ABSTRACT
Objective: The present study was designed to investigate the chemoprotective efficacy of an L-cysteine-based oxovanadium (IV) complex, namely, oxovanadium (IV)-L-cysteine methyl ester complex (VC-IV) against cisplatin (CDDP)-induced renal injury in Swiss albino mice.
Methods: CDDP was administered intraperitoneally (5 mg/kg body weight) and VC-IV was administered orally (1 mg/kg body weight) in concomitant and 7 days pre-treatment schedule.
Results: CDDP-treated mice showed marked kidney damage and renal failure. Administration of VC-IV caused significant attenuation of renal oxidative stress and elevation of antioxidant status. VC-IV also significantly decreased serum levels of creatinine and blood urea nitrogen, and improved histopathological lesions. Western blot analysis of the kidneys showed that VC-IV treatment resulted in nuclear translocation of nuclear factor E2-related factor 2 (Nrf2) through modulation of cytosolic Kelch-like ECH-associated protein 1. Thus, VC-IV stimulated Nrf2-mediated activation of antioxidant response element (ARE) pathway and promoted expression of ARE-driven cytoprotective proteins, heme oxygenase 1 and NAD(P)H:quinone oxidoreductase 1, and enhanced activity of antioxidant enzymes. Interestingly, VC-IV did not alter the bioavailability and renal accumulation of CDDP in mice.
Discussion: In this study, VC-IV exhibited strong nephroprotective efficacy by restoring antioxidant defense mechanisms and hence may serve as a promising chemoprotectant in cancer chemotherapy.
KEYWORDS: Vanadium, kidney damage, reactive oxygen species, Nrf2/ARE pathway, antioxidant enzymes, apoptosis, atomic absorption spectroscopy
Introduction
The serendipitously discovered cisplatin (CDDP) triggered the discovery of other metal-based drugs, but still remains one of the most widely used anti-cancer drugs worldwide [1]. Despite its remarkable anti-neoplastic properties, CDDP therapy is associated with several adverse effects, including nephrotoxicity, ototoxicity, myelosuppression, and neurotoxicity [2]. Amongst them, nephrotoxicity occurs in 25–33% of patients upon single dose and in 50–75% of patients upon multiple doses, which seriously impairs their quality of life and even become life-threatening [3]. The glomerular filtration rate decrease by 30% after only two doses, and treatments must often be stopped. In fact, only 60% of patients complete three of four cisplatin cycles [4]. This ultimately compromises the treatment outcome including disease control and survival in patients with curable malignancies. In this regard, screening of potential compounds is required to provide protection against CDDP-induced nephropathy, without interfering with its efficacy.
CDDP undergoes nonenzymatic hydrolysis to form aquated and electrophilic products through chloride ligand-exchange reactions upon uptake into the cell. Loss of labile chloride ligands results in nucleophilic substitution reactions with DNA and proteins, generation of oxidative stress, inflammation, and ultimately cell death [5,6]. Amongst the protective strategies, the most notable signaling pathway is the nuclear factor E2-related factor 2 (Nrf2)-mediated activation of antioxidant response element (ARE) pathway which can counteract against accumulating reactive oxygen species (ROS) and electrophiles [5,7]. Studies show that Nrf2-null mice have an impaired capacity to quench free radicals and electrophiles in the kidneys and thus kidneys become extremely susceptible to oxidative injury [5]. Moreover, CDDP-induced renal injury is exacerbated in Nrf2-knockout mice, whereas the pre-administration of Nrf2 inducers inhibit CDDP-mediated nephropathy [7]. Clinical trials of the potent Nrf2 activator bardoxolone methyl showed significant improvement in renal function in chronic kidney disease patients with type 2 diabetes [8]. These indicate the importance of Nrf2 in renal antioxidant defense and thus, activation of Nrf2/ARE pathway can be considered as an important molecular target to prevent CDDP-induced nephrotoxicity.
Vanadium is an essential trace element that is involved in numerous biological processes in plants, mammals and microorganisms [9]. Vanadium-based compounds exhibit a broad range of pharmacological activities, namely, antioxidant property, anticarcinogenic effect, lowering of cholesterol, triglycerides and glucose levels, diuretic effect, contraction of blood vessels, enhancement of oxygen-affinity of hemoglobin and myoglobin, etc [9,10]. Vanadium also functions as an oxidation–reduction catalyst [11]. Vanadium compounds have been found to increase the activity of antioxidant enzymes and glutathione (GSH) content through up-regulation of Nrf2 [12,13]. Among vanadium-based compounds, oxovanadium (IV) complexes (VOL2, where L is a bidentate anionic organic ligand) are more effective, better tolerated, and produce more reliable pharmacological effects than their inorganic counterparts [14]. Several oxovanadium (IV) complexes also have been reported to possess good antioxidant potential and exert protection against oxidative stress [15,16].
All these above stated facts motivated us to investigate the effect of an oxovanadium (IV) complex, namely, oxovanadium (IV)-L-cysteine methyl ester complex (VC-IV) against CDDP-induced nephrotoxicity in Swiss albino mice. For this purpose, we administered the VC-IV complex along with CDDP in concomitant- and 7 days pre-treatment schedules to find out any schedule-dependent difference in efficacy. We also treated a group of mice with the ligand L-cysteine methyl ester hydrochloride (LCME) to observe the effect of the ligand itself. In addition, one group of mice was also fed with VC-IV only in order to see whether the complex has any negative side effect. The nephroprotective efficacy of the complex was evaluated by means of renal function markers and histopathology. The study was also complemented with measurements of oxidative stress markers and antioxidant enzymes. To find out the probable protective mechanism, the influence of the oxovanadium (IV) complex on ARE pathway was studied by Western blot analysis of redox-sensitive transcription factor Nrf2 along with its downstream effectors heme oxygenase 1 (HO1) and NAD(P)H:quinone oxidoreductase 1 (NQO1).
Methods
Chemicals
Oxovanadium (IV)-L-cysteine methyl ester complex (VC-IV, Figure 1) was prepared following the literature procedure of Sakurai et al. [17,18]. All chemicals if not specified were obtained from Sigma-Aldrich Chemicals Private Limited (Bangalore, India). In situ cell death detection kit, AP was purchased from Roche Diagnostics India Private Limited (Kolkata, India). Nrf2, Keap1, HO1, NQO1, anti-rabbit IgG-HRP, anti-goat IgG-HRP, anti-mouse IgG-HRP, and Luminol were bought from Santa Cruz Biotechnology (Texas, USA). GAPDH and Histone H3 were purchased from Novus Biologicals (Colorado, USA). Blood urea nitrogen (BUN), creatinine, and Elyte 3 kit (Na+, K+, and Cl– Colorimetric) were purchased from Crest Biosystems (Goa, India).
Figure 1.
