Abstract
Background
Cyclophosphamide (CP) is widely used in the treatment of tumors and B-cell malignant disease, such as lymphoma, myeloma, chronic lymphocytic leukemia, and Waldenstrom's macroglobulinemia. Renal damage is one of the dose-limiting side effects of CP. Oxidative stress is reported to play important roles in CP-induced renal damage.
Aim
To find out whether aminoguanidine (AG) protects against CP-induced oxidative stress and renal damage.
Method
Renal damage was induced in the rats by administration of a single injection of CP at a dose of 150 mg/kg body weight intraperitoneally. For the AG pretreatment studies, the rats were injected intraperitoneally with AG at a dose of 200 mg/kg body weight 1 hour before administration of CP. The control rats received AG or saline alone. All the rats were killed 16 hours after the administration of CP or saline. The kidneys were used for histological examination by light microscopy and biochemical assays — malondialdehyde, protein carbonyl content, reduced glutathione (GSH), and the activities of antioxidant enzymes including glutathione peroxidase (GPx), glutathione S transferase (GSTase), catalase, glutathione reductase, and myeloperoxidase (MPO), a marker of neutrophil infiltration.
Results
Pretreatment with AG attenuated CP-induced renal damage histologically. Pretreatment with AG prevented CP-induced lipid peroxidation, protein oxidation, depletion of reduced GSH, and loss of activities of the antioxidant enzymes including GPx, catalase, and GSTase and also MPO activity.
Conclusion
The results of the present study reveal that AG can prevent CP-induced renal damage by inhibiting oxidative stress. Thus, AG may be useful for prevention of the nephrotoxicity of CP.
Keywords: Aminoguanidine, Cyclophospharmide, Oxideative stress, Renal damage, Rats
Introduction
Cyclophosphamide (CP) and its structural analog ifosfamide are highly effective cytostatic drugs. CP has been in the clinic since the late 1950s and is still one of the most widely used drugs in cancer chemotherapy.1,2 CP has a wide spectrum of clinical uses, and it has been proved to be effective in the treatment of cancer and non-malignant disease states such as rheumatoid arthritis.3 While both CP and ifosfamide have severe urotoxic side effects, only ifosfamide was thought to be nephrotoxic. The nephrotoxicity of CP is generally overlooked because plasma creatinine, an indicator of glomerular function of the kidney, is not altered significantly in patients on CP chemotherapy. However, in a recent study, we have demonstrated, using a rat model, that CP induces renal damage histologically, but the plasma creatinine, a reliable biochemical marker of renal dysfunction, remains unaltered.4
Studies have shown that CP can be nephrotoxic, both in humans and animal models. CP can result in glomerular dysfunction and tubular dysfunction.4–6 The elderly (due to progressive decline in renal function with aging) and children (due to toxic effect on the developing kidney) are susceptible to the nephrotoxicity of CP. The nephrotoxicity is dose related and includes a variable reduction of glomerular filtration rate (GFR) along with tubular dysfunction. Glomerular proteinuria, tubular proteinuria, reduction of GFR, and decrease in concentration function of kidney have been reported in children on chemotherapy.
Recent studies have indicated a role for oxidative stress in CP-induced renal damage. Intraperitoneal administration of CP has been reported to increase malondialdehyde (MDA), decrease in reduced glutathione (GSH), and decrease the activities of kidney's antioxidative enzymes, such as superoxide dismutase, glutathione peroxidase (GPx), glutathione reductase, and catalase.7–10
There is growing evidence for the role of aminoguanidine (AG) as an antioxidant. Many studies have demonstrated that AG acts as an antioxidant, and prevents the loss of antioxidant enzyme activities as well as cellular damage.11–16 In a recent study,17 AG has been shown to reduce cisplatin-induced nephrotoxicity due to its antioxidant effect. In another recent study,18 AG has been shown to increase the endogenous antioxidant defense mechanism in rats and protect the animals from radiation-induced lung toxicity. Abdel-Zaher et al.19 have shown that AG markedly inhibits acetaminophen-induced hepatic and renal depletion of antioxidants as well as nitric oxide overproduction.
As reactive oxygen species (ROS)-induced oxidative stress is reported to play an important role in CP-induced renal damage, and as AG has been recently reported to have antioxidant property the present study is aimed at investigating whether AG prevents CP-induced oxidative stress and renal damage.
Materials and methods
Animals
Adult male Wistar rats (200–250 g) were used for the experiments. The study was approved by the animal ethics Committee for the Purpose of Control and Supervision of Experimentation on Animals (CPCSEA), Government of India. The guidelines were followed. Dosage and route of administration of CP were determined from that described in the literature.20
Pilot study
A pilot study was performed to determine the optimal dose of AG needed to prevent CP-induced renal damage. The doses of AG tried were 50, 100, and 200 mg/kg body weight. The dose of 200 mg/kg body weight was found to offer maximal protection against CP-induced damage. Therefore, this dose was used in the study.
Animal treatment
The rats were divided into four groups and were treated as follows.
Group 1 rats received a single intraperitoneal injection of CP in saline at a dose of 150 mg/kg body weight. Group 2 rats were pretreated with AG at a dose of 200 mg/kg body weight intraperitoneally 1 hour before the administration of CP in saline at a dose of 150 mg/kg body weight. Group 3 rats received AG and saline. Finally, group 4 rats received saline alone. All the rats were killed 16 hours after CP or saline injection.
