Abstract
Objectives
Radioprotective potential of quercetin, a powerful free radical scavenger, was investigated in human red blood cells (RBCs) and in isolated RBC membranes exposed to γ-irradiation-induced oxidative stress.
Methods
RBCs and RBC membrane suspensions were irradiated (50 Gy) in the presence of quercetin (2–50 µM). Oxidative damage of the membranes was analysed by protein carbonyl measurement (enzyme-linked immunosorbent assay). In RBCs, the concentration of glutathione (GSH) was determined. Lipid peroxidation in RBCs, and for comparison in plasma and peripheral lymphocytes, was quantified by the amount of thiobarbituric acid-reactive substances (TBARS). Radiation-induced damage of the RBC membrane integrity was evaluated by the degree of haemolysis.
Results
Quercetin (50 µM) brought back the level of carbonyls to normal in γ-irradiated RBC membrane proteins and inhibited radiation-induced lipid peroxidation in plasma and lymphocytes, by 75 and 96%, respectively. However, it moderately decreased reduced/oxidized glutathione (GSH/GSSG) ratio and significantly increased TBARS concentrations, by 60 and 28% in irradiated and non-irradiated RBCs, respectively. Haemolysis rate was much higher in RBCs irradiated in the presence of quercetin vs. non antioxidant.
Discussion
In non-cellular systems (RBC membranes or plasma) and in lymphocytes, quercetin shows antioxidative/radioprotective activity but in whole RBCs it acts as a pro-oxidant and a cytotoxic substance. The possible mechanisms of such action are discussed.
Keywords: γ-Irradiation, Oxidative stress, Quercetin, Red blood cell membrane
Introduction
The effects of γ-irradiation, on the molecular level, are largely caused by water radiolysis, a process that produces reactive oxygen species (ROS) with hydroxyl radical being the most hazardous. ROS can interact with critical macromolecules, such as DNA, proteins, carbohydrates, and lipids inducing cell damage. In an enucleated cell, such as an erythrocyte (red blood cell; RBC), a cell membrane is the main target for γ-rays.1 Therefore, an erythrocyte is a good model system for studying mechanisms of membrane damage and protective agents. Circulating all over the body, RBCs are representative samples for the whole body exposure. They are easy to obtain and suitable for monitoring both the short- and long-term radiation effect. Storage conditions for RBCs, with maintaining their viability and function up to 42 days, have been well established in transfusion medicine. It has been generally accepted that peroxidation of RBC membrane lipids and proteins by radiation-induced ROS can damage membrane integrity resulting in severe cellular dysfunction.2–5 Evidence for such damage includes impaired aggregability, adherence, and deformability,6 enhanced haemoglobin and glutathione (GSH) oxidation,7 increased haemolysis and leakage of intracellular potassium, and lactate dehydrogenase.8–11
Plant compounds can protect cells against radiation-induced ROS-mediated damage.12–14 Quercetin, 3, 3′, 4′, 5, 7-pentahydroxyflavone, is one of the most abundant dietary polyphenolic substances occurring in fruits and vegetables. Quercetin is a strong antioxidant and it has the ability to act as an antimicrobial, anti-inflammatory/antiallergic, antimutagenic, and anticarcinogenic agent.15,16 Antioxidant activity of quercetin is based on its direct ROS scavenging ability (hydroxyl radical, superoxide anion, singlet oxygen, peroxyl radicals, and others). Quercetin can also prevent the formation of ROS through chelating of transition metal ions, and promote the enzymatic and non-enzymatic antioxidant defence system.17 Growing evidence suggests that quercetin can ameliorate the effects of radiation. Recently, quercetin was determined to inhibit UV-induced lipid peroxidation in vitro in liposomes.18 Quercetin decreased γ-radiation-induced damage in human peripheral blood lymphocytes and plasmid DNA19 as well as when given as pre-treatment, it protected against primary leucocyte DNA damage in γ-irradiated mice.20 There are various mechanisms by which quercetin can protect cells against the harmful effects of radiation. These include, e.g. absorption of radiation, antioxidant activity, and interaction with signal transduction pathways.15,21,22 However, the mechanisms of quercetin action are still not fully elucidated. The existing data on the effects of quercetin on γ-irradiation-induced oxidation and damage of cellular membrane are scarce. Moreover, in some experimental conditions, quercetin was found to show pro-oxidative and toxic properties.15
The aim of the present in vitro study was to investigate the radioprotective potential of quercetin, at the concentrations of 2–50 µM, on RBCs and on isolated RBC membranes exposed to γ-irradiation (50 Gy). The oxidative damage of RBC membranes was analysed by measurement of the carbonyl group concentration, a marker of protein oxidation. Lipid peroxidation levels in whole RBCs, and for comparison in plasma and in peripheral blood lymphocytes, were assessed by measurement of the amount of thiobarbituric acid-reactive substances (TBARS). The concentration of non-protein thiols (GSH) was determined as a marker of the endogenous antioxidant defence. Radiation-induced impairment of the cell membrane integrity was evaluated by the degree of haemolysis.
