Abstract
Objective
The goal of this study was to evaluate the antioxidant and antiproliferative activities of 10 traditional medicinal plants, Asclepias curassavica, Ophiorrhiza mungos Linn., Cynodon dactylon (L.) Pers, Costus speciosus (J. Koenig.) Smith Costaceae, Achyranthes aspera L., Amaranthus tristis Roxb., Blepharis maderaspatensis L., Merremia emerginata Hall.f., Aegle marmelos Corr., and Tabernaemontana heyneana Wall., used in the traditional Indian system of medicine as a cure for cancer. The present study focuses on the anticancer potential of traditional medicinal plants to induce apoptosis in cancer cell lines.
Methods
Plants were sequentially extracted with hexane, ethyl acetate, and methanol. The extract was concentrated to yield the crude extract, which was tested for antioxidant activity using 1,1-diphenyl-2-picrylhydrazyl, nitric oxide and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide assays on four cancer cell lines and a normal cell line. The anticancer potential of cytotoxic extracts was determined by the Annexin-fluorescein isothiocyanate-conjugated assay in human colon adenocarcinoma cell lines (COLO 320 DM).
Results
All the tested extracts showed significant antioxidant and antiproliferative activities in a concentration- and time-dependant manner in the following descending order: A. curassavica > C. dactylon > C. speciosus root > A. tristis > M. emarginata > O. mungos > T. Heyneana > B. maderaspatensis > A. marmelos > A. aspera.
Conclusion
The results of the present study support the need of further studies to isolate potential anticancer drug with cancer cell-specific cytotoxicity. Additionally, the study supports the anticancer property of medicinal plants used in the traditional Indian medicine system and further evaluation of the selected medicinal plants for an effective anticancer drug with minimal side effects.
Keywords: Asclepias curassavica, Cynodon dactylon, Costus speciosus, Amaranthus tristis, Merremia emarginata, Ophiorrhiza mungos, Tabernaemontana heyneana
Introduction
Lipid peroxidation has recently gained more importance due to its involvement in pathogenesis of many diseases, such as cancer, diabetes and ageing.1 Free radicals or reactive oxygen species (ROS) are produced in vivo from various biochemical reactions and from the respiratory chain as a result of occasional leakage. ROS are cytotoxic due to the intermediate formed during univalent reduction of molecular oxygen, including superoxide radical (O2·−), hydrogen peroxide (H2O2), and the hydroxyl radical (·OH). These oxygen intermediates differ significantly in their interactions and can cause extensive cellular damage such as nucleic acid strand scission, modification of polypeptides, and lipid peroxidation.2 These ROS cause destructive and irreversible damage to macromolecules such as lipids, proteins, and DNA in cells.3 Although normal cells possess antioxidant defence systems against ROS, the continuous accumulation of damage to the cells induces ageing and diseases such as cancer.4
Cancer is the second leading cause of death after cardiovascular diseases in India5. Medicinal plants are frequently used by traditional healers to treat a variety of ailments and symptoms including fever, cold, headache, diabetes, and cancer. It is well established that plants have been a useful source of clinically relevant antitumour compounds.6 According to the World Health Organization, approximately 70–95% of the developing world's populations rely on such traditional plant-based systems of medicine to provide them with primary healthcare.7
Cancer cells possess the ability to escape apoptosis by various ways. The aim of anticancer agents is to trigger the apoptosis signalling system in these cancer cells while disturbing their proliferation.8 There is accumulating evidence that these agents exert their cytotoxic effects mainly by inducing apoptosis in tumour cells. Impairment of apoptosis is known to be related to cell immortality and carcinogenesis; therefore, the induction of apoptosis in neoplastic cells is vital in cancer treatment. The chemotherapeutic drugs that have been observed to induce apoptosis in vitro include etoposide, camptothecin, VM26, vincristine, cis-platinum, cyclophosphamide, paclitaxel, 5-fluorouracil, and doxorubicin.9,10
Thus, identification of potential chemotherapeutic agents using mechanism-based studies holds great promise for elucidating mechanisms and devising more specific and effective treatments for cancer-related diseases. One of the approaches used in drug discovery is the ethnomedical data approach, in which the selection of a plant is based on prior information on the folk medicinal use of the plant. It is generally known that ethnomedical data substantially increase the chance of finding active plants relative to random approaches.11 The plants selected for the present study, Asclepias curassavica Linn. – leaves, Ophiorrhiza mungos Linn. – leaves, Cynodon dactylon (L.) Pers – root Poaceae, Costus speciosus (J. Koenig.) Smith Costaceae – root and leaves, Achyranthes aspera L. – leaves, Amaranthus tristis Roxb. – leaves, Blepharis maderaspatensis L. – leaves, Merremia emerginata Hall.f. – leaves, Aegle marmelos Corr. – leaves and Tabernaemontana heyneana Wall. Apocynaceae – leaf, are traditionally used as cure for cancer (Table 1);12–18 however, there are no recorded data for cytotoxicity against cancer cell lines and normal cell lines for these plants. Hence, in the present study, the solvent extracts of the above plants were screened for in vitro antioxidant, antiproliferative, and anticancer activities.
Table 1.