Tissue procurement
The rats were killed by exsanguination. The kidneys were removed, blotted dry, and used for light microscopic examination and biochemical studies.
Light microscopy
The tissues were fixed overnight in 10% buffered neutral formalin, processed to paraffin wax, sectioned at 5 µm, and stained with hematoxylin-eosin for examination by light microscopy
Biochemical studies
The biochemical assays were carried out on 10% (w/v) of kidney homogenates prepared in ice-cold 1.15% KCl.
(a) Malondialdehyde
Malondialdehyde content was measured as described by Ohkawa et al.21 The mixture consisted of 0.8 ml of sample, 0.2 ml of 8.1% sodium dodecylsulfate, 1.5 ml of 20% glacial acetic acid adjusted to pH 3.5, and 1.5 ml of 0.8% aqueous solution of thiobarbituric acid. The mixture was made up to 4 ml with distilled water and heated at 95°C for 60 minutes using a glass ball as condenser. After cooling with tap water, 1 ml distilled water and 5 ml n-butanol and pyridine mixture (15:1) were added and the solution was shaken vigorously. After centrifugation at 2000 × g for 10 minutes, the absorbance of the organic layer was measured at 532 nm. The amount of thiobarbituric reacting substances formed is calculated from a standard curve prepared using 1, 1, 3, 3-tetramethoxy propane and the values expressed as nmol per mg protein.
(b) Protein carbonyl content
Protein carbonyl content (PCo) was measured using dinitrophenyl hydrazine (DNPH) as described by Sohal et al.22 To 0.5 ml of sample, an equal volume of 10 mM DNPH in 2 N HCl was added and incubated for 1 hour shaking intermittently at room temperature. Corresponding blank was carried out by adding only 2 N HCl to the sample. After incubation, the mixture was precipitated with 10% trichloroacetic acid (TCA) (final concentration) and centrifuged. The precipitate was washed twice with ethanol:ethyl acetate (1:1) and finally dissolved in 1 ml of 6 M guanidine HCl, centrifuged at low speed (2000 × g) and the supernatant was read at 366 nm. The difference in absorbance between the DNPH-treated and HCl-treated sample is determined and expressed as nmol of carbonyl groups per mg of protein, using an extinction coefficient of 22 mM−1 cm−1.
(c) Glutathione
Non-protein thiol was determined by the method described by Sedlak and Lindsay.23 Briefly, proteins were removed by the addition of 21 µl 50% TCA to 400 µl plasma. Then, samples were centrifuged at 12 000 rpm for 10 minutes. Then, 50 µl of the obtained supernatant and 100 µl 6 M dithio-nitrobenzene (Ellman's reagent) were added successively to 850 µl 0.2 M phosphate buffer, pH 8.2, and after 1 hour the absorbance was measured at 412 nm. The results were read from a standard curve prepared from 1 M solution of reduced GSH.
(d) Myeloperoxidase
MPO activity was measured with O-dianisidine-H2O2 assay.24 The rate of decomposition of H2O2 by MPO was determined by measuring the rate of color development at 460 nm. To 10 µl of sample, 11 µl of H2O2, 17 µl of O-dianisidine, and 962 µl of phosphate buffer were added and the color read at 460 nm at an interval of 30 seconds for 4 minutes and the rate of change/minute was determined. Extinction coefficient of 1.13 × 104 cm−1 was used for the calculation. One unit is the amount of enzyme decomposing 1 µmol of peroxide per minute.
(e) Catalase
Catalase activity was estimated by measuring the change in absorption at 240 nm using H2O2 as substrate.25 To 1 ml of 30 mM buffered H2O2, the enzyme (sample) was added to start the reaction. The final volume was made up to 2 ml with 0.05 M phosphate buffer pH 7.0. Change in optical density (OD) was observed for 2 minutes at 240 nm. One unit is the activity that disproportionates H2O2 at the rate of 10−3 absorbance/second.
(f) Glutathione S transferase
The activity of glutathione S transferase (GSTase) was measured spectrophotometrically using the substrate 1-chloro-2, 4-dinitrobenzene (CDNB).26 To 0.1 ml of 1 M potassium phosphate buffer pH 6.5, following reagents were added: 0.1 ml of 10 mM GSH, 0.05 ml 20 mM CDNB, and water and made up the volume to 1 ml. The reaction was started by adding the enzyme and the change in OD at 340 nm was measured for 1–2 minutes. One unit of enzyme is the amount required to conjugate 1 mmol of substrate with GSH in 1 minute.
(g) Glutathione reductase
In the presence of enzyme, hydrogen is transferred from reduced nicotinamide adenine dinucleotide phosphate (NADPH) to oxidized glutathione (GSSG) and the reaction is measured at 340 nm.27 To the reaction mixture containing 0.05 ml of 1 M phosphate buffer pH 7.6, 0.15 ml of 10 mM ethylene diamine tetraacetic acid (EDTA), 0.1 ml of 1 mM NADPH, and 0.1 ml 10 mM GSSG, the enzyme was added. The volume was made up to 1 ml and the decrease in OD at 340 nm was measured for 2–3 minutes. One unit is the amount of enzyme needed to oxidize 1 mmol of NADPH/minute.