Materials and methods
Chemicals
Quercetin, dimethyl sulfoxide (DMSO), bovine serum albumin (BSA), α-cellulose, 5, 5′-dithio-bis-(2-nitrobenzoic acid), Histopaque 1077, sodium dodecyl sulphate, sodium borohydride, hypochlorous acid (HOCl), thiobarbituric acid (TBA), rabbit anti-dinitrophenylhydrazine (anti-DNP) antibody, and horseradish peroxidase (HRP)-conjugated anti-rabbit antibody, were purchased from Sigma-Aldrich Chemical Co., Warsaw, Poland. Other chemicals, all of analytical grade, were obtained from POCh, Gliwice, Poland.
Isolation and γ-irradiation of cells and RBC membranes
Human whole blood units from single healthy, adult donors who passed the routine selection criteria and before donation were not exposed to oxidative stress due to cigarette smoking, diet or drug therapy, and fresh frozen plasma were obtained from the Regional Center for Transfusion Medicine in Lodz (Poland). Whole blood (450 ml) was collected in acid-citrate-dextrose (ACD-A) solution. This study was approved by the local Ethics Committee (no. KBBN-UŁ/I/3/2012).
RBCs were separated from blood plasma and leucocytes by centrifugation (1800 g for 10 minutes), washed three times with phosphate-buffered saline (PBS) (140 mM NaCl in 10 mM Na-phosphate, pH 7.4) and passed through α-microcrystalline cellulose column to remove the residual leucocytes. The RBC ghost membranes were prepared from washed cells according to Dodge et al.23 The protein concentration in RBC membrane suspension was determined by the Lowry method.24 Lymphocytes were isolated from blood using density gradient (Histopaque), then the cells were washed twice with PBS. The number of lymphocytes was counted under the microscope; the viability of the cells was assayed by the trypan blue exclusion test.
RBCs and isolated RBC membrane suspensions as well as lymphocytes and plasma were irradiated at the Institute of Applied Radiation Chemistry (Technical University, Lodz, Poland) by using a 60Co source (dose rate 0.392 ± 0.027 Gy/minute; first category Irradiator BK-10000 ZZUJ Polon, Poznan, Poland). RBCs (haematocrit (Ht) = 80%) or lymphocytes (1 × 106 cells/ml) were divided into eight equal aliquots in polystyrene tubes. The first aliquot was used as non-irradiated control, second as irradiated control, the other three were irradiated in the presence of different quercetin concentrations and three were used to test the effect of different quercetin concentrations in non-irradiated samples. Quercetin, dissolved in 50% DMSO, was added to the cells. The final DMSO concentration in control and quercetin containing samples was 0.5%. The lymphocytes were irradiated after 2-hour incubation with flavonoid at ambient temperature; the RBCs were stored for 24 hours at 4°C. After incubation, the RBCs were irradiated and analysed on the same day. The RBC membrane suspensions (volume 100 µl, protein concentration approximately 4 mg/ml) and plasma were irradiated in the presence of quercetin or in the absence of the antioxidant. Non-irradiated membrane suspension or plasma served as controls. Until analysis, the RBC membrane and plasma samples were stored at −20°C.
Determination of protein carbonyl groups
Concentration of protein carbonyls in RBC membranes was measured by enzyme-linked immunosorbent assay according to Buss et al.25 with modifications as described by Alamdari et al.26 Briefly, carbonyls of membrane protein samples were derivatized with DNP and probed with anti-DNP antibody followed by a second antibody conjugated with HRP. Freshly prepared substrate solution (SIGMA Fast o-Phenylenediamine Dihydrochloride Tablet Sets) was added; the absorbance was read at 490 nm (Bio-Rad Microplate Reader, model 550, Poland, Warsaw). Standard curves (1–10 nmol CO per mg of protein) were prepared by mixing varying proportions (0–100%) of HOCl-oxidized BSA with fully reduced (by sodium borohydride) BSA while maintaining a constant total protein concentration. The amount of carbonyl groups in the oxidized BSA was determined spectrophotometrically as described by Levine et al.27
GSH measurement
GSH amount was measured spectrophotometrically with Ellman's reagent. The GSH concentration was calculated by using the molar extinction coefficient (ɛ = 13 600 M−1 cm−1).