Chemical compounds isolated and the medicinal properties of the selected plants
| Plant | Compounds isolated | Medical use |
|---|---|---|
| Achyranthes aspera | Oleanolic acid, achyranthine, and betaine | Stomach troubles, stones in the bladder, opacity of the cornea, wounds, piles, pneumonia, renal dropsy, leprosy, tetanus, gonorrhoea, and cancer |
| Aegle marmelos | Allo-imperatorin, beta-sitosterol, marmelosin, tannic acid, auraptene, marmin, umbelliferone, and lupeol | Improve appetite, griping pain in the loins, constipation, gas, colic, sprue, scurvy, wheezing, and respiratory spasms |
| Amaranthus tristis | Amarantin, isoamarantin, and betanin | Bilious disorders, appetizer, intestinal and urinary discharges, cough, bronchitis, blood purifier, dropsy, astringent, dysentery, and diuretic |
| Asclepias curassavica | Oleanolic acid, uzarigenin, calactin, calotropin, coroglaucigenin, calotropagenin, uzarin, clepogenin, asclepogenin, curassavogenin, and ascurogenin | Piles, gonorrhoea, roundworm infection, and abdominal tumours |
| Blepharis maderaspatensis | – | Wounds, headache, and diuretic |
| Costus speciosus | Pinocarveool, cadinene, cineol, p-methoxybenzophenone, carvacrol, glyceollins II and III, diosgenin, tigogenin, lanosterol, stigmasterol, gracillin, and costusoside | Stomach troubles, asthma, cold, cholera, and dysentery |
| Cynodon dactylon | Campothecin, arundoin, furfural, furfural alcohol, beta-ionone, 2-(4′-hrdroxyphenyl) propionic acid, phytol, beta-sitosterol, stigmasterol, and 4-hydroxybenzoic acids | Leprosy and skin-related diseases, wound healing, and piles |
| Merremia emerginata | Rheumatism, neuralgia, treat cough, and ear sores | |
| Ophiorrhiza mungos | Beta-sitosterol, 5α-ergost-7-en-3β-ol, 5α-ergost-8-(14)en-3β-ol, and hydrocyanic acid | Snakebite, scorpion sting, bites of mad dogs, stomach disorders, dressing ulcers, and cancer |
| Tabernaemontana heyneana | Myristic acid, palmitic acid, oleic acid, and linolenic acid | Inflammation of the cornea |
Materials and methods
Plant materials and extract preparation of extract
The medicinal plants selected for the present study were collected from various places in and around Tamil Nadu – A. curassavica, O. mungos, C. speciosus, T. heyneana, and B. maderaspatensis from Western Ghats; and C. dactylon, A. aspera, A. tristis, M. emerginata, and A. marmelos were collected from in and around Chennai and brought to the institute, washed twice with double-distilled water and allowed to shade dry for aapproximately 10 days with free aeration. The plants were authenticated by a plant taxonomist at the Department of Botany, Loyola College, Chennai. Voucher specimens were preserved in an institutional herbarium. Shade-dried plant parts were powdered using an electric blender and the powder was extracted with three types of solvents (hexane, ethyl acetate, and methanol) using cold percolation method. The powdered plant material (500 g) was soaked in 2.5 l of hexane for 48 hours with occasional shaking, filtered, and concentrated using a rotary evaporator under reduced pressure. Next, ethyl acetate (2.5 l) and methanol (2.5 l) were added sequentially and extracted as above. The crude extracts were dissolved in DMSO and used as a stock solution. Extracts were filter sterilized (0.22 µm) before the experiments.
1,1-Diphenyl-2-picrylhydrazyl free radical scavenging assay
The hydrogen-donating abilities of the extracts were examined using the method of Blois19 in the presence of 1,1-diphenyl-2-picrylhydrazyl (DPPH), a stable free radical. DPPH offers a convenient and accurate method for titrating the oxidisable groups of natural or synthetic antioxidants. The samples and the positive control, Vitamin C, were diluted in ethanol to prepare sample solutions equivalent to 400, 200, 100, 50, and 25 µg of dried extract/ml solution. The reaction mixture contained 1000 µl of 0.25 mM DPPH with various concentrations of the extracts in 1 ml of ethanol. After incubating for 20 minutes at room temperature, the absorbance at 517 nm was recorded using a Hitachi U – 2000 spectrophotometer (Hitachi, Tokyo, Japan). The inhibition percentage (%) of radical scavenging activity was calculated using the following equation
where A0 is absorbance of the control and As is absorbance of the sample at 517 nm. From the inhibition (%), the amount of the samples (μg) required to reduce the absorbance by 50% (IC50) was determined.
Nitric oxide scavenging assay
In the nitric oxide (NO) radical inhibition assay, sodium nitroprusside in aqueous solution at physiological pH, spontaneously generates NO, which interacts with oxygen to produce nitrite ions, which can be estimated by the Griess Illosvoy reaction.20 In this study, Griess Illosvoy reagent was modified using naphthylethylenediamine dihydrochloride (0.1% w/v) instead of 1-naphthylamine (5%). Scavengers of NO compete with oxygen and reduce the production of NO. The reaction mixture (3 ml) containing sodium nitroprusside (10 mM, 2 ml), phosphate-buffered saline (PBS; 0.5 ml), and extract or standard solution (0.5 ml) was incubated at 25°C for 150 minutes. After incubation, 0.5 ml of the reaction mixture containing nitrite was mixed with 1 ml of sulphanilic acid reagent (0.33% in 20% glacial acetic acid) and allowed to stand for 5 minutes to enable complete diazotisation. Then, 1 ml of naphthylethylenediamine dihydrochloride (0.1% w/v) was added. The solution was mixed and allowed to stand for 30 minutes. A pink-coloured chromophore formed in diffuse light. The absorbance of these solutions was measured at 540 nm against the corresponding blank solutions in a microtitre plate using an enzyme-linked immnunosorbent assay reader. The IC50 value is the concentration of sample required to inhibit the production of NO radical by 50%.
Cell lines and culture medium
COLO 320 DM cells (human colon cancer cell line) were purchased from the National Centre for Cell Science (NCCS, Pune) and cultured using RPMI 1640 media (Sigma, St Louis, MO, USA) supplemented with 10% fetal calf serum (Gibco-Invitrogen, Carlsbad, CA, USA), 100 U/ml penicillin (Gibco), 100 mg/ml streptomycin (Gibco), and amphotericin B (Gibco).
MCF-7 cells (human breast cancer cell line), AGS (human stomach cancer cells), A549 (human lung cancer cells) and VERO (monkey normal kidney epithelial) cells were purchased from King Institute, Chennai. The cells were cultured in Dulbecco's modified Eagle's media (Sigma) supplemented with 10% fetal calf serum (Gibco), 100 U/ml penicillin (Gibco), 100 mg/ml streptomycin (Gibco), and amphotericin B (Gibco). The cell lines used in the present study were cultured as monolayers in 25 cm2 plastic tissue culture flasks at 37°C under a humidified atmosphere of 5% CO2 in air.
Cytotoxicity studies
The antiproliferative potential of the extracts was determined by the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) assay as described by Mossman.21 Briefly, the tetrazolium salt MTT (Sigma) solution was prepared fresh as 0.5 mg/ml in PBS (Gibco) just before use. Cells were seeded onto 96-well plates (1 × 105 cells/well) and allowed to attach for 6 hours. The cells were then treated with various concentrations of extract (3.125, 6.25, 12.5, 25, 50, 100, and 200 µg/ml) for 24 hours, and 100 µl of MTT dye was added to each well. The plates were incubated in a CO2 incubator for 4 hours, and the optical density was determined by eluting the dye with DMSO and recording the absorbance at 560 nm using a 96-well plate reader.