(h) Glutathione peroxidase
Total peroxidase was determined by following the oxidation of NADPH at 340 nm using hydrogen peroxide.28 To 0.25 ml of 0.4 M phosphate buffer, 0.2 ml of 4 mM EDTA, 0.2 ml of 10 mM GSH, 0.2 ml of sodium azide, 0.2 ml of 1.6 mM NADPH, 0.03 ml glutathione reductase (one unit), and the enzyme (sample) were added. The total volume was made up to 2 ml with water. Reaction was started by adding 0.2 ml of H2O2 and change in OD at 340 nm was followed. Extinction coefficient of 6.1 mm−1 was used for the calculation. One unit of GPx activity is defined as the amount of enzyme needed to oxidize 1 nmol of NADPH/minute.
Protein content in the homogenates was measured by the method of Lowry et al.29
Statistical analysis
The results are expressed as mean ± SD. Comparison between groups was done using analysis of variance. Student's t-test with Bonferroni correction was used to compare individual means in the case of a significant F.
Results
Light microscopy (Fig. 1)
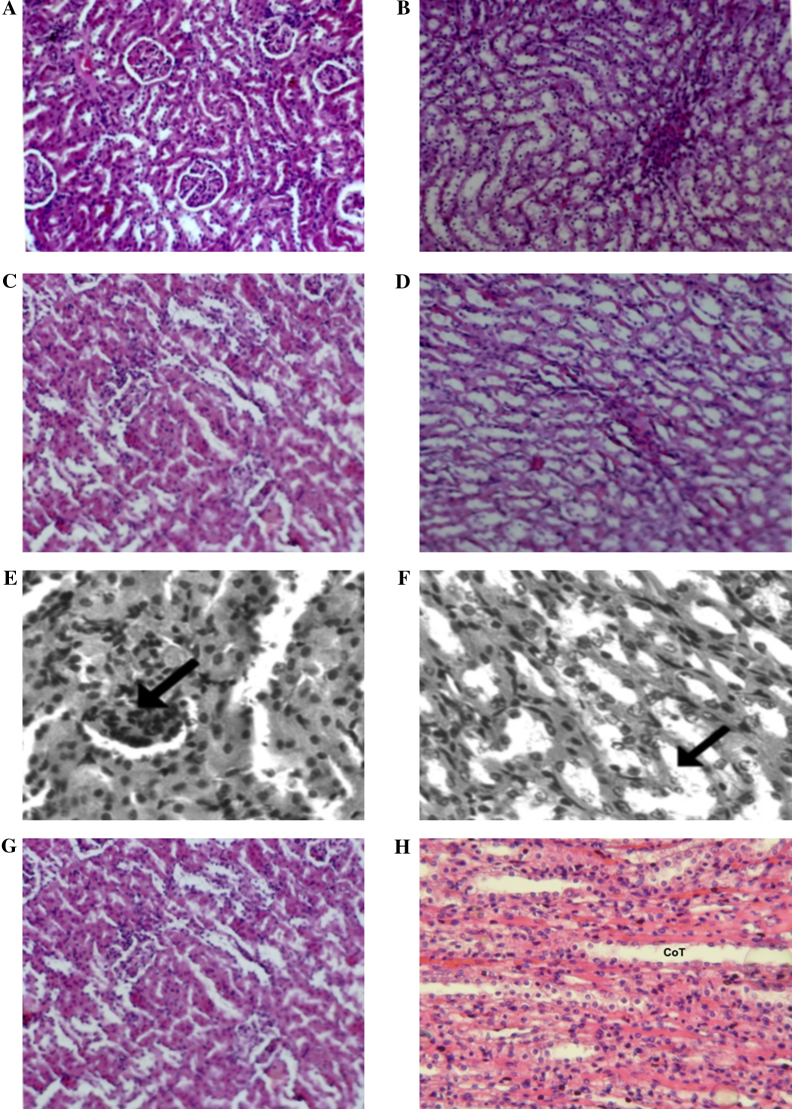
Figure 1.
(A) Renal cortex of a control rat showing normal architecture (H&E, ×100). (B) Renal medulla of a control rat showing normal architecture (H&E, ×100). (C) Renal cortex of an AG-treated rat showing normal architecture (H&E, ×100). (D) Renal medulla of an AG-treated rat showing normal morphology (H&E, ×100). (E) Renal cortex of a CP-treated rat. Diffuse glomerular and tubular nephritis was observed (H&E, ×100). (F) Renal medulla of CP-treated rat. Tubules in the medulla were enlarged with edema (arrow) with hypertrophied lining epithelial cells (H&E, ×100). (G) Kidney cortex of AG + CP-treated rat. The cortex showed almost normal architecture (H&E, ×100). (H) Kidney medulla of AG + CP-treated rat. The medulla showed normal architecture (H&E, ×100).
The kidneys of the control rats and AG-treated rats showed normal architecture (Figs. 1A–D). The toxic effect of CP on the kidney involved the glomeruli, the tubules, and the interstitium. Glomerular nephritis was observed as seen by increased cellular contents within the capillary loops (Fig. 1E). The lining epithelial cells of the tubules of the medulla were hypertrophied and there was edema of the tubules (Fig. 1F). The kidneys of AG + CP-treated rats showed almost normal architecture (Figs.Fig. 1G and H).