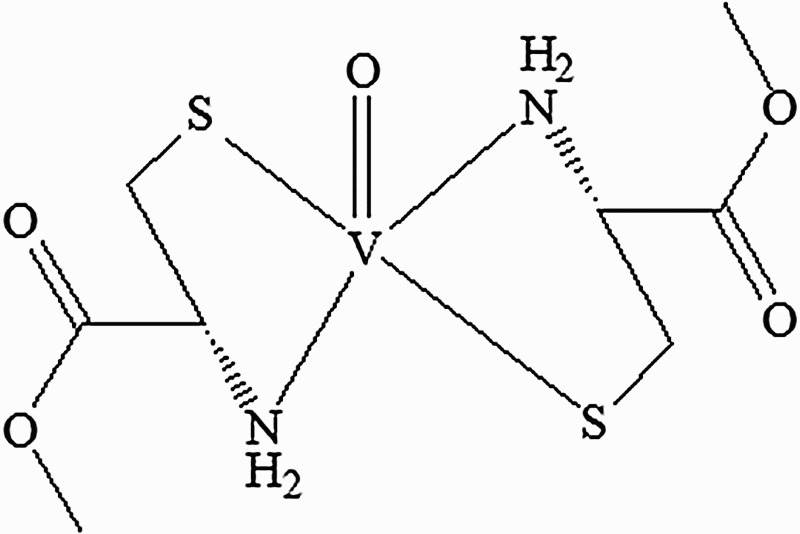
Structure of oxovanadium (IV)-L-cysteine methyl ester (VC-IV) complex.
Animals
Adult (7–8 weeks old) Swiss albino female mice (25 ± 2 g b.w.), bred in the animal colony of Chittaranjan National Cancer Institute (Kolkata, India) were used for this study. The mice were kept at controlled temperature (22 ± 2°C) and humidity (60 ± 5%) under alternating light and dark conditions (12 hours/12 hours). Standard food pellets and drinking water were provided ad libitum. The experiments were carried out strictly following the guidelines of Institutional Animal Ethics Committee (CPCSEA Registration No. 1774/GO/RBi/S/14/CPCSEA).
Experimental design
The organovanadium compound was administered as a suspension using 5.5% propylene glycol in water. In the present study the animals were divided into six groups containing six animals (n = 6) in each group (Figure 2). The groups were:
Vehicle-treated group [Vehicle]: Each animal was given 5.5% propylene glycol in water by oral gavage for 9 consecutive days.
Only VC-IV-treated group [VC-IV]: Each animal was treated only with VC-IV (1 mg/kg b.w., p.o.) throughout the experimental period.
CDDP-treated group [CDDP]: Each animal was injected with CDDP (5 mg/kg b.w., i.p.) for consecutive 5 days (day 1 to day 5).
CDDP + L-cysteine methyl ester-treated group [CDDP + LCME]: LCME was administered orally at the dose of 0.85 mg/kg b.w. (comparable with the amount of ligand content in the complex at the dose of 1 mg/kg b.w.) in water 7 days prior to CDDP treatment and then continued up to day 9 and CDDP was given as in CDDP-treated group.
CDDP + VC-IV concomitant-treatment group [CDDP + VC-IV Con]: VC-IV (1 mg/kg b.w., p.o.) was administered from day 1 to 9 and CDDP was given as in CDDP-treated group.
CDDP + VC-IV pre-treatment group [CDDP + VC-IV Pre]: VC-IV (1 mg/kg b.w., p.o.) was administered 7 days prior to CDDP treatment and then continued up to day 9, and CDDP was given as in CDDP-treated group.
Figure 2.
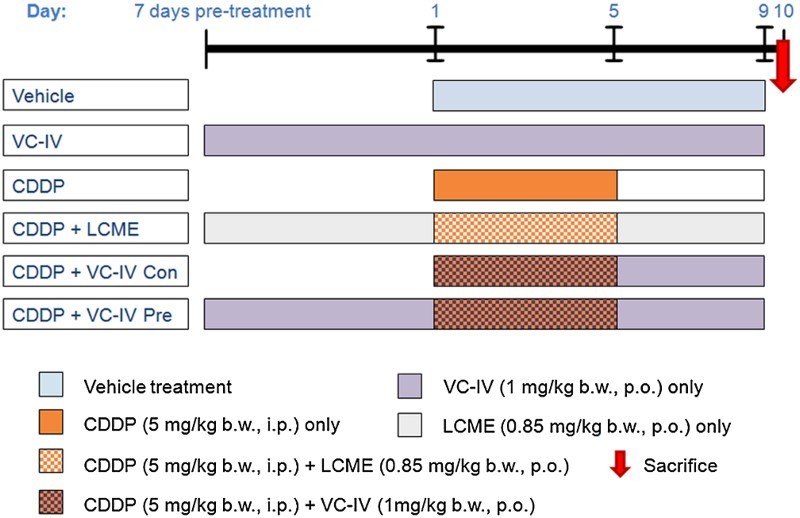
Experimental treatment schedule to study the protective effect of VC-IV against CDDP-induced nephrotoxicity. In the first group the vehicle (5.5% propylene glycol aqueous suspension) was administered for 9 consecutive days. In the VC-IV-treated group, VC-IV was administered at the dose of 1 mg/kg b.w., p.o. throughout the period (16 days). In the CDDP-treated group, CDDP (5 mg/kg b.w., i.p.) was administered for consecutive 5 days (day 1 to day 5). In the fourth group, the ligand LCME (0.85 mg/kg b.w., p.o.) was administered along with CDDP in order to study the effect of the ligand only. In the fifth and sixth group, the organovanadium complex (1 mg/kg b.w., p.o.) was administered along with CDDP in concomitant-treatment and 7 days pre-treatment schedule, respectively. VC-IV: oxovanadium (IV)-L-cysteine methyl ester complex; CDDP: cisplatin; LCME: L-cysteine methyl ester hydrochloride.
The mice were euthanized on day 10, 4 days after last injection of CDDP.
Sample collection
Before euthanasia, all animals were fasted for 4 hours and then blood samples were collected from the retro-orbital venous plexus under anesthesia (ketamine-HCl, 24 mg/kg b.w., i.m.) and after blood collection they were euthanized by decapitation. Following clot formation, the serum was separated by centrifugation at 2000×g for 10 minutes and stored at –20°C. The kidneys of each animal were quickly excised, washed with ice-cold isotonic saline (0.9% NaCl) and blotted dry. One part of the kidneys (2–3 mm) were fixed in 10% neutral buffered formalin for histopathological and immunohistochemical studies. The rest of the kidney tissues were immediately weighed and stored at –80°C for further biochemical and Western blot analysis.