Lipid peroxidation measurement
Lipid peroxidation in RBCs and in plasma was quantified by measuring the concentration of the TBARS.28 Briefly, equal volumes of the sample (RBC suspension, Ht = 4% or plasma), 15% (m/v) trichloroacetic acid containing 0.25 M HCl, and 0.375% (m/v) TBA containing 0.25 M HCl were mixed, incubated at 95°C for 10 minutes, and cooled. The sample was centrifuged at 7000 g for 10 minutes and the absorbance was measured at 535 nm (Spectrophotometer UV/Vis Helios alpha, Unicam, England, Cambridge). The TBARS concentration was calculated by using the molar extinction coefficient (ɛ = 156 000 M−1 cm−1). In lymphocytes, TBARS concentration was determined by the method described by Halliwell and Gutteridge.29
Haemolysis
Haemolysis of RBCs was determined by measurement of the amount of haemoglobin released from cells relative to the total cellular haemoglobin content. Ten microlitres of erythrocytes (Ht = 80%) were incubated with 5 ml of normal saline or distilled water for 30 minutes. The samples were centrifuged at 3000 rpm for 10 minutes, and the supernatants were measured spectrophotometrically at 540 nm (Spectrophotometer UV/Vis Helios alpha, Unicam). The percentage of haemolysis was calculated as follows:
where Asample and A100%lysis are the absorbances of the haemoglobin released from RBC in normal saline and after complete haemolysis in distilled water, respectively.
Statistical analysis
The data point in figures are the means of n = 4–6 independent experiments, each performed in triplicate. The data were analyzed with statistical software StatSoft Polska Inc. (Poland, Krakow) ‘Statistica’ v.10. The results were tested for normal distribution by means of the Kolmogorov–Smirnov test. The differences between groups were analysed by one-way analysis of variance (ANOVA) followed by the post hoc Tukey's test or by the non-parametric Kruskal–Wallis test. The significance of differences between the mean values of irradiated vs. appropriate non-irradiated samples was analysed by paired Student's t-test. A level P < 0.05 was accepted as statistically significant.
Results
Effects of quercetin on radiation-induced carbonyl generation in the RBC membrane protein
In our study, protein carbonyl concentration measurement was a method of choice to assess the extent of radiation-induced oxidative damage in RBC isolated membranes. Proteins constitute half of the membrane mass in human RBCs; the remaining half consists of lipids and carbohydrates (approximately 42 and 8%, respectively). ROS generated by γ-irradiation affect all types of biomolecules and oxidative damage to lipids and proteins can be concomitant. Therefore, products of both protein oxidation and lipid peroxidation can equally be used as oxidative stress markers. Protein carbonyl content is the most general and well-used biomarker of severe oxidative protein damage. Oxidized proteins are generally more stable than lipid peroxidation products and carbonyl measurement seemed to be more suitable when oxidative damage was evaluated in RBC ghost membranes subjected to extensive washing procedure.
Ionizing radiation at the dose of 50 Gy resulted in elevated carbonyl group level in the RBC membrane protein (Fig. 1). Incubation with quercetin at the concentration of 50 µM prior to irradiation brought back the level of carbonyls to normal. The measured values were 1.38 ± 0.075 and 1.36 ± 0.053 nmol/mg of protein for the membranes irradiated in the presence of quercetin and for a non-irradiated control, respectively. Quercetin did not significantly influence protein carbonyl levels in non-irradiated membrane suspensions (P > 0.05).
Figure 1.
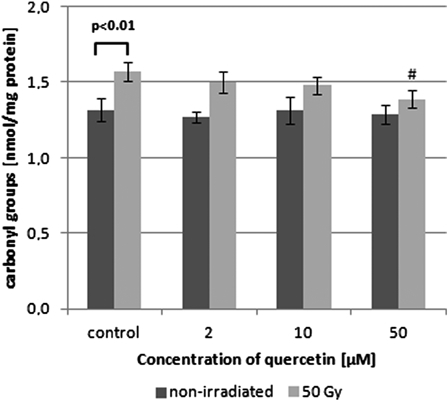
Changes in the levels of protein carbonyl groups of control, γ-irradiated, and quercetin-pretreated prior irradiation isolated erythrocyte membranes. Values are given as mean ± SD of six independent experiments in each group; #P < 0.05, compared with irradiated control (in the absence of quercetin), one-way ANOVA followed by the Tukey's post hoc test. The differences between control non-irradiated vs. irradiated RBC membranes were analysed by paired Student's t-test (P < 0.01).