Flow cytometric analysis
Apoptosis was determined using fluorescein isothiocyanate-conjugated (FITC)-labelled Annexin V antibody by flow cytometry.22 The fraction of the cell population in each quadrant was analysed using quadrant statistics. Cells in the lower right quadrant represented apoptotic cells, and those in the upper right quadrant represented necrotic or post-apoptotic necrotic cells.23 COLO 320 DM (1 × 107) cells were treated with 3.15, 6.25, 12.5, and 25 µg/ml of the extracts possessing cytotoxic potential for 24 hours. At the end of the treatment, cells were washed with PBS and resuspended in binding buffer (10 mM HEPES/NaOH pH 7.4, 140 mM NaCl, 2.5 mM CaCl2). Aliquots of cells (100 µl) were incubated with 5 µl of Annexin V-FITC, mixed and incubated for 15 minutes at room temperature in dark and stained with propidium iodide (PI; 5 mg/ml). The cells were then gently vortexed, and 10 000 events were acquired and analysed using a Becton Dickinson FACSCalibur (Becton Dickinson, USA). In brief, early apoptotic cells were defined as FITC-annexin-V-positive cells and necrotic cells were defined as PI-negative cells.
Statistical analysis
Antiproliferative studies were performed in six replicates, and the results were expressed as percentage growth inhibition of control. IC50 values for growth inhibitions were derived from a nonlinear regression model (curve fit) based on a sigmoidal dose response curve (variable) and computed using GraphPad Prism (La Jolla, CA, USA). Data are given as the mean ± SEM. Statistical significance was set at P < 0.05. Statistical evaluation was performed using one-way analysis of variance followed by Duncan's multiple range test (DMRT).
Results
Antioxidant activity
The solvent extracts of all the plants analysed in this study exhibited antioxidant potential in a concentration-dependent manner as evident from in vitro DPPH and NO scavenging assays. Table 2 depicts the IC50 values of selected extracts for inhibition of DPPH free radical and NO production. Among the extracts tested, A. curassavica ethyl acetate extract exhibited the highest NO scavenging activity with IC50 values of 35.57 and 62.85 µg/ml for DPPH and NO scavenging assays, respectively. In addition, the A. curassavica ethyl acetate extract, methanolic extract of C. dactylon (63.03:98.89 µg/ml), ethyl acetate extract of A. marmelos (64.01:111.22 µg/ml), and methanolic extract of O. mungos (64.01:115.69 µg/ml) exhibited strong antioxidant activity in the DPPH and NO scavenging assays compared to the other extracts. The results of the present study show that extracts of A. curassavica, O. mungos, B. maderaspatensis, C. dactylon, A. tristis, and A. marmelos exhibited strong DPPH and NO scavenging abilities.
Table 2.
DPPH and NO scavenging property of selected medicinal plants
| IC50 (μg/ml) DPPH | IC50 (μg/ml) NO | |
|---|---|---|
| A. aspera | ||
| Hexane | 953.16 ± 72.58 | ND |
| Ethyl acetate | 613.10 ± 46.69 | 892.69 ± 67.97 |
| Methanol | 753.13 ± 57.35 | ND |
| A. marmelos | ||
| Hexane | 124.02 ± 9.44 | 213.64 ± 16.27 |
| Ethyl acetate | 64.01 ± 4.87 | 111.22 ± 8.47 |
| Methanol | 83.01 ± 6.32 | 123.45 ± 9.40 |
| A. tristis | ||
| Hexane | 142.02 ± 10.81 | 284.17 ± 21.64 |
| Ethyl acetate | 93.02 ± 7.08 | 128.32 ± 9.77 |
| Methanol | 532.09 ± 40.52 | 736.10 ± 56.05 |
| A. curassavica | ||
| Hexane | 1003.14 ± 76.39 | ND |
| Ethyl acetate | 35.57 ± 2.71 | 62.87 ± 4.79 |
| Methanol | 625.22 ± 47.61 | ND |
| B. maderaspatensis | ||
| Hexane | 987.16 ± 75.17 | ND |
| Ethyl acetate | 372.06 ± 28.33 | 428.27 ± 32.61 |
| Methanol | 127.02 ± 9.67 | 175.06 ± 13.37 |
| C. speciosus leaves | ||
| Hexane | 745.48 ± 56.77 | 983.86 ± 74.92 |
| Ethyl acetate | 625.22 ± 47.61 | 843.64 ± 64.24 |
| Methanol | 897.73 ± 68.36 | ND |
| C. speciosus roots | ||
| Hexane | 845.28 ± 64.55 | ND |
| Ethyl acetate | 320.05 ± 24.37 | 550.28 ± 42.12 |
| Methanol | 234.04 ± 17.82 | 387.06 ± 29.47 |
| C. dactylon | ||
| Hexane | 874.15 ± 66.56 | ND |
| Ethyl acetate | 311.05 ± 23.69 | 450.08 ± 34.27 |
| Methanol | 63.03 ± 4.82 | 98.89 ± 7.53 |
| M. emerginata | ||
| Hexane | 150.39 ± 11.45 | 206.13 ± 15.70 |
| Ethyl acetate | 357.06 ± 27.19 | 512.77 ± 39.05 |
| Methanol | 514.09 ± 39.15 | 756.13 ± 57.58 |
| O. mungos | ||
| Hexane | 325.05 ± 24.75 | 563.09 ± 42.88 |
| Ethyl acetate | 96.02 ± 7.31 | 155.43 ± 11.84 |
| Methanol | 64.01 ± 4.87 | 115.69 ± 8.81 |
| T. heyneana | ||
| Hexane | 254.04 ± 19.34 | 400.07 ± 30.46 |
| Ethyl acetate | 340.17 ± 26.04 | 612.20 ± 46.62 |
| Methanol | 220.07 ± 16.75 | 361.06 ± 27.49 |
| Vitamin C | 5 ± 0.38 | 25.00 ± 1.90 |
ND, not detectable.
Each value represents the mean ± SD for six replications in each group.
Antiproliferative effect
The extracts were tested in vitro for their ability to inhibit the growth of the human tumour cell lines AGS, A549, MCF-7, and COLO 320 DM and the normal cell line VERO. The MTT assay, a non-radioactive, fast, and economical assay that is widely used to quantify cell viability and proliferation was used to determine IC50 values for the extracts of the 10 selected plants for three different time intervals. The preliminary screening cytotoxicity results of the extracts at various time intervals are summarized in Fig. 1 and in Tables 3Table 4.5. Of the plants screened, seven had IC50 values of less than 50 µg/ml towards cancer cells with no or minimal toxicity towards normal cell line after 72 hours treatment. Among the 33 extracts studied, the ethyl acetate extract of A. curassavica and A. tristis; ethyl acetate and methanolic extract of C. speciosus root, C. dactylon and T. heyneana; hexane extract of M. emarginata, and methanolic extract of O. mungos exhibited significant (P < 0.05) cytotoxic effects in the tested cell lines.