Biochemical assays
Creatinine, BUN and electrolytes, Na+, K+, and Cl–, were measured spectrophotometrically (Infinite® 200 PRO, TECAN, Switzerland) from serum samples using commercially available kits (Crest Biosystems, India) according to the manufacturer’s protocol. The intracellular amounts of reactive oxygen species (ROS) were measured in kidney tissue homogenate by a fluorometric assay with 2′,7′-dichlorofluorescin diacetate (DCFH-DA) [19]. Nitric oxide (NO) production in kidney tissue homogenate was determined by estimating the level of stable NO metabolites, namely, nitrate (NO3–) and nitrite (NO2–) ions by reaction with Griess reagent using NaNO2 as standard [20]. Renal lipid peroxidation (LPO) level was measured by estimating the formation of thiobarbituric acid reactive substances (TBARS) [21]. Renal concentrations of reduced glutathione (GSH) and oxidized glutathione (GSSG) were assessed by a kinetic assay in which catalytic amounts of GSH caused a continuous reduction of 5,5′-dithiobis-2-nitrobenzoic acid (DTNB) to 5,5′-thiobis-2-nitrobenzoic acid (TNB) at 412 nm [22]. Superoxide dismutase (SOD) activity was determined in kidney cytosol by monitoring the auto-oxidation of pyrogallol (20 mM) at 420 nm [23]. Catalase (CAT) activity in kidney cytosol was quantified spectrophotometrically at 240 nm using H2O2 as the substrate [24]. Glutathione peroxidase (GPx) activity in kidney cytosol was estimated by NADPH oxidation using a coupled reaction system consisting of GSH (10 mM), glutathione reductase (2.4 unit), and H2O2 (12 mM) [25]. Glutathione-S-transferase (GST) activity in kidney cytosol was assessed by the increase in absorbance at 340 nm with 1-chloro-2,4-dinitrobenzene (30 mM) as the substrate [26]. Protein content in kidney homogenate was measured by the method of Lowry et al. [27], with bovine serum albumin (BSA) as the standard using spectrophotometer.
Histopathology
After 24 hours of fixation, the tissue samples were processed and embedded in paraffin blocks, sectioned at 5 μm, and stained with hematoxylin–eosin and observed under light microscope (DM 1000, Leica, Germany). Histological changes were evaluated semiquantitatively by a pathologist unaware of the type of treatment. A minimum of 10 fields for each kidney slide was examined and assigned for severity of changes using the following scale: −, none; +, mild damage; ++, moderate damage; and +++, severe damage.
Immunoblotting
Nuclear and cytoplasmic protein fractions from the kidneys were isolated using nuclear and cytoplasmic extraction buffer containing protease inhibitor. Equal amount of protein samples (50 μg/lane) were loaded into a 10–15% SDS–polyacrylamide gel. After electrophoresis the gels were transferred to PVDF membranes. Then, the membranes were blocked with 5% BSA in TBST buffer (20 mM Tris–HCl, pH 7.4, 150 mM NaCl, 0.1% Tween-20) for 1 hour. After blocking, the membranes were incubated with specific primary antibodies (1:1000 dilution, 3 hours at room temperature) specific for Nrf2, Keap1, HO1, and NQO1. The membranes were then treated with horseradish peroxidase (HRP) conjugated secondary antibody (1:2000 dilution, 1 hour at room temperature). Then the immunoreactive protein bands were visualized with chemiluminescence [28]. Densitometric analysis of Western blots was performed (Gel Doc™ XR + system, BioRad, USA) and the protein levels were normalized by comparison with Histone H3 (for Nrf2) and GAPDH (for Keap1, HO1, and NQO1).
In situ cell death
Apoptosis of the kidney sections were determined by using the terminal deoxynucleotidyl transferase (TdT) mediated dUTP nick end labeling (TUNEL) method with the help of in situ cell death detection kit, according to the manufacturer’s instructions [29]. Briefly, the slides were incubated with TUNEL reaction mixture containing TdT and fluorescein-dUTP at 37°C for 60 minutes in a humidified chamber and further treated with anti-fluorescein antibody conjugated with alkaline phosphatase (37°C for 30 minutes in a humidified chamber). The bound alkaline phosphatase was then stained with BCIP (5-bromo-4-chloro-3-indolyl-phosphate)/NBT (nitro blue tetrazolium) and observed under light microscope (DM 1000, Leica, Germany).
Atomic absorption spectroscopy
Platinum (Pt) concentration in serum samples and kidney tissues were determined by flame atomic absorption spectroscopy (AAS; AA240FS Varian, Agilent Technologies, USA). Briefly, samples were digested in aqua regia (HCl:HNO3 = 3:1) at 180°C for 20 minutes using microwave accelerated digestion system (MARS 5, Cem Corporation, USA). Then the samples were run on AAS. A wavelength of 266.0 nm with a lamp current of 10 mA and spectral bandwidth of 0.2 nm was employed in air-acetylene flames [30].
Statistical analysis
All data were expressed as mean ± SD, n = 6 mice per group. Statistical analysis was performed using one-way ANOVA (GraphPad Prism, version 5.0; GraphPad Software, Inc., CA, USA) followed by Tukey’s multiple comparison test (post hoc test) with the help of critical difference or least significant difference at 5 and 1% level of significance to compare the mean values. Significant difference was indicated when the P value was <0.05.
Results
Amelioration of renal dysfunction by VC-IV
CDDP administration caused severe nephrotoxicity as the levels of serum creatinine and BUN were elevated significantly (P < 0.05) by 397.95 and 113.67%, respectively, compared to vehicle-treated group (Table 1). Administration of VC-IV in concomitant-treatment schedule reduced (P < 0.05) serum creatinine and BUN levels by 45.31 and 32.40%, respectively, compared to CDDP-treated group. Pre-treatment with VC-IV also significantly (P < 0.05) reduced the elevated levels of creatinine and BUN by 56.33 and 37.92%, respectively, compared to CDDP-treated group. Treatment with the ligand LCME could not provide any protection against CDDP-induced elevation of renal function markers. In addition, we did not find any significant alternation (P > 0.05) in the serum levels of Na+, K+, and Cl– ions in only vehicle/VC-IV/CDDP-treated mice or the combination treatment groups (Table 1).
Table 1. Renal functional index in mice serum of different treatment groups.
| Groups | Creatinine (mg/dl) | BUN (mg/dl) | Na+ (mM/l) | K+ (mM/l) | Cl– (mM/l) |
|---|---|---|---|---|---|
| Vehicle | 0.49 ± 0.04 | 20.99 ± 1.63 | 141.86 ± 18.21 | 4.16 ± 0.45 | 101.29 ± 4.54 |
| VC-IV | 0.48 ± 0.03 | 20.82 ± 0.78 | 142.49 ± 11.96 | 4.49 ± 0.57 | 106.16 ± 3.85 |
| CDDP | 2.45 ± 0.16ab | 44.85 ± 3.57ab | 139.13 ± 6.34 | 4.47 ± 0.41 | 103.54 ± 7.85 |
| CDDP + LCME | 2.33 ± 0.15ab | 44.35 ± 4.04ab | 146.02 ± 5.79 | 4.35 ± 0.42 | 101.87 ± 5.48 |
| CDDP + VC-IV Con | 1.34 ± 0.12abcd | 30.32 ± 2.38abcd | 143.32 ± 5.16 | 4.34 ± 0.49 | 107.38 ± 11.06 |
| CDDP + VC-IV Pre | 1.07 ± 0.09abcde | 27.84 ± 1.81abcd | 138.17 ± 8.49 | 4.33 ± 0.53 | 103.83 ± 5.42 |
Vehicle: vehicle-treated group; VC-IV: only VC-IV-treated group; CDDP: only CDDP-treated group; CDDP + LCME: CDDP + L-cysteine methyl ester hydrochloride treated group; CDDP + VC-IV Con: CDDP + VC-IV concomitant-treatment group; CDDP + VC-IV Pre: CDDP + VC-IV pre-treatment group. Data were represented as mean ± S.D. asignificantly (P < 0.05) different from vehicle-treated group, bsignificantly (P < 0.05) different from VC-IV-treated group, csignificantly (P < 0.05) different from CDDP-treated group, dsignificantly (P < 0.05) different from CDDP + LCME-treated group and esignificantly (P < 0.05) different from CDDP + VC-IV concomitant-treatment group.