Effects of quercetin on radiation-induced GSH oxidation in RBCs
Ellman's reagent reacts with all non-protein cellular thiols. In the RBC, GSH represents over 98% of the all low-molecular weight thiols, and therefore determination of their concentration can be approximately related to the concentration of GSH.
Our results showed a statistically significant decrease of GSH concentration in RBCs subjected to 50 Gy irradiation when compared with non-irradiated cells (by approximately 10%, P < 0.05), reflecting radiation-induced decrease of the GSH/GSSG (glutathione reduced/oxidized) ratio. In the presence of quercetin, the GSH concentration was reduced both in irradiated and non-irradiated erythrocytes, compared with their corresponding controls, not containing quercetin (Fig. 2). Only the lowest quercetin concentration used (2 µM) did not significantly influence the GSH concentration in non-irradiated erythrocytes as compared with the control (no antioxidant).
Figure 2.
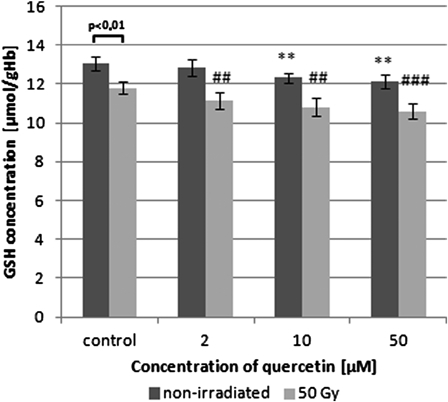
Changes in GSH concentration of control, γ-irradiated, and quercetin-pretreated prior irradiation erythrocytes. Values are given as mean ± SD of five independent experiments in each group; **P < 0.01 compared with non-irradiated control, ##P < 0.01, ###P < 0.001 compared with irradiated control (in the absence of quercetin), one-way ANOVA followed by the Tukey's post hoc test. The differences between control non-irradiated vs. irradiated RBCs were analysed by paired Student's t-test (P < 0.01).
Effects of quercetin on radiation-induced lipid peroxidation and haemolysis
Lipid peroxidation level in pre-incubated with quercetin (24 hours at 4°C) non-irradiated and γ-irradiated RBCs is shown in Fig. 3A. In irradiated cells, at the dose of 50 Gy, there was a significant increase in the concentration of TBARS (by above 40% vs. control; P < 0.01). Moreover, pre-treatment of RBCs with quercetin increased the TBARS levels in irradiated erythrocytes even more (by approximately 45 and 60% at quercetin concentrations 10 and 50 µM, respectively). Quercetin at the highest used concentration significantly enhanced lipid peroxidation in non-irradiated RBCs (by 28% vs. control; P < 0.05). To compare the effect of quercetin on radiation-induced lipid peroxidation in other non-cellular and cellular systems in our experimental conditions, TBARS concentrations were determined in plasma and in peripheral blood lymphocytes irradiated in the presence of the flavonoid. In contrast, our results showed that quercetin (10–40 µM) in a concentration-dependent manner inhibited TBARS generation in irradiated (100 Gy) plasma (Fig. 3B) and in irradiated (25 Gy) lymphocytes (Fig. 3C). There was also a decrease in TBARS concentration in quercetin-treated non-irradiated plasma, but the presence of quercetin did not influence the amount of TBARS in non-irradiated lymphocytes.
Figure 3.
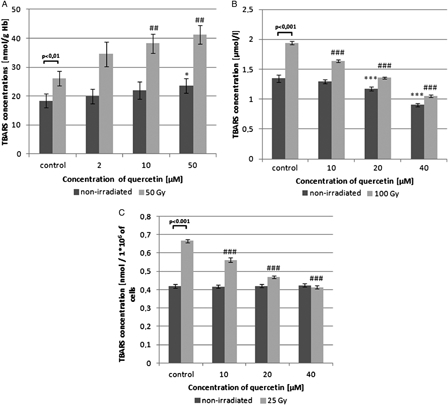
Changes in lipid peroxidation level of control, γ-irradiated, and quercetin-pretreated prior irradiation erythrocytes (A), plasma (B), and mononuclear cells of peripheral blood (C). Values are given as mean ± SD of six independent experiments in each group; *P < 0.05, ***P < 0.001 compared with non-irradiated control, ##P < 0.01, ###P < 0.001 compared with irradiated control (in the absence of quercetin), one-way ANOVA followed by the Tukey's post hoc test. The differences between control non-irradiated vs. irradiated RBCs were analysed by paired Student's t-test (P < 0.01 and P < 0.001 in Fig. 3A, B, and C, respectively).