Biochemical assays
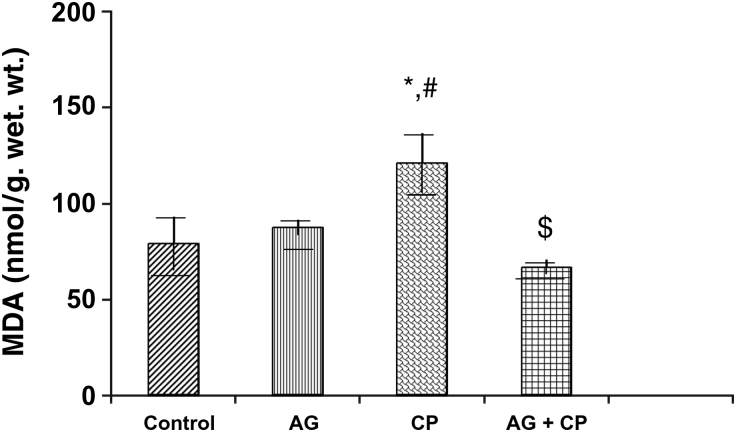
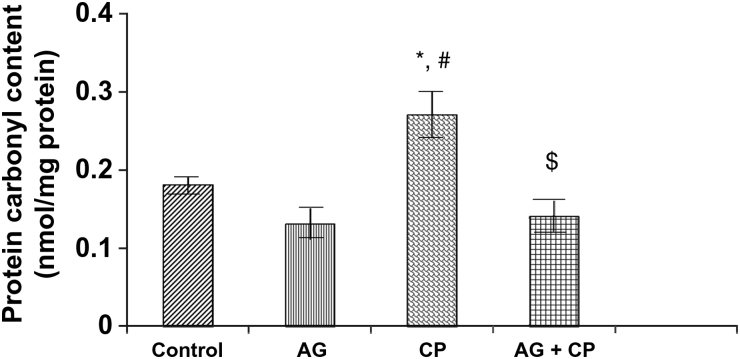
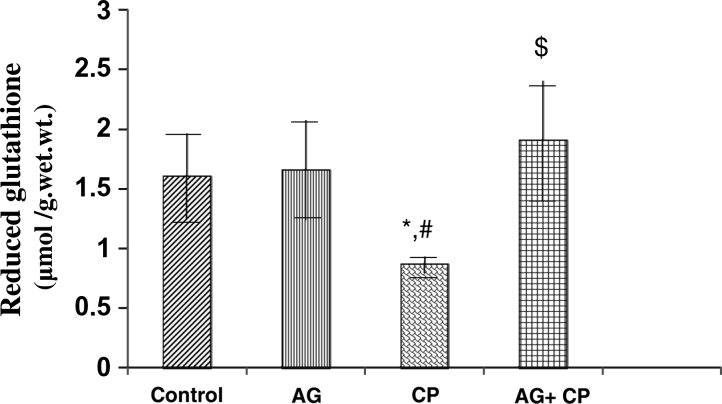
Treatment with AG alone did not affect the biochemical parameters significantly (Figs. 2–9). The biochemical results obtained for AG-treated rats were not statistically significant from that of the control rats. MDA, an indicator of lipid peroxidation, was increased by 52% in the kidneys of CP as compared with the control. Pretreatment with AG attenuated CP-induced MDA elevation (Fig. 2). PCo, a sensitive indicator of protein oxidation, was increased by 50% in the kidneys of CP-treated rats as compared with the control. Pretreatment with AG prevented CP-induced protein oxidation (Fig. 3). GSH, the most important intracellular antioxidant, was decreased by 46% as compared with the control. Pretreatment with AG completely restored the GSH levels (Fig. 4).
Figure 2.
MDA levels in the kidneys of AG-treated rats and CP-treated rats. Data represent mean ± SD of 5–7 rats. *P < 0.02 vs. control, #P < 0.02 vs. AG, $P < 0.01 vs. CP.
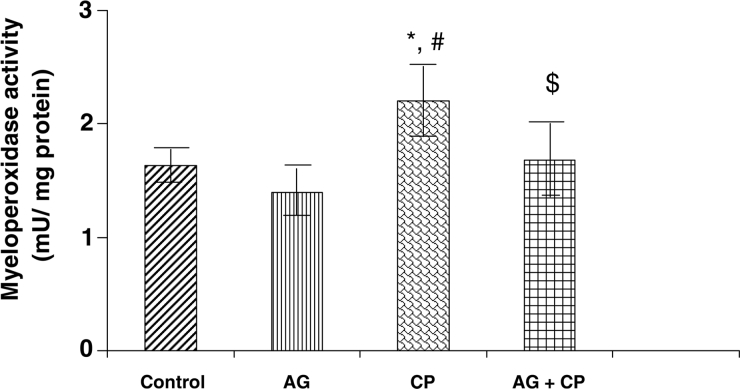
Figure 9.
MPO activity in the kidneys of AG-treated rats and CP-treated rats. Data represent mean ± SD of 5–7 rats. *P < 0.01vs. control, #P < 0.01 vs. AG, $P < 0.05 vs. CP.
Figure 3.
PCo in the kidneys of AG-treated rats and CP-treated rats. Data represent mean ± SD of 5–7 rats. *P < 0.01 vs. control, #P < 0.002 vs. AG, $P < 0.002 vs. CP.
Figure 4.
Reduced GSH levels in the kidneys of AG-treated rats and CP-treated rats. Data represent mean ± SD of 5–7 rats. *P < 0.01vs. control, #P < 0.01vs. AG, $P < 0.001 vs. CP.