Attenuation of oxidative stress by VC-IV
Administration of CDDP caused renal oxidative burst as indicated by raised levels of ROS (by 125.53%), NO (by 52.08%) and LPO (by 111.67%) compared to vehicle-treated mice (Figure 3(a–c)). Concomitant-treatment with the vanadium compound resulted in significant (P < 0.05) reduction in the levels of ROS, NO, and LPO by 24.97, 15.41, and 29.12%, respectively, in comparison to the CDDP-treated group. In case of VC-IV pre-treatment group the reduction (P < 0.05) in the levels of ROS, NO, and LPO were found to be 34.20, 21.35, and 35.80%, respectively, in comparison to the CDDP-treated group. However, administration of LCME in CDDP-treated mice failed to reduce (P > 0.05) the levels of oxidative stress markers.
Figure 3.
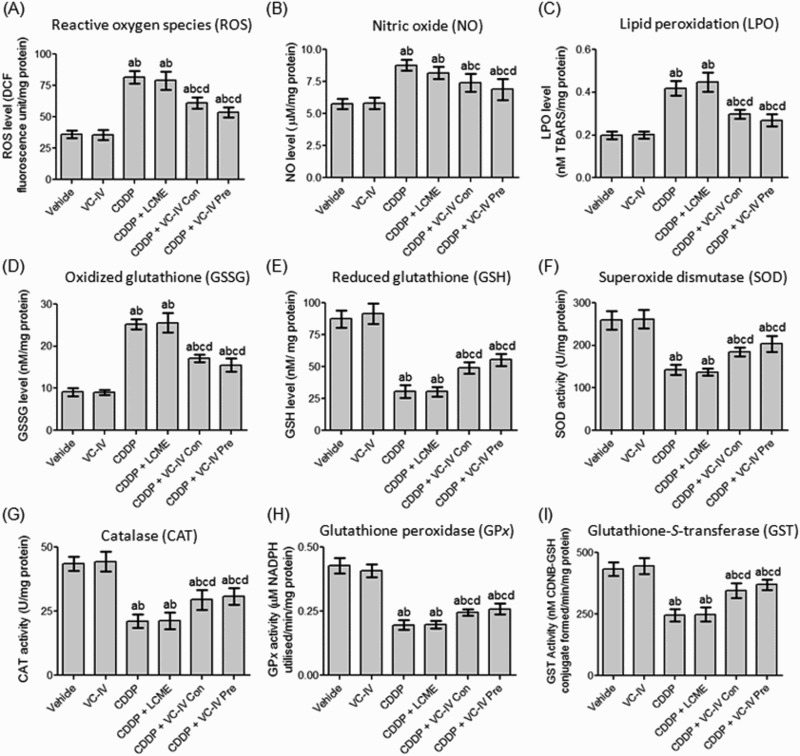
VC-IV modulated CDDP-induced alterations of renal redox status in mice. VC-IV reduced (a) ROS level, (b) NO level, (c) LPO level, (d) GSSG level and enhanced (e) GSH level, (f) SOD activity, (g) CAT activity, (h) GPx activity, (i) GST activity in the kidneys of mice. Data were represented as mean ± S.D. asignificantly (P < 0.05) different from vehicle-treated group, bsignificantly (P < 0.05) different from VC-IV-treated group, csignificantly (P < 0.05) different from CDDP-treated group, dsignificantly (P < 0.05) different from CDDP + LCME-treated group, and esignificantly (P < 0.05) different from CDDP + VC-IV concomitant-treatment group.
Modulation of antioxidant system by VC-IV
CDDP treatment resulted in significant (P < 0.05) depletion in the level of GSH (by 65.07%) along with significant (P < 0.05) rise in the level of GSSG (by 175.03%) in mice kidneys compared to vehicle-treated group (Figure 3(d,e)). Concomitant-treatment and pre-treatment with VC-IV increased the renal level of GSH by 60.22% (P < 0.05) and 81.59% (P < 0.05), respectively, in comparison to the CDDP-treated group. Additionally, concomitant-treatment and 7 days pre-treatment with the vanadium compound significantly (P < 0.05) reduced the elevated level of GSSG by 32.08 and 38.38%, respectively, compared to CDDP-treated group. The activity of antioxidant enzymes, namely, SOD, CAT, GPx, and GST, in mice kidneys were decreased significantly (P < 0.05) due to administration of CDDP, compared to vehicle-treated mice (Figure 3(f–i)). Treatment with only LCME in the CDDP-treated mice could not prevent the depletion of antioxidant enzyme system. Concomitant-treatment with the organovanadium compound resulted in significant (P < 0.05) elevation in the activity of SOD, CAT, GPx, and GST by 28.93, 37.87, 25.0, and 41.24%, respectively, compared to CDDP-treated mice. Pre-treatment with VC-IV also resulted in significant (P < 0.05) increase in the activity of SOD, CAT, GPx, and GST by 42.53, 46.53, 32.13, and 50.82%, respectively, compared to CDDP-treated mice.
Mitigation of CDDP-induced histological alterations by VC-IV
The kidney histology of CDDP-treated mice showed sloughing and loss of brush border of tubular epithelial cells, interstitial hemorrhage, atrophy, and tubular dilatation (Figure 4(a)). The microphotographs of TUNEL assay also illustrated high incidence of cell death around glomerulus and proximal convulated tubule (Figure 4(b)). Administration of the vanadium compound in both concomitant and pre-treatment schedule mitigated CDDP-induced histopathological lesions as evident from considerable decrease in semiquantitative histological damage scores, of which pre-treatment group showed better result (Table 2). Treatment with LCME could not ameliorate CDDP-induced renal damages.
Figure 4.