Haemolysis measurement reflects the erythrocyte membrane damage. There was a significant concentration-dependent haemolysis enhancement in the erythrocytes irradiated in the presence of quercetin (up to 85%) vs. irradiated control (Fig. 4). Haemolysis in pre-treated with quercetin non-irradiated and irradiated in the absence of quercetin erythrocytes was not observed.
Figure 4.

Haemolysis in control, γ-irradiated, and quercetin-pretreated prior irradiation erythrocytes. Values are given as mean ± SD of four independent experiments in each group; #P < 0.05, ##P < 0.01, ###P < 0.001 compared with irradiated control (in the absence of quercetin). Owing to the non-normal distribution data the Kruskal–Wallis one-way ANOVA and Wilcoxon test were performed.
Discussion
The development of effective radioprotectors is important for safe application of ionizing radiation in medical practices, e.g. radiotherapy, nuclear medicine, and transfusion medicine (irradiation of blood components to prevent Transfusion-Associated Graft versus Host Disease). In the present study, the radioprotective efficacy of quercetin on human RBCs and their membranes in vitro were investigated. The preventative effects of aglycone quercetin are seen in vitro at approximately 1–40 µM and similar concentration range (2–50 µM) was used in our study. Although quercetin concentration derived from dietary assumptions and plasma bioavailability is lower, it is likely that these concentrations could be achieved through dietary supplementation or intravenous administration of quercetin.30,31
Amino acid residues in the membrane proteins are highly susceptible to oxidation by ROS that are formed during exposure to ionizing irradiation. Our earlier studies have revealed that protein carbonyl groups are good measures of irradiation-induced plasma protein oxidation and that antioxidant vitamins, i.e. vitamin C, may protect proteins against such oxidative damage.32 In the current study, it was shown that quercetin (50 µM) efficiently protected RBC membrane proteins against irradiation-induced (50 Gy) carbonyl formation (Fig. 1). Lately, Pandey and Rizvi16 have revealed a protection against membrane protein carbonylation by quercetin in erythrocytes subjected to oxidative stress caused by t-buthylhydroperoxide. In their study, quercetin at the concentration of 1 µM showed beneficial effect, however, the most effective protective result was obtained for the concentration 100-fold higher.
Radioprotective effectiveness of quercetin, in the majority on DNA level, was reported earlier.19 The authors demonstrated that quercetin (24 µM) added to human peripheral blood lymphocytes prior to γ-irradiation (1–4 Gy) reduced DNA damage, decreased TBARS concentration, and improved antioxidant status. Similarly, in our study, quercetin (10–40 µM) efficiently inhibited radiation-induced lipid peroxidation in human plasma (Fig. 3B) and in peripheral lymphocytes (Fig. 3C). It is believed that antioxidant capacity of polyphenols, including quercetin, is due to their stabilizing effect on the cell membranes.33,34 Strong antioxidant activity of polyphenols and scavenging of radiation-induced ROS appears to be contributing towards their overall radioprotective ability.35 However, quercetin was not able to protect whole RBCs against γ-irradiation in our study. Instead, the presence of quercetin resulted in pro-oxidative effects since it decreased the GSH/GSSG ratio and increased lipid peroxidation, predominantly in irradiated RBCs (Figs. 2 and 3A). Contrary to these results, Broncel et al.36 have demonstrated significant decrease of TBARS concentration by quercetin at similar concentration range (10–100 µM) in erythrocytes of healthy donors. Comparing their experimental conditions to ours, these authors used much less erythrocytes per tested tube (Ht = 2 vs. 80%). The authors also did not specify the solvent they used to prepare a stock solution of quercetin, which could influence a discrepancy between the results.
One possible explanation how quercetin increased oxidative stress in RBCs is that, the C4-keto moiety and the C2=C3 double bond in flavonoid molecule can facilitate the formation of quinoid-type metabolites, o-quinones, and p-quinone methides.37 These oxidation products of quercetin display some toxic effects due to their ability of arylating protein thiols. Quinones very easily react with thiols and can form a reversible adduct with GSH, the most abundant RBC thiol. When the GSH concentration is low quinones can react with other thiols. Binding of quinones to RBC membrane protein thiols can lead to various toxic effects, i.e. increased membrane permeability or dysfunction of enzymes comprising a critical –SH group.