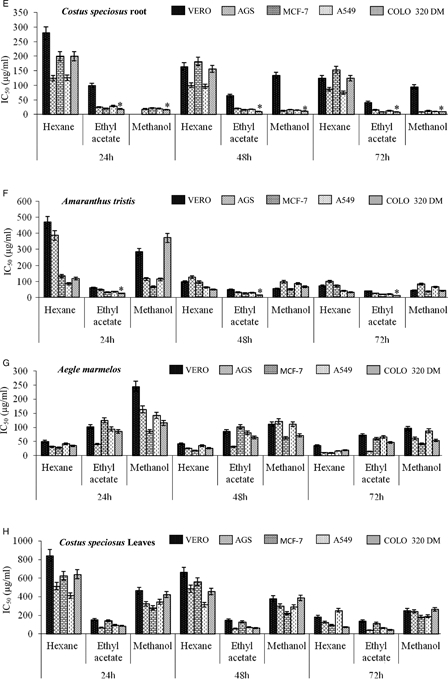
Figure 1.
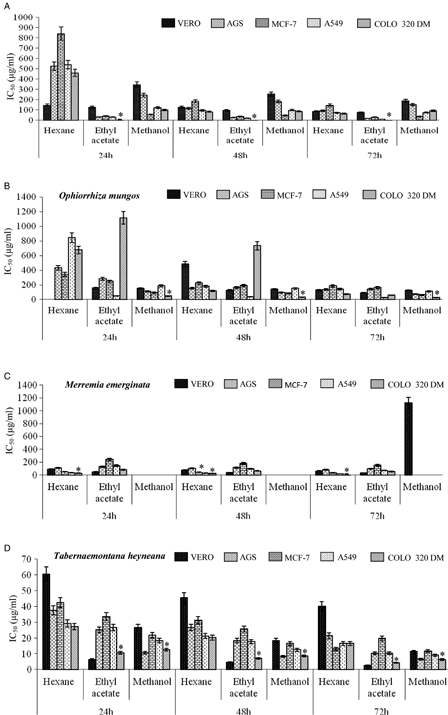
Cytotoxic effects of the extracts of medicinal plants on four different cancer cell lines and a normal cell line at various time intervals. (A) A. curassavica; (B) O. mungos; (C) M. emerginata; (D) T. heyneana; (E) C. speciosus root; (F) A. tristis; (G) A. marmelos; (H) C. speciosus leaves. *P < 0.05 compared to other cell lines.
Table 3.
Cytotoxic effects of the extracts of C. dactylon root on four different cancer cell lines and a normal cell line at various time intervals
| IC50 (μg/ml) | |||||
|---|---|---|---|---|---|
| VERO | AGS | MCF-7 | A 549 | COLO 320 DM | |
| C. dactylon | |||||
| 24 hours | |||||
| Hexane | 99 ± 7a | 45 ± 3d | 52 ± 3c | 60 ± 4b | 50 ± 3cd |
| Ethyl acetate | 25 ± 1b | 23 ± 1c | 28 ± 2a | 25 ± 1b | 22 ± 1c |
| Methanol | NT | 21 ± 1b | 37 ± 2a | 15 ± 1c | 9 ± 0bd |
| 48 hours | |||||
| Hexane | 83 ± 6a | 31 ± 2d | 49 ± 3b | 52 ± 4b | 43 ± 3c |
| Ethyl acetate | 20 ± 1a | 15 ± 1b | 12 ± 0c | 16 ± 1b | 10 ± 0d |
| Methanol | ND | 11 ± 0b | 20 ± 1a | 8 ± 0c | 6 ± 0d |
| 72 hours | |||||
| Hexane | 54 ± 4a | 28 ± 2d | 36 ± 2c | 43 ± 3b | 36 ± 2c |
| Ethyl acetate | 16 ± 1a | 10 ± 0b | 9 ± 0c | 11 ± 0b | 8 ± 0c |
| Methanol | 684 ± 52a | 8 ± 0b | 15 ± 1b | 6 ± 0b | 4 ± 0b |
ND, not detectable.
Each value represents the mean ± SD for six replicates in each group.
Different letters in a row indicate P < 0.05 by DMRT.
Table 4.
Cytotoxic effects of the extracts of A. aspera on four different cancer cell lines and a normal cell line at various time intervals
| IC50 (μg/ml) | |||||
|---|---|---|---|---|---|
| VERO | AGS | MCF-7 | A 549 | COLO 320 DM | |
| A. aspera | |||||
| 24 hours | |||||
| Hexane | 221 ± 16b | 142 ± 10d | 98 ± 7e | 182 ± 13c | 241 ± 18a |
| Ethyl acetate | 741 ± 56a | 346 ± 26d | 421 ± 32c | 515 ± 39b | 441 ± 33c |
| Methanol | 682 ± 51a | 224 ± 17c | 315 ± 24b | 285 ± 21b | 321 ± 24b |
| 48 hours | |||||
| Hexane | 185 ± 14b | 115 ± 8d | 82 ± 6e | 152 ± 11c | 212 ± 16a |
| Ethyl acetate | 687 ± 52a | 291 ± 22e | 342 ± 26d | 468 ± 35b | 386 ± 29c |
| Methanol | 412 ± 31a | 196 ± 14c | 286 ± 21b | 206 ± 15c | 294 ± 22b |
| 72 hours | |||||
| Hexane | 156 ± 11b | 84 ± 6d | 63 ± 4e | 131 ± 10c | 189 ± 14a |
| Ethyl acetate | 212 ± 16d | 246 ± 18c | 254 ± 19b | 183 ± 14 e | 266 ± 20 a |
| Methanol | 394 ± 30a | 152 ± 11c | 192 ± 14d | 143 ± 10 c | 235 ± 17 b |
Each value represents the mean ± SD for six replicates in each group.
Different letters in a row indicate P < 0.05 by DMRT.
Table 5.