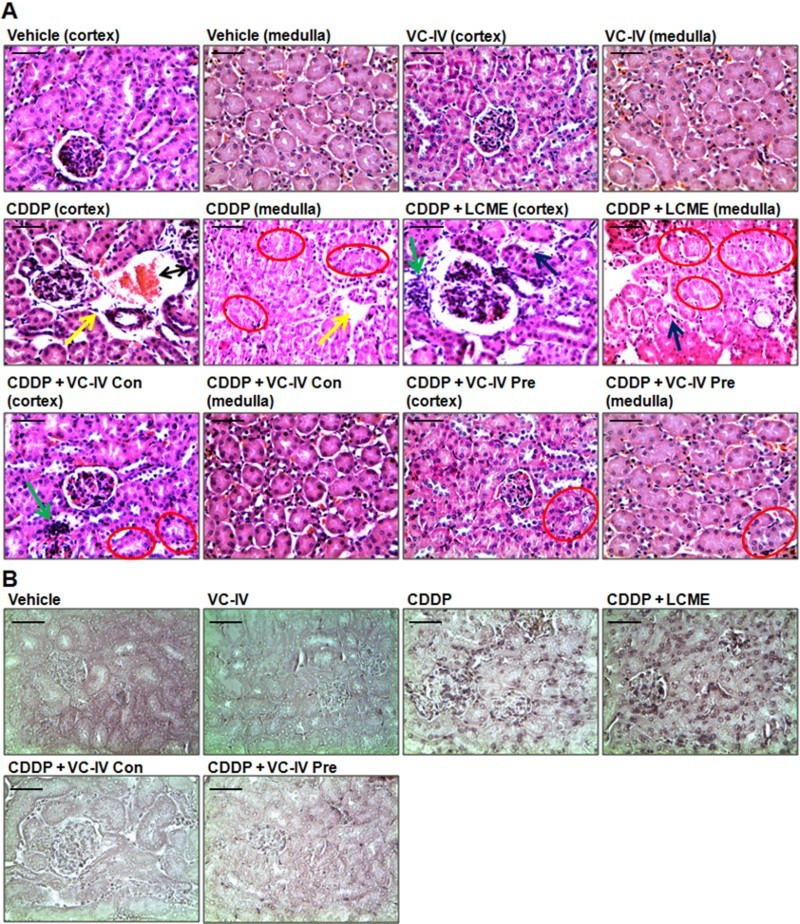
VC-IV reduced CDDP-induced renal damage in mice. (a) Photomicrographs of kidney sections of mice stained with hematoxylin and eosin (H and E). Normal histology with renal corpuscle, proximal, and distal convoluted tubules was observed in vehicle-treated and only VC-IV-treated group. Large area of interstitial hemorrhage (black color double headed arrow), atrophy (yellow color arrow) and loss of brush border (red color oval) were found in CDDP-treated group. Loss of brush border (red color oval), tubular dilatation (blue color arrow), and leukocyte infiltration (green color arrow) were found in CDDP + LCME-treated group. CDDP + VC-IV concomitant-treatment group showed moderate loss of brush border (red color oval) and mild leukocyte infiltration (green color border). Quite normal histology was observed in CDDP + VC-IV pre-treatment group except mild loss of brush border. (b) Photomicrographs of kidney sections of mice stained with TUNEL reagent (BCIP/NBT), × 400 magnification, scale bar = 50 μm. CDDP treatment resulted in apoptosis (TUNEL-labelled cells) around glomerulus and proximal convulated tubules. CDDP + LCME-treated group also showed apoptosis around glomerulus and proximal convulated tubules. Treatment with VC-IV in concomitant- and pre-treatment schedule showed null or minimal presence of apoptotic cells.
Table 2. Effect of VC-IV administration on morphological changes as assessed by histopathological analysis of kidneys of mice treated with CDDP.
| Pathological changes | Groups | |||||
|---|---|---|---|---|---|---|
| Vehicle | VC-IV | CDDP | CDDP + LCME | CDDP + VC-IV Con | CDDP + VC-IV Pre | |
| Interstitial hemorrhage | – | – | +++ | +++ | + | + |
| Glomerular atrophy | – | – | +++ | ++ | +/– | – |
| Tubular brush border loss | – | – | +++ | +++ | ++ | + |
| Tubular atrophy | – | – | ++ | ++ | + | – |
| Cell infiltration | – | – | +++ | +++ | ++ | +/– |
| Cell death (TUNEL) | – | – | +++ | +++ | +/– | – |
Vehicle: vehicle-treated group; VC-IV: only VC-IV-treated group; CDDP: only CDDP-treated group; CDDP + LCME: CDDP + L-cysteine methyl ester hydrochloride-treated group; CDDP + VC-IV Con: CDDP + VC-IV concomitant-treatment group; CDDP + VC-IV Pre: CDDP + VC-IV pre-treatment group. −: none; +: mild damage; ++: moderate damage; and +++: severe damage are semiquantitative scores by a pathologist unaware of the type of treatment.
Induction of ARE pathway by VC-IV
Western blot analysis showed that the cytosolic expression of HO1 and NQO1 were significantly (P < 0.05) lower in the kidneys of CDDP-treated mice (Figure 5(a), lane 3) than that of vehicle-treated mice (lane 1). On the other hand, the cytosolic Keap1 expression was found to be increased (P < 0.05) in CDDP-treated group compared to vehicle-treated mice. In contrast, VC-IV enhanced (P < 0.05) nuclear localization of Nrf2 and cytosolic expression of HO1 and NQO1 in comparison to CDDP-treated group in a schedule-dependent manner (lane 5 and 6). Interestingly, VC-IV treatment also reduced (P < 0.05) Keap1 expression (cytosolic) compared to CDDP-treated mice. Treatment with the ligand LCME could not provide any alterations in the expression profiles of the proteins compared to CDDP-treated mice (lane 4). Figure 5(b–e) also represents the quantitative relative expressions of the proteins.
Figure 5.