On the other hand, our results agree with earlier reports by other authors who showed that in a Fenton reaction system, some quercetin derivatives with free catechol moiety or free hydroxyl group in position 3 (or both) were pro-oxidant, through superoxide radical and hydrogen peroxide production.38 Pro-oxidative effects of quercetin on A549 cells (decrease in thiol content, total antioxidant capacity, cell proliferation, and induction of cell apoptosis and necrosis), at higher concentrations of the flavonoid (>50 µM), due to ROS formation were also reported by Robaszkiewicz et al.39 Similarly, Yen et al.40 monitored pro-oxidant properties of quercetin, naringenin, hesperetin, and morin (at concentrations of 25–200 µM) in human lymphocytes, manifested by the generation of hydrogen peroxide and the superoxide anion, TBARS production, and depletion of cell membrane protein thiols.
Both the above mechanisms, a production of quercetin metabolites and/or ROS, can possibly be undergone in quercetin-treated RBCs. Predictably, the pro-oxidative and cytotoxic effects of quercetin were enhanced by γ-irradiation. It cannot be excluded that irradiation of quercetin leads to flavonoid decomposition accompanied by the generation of some toxic radiation products. Lipid peroxidation is one of the major forms of cellular damage induced by radiation. By-products of lipid peroxidation have been shown to cause deep changes in the structural organization and functions of the cell membrane including decreased membrane fluidity, increased membrane permeability, inactivation of membrane bound enzymes, and loss of essential fatty acids.41 In the present study, irradiation in the presence of quercetin induced haemolysis of RBCs (Fig. 4). RBC membranes, in particular, are vulnerable to lipid peroxidation due to constant exposure to high oxygen tension and richness in polyunsaturated fatty acids. Moreover, RBCs are especially susceptible to oxidative damage due to the ability of haemoglobin to participate in generation of hydroxyl radicals mediated by redox metals.42 Attack of toxic metabolites and oxidants on RBC membrane can finally lead to diminished RBC survival.
In summary, our results indicate that in a Fenton reaction prone system such as RBCs, unlike in lymphocytes or the non-cellular systems (plasma, isolated RBC membrane) quercetin acts as a pro-oxidant. Quercetin and its glycosides are used as therapeutic agents in the treatment of diseases involving free radicals. One could hypothesize that the capacity of flavonoids to induce detoxifying enzymes is a major mechanism by which these compounds exert their preventive action. It has to be underlined, however, that flavonoids neither in vitro nor in vivo can be considered solely as antioxidants, since under certain reaction conditions they can also display pro-oxidant activity. Antioxidative properties of quercetin in vitro depend on various experimental conditions, i.e. flavonoid concentration, biological system, incubation time, pH, and the presence of toxic metabolic by-products. The issue why and to which extent is quercetin able to act as anti- or pro-oxidant is still poorly understood and requires further studies.
Acknowledgements
This study was supported by grant 506/810 from the University of Lodz.
References
- 1.Prasad NR, Menon VP, Vasudev V, Pugalendi KV. Radioprotective effect of sesamol on gamma-radiation induced DNA damage, lipid peroxidation and antioxidants levels in cultured human lymphocytes. Toxicology 2005;209(3):225–35. [DOI] [PubMed] [Google Scholar]
- 2.Anand AJ, Dzik WH, Imam A, Sadrzadeh SM. Radiation-induced red cell damage: role of reactive oxygen species. Transfusion 1997;37(2):160–5. [DOI] [PubMed] [Google Scholar]
- 3.Sharifi S, Dzik WH, Sadrzadeh SMH. Human plasma and tirilazad mesylate protect stored human erythrocytes against the oxidative damage of gamma-irradiation. Transfus Med 2000;10(2):125–30. [DOI] [PubMed] [Google Scholar]