Cytotoxic effects of the extracts of B. maderaspatensis on four different cancer cell lines and a normal cell line at various time intervals
| IC50 (μg/ml) | |||||
|---|---|---|---|---|---|
| VERO | AGS | MCF-7 | A 549 | COLO 320 DM | |
| B. maderaspatensis | |||||
| 24 hours | |||||
| Hexane | 325 ± 24a | ND | ND | ND | ND |
| Ethyl acetate | 150 ± 11c | 95 ± 7d | 145 ± 11c | 239 ± 18b | 624 ± 47a |
| Methanol | 89 ± 6d | 354 ± 27a | 245 ± 18b | 185 ± 14c | 106 ± 8d |
| 48 hours | |||||
| Hexane | 265 ± 20a | ND | ND | ND | ND |
| Ethyl acetate | 143 ± 10b | 86 ± 6d | 94 ± 7d | 184 ± 14a | 112 ± 8bc |
| Methanol | 72 ± 5c | 261 ± 19a | 135 ± 10b | 126 ± 9b | 79 ± 6c |
| 72 hours | |||||
| Hexane | 182 ± 13a | ND | ND | ND | 3334 ± 253b |
| Ethyl acetate | 86 ± 6c | 72 ± 5b | 80 ± 6bc | 153 ± 11a | 45 ± 3d |
| Methanol | 66 ± 5d | 221 ± 16a | 116 ± 8b | 91 ± 6c | 55 ± 4d |
ND, not detectable.
Each value represents the mean ± SD for six replicates in each group.
Different letters in a row indicate P < 0.05 by DMRT.
The ethyl acetate extract of A. curassavica exhibited antiproliferative activity towards the tested cancer cell lines with minimal toxicity towards the normal cells in a time- and dose-dependent manner (Fig. 1A). Significantly higher effects were seen in COLO 320 DM and A549 cell lines with IC50 < 50 µg/ml. The extracts of O. mungos exhibited antiproliferative effects towards all the tested cell lines in a time- and concentration-dependant manner. The methanolic extract of O. mungos exhibited cytotoxic effects with significantly low IC50 values (26.37 µg/ml) towards COLO 320 DM cells compared to the other cancer and normal cell lines. The hexane and ethyl acetate extracts of O. mungos exhibited toxicity towards all the tested cell lines with very high IC50 values for 72 hours incubation (Fig. 1B).
The M. emarginata hexane extract inhibited the proliferation of all the tested cell lines. In particular, this extract significantly inhibited proliferation of the COLO 320 DM and A549 cells in a time- and concentration-dependent manner with an IC50 of 15.54 and 18.43 µg/ml, respectively, for 72 hours treatment. The IC50 for VERO cells was 65.15 µg/ml when treated with the M. emarginata hexane extract (Fig. 1C).
The T. heyneana organic solvent extract also inhibited the proliferation of the tested cell lines (Fig. 1D). All the extracts of T. heyneana were cytotoxic towards the cancer cells with low IC50 values of <20 µg/ml. The plant was also cytotoxic towards VERO cells with IC50 values of 39.93, 2.56 and 11.24 µg/ml for hexane, ethyl acetate, and methanol extracts, respectively.
The C. dactylon extracts exhibited antiproliferative activity towards the selected cell lines (AGS, MCF-7, A549, COLO 320 DM, and VERO) with an IC50 < 50 µg/ml for 72 hours incubation. The methanolic extract of C. dactylon exhibited low IC50 values of 8.54, 15.24, 6.24, and 4.76 µg/ml towards the AGS, A549, MCF-7, and COLO 320 DM cells, respectively, with an IC50 of approximately 684 µg/ml in VERO cells for 72 hours incubation (Table 3).
The ethyl acetate and methanol extracts of C. speciosus root exhibited time- and concentration-dependant cytotoxic effects towards the cancer cell lines tested. The IC50 of the ethyl acetate and methanol extracts of C. speciosus were 41.88 and 94.78 µg/ml in VERO cells, respectively. The methanolic extract of C. speciosus exhibited significant (P < 0.05) cytotoxic effects towards all the cancer cell lines (AGS, A549, MCF-7, and COLO 320-DM) when compared to the normal VERO cells. The ethyl acetate extract of C. speciosus significantly (P < 0.05) reduced the viability of COLO 320 DM and MCF-7 cells in a time- and concentration-dependant manner (Fig. 1E).
The A. tristis ethyl acetate extract exhibited cytotoxic activity in all the tested cell lines with an IC50 of 10.32, 19.21, 21.18, and 26.16 µg/ml for COLO 320 DM, MCF-7, A549, and AGS cell lines, respectively, for the 72 hours incubation period. The extract possessed significant toxicity towards the cancer cell lines compared to the normal VERO cell line with an IC50 of 39.44 µg/ml for 72 hours incubation (Fig. 1F).
The hexane extract of A. marmelos exhibited significant antiproliferative activity towards the four different cancer cell lines (AGS, A549, MCF-7, and COLO 320 DM) compared to the normal VERO cells with an IC50 of 35.17 µg/ml. The ethyl acetate extract of A. marmelos exhibited significant toxicity towards AGS and COLO 320 DM cells compared to the VERO cells with an IC50 of 14.65 and 46.44 µg/ml, respectively, after 72 hours of treatment (Fig. 1G).
Fig. 1H depicts the inhibition of proliferation by the organic extracts of C. speciosus leaves towards the tested cell lines. The extracts of C. speciosus inhibited the proliferation of cell lines at very high doses with higher IC50 values than the other extracts towards all the tested cell lines. The extracts of A. aspera exhibited antiproliferative activity towards all the tested cell lines. The hexane extract of A. aspera had a significantly low IC50 towards AGS and MCF-7 cells (84.27 and 63.43 µg/ml, respectively) (Table 4). The B. maderaspatensis hexane extract was not cytotoxic towards the tested cell lines upto to the maximum tested concentration of 200 µg/ml (Table 5).
Among the 33 crude extracts studied, the ethyl acetate extract of A. curassavica and A. tristis, ethyl acetate and methanolic extract of C. speciosus root, C. dactylon, and T. heyneana, hexane extract of M. emarginata, and methanolic crude of O. mungos exhibited significant cytotoxic effects on the tested cell lines. Different extracts possessed different toxicity profiles in the tested cell lines. Most of the extracts were active in colon cancer cells; hence, subsequent experiments were carried in the COLO 320 DM cells and VERO cells for the 24 hours incubation period to determine the active components.