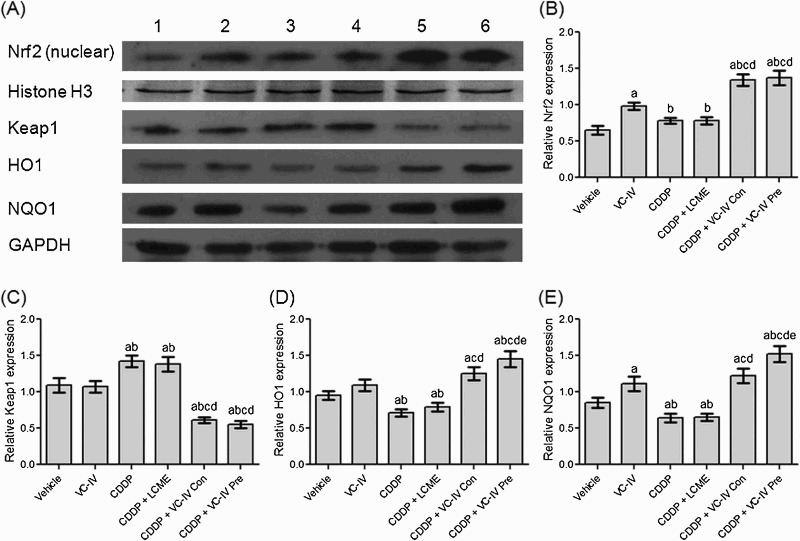
VC-IV induced Nrf2-mediated activation of ARE pathway. (a) Expression profile of Nrf2 (nuclear), Histone H3, Keap1, HO1, NQO1 and GAPDH in mice kidneys following different treatments. Lane 1: Vehicle-treated group, lane 2: VC-IV-treated group, lane 3: CDDP-treated group, lane 4: CDDP + LCME-treated group, lane 5: CDDP + VC-IV concomitant-treatment group, and lane 6: CDDP + VC-IV pre-treatment group. The bar diagrams showed relative band intensity of (b) Nrf2 (nuclear), (c) Keap1, (d) HO1, and (e) NQO1. The results were normalized with Histone H3 for Nrf2 and GAPDH for Keap1, HO1, and NQO1. Data were represented as mean ± S.D, n = 4. asignificantly (P < 0.05) different from vehicle-treated group, bsignificantly (P < 0.05) different from VC-IV-treated group, csignificantly (P < 0.05) different from CDDP-treated group, dsignificantly (P < 0.05) different from CDDP + LCME-treated group, and esignificantly (P < 0.05) different from CDDP + VC-IV concomitant-treatment group.
Platinum concentration
The Pt concentration obtained in the CDDP-treated mice was 7.53 µg/ml serum in serum and 18.93 µg/g tissue in kidneys. VC-IV and LCME treatment in combination with CDDP did not significantly (P > 0.05) alter the Pt concentration compared to only CDDP-treated group (Figure 6(a,b)).
Figure 6.
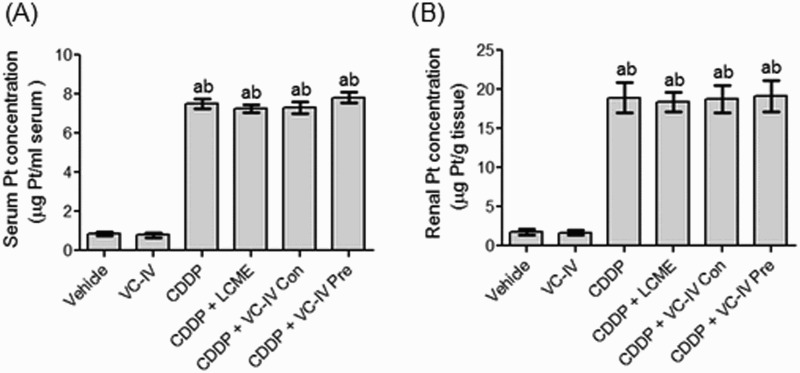
Atomic absorption spectroscopic analysis of Pt. Quantitative estimation of (a) serum Pt concentration and (b) kidney Pt concentration in mice of different treatment groups. Data were represented as mean ± S.D. asignificantly (P < 0.05) different from vehicle-treated group, bsignificantly (P < 0.05) different from VC-IV-treated group, csignificantly (P < 0.05) different from CDDP-treated group, dsignificantly (P < 0.05) different from CDDP + LCME-treated group, and esignificantly (P < 0.05) different from CDDP + VC-IV concomitant-treatment group.
Discussion
CDDP is an effective broad-spectrum chemotherapeutic agent. However, CDDP therapy is limited by the emergence of irreversible renal injury [6]. Amongst current management strategies, saline hydration is one which reduces the nephrotoxicity and allows an increase of the dose to achieve therapeutic levels. But, even with vigilant hydration, approximately one-third of the patients have a transient elevation of BUN levels and/or other evidences of kidney damage in the days following the CDDP treatment [31]. On the other hand, amifostine represents the most promising nephroprotectant in clinical practice approved by USFDA. However, intravenous administration of amifostine in a phase III clinical trial resulted in toxicities in 41% of head and neck cancer patients, including hypotension, hypocalcemia, nausea, vomiting, and allergy, and hence necessitated withdrawal of treatment [32]. Thus, development of non-toxic chemoprotectants is essential to provide protection against CDDP-induced renal damage.
In this study we investigated whether an oxovanadium (IV) compound could provide sufficient protection against CDDP-induced kidney injury, using a preclinical mouse model of CDDP-induced nephropathy. We found that CDDP-induced severe renal injury by multiple endpoints (levels of creatinine and BUN, and histopathology). The histopathological features include sloughing and loss of brush border of tubular epithelial cells, interstitial hemorrhage, inflammatory cell infiltration and apoptosis. Co-administration of VC-IV in concomitant and pre-treatment schedule ameliorated CDDP-induced alterations in kidney functioning and minimized renal tubular injury as well as cell death. VC-IV-induced modification of the alterations in renal function and structure was more prominent in the pre-treatment group than the concomitant-treatment group. We further investigated the status of oxidative stress markers and antioxidant enzymes in the kidneys of CDDP-treated and combined treatment groups, particularly focussing on Nrf2-mediated signaling pathway.
Oxidative stress plays a major role in CDDP-induced kidney injury. Several preclinical models have shown that CDDP treatment augments the generation of ROS and reactive nitrogen species (RNS), such as, superoxide anions, hydroxyl radicals, hydrogen peroxide, and nitric oxide [5,6]. In this study, CDDP treatment also resulted in pronounced renal oxidative stress and diminution of the activity of antioxidant enzymes. In contrast, administration of VC-IV caused mitigation in the levels of ROS, NO, and LPO in the mice kidneys and enhanced the renal antioxidant status. In order to explore the mechanism, the influence of VC-IV on Nrf2/ARE pathway was examined in this study. Under homeostatic conditions, Nrf2 is present in the cytoplasm attaching to a cytosolic protein Keap1, which functions as a suppressor of Nrf2 by retaining it in cytosol and enhancing its proteasomal degradation via ubiquitination [33,34]. But upon activation, Nrf2 acts as a key transcriptional factor that triggers the ARE pathway, and in turn regulates the expression of several antioxidant and phase II detoxifying enzymes [33]. Consistent with the results from other studies, the results of the present study showed that HO1 and NQO1 expression in the CDDP-treated group was lower than those of vehicle-treated mice. Interestingly, VC-IV co-administration induced localization of Nrf2 in nucleus and enhanced expression of HO1 and NQO1. In this study, VC-IV probably modify interactions between Keap1 and Nrf2, allowing nuclear translocation of Nrf2 and thereby promote induction of HO1 and NQO1 expression. We selected these two proteins for analysis because of their central role in protection against oxidative stress, and because they are signatures of Nrf2 activity [34]. Moreover, the enhancement in the activities of other antioxidant enzymes, such as, SOD, CAT, GPx, and GST also validated the involvement of Nrf2-mediated activation of ARE pathway in VC-IV-mediated nephroprotection. Thus, this study proved that VC-IV augmented antioxidant enzyme system in the kidneys of CDDP-treated mice and provided the necessary protection through Nrf2 activation.