- 4.Zbikowska HM, Antosik A. Irradiation dose-dependent oxidative changes in red blood cells for transfusion. Int J Radiat Biol 2012;88(9):654–60. [DOI] [PubMed] [Google Scholar]
- 5.Mead JF. Free radical mechanisms of damage and consequences for cellular membranes: Free Radicals in Biology. Pryor WA, (ed.). New York: Academic Press; 1991. p. 51–67 [Google Scholar]
- 6.Relevy H, Koshkaryev A, Manny N, Yedgar S, Barshtein G. Blood banking-induced alteration of red blood cell flow properties. Transfusion 2008;48(1):136–46. [DOI] [PubMed] [Google Scholar]
- 7.Kanias T, Acker JP. Biopreservation of red blood cells – the struggle with hemoglobin oxidation. FEBS J 2010;277(2):343–56. [DOI] [PubMed] [Google Scholar]
- 8.Hirayama J, Abe H, Azuma H, Ikeda H. Leakage of potassium from red blood cells following gamma ray irradiation in the presence of dipyridamole, trolox, human plasma or mannitol. Biol Pharm Bull 2005;28(7):1318–20. [DOI] [PubMed] [Google Scholar]
- 9.Weiskopf RB, Schnapp S, Rouine-Rapp K, Bostrom A, Toy P. Extracellular potassium concentrations in red blood cell suspensions after irradiation and washing. Transfusion 2005;45(8):1295–301. [DOI] [PubMed] [Google Scholar]
- 10.Sowemimo-Coker SO. Red blood cell hemolysis during processing. Transfus Med Rev 2002;16(1):46–60. [DOI] [PubMed] [Google Scholar]
- 11.Zimmermann R, Wintzheimer S, Weisbach V, Strobel J, Zingsem J, Eckstein R. Influence of prestorage leukoreduction and subsequent irradiation on in vitro red blood cell (RBC) storage variables of RBCs in additive solution saline-adenine-glucose-mannitol. Transfusion 2009;49(1):75–80. [DOI] [PubMed] [Google Scholar]
- 12.Jagetia GC. Radioprotective potential of plants and herbs against the effects of ionizing radiation. J Clin Biochem Nutr 2007;40(2):74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maurya DK, Devasagayam TP, Nair CK. Some novel approaches for radioprotection and the beneficial effect of natural products. Indian J Exp Biol 2006;44(2):93–114. [PubMed] [Google Scholar]
- 14.Weiss JF, Landauer MR. Protection against ionizing radiation by antioxidant nutrients and phytochemicals. Toxicology 2003;189(1–2):1–20. [DOI] [PubMed] [Google Scholar]
- 15.Boots AW, Haenen GR, Bast A. Health effects of quercetin: from antioxidant to nutraceutical. Eur J Pharmacol 2008;585(2–3):325–37. [DOI] [PubMed] [Google Scholar]
- 16.Pandey KB, Rizvi SI. Protection of protein carbonyl formation by quercetin in erythrocytes subjected to oxidative stress. Med Chem Res 2010;19:186–92. [Google Scholar]
- 17.Vasquez-Garzon VR, Arellanes-Robledo J, Garcia-Roman R, Aparicio-Rautista DI, Villa-Trevino S. Inhibition of reactive oxygen species and pre-neoplastic lesions by quercetin through an antioxidant defense mechanism. Free Radic Res 2009;43(2):128–37. [DOI] [PubMed] [Google Scholar]
- 18.Fahlman BM, Krol ES. Inhibition of UVA and UVB radiation-induced lipid oxidation by quercetin. J Agric Food Chem 2009;57(12):5301–5. [DOI] [PubMed] [Google Scholar]
- 19.Devipriya N, Sudheer AR, Srinivasan M, Menon VP. Quercetin ameliorates gamma radiation-induced DNA damage and biochemical changes in human peripheral blood lymphocytes. Mutat Res 2008;654(1):1–7. [DOI] [PubMed] [Google Scholar]
- 20.Benkovic V, Knezevic AH, Dikic D, Lisicic D, Orsolic N, Basic I,. et al. Radioprotective effects of quercetin and ethanolic extract of propolis in gamma-irradiated mice. Arh Hig Rada Toksikol 2009;60(2):129–38. [DOI] [PubMed] [Google Scholar]
- 21.Casagrande R, Georgetti SR, Verri WA, Dorta DJ, dos Santos AC, Fonseca MJV. Protective effect of topical formulations containing quercetin against UVB-induced oxidative stress in hairless mice. J Photochem Photobiol B 2006;84(1):21–7. [DOI] [PubMed] [Google Scholar]
- 22.Murota K, Terao J. Antioxidative flavonoid quercetin: implication of its intestinal absorption and metabolism. Arch Biochem Biophys 2003;417(1):12–7. [DOI] [PubMed] [Google Scholar]
- 23.Dodge JT, Mitchell C, Hanahan DJ. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys 1963;100:119–30. [DOI] [PubMed] [Google Scholar]