Flow cytometric analysis
Table 6 depicts the flow cytometric analysis of the crude extracts from the cytotoxic plants at four different concentrations after 24 hours of treatment. The extracts possessing cytotoxic potential towards the cancer cell lines with minimal or no toxicity for normal VERO cells were selected to determine the mechanism of cell death. Each extract was studied at four different concentrations (25, 12.5, 6.25, and 3.15 µg/ml) for a treatment period of 24 hours. Among the 33 extracts, only 9 were found to be active against cancer cell lines. A pronounced effect was observed in COLO 320 DM cells. Hence, further studies to determine the mechanism of cell death in cancer cells were restricted to COLO 320 DM cells.
Table 6.
Percentage of apoptosis induction of selected plant extracts in COLO 320 DM cells measured by flow cytometry using Annexin V FITC/PI
| Extract | Concentration (μg/ml) | Viable cells | Necrotic cells | Late apoptotic | Early apoptotic | |
|---|---|---|---|---|---|---|
| Control | 100 | – | – | – | ||
| A. tristis | Ethyl acetate | 25 | 67.10 | 23.15 | 8.43 | 1.32 |
| 12.5 | 74.65 | 17.72 | 6.97 | 0.66 | ||
| 6.25 | 89.13 | 6.13 | 4.21 | 0.53 | ||
| 3.15 | 97.22 | 0.66 | 1.87 | 0.25 | ||
| A. curassavica | Ethyl acetate | 25 | 53.63 | 13.48 | 21.46 | 11.43 |
| 12.5 | 59.48 | 14.78 | 16.53 | 9.21 | ||
| 6.25 | 66.27 | 11.53 | 13.94 | 8.26 | ||
| 3.15 | 74.27 | 7.86 | 10.56 | 7.31 | ||
| C. speciosus root | Ethyl acetate | 25 | 60.24 | 20.79 | 14.65 | 4.32 |
| 12.5 | 68.46 | 17.38 | 11.53 | 2.63 | ||
| 6.25 | 73.68 | 18.32 | 6.84 | 1.16 | ||
| 3.15 | 80.86 | 19.14 | 5.90 | 0.95 | ||
| Methanol | 25 | 59.68 | 13.26 | 17.43 | 9.63 | |
| 12.5 | 70.20 | 7.39 | 14.87 | 7.54 | ||
| 6.25 | 81.13 | 1.75 | 11.76 | 5.36 | ||
| 3.15 | 89.57 | 0.36 | 7.95 | 2.12 | ||
| C. dactylon | Ethyl acetate | 25 | 64.58 | 14.72 | 15.94 | 4.76 |
| 12.5 | 77.33 | 7.78 | 11.43 | 3.46 | ||
| 6.25 | 84.05 | 5.33 | 8.64 | 1.98 | ||
| 3.15 | 91.55 | 3.18 | 4.59 | 0.68 | ||
| Methanol | 25 | 76.66 | 1.07 | 15.43 | 6.84 | |
| 12.5 | 81.95 | – | 13.57 | 4.48 | ||
| 6.25 | 88.25 | – | 9.21 | 2.54 | ||
| 3.15 | 95.79 | – | 3.15 | 1.06 | ||
| M. emerginata | Hexane | 25 | 69.26 | 21.16 | 7.23 | 2.35 |
| 12.5 | 76.92 | 18.53 | 4.42 | 0.13 | ||
| 6.25 | 84.69 | 12.48 | 2.83 | – | ||
| 3.15 | 93.58 | 5.25 | 1.17 | – | ||
| O. mungos | Methanol | 25 | 56.24 | 23.98 | 17.04 | 2.74 |
| 12.5 | 70.68 | 13.74 | 13.53 | 2.05 | ||
| 6.25 | 82.36 | 7.12 | 9.28 | 1.24 | ||
| 3.15 | 87.47 | 5.34 | 6.32 | 0.87 | ||
| T. heyneana | Ethyl acetate | 25 | 57.61 | 39.96 | 2.31 | 0.12 |
| 12.5 | 67.71 | 30.29 | – | – | ||
| 6.25 | 77.13 | 22.87 | – | – | ||
| 3.15 | 89.55 | 10.45 | – | – |
Values represent the percentage of cells.
–, No cells present.
Among the tested plants, almost all the extracts were able to induce apoptosis in a time- and concentration-dependant manner. Incubation of COLO 320 DM cells with 25 µg/ml of the A. curassavica crude ethyl acetate extract induced apoptosis (early apoptotic, 11.43% and late apoptotic, 21.46%) with minimal necrotsis (13.48%)
The methanolic extract of C. speciosus root (25 µg/ml) also exhibited a similar pattern of apoptotic cell death with 9.63% cells in early apoptotsis and 17.43% cells in late apoptosis; 13.26% of cells were necrotic, and the remaining were viable. All the other tested cytotoxic plant extracts induced apoptotic cell death in COLO 320 DM cells with an increase in the percentage of necrotic cells and decrease in apoptotic cell percentage. The methanolic and ethyl acetate extracts of C. dactylon exhibited apoptotic cell death in a dose-dependent manner with 6.84% and 4.76% of cells in early apoptosis, respectively at 25 µg/ml. The C. speciosus ethyl acetate and O. mungos methanol extracts (25 µg/ml) induced apoptosis in COLO 320 DM cells with 4.32 and 2.74% cells in early apoptosis.
The ethyl acetate extract of A. tristis (25 µg/ml) induced apoptotic cell death in COLO 320 DM cells in a concentration-dependent manner with 8.43% of cells exhibiting annexin FITC+/PI+ and 1.32% of cells with Annexin FITC+/PI−. M. emarginata and T. heyneana were found to induce cell death via necrosis; most of the cells were stained with PI alone, indicating dead cells.
Discussion
A number of commercially proven anticancer drugs used in modern medicine were initially used in crude form in traditional or folk healing practices or for other purposes that suggested potentially useful biological activity. It is estimated that approximately 75% of the 120 biologically active plant-derived compounds presently in use worldwide have been derived through follow-up studies to verify the authenticity of data from folk and ethnomedical uses, and there is great potential for new drug discoveries based on traditional plant uses.24 Natural antioxidants have a wide range of biochemical activities, including inhibiting ROS generation, direct or indirect scavenging of free radicals, and alterating the intracellular redox potential.25 Antioxidants have been used to inhibit apoptosis because apoptosis was initially thought to be mediated by oxidative stress.26 Anticancer agents have been isolated from many plants such as Andrographis paniculata, Phyllanthus amarus, Piper longum, Semecarpus anacardium, Withanica somnifera, Moringa oleifera, Aloe vera, Curcuma longa, Allium sativum, and Tinospora cordifolia.
