reach the statistically significant level. These findings suggest that dietary TAC might be affected by phytochemicals with antioxidant properties other than vitamin C and carotenoids.
In conclusion, intake of dietary vitamin C and carotenoids and also serum TAC levels are correlated with depression scales in young university male students. Dietary TAC assessed by FFQ-based FRAP and TEAC scores did not show any significant association with depressive symptoms, serum TAC, and hs-CRP levels. The lower antioxidant status in students with depression suggests paying more attention to dietary modifications regarding consumption of antioxidant-rich food items. As a limitation, our study was carried out on male subjects suggesting further studies with higher sample size on both genders to determine factors affecting serum and dietary TAC and their relationship with depression.
Acknowledgement
This study was a part of the Master of Science thesis of Mohammad Prohan, Nutrition Department, School of Paramedicine, Jundishapur University of Medical Sciences, Ahvaz, Iran. The costs of laboratory tests were covered by a grant from Vice Chancellor for Research Affairs of Ahvaz Jundishapur University of Medical Sciences.
References
- 1.Lecomte D, Fornes P. Suicide among youth and young adults, 15 through 24 years of age. A report of 392 cases from Paris, 1989–1996. J Forensic Sci 1998;43(5):964–8. [PubMed] [Google Scholar]
- 2.Murray CJ, Lopez AD. Global mortality, disability, and the contribution of risk factors: Global Burden of Disease Study. Lancet 1997;349(9063):1436–42. [DOI] [PubMed] [Google Scholar]
- 3.Maes M, Galecki P, Chang YS, Berk M. A review on the oxidative and nitrosative stress (O&NS) pathways in major depression and their possible contribution to the (neuro)degenerative processes in that illness. Prog Neuropsychopharmacol Biol Psychiatry 2011;35(3):676–92. [DOI] [PubMed] [Google Scholar]
- 4.Ng F, Berk M, Dean O, Bush AI. Oxidative stress in psychiatric disorders: evidence base and therapeutic implications. Int J Neuropsychopharmacol 2008;11(6):851–76. [DOI] [PubMed] [Google Scholar]
- 5.Cumurcu BE, Ozyurt H, Etikan I, Demir S, Karlidag R. Total antioxidant capacity and total oxidant status in patients with major depression: impact of antidepressant treatment. Psychiatry Clin Neurosci 2009;63(5):639–45. [DOI] [PubMed] [Google Scholar]
- 6.Park JY, You JS, Chang KJ. Dietary taurine intake, nutrients intake, dietary habits and life stress by depression in Korean female college students: a case-control study. J Biomed Sci 2010;17(Suppl 1):S40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Payne ME, Steck SE, George RR, Steffens DC. Fruit, vegetable, and antioxidant intakes are lower in older adults with depression. J Acad Nutr Diet 2012;112(12):2022–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lindsay DG, Astley S. European research on the functional effects of dietary antioxidants—EUROFEDA. Mol Aspects Med 2002;23(1–3):1–38. [DOI] [PubMed] [Google Scholar]
- 9.Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J Pers Assess 1996;67(3):588–97. [DOI] [PubMed] [Google Scholar]
- 10.Ghassemzadeh H, Mojtabai R, Karamghadiri N, Ebrahimkhani N. Psychometric properties of a Persian-language version of the Beck Depression Inventory–Second edition: BDI-II-PERSIAN. Depress Anxiety 2005;21(4):185–92. [DOI] [PubMed] [Google Scholar]
- 11.Esfahani FH, Asghari G, Mirmiran P, Azizi F. Reproducibility and relative validity of food group intake in a food frequency questionnaire developed for the Tehran Lipid and Glucose Study. J Epidemiol 2010;20(2):150–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mirmiran P, Esfahani FH, Mehrabi Y, Hedayati M, Azizi F. Reliability and relative validity of an FFQ for nutrients in the Tehran lipid and glucose study. Public Health Nutr 2010;13(5):654–62. [DOI] [PubMed] [Google Scholar]
- 13.Ghafarpour M, Houshiar-Rad A, Kianfar H. The manual for household measures, cooking yields factors and edible portion of food. Tehran: Keshavarzi Press; 1999. (in Persian). [Google Scholar]
- 14.Halvorsen BL, Holte K, Myhrstad MC, Barikmo I, Hvattum E, Remberg SF,. et al. A systematic screening of total antioxidants in dietary plants. J Nutr 2002;132(3):461–71. [DOI] [PubMed] [Google Scholar]
- 15.Pellegrini N, Serafini M, Salvatore S, Del Rio D, Bianchi M, Brighenti F. Total antioxidant capacity of spices, dried fruits, nuts, pulses, cereals and sweets consumed in Italy assessed by three different in vitro assays. Mol Nutr Food Res 2006;50(11):1030–8. [DOI] [PubMed] [Google Scholar]