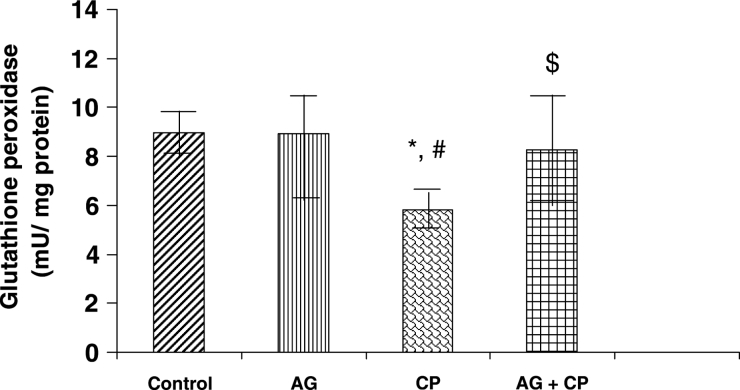
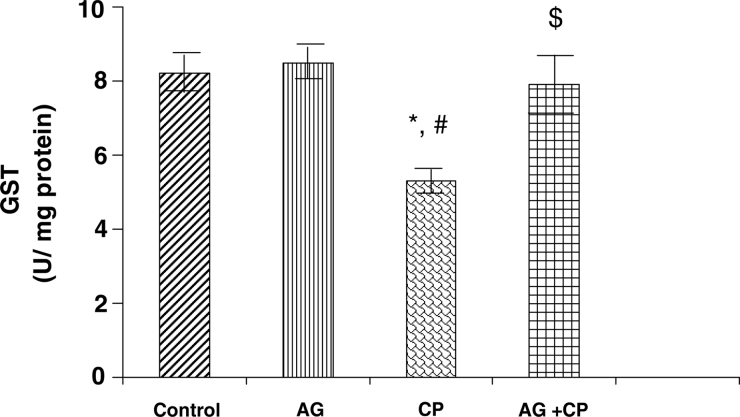
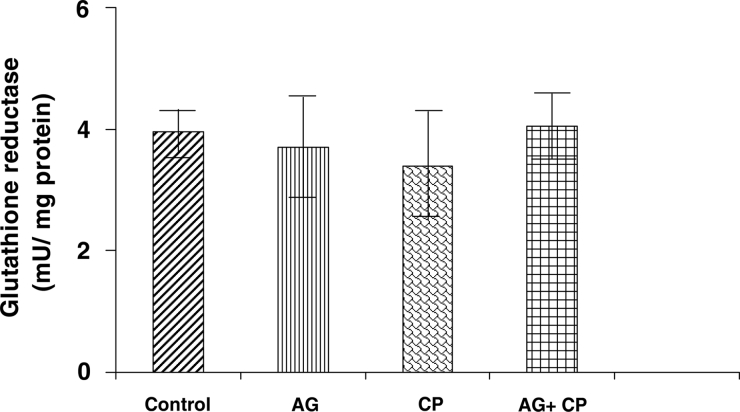
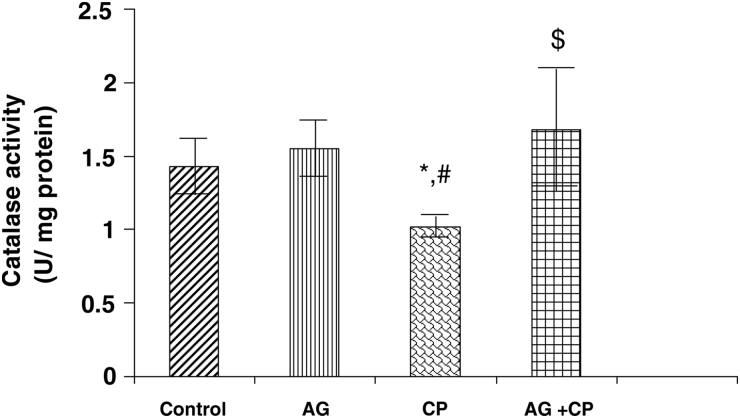
With regard to the activities of the antioxidant enzymes, the GSH-dependent enzymes GPx and GSTase were decreased by 27 and 35%, respectively, in the kidneys of CP-treated rats as compared with the control. Pretreatment with AG almost completely restored their activities (Figs. 5 and 6). The activity of glutathione reductase, the enzyme that regenerates reduced GSH from GSSG, was not significantly altered in the kidneys of CP-treated rats as compared with the control (Fig. 7). With respect to the activity of catalase, an important enzyme that detoxifies the potent ROS hydrogen peroxide was decreased by 29% in the kidneys of CP-treated rats as compared with the control. Pretreatment with AG completely restored the activity of catalase (Fig. 8).
Figure 5.
GPx activity in the kidneys of AG-treated rats and CP-treated rats. Data represent mean ± SD of 5–7 rats. *P < 0.05 vs. control, #P < 0.05 vs. AG, $P < 0.05 vs. CP.
Figure 6.
GST activity in the kidneys of AG-treated rats and CP-treated rats. Data represent mean ± SD of 5–7 rats. *P < 0.02 vs. control, #P < 0.02 vs. AG, $P < 0.05 vs. CP.
Figure 7.