Traditional medicines are commonly made by boiling the plant material in water or by soaking in alcohol. The plants selected for this study were mostly used as alcoholic extracts; hence, the plants were sequentially extracted with three different types of solvents. Preliminary phytochemical screening of the extracts revealed the presence of flavonoids, alkaloids, phenol, and acid (data not shown). The phytochemicals present in the crude extract may be responsible for the antioxidative and antiproliferative activity of the plant. Flavonoids and alkaloids have been shown to induce apoptosis both in vivo and in vitro and to promote antioxidative properties. Flavonoids have potent antiproliferative, antineoplastic, and antioxidant activities, and it has been suggested that they prevent chronic diseases such as cancer.27
The antioxidant activities of the selected medicinal plants were studied by monitoring the ability of the plant extracts to scavenge free radicals generated in vitro using two different methods (DPPH and NO scavenging assay). NO, a free radical produced in mammalian cells, regulates various physiological processes, and excess production of NO is associated with cancer.28 The inactivation of free radicals is an effective method of controlling oxidative damage. Therefore, determining the radical scavenging activity is a means to evaluate the antioxidant potential of a plant. The DPPH radical, which bears a deep purple (violet) colour, is one of the few organic nitrogen radicals. When the DPPH radical reacts with suitable reducing agents, the electrons off, and the solution loses its colour in a stoichiometric manner, depending on the number of electrons taken up.29 NO reacts with oxygen to produce stable products nitrate and nitrite through intermediates NO2, N2O4, and N3O4 estimated by Griess reagent. In the presence of a radical scavenger, the amount of nitrous acid will decrease.
The ethyl acetate extract of A. curassavica, methanolic extract of C. dactylon, ethyl acetate and methanol extracts of A. marmelos, ethyl acetate and methanol extracts of O. mungos, methanol extract of B. maderaspatensis, and hexane extract of M. emarginata exhibited strong antioxidant activities as demonstrated by the ability to scavenge DPPH and NO radicals generated in vitro. The DPPH antiradical activity of the plants is due to the ability of the extracts to donate hydrogen atom to the free radical (DPPH) and convert the free radical to an inactive nonradical. Similar effects were observed in the NO scavenging assay, where the production of nitrite by the incubation of sodium nitroprusside in standard PBS at 25 °C was reduced by the extracts of the selected plants due to competition between the extract and oxygen to interact with NO.
A large number of medicinal plants and their purified constituents have shown beneficial therapeutic potentials. Various plants have been reported to exhibit antioxidant activity, including Ocimum sanctum, Piper cubeba, A. sativum, Terminalia bellerica, Camellia sinensis, Zingiber officinale, and several Indian and Chinese traditional medicinal plants. The majority of the antioxidant activity is due to the flavones, isoflavones, flavonoids, anthocyanin, coumarin, lignans, catechins, and isocatechins.30 Many plant extracts have been reported to induce apoptosis in cell lines due to their ability to scavenge NO radicals in cell-free systems.31
Cytotoxicity screening models provide important preliminary data to select plant extracts with potential antineoplastic properties for future work.32 The ethyl acetate extract of A. curassavica and A. tristis, ethyl acetate and methanolic extract of C. speciosus root and C. dactylon, hexane extract of M. emarginata, and methanolic extract of O. mungos and T. heyneana exhibited significant cancer cell-specific cytotoxic effects in the tested cell lines in a time- and dose-dependent manner with minimal toxicity towards VERO cells. The cytotoxic activities might be due to the antiproliferative potential of the extracts to exhibit cancer cell-specific cell death. If an extract possesses significant toxicity towards cancer cells with no or minimal toxicity towards normal cells, the extract may prove useful for finding new treatments for cancer.33,34
The ethyl acetate extract of A. curassavica, methanolic and ethyl acetate extracts of C. speciosus root and C. dactylon, methanolic extract of O. mungos, hexane extract of M. emerginata, and ethyl acetate extract of A. tristis and T. heyneana induced apoptotic cell death of COLO 320 DM cells in a concentration-dependant manner due to the synergistic activity of the phytochemicals present in the extracts. Recent studies have demonstrated that flavonoides, alkaloids, and sterols in extract cause apoptotic cell death in vitro.
Considerable attention has focused on the role of apoptosis or programmed cell death in the pathogenesis and treatment of human cancer.35 Indeed, a variety of cytotoxic drugs have been reported to induce apoptosis in malignant cells in vitro.36 The antioxidant and anticancer potential of the total methanol extract of Betula platyphylla was due to its DPPH radical scavenging activity.35 The aqueous and methanolic extracts of Wasabia japonica root exhibited anticancer potential by inducing apoptosis in RAW264.7 cells due to its ability to scavenge the NO radicals in cell-free systems.31 We have identified the bioactive components from A. curassavica and O. mungos as beta-sitosterol and luteolin-7-glucoside, respectively, with potential chemopreventive activity in 1,2-dimethylhydrazine-induced colon carcinogenesis in rats.37,38 The present study emphasises the importance of the selected plants as potential sources for anticancer drug discovery.
The results of the present study support the claim by traditional healers that the ethyl acetate extract of A. curassavica, methanolic and ethyl acetate extracts of C. speciosus root and C. dactylon, methanolic extract of O. mungos, hexane extract of M. emerginata, and ethyl acetate extracts of A. tristis and T. heyneana have anticancer activities, which has been partially validated by identifying the extracts in the plants and their potent proapoptotic activity. The mechanism of apoptosis is not fully understood, but one could speculate that these extracts may prevent proliferation of cancer cells by suppressing proliferative markers. Hence, some of the compounds in these extracts, if structurally identified and characterized, may be a potent candidates for anticancer drug development.
Acknowledgements
All authors contributed to the study and/or manuscript. All authors read and approved the findings of the study. Support from the National Nutrition Policy Chair, King Saud University, Riyadh, Saudi Arabia, is gratefully acknowledged.