In order to specifically observe the effect of the complexation, the ligand LCME was administered in 7 days pre-treatment schedule along with CDDP in mice. The dose of LCME was calculated according to the LCME content in the oxovanadium (IV) complex. However, LCME treatment alone did not offer any protection to CDDP-induced renal and genetic damages. This proved the specificity of the oxovanadium (IV) species in providing the protective efficacy towards CDDP-induced toxicity. Another objective of this study was to detect any interaction between CDDP and the vanadium compound. For that, Pt concentration in serum and kidneys was estimated by atomic absorption spectroscopy in all treatment groups of mice. No difference in the content of Pt was found between only CDDP and CDDP combination with VC-IV treated mice. This observation indicated that VC-IV did not produce any influence in the bioavailability and renal accumulation of CDDP in mice.
Conclusion
The present study demonstrated for the first time that an oxovanadium (IV) complex could exert a protective role in the amelioration of CDDP-induced renal injury in mice. The observed protective efficacy was attributed to the oxovanadium (IV) complex-mediated mitigation of oxidative and nitrosative stress, and enhancement of the activities of antioxidant and detoxifying enzymes through Nrf2 activation. Previously we have observed that VC-IV can provide adequate protection against CDDP-induced myelotoxicity and DNA damage in murine bone marrow cells [35]. This study further elaborates its protective efficacy against CDDP-induced nephrotoxicity. Hence, VC-IV can serve as a promising chemoprotectant to overcome CDDP-induced severe complications and may increase the therapeutic window of CDDP in cancer patients.
Notes on contributors
Abhishek Basu is the Research Associate (RA) in the Department of Cancer Chemoprevention, Chittaranjan National Cancer Institute, Kolkata, India awarded by Council of Scientific and Industrial Research (CSIR), New Delhi, India. His areas of expertise are pharmacology, toxicology and cancer biology. His research work deals with the evaluation of organovanadium compounds as a suitable chemoprotectant and sensitizing agent in cancer chemotherapy in order to establish a better chemotherapeutic regimen with the aim to improve patients’ quality of life.
Arin Bhattacharjee is the Senior Research Fellow (SRF) in the Department of Cancer Chemoprevention, Chittaranjan National Cancer Institute, Kolkata, India awarded by Indian Council of Medical Research (ICMR), New Delhi, India. His areas of expertise are medicinal chemistry, nanotechnology and cancer chemotherapy. His research work deals with the evaluation of nano-selenium as an adjuvant in cancer chemotherapy in order to reduce the toxic manifestations of chemotherapy and simultaneously to enhance therapeutic efficacy of conventional antineoplastic agents.
Subhadip Hajra is the Young Scientist in the Department of Cancer Chemoprevention, Chittaranjan National Cancer Institute, Kolkata, India awarded by Department of Science and Technology (DST), New Delhi, India. His areas of expertise are microbiology, phytology, pharmacology and cancer biology. He has 16 publications and several book chapters. His research work includes phytochemistry, pharmacological screening of natural products, redox regulation in pathological conditions, and genotoxicology. He currently aims to establish natural product-based cost-effective chemotherapeutic regimen.
Amalesh Samanta is the Professor in the Division of Microbiology, Department of Pharmaceutical Technology, Jadavpur University, Kolkata. His areas of expertise are formulation designing, antimicrobial and pharmacological screening of phytoconstituents, polymer science and prebiotic evaluation from natural polysaccharides as an immune booster and anticancer agent. He has 78 publications in national and international journals of repute. His research work deals with the development of natural materials to address modern day diseases like colon cancer, food poisoning and enteric diseases.
Sudin Bhattacharya is the Head, Department of Cancer Chemoprevention, Chittaranjan National Cancer Institute, Kolkata, India. His areas of expertise are medicinal chemistry, anticancer drug development, drug designing, pharmacology, toxicology and cancer biology. He has 76 publications including book chapters. His research work includes synthesis and evaluation of antioxidative as well as cancer chemopreventive properties of small molecules and reduction of the toxic manifestation imparted by different cancer chemotherapeutic drugs by organic small molecules, nano-selenium and complementary alternative medicines.
Funding Statement
Abhishek Basu and Arin Bhattacharjee gratefully acknowledge Indian Council of Medical Research (ICMR) for Senior Research Fellowship (no. 3/2/2/58/2011/NCD-III and no. 45/36/2008/PHA-BMS). Subhadip Hajra also gratefully acknowledges Science and Engineering Research Board (SERB), Department of Science and Technology (DST) for Start-Up Research Grant (no. SB/YS/LS-121/2014).
Acknowledgement
The authors appreciate the help from Dr Syamsundar Mandal, Department of Epidemiology and Biostatistics, Chittaranjan National Cancer Institute for his guidance on the statistical analysis of this study. The authors wish to thank the Prof (Dr) Jaydip Biswas, Director, CNCI for the support in this study.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Sooriyaarachchi M, White WM, Narendran A, et al. Chemoprotection by D-methionine against cisplatin-induced side-effects: insight from in vitro studies using human plasma. Metallomics. 2014;6(3):532–541. doi: 10.1039/C3MT00238A [DOI] [PubMed] [Google Scholar]
- [2].Florea AM, Büsselberg D. Cisplatin as an anti-tumor drug: cellular mechanisms of activity, drug resistance and induced side effects. Cancers. 2011;3(1):1351–1371. doi: 10.3390/cancers3011351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ulu R, Doğukan A, Tuzcu M, et al. Modulation of Nrf2/HO-1 by thymoquinone during cisplatin-induced nephrotoxicity. Turk Neph Dial Transpl. 2013;22(2):182–187. doi: 10.5262/tndt.2013.1002.09 [DOI] [Google Scholar]
- [4].Camano S, Lazaro A, Moreno-Gordaliza E, et al. Cilastatin attenuates cisplatin-induced proximal tubular cell damage. J Pharmacol Exp Ther. 2010;334(2):419–429. doi: 10.1124/jpet.110.165779 [DOI] [PubMed] [Google Scholar]
- [5].Aleksunes LM, Goedken MJ, Rockwell CE, et al. Transcriptional regulation of renal cytoprotective genes by Nrf2 and its potential use as a therapeutic target to mitigate cisplatin-induced nephrotoxicity. J Pharmacol Exp Ther. 2010;335(1):2–12. doi: 10.1124/jpet.110.170084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Pabla N, Dong Z. Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int. 2008;73(9):994–1007. doi: 10.1038/sj.ki.5002786 [DOI] [PubMed] [Google Scholar]