Glutathione reductase activity in the kidneys of AG-treated rats and CP-treated rats. Data represent mean ± SD of 5–7 rats.
Figure 8.
Catalase activity in the kidneys of AG-treated rats and CP-treated rats. Data represent mean ± SD of 5–7 rats. *P < 0.02 vs. control, #P < 0.02 vs. AG, $P < 0.05 vs. CP.
MPO, a sensitive indicator of neutrophil infiltration, was increased by 37% in the kidneys of CP-treated rats as compared with the control. Pretreatment with AG attenuated CP-induced increase in MPO activity (Fig. 9).
Discussion
Oxidative stress is known to play an important role in CP-induced renal damage.5,6 Accordingly, in the present study the parameters of oxidative stress, namely, MDA and PCo were found to be increased in the kidneys of CP-treated rats, suggesting that CP treatment caused oxidative damage to the lipids and proteins of the kidney. AG pretreatment prevented CP-induced elevation in these oxidative stress markers.
Antioxidant enzymes protect tissue against oxidative injury. Tissue damage caused by ROS is reported to occur after the cellular antioxidants are depleted. In the present study, a decrease in the activities of the important antioxidant enzymes, namely catalase, GPO, and GSTase, was observed in the kidneys of CP-treated rats. AG pretreatment restored the activities of the antioxidant enzymes significantly. AG pretreatment attenuated CP-induced renal damage as well.
The protective effect of AG in CP-induced renal damage may be achieved by two mechanisms. Firstly, AG reduces the formation of potent oxidant peroxynitrite. Most of the deleterious effects of nitric oxide are believed to be produced by peroxynitrite, a potent oxidant that is produced by the reaction of nitric oxide with superoxide anion.30,31 Mitochondria in vivo produce superoxide and in pathological situations the amount of superoxide produced increases; therefore, in the presence of nitric oxide, mitochondria will be a major site of peroxynitrite formation.32 Peroxynitrite is a potent oxidant that depletes the antioxidant defenses including GSH, protein thiol, and antioxidant enzymes.33–35 Insufficiency of the protective antioxidant system results in enhanced lipid peroxidation and tissue injury. When the production of nitric oxide is decreased by the administration of nitric oxide synthase inhibitor AG, the source of peroxynitrite is reduced. Thus, less peroxynitrite is produced, the oxidative damage to the lipids and proteins is decreased and the cellular antioxidant systems are spared. In a recent study, we have demonstrated that AG pretreatment attenuates CP-induced elevation in nitrite levels, protein nitration, PARP(S) activation, and renal damage (unpublished data). In addition, in the present study we observed that AG pretreatment reduced CP-induced oxidative stress and restored the antioxidant activity in the kidney.
Secondly, the protective effect of AG may result from direct antioxidant effects of AG. There is growing evidence for the role of AG as an antioxidant. AG has been reported to be an effective hydroxyl radical scavenger.36 AG exhibits a significant dose-dependent effect against free radical damage. AG has been shown to exhibit trapping activity towards the lipid-derived aldehydes such as MDA and 4-hydroxynonenal.37 Several studies have shown that AG acts as an antioxidant and can restore the antioxidants in the tissues. AG has been reported to prevent gentamicin-induced renal damage by acting as a potent free radical scavenger and prevents the depletion of cellular antioxidants.38 A study has shown that the protective effect of AG against nephrotoxicity induced by cisplatin in normal rats is by its capacity to inhibit lipid peroxidation.39 Kedziora-kornatowska et al.40 have demonstrated that AG can prevent oxidative stress in the peripheral blood granulocytes of rats with streptozotocin-induced diabetes. Besides, AG prevents impairment of blood antioxidant systems in insulin-dependent diabetic rats.41 A recent study shows that AG improves antioxidant status and attenuates lipid peroxidation in the brain of rats exposed to chronic stress.42 Another recent study demonstrates that AG can reduce the oxidative damage to proteins and hence PCo.43
We have earlier demonstrated that AG pretreatment prevents CP-induced nitrosative stress, oxidative stress, and hemorrhagic cystitis in rats.44 Two other studies have reported similar results.45,46
Clinical trials have shown that AG has very low toxicity level and is safe to use in dosages of 150–300 mg/day. Side effects in human trials have been limited to nausea and headache.47
The results of the present study show that AG protects against CP-induced oxidative stress and renal damage. Thus, AG may be effective in preventing CP-induced renal damage in humans. If proven so, AG may be a promising drug for the prevention of CP-induced renal damage in cancer patients on chemotherapy. Thus, the therapeutic index of CP can be improved.