- 16.Pellegrini N, Serafini M, Colombi B, Del Rio D, Salvatore S, Bianchi M,. et al. Total antioxidant capacity of plant foods, beverages and oils consumed in Italy assessed by three different in vitro assays. J Nutr 2003;133(9):2812–9. [DOI] [PubMed] [Google Scholar]
- 17.Payne ME, Hybels CF, Bales CW, Steffens DC. Vascular nutritional correlates of late-life depression. Am J Geriatr Psychiatry 2006;14(9):787–95. [DOI] [PubMed] [Google Scholar]
- 18.Averina M, Nilssen O, Brenn T, Brox J, Arkhipovsky VL, Kalinin AG. Social and lifestyle determinants of depression, anxiety, sleeping disorders and self-evaluated quality of life in Russia. Soc Psychiatry Psychiatr Epidemiol 2005;40(7):511–8. [DOI] [PubMed] [Google Scholar]
- 19.Jacka FN, Pasco JA, Mykletun A, Williams LJ, Hodge AM, O'Reilly SL,. et al. Association of Western and traditional diets with depression and anxiety in women. Am J Psychiatry 2010;167(3):305–11. [DOI] [PubMed] [Google Scholar]
- 20.Rienks J, Dobson AJ, Mishra GD. Mediterranean dietary pattern and prevalence and incidence of depressive symptoms in mid-aged women: results from a large community-based prospective study. Eur J Clin Nutr 2013;67(1):75–82. [DOI] [PubMed] [Google Scholar]
- 21.Nanri A, Kimura Y, Matsushita Y, Ohta M, Sato M, Mishima N,. et al. Dietary patterns and depressive symptoms among Japanese men and women. Eur J Clin Nutr 2010;64(8):832–9. [DOI] [PubMed] [Google Scholar]
- 22.Akbaraly TN, Brunner EJ, Ferrie JE, Marmot MG, Kivimaki M, Singh-Manoux A. Dietary pattern and depressive symptoms in middle age. Br J Psychiatry 2009;195(5):408–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA 2007;297(8):842–57. [DOI] [PubMed] [Google Scholar]
- 24.Merrill RM, Taylor P, Aldana SG. Coronary Health Improvement Project (CHIP) is associated with improved nutrient intake and decreased depression. Nutrition 2008;24(4):314–21. [DOI] [PubMed] [Google Scholar]
- 25.Khanzode SD, Dakhale GN, Khanzode SS, Saoji A, Palasodkar R. Oxidative damage and major depression: the potential antioxidant action of selective serotonin re-uptake inhibitors. Redox Rep 2003;8(6):365–70. [DOI] [PubMed] [Google Scholar]
- 26.Beydoun MA, Beydoun HA, Boueiz A, Shroff MR, Zonderman AB. Antioxidant status and its association with elevated depressive symptoms among US adults: National Health and Nutrition Examination Surveys 2005–6. Br J Nutr 2013;109(9):1714–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Craft NE, Haitema TB, Garnett KM, Fitch KA, Dorey CK. Carotenoid, tocopherol, and retinol concentrations in elderly human brain. J Nutr Health Aging 2004;8(3):156–62. [PubMed] [Google Scholar]
- 28.Gałecki P, Szemraj J, Bieńkiewicz M, Florkowski A, Gałecka E. Lipid peroxidation and antioxidant protection in patients during acute depressive episodes and in remission after fluoxetine treatment. Pharmacol Rep 2009;61(3):436–47. [DOI] [PubMed] [Google Scholar]
- 29.Sarandol A, Sarandol E, Eker SS, Erdinc S, Vatansever E, Kirli S. Major depressive disorder is accompanied with oxidative stress: short-term antidepressant treatment does not alter oxidative-antioxidative systems. Hum Psychopharmacol 2007;22(2):67–73. [DOI] [PubMed] [Google Scholar]
- 30.Rautiainen S, Serafini M, Morgenstern R, Prior RL, Wolk A. The validity and reproducibility of food-frequency questionnaire-based total antioxidant capacity estimates in Swedish women. Am J Clin Nutr 2008;87(5):1247–53. [DOI] [PubMed] [Google Scholar]
- 31.Pellegrini N, Salvatore S, Valtueña S, Bedogni G, Porrini M, Pala V,. et al. Development and validation of a food frequency questionnaire for the assessment of dietary total antioxidant capacity. J Nutr 2007;137(1):93–8. [DOI] [PubMed] [Google Scholar]
- 32.Steptoe A, Kunz-Ebrecht SR, Owen N. Lack of association between depressive symptoms and markers of immune and vascular inflammation in middle-aged men and women. Psychol Med 2003;33(4):667–74. [DOI] [PubMed] [Google Scholar]
- 33.Ezat M, Zahra A, Hassona A, Obeah E. The relation between major depression, plasma cytokines and highly sensitive CRP. Curr Psychiatry 2006;13(2):226–39. [Google Scholar]
- 34.Miller GE, Stetler CA, Carney RM, Freedland KE, Banks WA. Clinical depression and inflammatory risk markers for coronary heart disease. Am J Cardiol 2002;90(12):1279–83. [DOI] [PubMed] [Google Scholar]