- 24.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 1951;193(1):265–75. [PubMed] [Google Scholar]
- 25.Buss H, Chan TP, Sluis KB, Domigan NM, Winterbourn CC. Protein carbonyl measurement by a sensitive ELISA method. Free Radic Biol Med 1997;23(3):361–6. [DOI] [PubMed] [Google Scholar]
- 26.Alamdari DH, Kostidou E, Paletas K, Sarigianni M, Konstas AG, Karapiperidou A,. et al. High sensitivity enzyme-linked immunosorbent assay (ELISA) method for measuring protein carbonyl in samples with low amounts of protein. Free Radic Biol Med 2005;39(10):1362–7. [DOI] [PubMed] [Google Scholar]
- 27.Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz AG,. et al. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol 1990;186:464–78. [DOI] [PubMed] [Google Scholar]
- 28.Rice-Evans C, Diplock A, Symons M. Techniques in free radical research: Laboratory Techniques in Biochemistry and Molecular Biology. Burdon R, van Knippenberg P, (eds.) Amsterdam, London, New York, Tokyo: Elsevier; 1991. p. 143–7. [Google Scholar]
- 29.Halliwell B, Gutteridge JM. Formation of thiobarbituric-acid-reactive substance from deoxyribose in the presence of iron salts: the role of superoxide and hydroxyl radicals. FEBS Lett 1981;128(2):347–52. [DOI] [PubMed] [Google Scholar]
- 30.Manach C, Williamson G, Morand C, Scalbert A, Remesy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr 2005;81(1 Suppl):230S–42S. [DOI] [PubMed] [Google Scholar]
- 31.Vargas AJ, Burd R. Hormesis and synergy: pathways and mechanisms of quercetin in cancer prevention and management. Nutr Rev 2010;68(7):418–28. [DOI] [PubMed] [Google Scholar]
- 32.Zbikowska HM, Nowak P, Wachowicz B. Protein modification caused by a high dose of gamma irradiation in cryo-sterilized plasma: protective effects of ascorbate. Free Radic Biol Med 2006;40(3):536–42. [DOI] [PubMed] [Google Scholar]
- 33.Margina D, Ilie M, Gradinaru D. Quercetin and epigallocatechin gallate induce in vitro a dose-dependent stiffening and hyperpolarizing effect on the cell membrane of human mononuclear blood cells. Int J Mol Sci 2012;13(4):4839–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Margina D, Ilie M, Manda G, Neagoe I, Mocanu M, Ionescu D,. et al. Quercetin and epigallocatechin gallate effects on the cell membranes biophysical properties correlate with their antioxidant potential. Gen Physiol Biophys 2012;31(1):47–55. [DOI] [PubMed] [Google Scholar]
- 35.Chaudhary P, Shukla SK, Kumar IP, Namita I, Afrin F, Sharma RK. Radioprotective properties of apple polyphenols: an in vitro study. Mol Cell Biochem 2006;288(1–2):37–46. [DOI] [PubMed] [Google Scholar]
- 36.Broncel M, Franiak I, Koter-Michalak M, Duchnowicz P, Chojnowska-Jezierska J. [The comparison in vitro the effects of pravastatin and quercetin on the selected structural parameters of membrane erythrocytes from patients with hypercholesterolemia]. Pol Merkur Lekarski 2007;22(128):112–6. [PubMed] [Google Scholar]
- 37.Awad HM, Boersma MG, Boeren S, van Bladeren PJ, Vervoort J, Rietjens IM. Structure-activity study on the quinone/quinone methide chemistry of flavonoids. Chem Res Toxicol 2001;14(4):398–408. [DOI] [PubMed] [Google Scholar]
- 38.Kessler M, Ubeaud G, Jung L. Anti- and pro-oxidant activity of rutin and quercetin derivatives. J Pharm Pharmacol 2003;55(1):131–42. [DOI] [PubMed] [Google Scholar]
- 39.Robaszkiewicz A, Balcerczyk A, Bartosz G. Antioxidative and prooxidative effects of quercetin on A549 cells. Cell Biol Int 2007;31(10):1245–50. [DOI] [PubMed] [Google Scholar]
- 40.Yen GC, Duh PD, Tsai HL, Huang SL. Pro-oxidative properties of flavonoids in human lymphocytes. Biosci Biotechnol Biochem 2003;67(6):1215–22. [DOI] [PubMed] [Google Scholar]
- 41.van Ginkel G, Sevanian A. Lipid peroxidation-induced membrane structural alterations. Methods Enzymol 1994;233:273–88. [DOI] [PubMed] [Google Scholar]
- 42.Van Dyke BR, Saltman P. Hemoglobin: a mechanism for the generation of hydroxyl radicals. Free Radic Biol Med 1996;20(7):985–9. [DOI] [PubMed] [Google Scholar]