References
- 1.Fleming RA. An overview of cyclophosphamide and ifosfamide pharmacology. Pharmacotherapy 1997;17:146–54S. [PubMed] [Google Scholar]
- 2.Boddy AV, Yule SM. Metabolism and pharmacokinetics of oxazaphosphorines. Clin pharmacokinetics 2000;38:291–304. [DOI] [PubMed] [Google Scholar]
- 3.Trefler J, Matyska-Piekarska E, Łacki JK. Cyclophosphamide in the therapy of rheumatoid arthritis and its complications (in Polish). Pol Merkur Lekarski 2007;22:566–70. [PubMed] [Google Scholar]
- 4.Sugumar E, Kanakasabapathy I, Abraham P. Normal plasma creatinine level despite histological evidence of damage and increased oxidative stress in the kidneys of cyclophosphamide treated rats. Clin Chim Acta 2007;376:244–5. [DOI] [PubMed] [Google Scholar]
- 5.Ghosh S, Ghosh D, Chattopadhyay S, Debnath J. Effect of ascorbic acid supplementation on liver and kidney toxicity in cyclophosphamide-treated female albino rats. J Toxicol Sci 1999;24:141–4. [DOI] [PubMed] [Google Scholar]
- 6.Senthilkumar S, Devaki T, Manohar BM, Babu MS. Effect of squalene on cyclophosphamide-induced toxicity. Clin Chim Acta 2006;364:335–42. [DOI] [PubMed] [Google Scholar]
- 7.Stankiewicz A, Skrzydlewska E. Protection against cyclophosphamide-induced renal oxidative stress by amifostine: the role of antioxidative mechanisms. Toxicol Mech Methods 2003;13:301–8. [DOI] [PubMed] [Google Scholar]
- 8.Haque R, Bin-Hafeez B, Parvez S, Pandey S, Sayeed I, Ali M, et al.. Aqueous extract of walnut (Juglans regia L.) protects mice against cyclophosphamide-induced biochemical toxicity. Hum Exp Toxicol 2003;22:473–80. [DOI] [PubMed] [Google Scholar]
- 9.Manda K, Bhatia AL. Prophylactic action of melatonin against cyclophosphamide-induced oxidative stress in mice. Cell 2003;19:367–72. [DOI] [PubMed] [Google Scholar]
- 10.Sayed-Ahmed MM. Progression of cyclophosphamide-induced acute renal metabolic damage in carnitine-depleted rat model. Clin Exp Nephrol 2010;14:418–26. [DOI] [PubMed] [Google Scholar]
- 11.Kedziora-Kornatowska KZ, Luciak M, Blaszczyk J, Pawlak W. Effect of aminoguanidine on erythrocyte lipid peroxidation and activities of antioxidant enzymes in experimental diabetes. Clin Chem Lab Med 1998;36:771–5. [DOI] [PubMed] [Google Scholar]
- 12.Di Paola R, Marzocco S, Mazzon E. Effect of aminoguanidine in ligature-induced periodontitis in rats. J Dent Res 2004;83:343–8. [DOI] [PubMed] [Google Scholar]
- 13.Baynes JW, Thorpe SR. Glycoxidation and lipoxidation in atherogenesis. Free Radic Biol Med 2000;28:1708–16. [DOI] [PubMed] [Google Scholar]
- 14.Abd El-Gawad HM, El-Sawalhi MM. Nitric oxide and oxidative stress in brain and heart of normal rats treated with doxorubicin: role of aminoguanidine. J Biochem Mol Toxicol 2004;18:69–77. [DOI] [PubMed] [Google Scholar]
- 15.Hung CR. Role of gastric oxidative stress and nitric oxide in formation of hemorrhagic erosion in rats with ischemic brain. World J Gastroenterol 2006;12:574–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ara C, Karabulut AB, Kirimlioglu H. Protective effect of aminoguanidine against oxidative stress in an experimental peritoneal adhesion model in rats. Cell Biochem Funct 2006;24:443–8. [DOI] [PubMed] [Google Scholar]
- 17.Atasayar S, Gürer-Orhan H, Orhan H, Gürel B, Girgin G, Ozgüneş H. Preventive effect of amino guanidine compared to vitamin E and C on cisplatin-induced nephrotoxicity in rats. Exp Toxicol Pathol 2009;61:23–32. [DOI] [PubMed] [Google Scholar]
- 18.Eroglu C, Yildiz OG, Saraymen R, Soyuer S, Kilic E, Ozcan S. Aminoguanidine ameliorates radiation-induced oxidative lung damage in rats. Clin Invest Med 2008;31:E182–8. [DOI] [PubMed] [Google Scholar]
- 19.Abdel-Zaher AO, Abdel-Rahman MM, Hafez MM, Omran FM. Role of nitric oxide and reduced glutathione in the protective effects of aminoguanidine, gadolinium chloride and oleanolic acid against acetaminophen-induced hepatic and renal damage. Toxicology 2007;234:124–34. [DOI] [PubMed] [Google Scholar]
- 20.Ahluwalia A, Maggi CA, Santicioli P, Lecci A, Giuliani S. Characterizationof the capsaicin-sensitive component of cyclophosphamide induced inflammation in the rat urinary bladder. Br J Pharmacol 1994;111:1017–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 1979;95:351–8. [DOI] [PubMed] [Google Scholar]
- 22.Sohal RS, Agarwal S, Dubey A, Orr WC. Protein oxidative damage is associated with life expectancy of houseflies. Proc Natl Acad Sci USA 1993;90:7255–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sedlak J, Lindsay RH. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal Biochem 1968;25:192–205. [DOI] [PubMed] [Google Scholar]