- [7].Shelton LM, Park BK, Copple IM. Role of Nrf2 in protection against acute kidney injury. Kidney Int. 2013;84(6):1090–1095. doi: 10.1038/ki.2013.248 [DOI] [PubMed] [Google Scholar]
- [8].Ruiz S, Pergola PE, Zager RA, et al. Targeting the transcription factor Nrf2 to ameliorate oxidative stress and inflammation in chronic kidney disease. Kidney Int. 2013;83(6):1029–1041. doi: 10.1038/ki.2012.439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bishayee A, Waghray A, Patel MA, et al. Vanadium in the detection, prevention and treatment of cancer: the in vivo evidence. Cancer Lett. 2010;294(1):1–12. doi: 10.1016/j.canlet.2010.01.030 [DOI] [PubMed] [Google Scholar]
- [10].Fedorova EV, Buryakina AV, Zakharov AV, et al. Design, synthesis and pharmacological evaluation of novel vanadium-containing complexes as antidiabetic agents. PLoS One. 2014;9(7):e100386. doi: 10.1371/journal.pone.0100386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Sankar Ray R, Roy S, Ghosh S, et al. Suppression of cell proliferation, DNA protein cross-links, and induction of apoptosis by vanadium in chemical rat mammary carcinogenesis. Biochim Biophys Acta. 2004;1675(13):165–173. doi: 10.1016/j.bbagen.2004.09.004 [DOI] [PubMed] [Google Scholar]
- [12].Kim AD, Zhang R, Kang KA, et al. Increased glutathione synthesis following Nrf2 activation by vanadyl sulfate in human change liver cells. Int J Mol Sci. 2011;12(12):8878–8894. doi: 10.3390/ijms12128878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kim AD, Zhang R, Kang KA, et al. Jeju ground water containing vanadium enhances antioxidant systems in human liver cells. Biol Trace Elem Res. 2012;147(1–3):16–24. doi: 10.1007/s12011-011-9277-5 [DOI] [PubMed] [Google Scholar]
- [14].Sanna D, Ugone V, Micera G, et al. Temperature and solvent structure dependence of VO2+ complexes of pyridine-N-oxide derivatives and their interaction with human serum transferrin. Dalton Trans. 2012;41(24):7304–7318. doi: 10.1039/c2dt12503j [DOI] [PubMed] [Google Scholar]
- [15].Sheela A, Sarada NC, Vijayaraghavan R. A possible correlation between antioxidant and antidiabetic potentials of oxovanadium (IV) complexes. Med Chem Res. 2013;22(6):2929–2937. doi: 10.1007/s00044-012-0287-4 [DOI] [Google Scholar]
- [16].Francik R, Krośniak M, Barlik M, et al. Impact of vanadium complexes treatment on the oxidative stress factors in Wistar rats plasma. Bioinorg Chem Appl. 2011;2011:206316. doi: 10.1155/2011/206316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sakurai H, Hamada Y, Shimomura S, et al. Cysteine methyl ester-oxovanadium (IV) complex, preparation and characterization. Inorg Chim Acta. 1980;46:L119–L120. doi: 10.1016/S0020-1693(00)84158-4 [DOI] [Google Scholar]
- [18].Basu A, Bhattacharjee A, Samanta A, et al. Prevention of cyclophosphamide-induced hepatotoxicity and genotoxicity: effect of an L-cysteine based oxovanadium (IV) complex on oxidative stress and DNA damage. Environ Toxicol Pharmacol. 2015;40(3):747–757. doi: 10.1016/j.etap.2015.08.035 [DOI] [PubMed] [Google Scholar]
- [19].De S, Sen T, Chatterjee M. Reduction of oxidative stress by an ethanolic extract of leaves of Piper beetle (Paan) Linn. decreased methotrexate-induced toxicity. Mol Cell Biochem. 2015;409(1–2):191–197. doi: 10.1007/s11010-015-2524-x [DOI] [PubMed] [Google Scholar]
- [20].Coşkun S, Gönül B, Ozer C, et al. The effects of dexfenfluramine administration on brain serotonin immunoreactivity and lipid peroxidation in mice. Cell Biol Toxicol. 2007;23(2):75–82. doi: 10.1007/s10565-006-0107-z [DOI] [PubMed] [Google Scholar]
- [21].Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95(2):351–358. doi: 10.1016/0003-2697(79)90738-3 [DOI] [PubMed] [Google Scholar]
- [22].Rahman I, Kode A, Biswas SK. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat Protoc. 2006;1(6):3159–3165. doi: 10.1038/nprot.2006.378 [DOI] [PubMed] [Google Scholar]
- [23].McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969;244(22):6049–6055. [PubMed] [Google Scholar]
- [24].Luck HA. Spectrophotometric method for estimation of catalase. In: Bergmeyer HV, editor, Methods of enzymatic analysis. New York (NY: ): Academic Press; 1963. p. 886–888 [Google Scholar]
- [25].Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;70(1):158–169. [PubMed] [Google Scholar]
- [26].Habig WH, Pabst MJ, Jakoby WB. Glutathione-S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249(22):7130–7139. [PubMed] [Google Scholar]
- [27].Lowry OH, Rosenbrough NJ, Farr AL, et al. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193(1):265–276. [PubMed] [Google Scholar]
- [28].Ghosh P, Singha Roy S, Basu A, et al. Sensitization of cisplatin therapy by a naphthalimide based organoselenium compound through modulation of antioxidant enzymes and p53 mediated apoptosis. Free Radic Res. 2015;49(4):453–471. doi: 10.3109/10715762.2015.1012079 [DOI] [PubMed] [Google Scholar]
- [29].Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119(3):493–501. doi: 10.1083/jcb.119.3.493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Pitts AE, Van Loon JC, Beamish FE. The determination of platinum by atomic absorption spectroscopy: part I. Air-acetylene flames. Anal Chim Acta. 1970;50(2):181–194. [Google Scholar]
- [31].Wang H, Jia Z, Sun J, et al. Nitrooleic acid protects against cisplatin nephropathy: role of COX-2/mPGES-1/PGE2 cascade. Mediators Inflamm. 2015;2015:293474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Das B, Antoon R, Tsuchida R, et al. Squalene selectively protects mouse bone marrow progenitors against cisplatin and carboplatin-induced cytotoxicity in vivo without protecting tumor growth. Neoplasia. 2008;10(10):1105–1119. doi: 10.1593/neo.08466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ejitha S, Prathibha P, Indira M. Nrf2-mediated antioxidant response by ethanolic extract of Sida cordifolia provides protection against alcohol-induced oxidative stress in liver by upregulation of glutathione metabolism. Redox Rep. 2015;20(2):75–80. doi: 10.1179/1351000214Y.0000000108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Tan SM, de Haan JB. Combating oxidative stress in diabetic complications with Nrf2 activators: how much is too much? Redox Rep. 2014;19(3):107–117. doi: 10.1179/1351000214Y.0000000087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Basu A, Bhattacharjee A, Samanta A, Bhattacharya S. An oxovanadium(IV) complex protects murine bone marrow cells against cisplatin-induced myelotoxicity and DNA damage. Drug Chem Toxicol. Forthcoming. DOI: 10.1080/01480545.2016.1237522. [DOI] [PubMed] [Google Scholar]