- 24.Diaz-Granados N, Howe K, Lu J, McKay DM. Dextran sulfate sodium-induced colonic histopathology, but not altered epithelial ion transport, is reduced by inhibition of phosphodiesterase activity. Am J Pathol 2000;156:2169–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aebi IH. Catalase in vitro. Methods Enzymol 1984;105:121–6. [DOI] [PubMed] [Google Scholar]
- 26.Awasthi YC, Dao DD, Saneto RP. Interrelationship between anionic and cationic forms of glutathione S-transferases of human liver. Biochem J 1980;191:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Racker E. Glutathione reductase from bakers’ yeast and beef liver. J Biol Chem 1955;217:855–65. [PubMed] [Google Scholar]
- 28.Nakamura W, Hosada S. Purification and properties of rat liver glutathione peroxidase. Biochim Biophys Acta 1974;358:251–61. [Google Scholar]
- 29.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem 1951;93:265–75. [PubMed] [Google Scholar]
- 30.Radi R, Beckman JS, Bush KM. Peroxynitrite-induced membrane lipid peroxidation: the cytotoxic potential of superoxide and nitric oxide. Arch Biochem Biophys 1991;288:481–5. [DOI] [PubMed] [Google Scholar]
- 31.Radi R. Nitric oxide, oxidants, and protein tyrosine nitration. Proc Natl Acad Sci USA 2004;101:4003–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Radi R, Cassina A, Hodara R, Quijano C, Castro L. Peroxynitrite reactions and formation in mitochondria. Free Radic Biol Med 2002;33:1451–64. [DOI] [PubMed] [Google Scholar]
- 33.Olas B, Nowak P, Wachowicz B. Resveratrol protects against peroxynitrite-induced thiol oxidation in blood platelets. Cell Mol Biol Lett 2004;9:577–87. [PubMed] [Google Scholar]
- 34.Varma SD, Hegde KR. Lens thiol depletion by peroxynitrite. Protective effect of pyruvate. Mol Cell Biochem 2007;298:199–204. [DOI] [PubMed] [Google Scholar]
- 35.Reinartz M, Ding Z, Flögel U, Gödecke A, Schrader J. Nitrosative stress leads to protein glutathiolation, increased s-nitrosation, and up-regulation of peroxiredoxins in the heart. J Biol Chem 2008;283:17440–9. [DOI] [PubMed] [Google Scholar]
- 36.Courderot-Masuyer C, Dalloz F, Maupoil V, Rochette L. Antioxidant properties of aminoguanidine. Fundam Clin Pharmacol 1999;13:535–40. [DOI] [PubMed] [Google Scholar]
- 37.Burcham PC, Kaminskas LM, Fontaine FR, Petersen DR, Pyke SM. Aldehyde-sequestering drugs: tools for studying protein damage by lipid peroxidation products. Toxicology 2002;181–182:229–36. [DOI] [PubMed] [Google Scholar]
- 38.Polat A, Parlakpinar H, Tasdemir S. Protective role of aminoguanidine on gentamicin-induced acute renal failure in rats. Acta Histochem 2006;108:365–71. [DOI] [PubMed] [Google Scholar]
- 39.Mansour MA, Mostafa AM, Nagi MN, Khattab MM, Al-Shabanah OA. Protective effect of aminoguanidine against nephrotoxicity induced by cisplatin in normal rats. Comp Biochem Physiol Toxicol Pharmacol 2002;132:123–8. [DOI] [PubMed] [Google Scholar]
- 40.Kedziora-Kornatowska KZ, Luciak M, Błaszczyk J, Pawlak W. Effect of aminoguanidine on the generation of superoxide anion and nitric oxide by peripheral blood granulocytes of rats with streptozotocin induced diabetes. Clin Chim Acta 1998;278:45–53. [DOI] [PubMed] [Google Scholar]
- 41.Stoppa GR, Cesquini M, Roman EA, Ogo SH, Torsoni MA. Aminoguanidine prevented impairment of blood antioxidant system in insulin dependent diabetic rats. Life Sci 2006;78:1352–61. [DOI] [PubMed] [Google Scholar]
- 42.Apinar D, Yargicoglu P, Derin N, Aslan M, Agar A. Effect of aminoguanidine on visual evoked potentials (VEPs), antioxidant status and lipid peroxidation in rats exposed to chronic restraint stress. Brain Res 2007;1186:87–94. [DOI] [PubMed] [Google Scholar]
- 43.Brodiak IV, Sybirna NO. Effect of aminoguanidine on oxidative modification of proteins in experimental diabetes mellitus in rats [Article in Ukrainian]. Ukr Biokhim Zh 2006;78:114–9. [PubMed] [Google Scholar]
- 44.Abraham P, Rabi S, Kulothungan P. Aminoguanidine, selective nitric oxide synthase inhibitor, ameliorates cyclophosphamide-induced hemorrhagic cystitis by inhibiting protein nitration and PARS activation. Urology 2009;73:1402–6. [DOI] [PubMed] [Google Scholar]
- 45.Abraham P, Rabi S, Selvakumar D. Protective effect of aminoguanidine against oxidative stress and bladder injury in cyclophosphamide-induced hemorrhagic cystitis in rat. Cell Biochem Funct 2009;27:56–62. [DOI] [PubMed] [Google Scholar]
- 46.Korkmaz A, Oter S, Sadir S, Coskun O, Topal T, Ozler M, et al.. Peroxynitrite may be involved in bladder damage caused by cyclophosphamide in rats. J Urol 2005;173:1793–6. [DOI] [PubMed] [Google Scholar]
- 47.Bolton WK, Cattran DC, Williams ME, Adler SG, Appel GB, Cartwright K, et al.. Randomized trial of an inhibitor of formation of advanced glycation end products in diabetic nephropathy. Am J Nephrol 2004;24:32–40. [DOI] [PubMed] [Google Scholar]