References
- 1.Halliwell B, Gutteridge JMC, Cross CE. Free radicals, antioxidants and human disease: where are we now. J Lab Clin Med. 1992;119:598–620. [PubMed] [Google Scholar]
- 2.Veerapur VP, Prabhakar KR, Vipankumar P, Kandadi MR, Ramakrishana S, Mishra B, et al.. Ficus racemosa stem bark extract: a potent antioxidant and a probable natural radioprotector. eCAM. 2009;6:317–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Cancer Society Global cancer facts & figures. 2nd edn, American Cancer Society, Atlanta, 2011. Available from: http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-027766.pdf. [Google Scholar]
- 4.Lopaczyski W, Zeisel SH. Antioxidant, programmed cell death, and cancer. Nutr Res. 2001;21:295–307. [Google Scholar]
- 5.Mates JM, Sanchez-Jimenez FM. Role of reactive oxygen species in apoptosis: implications for cancer therapy. Int J Biochem Cell Biol. 2000;32:157–70. [DOI] [PubMed] [Google Scholar]
- 6.Ravi RG, Harikesh D, Chandrasekhar TR, Pramod YG, Angad PM. Cytotoxic activity of ethanolic root extract of Calotropis gigantea Linn. Int J Drug Dev Res 2011;3:101–8. [Google Scholar]
- 7.Robinson MM, Zhang X. The world medicines situation 2011. Traditional medicines: global situation, issues and challenges. WHO/EMP/MIE/2011.2.3. Available from: http://apps.who.int/medicinedocs/documents/s18063en/s18063en.pdf.
- 8.Baskar AA, Ignacimuthu S. Chemopreventive effectof Cynodon dactylon (L.) Pers.extract against DMH-induced colon carcinogenesis in experimental animals. Exp Pathol 2010;62:423–31. [DOI] [PubMed] [Google Scholar]
- 9.Tan ML, Tengku Muhammad TS, Najimudin N, Sulaiman SF. Growth arrest and non-apoptotic programmed cell death associated with the up-regulation of c-myc mRNA expression in T-47D breast tumor cells following exposure to Epipremnum pinnatum (L.) Engl. hexane extract. J Ethnopharmacol 2005;96:375–83. [DOI] [PubMed] [Google Scholar]
- 10.Hazalin NAMN, Kalavathy R, Lim SM, Anthony LJC, Majeed ABA. Induction of apoptosis against cancer cell lines by four ascomycetes (endophytes) from Malaysian rainforest. Phytomedicine 2012;19:609–617. [DOI] [PubMed] [Google Scholar]
- 11.Abdol MMH, Fouladdel S, Shafiee A, Amin G, Ghaffari SM, Azizi E. Antiproliferative and apoptotic effect of Astrodaucus orientalis (L.) drude on T47D human breast cancer cell line: potential mechanisms of action. Afr J Biotechnol 2009;8:4265–76. [Google Scholar]
- 12.Nadkarni KM. Indian materia medica. Bombay, India:Popular Prakashan, 1976. [Google Scholar]
- 13.Rastogi RP, Mehrotra BN. Compendium of Indian medicinal plants. Vol. II Lucknow: Central Drug Research Institute,; New Delhi: National Institute of Science Communication, 1991, p. 1–833. [Google Scholar]
- 14.Rastogi RP, Mehrotra BN. Compendium of Indian medicinal plants. Vol. III Lucknow: Central Drug Research Institute; New Delhi: National Institute of Science Communication, 1993, p. 1–831. [Google Scholar]
- 15.Rastogi RP, Mehrotra BN. Compendium of Indian medicinal plants. Vol. IV Lucknow: Central Drug Research Institute; New Delhi: National Institute of Science Communication, 1995, p. 1–930. [Google Scholar]
- 16.Rastogi RP, Mehrotra BN. Compendium of Indian medicinal plants. Vol. V Lucknow: Central Drug Research Institute; New Delhi: National Institute of Science Communication, 1998a, p. 1–831. [Google Scholar]
- 17.Rastogi RP, Mehrotra BN. Compendium of Indian medicinal plants. Vol. VILucknow: Central Drug Research Institute,; New Delhi: National Institute of Science Communication, 1998b, p. 1–177. [Google Scholar]
- 18.Rastogi RP, Mehrotra BN. Compendium of Indian medicinal plants. Vol. I Lucknow: Central Drug Research Institute,; New Delhi: National Institute of Science Communication, 1990, p. 1–497. [Google Scholar]
- 19.Blois MS. Antioxidant determinations by the use of a stable free radical. Nature 1958;181:1199–200. [Google Scholar]
- 20.Hazra B, Biswas S, Mandal N. Antioxidant and free radical scavenging activity of Spondias pinnata. BMC Complement Altern Med 2008;8:63, doi: 10.1186/1472-6882-8-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mossman T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 1983;65:55–63. [DOI] [PubMed] [Google Scholar]
- 22.Gao LL, Li F, Jiao P, Yang MF, Zhou XJ, Si YH, et al.. Paris chinensis dioscin induces G2/M cell cycle arrest and apoptosis in human gastric cancer SGC-7901 cells. World J Gastroenterol 2011;17:4389–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhushan S, Singh J, Rao MJ, Saxena AK, Qazi GN. A novel lignan composition from Cedrus deodara induces apoptosis and early nitric oxide generation in human leukemia Molt-4 and HL-60 cells. Nitric Oxide 2006;14:72–88. [DOI] [PubMed] [Google Scholar]
- 24.Pushpangadan P, Kumar BM. Ethnobotany, CBD, WTO and the Biodiversity Act of India. Ethnobotany 2005;17:2–12. [Google Scholar]
- 25.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature 2000;408:239–47. [DOI] [PubMed] [Google Scholar]
- 26.Mohan M, Taneja TK, Sahdev S, Mohareer K, Begum R, Athar M, et al.. Antioxidants prevent UV-induced apoptosis by inhibiting mitochondrial cytochrome c release and caspase activation in Spodoptera frugiperda (Sf9) cells. Cell Biol Int 2003;27:483–90. [DOI] [PubMed] [Google Scholar]
- 27.Susantia D, Sirata HM, Farediah A, Rasadah MA, Norio A, Mariko K. Antioxidant and cytotoxic flavonoids from the flowers of Melastoma malabathricum L. Food Chem 2007;103:710–6. [Google Scholar]
- 28.Sreevidya N, Raghavan G, Madhavan V, Shanta M. Free radical scavenging potential of Chlorophytum tuberosum baker. J Ethnopharmacol 2006;104:423–5. [DOI] [PubMed] [Google Scholar]
- 29.Bondent V, Brand-Williams W, Bereset C. Kinetics and mechanism of antioxidant activity using the DPPH free radical methods. Lebenson Wiss Technol 1997;30:609–15. [Google Scholar]
- 30.Aqil F, Ahmed I, Mehmood Z. Antioxidant and free radical scavenging properties of twelve traditionally used Indian medicinal plants. Turk J Biol 2006;30:177–83. [Google Scholar]
- 31.Lee YS, Yang JH, Bae MJ, Yoo WK, Ye S, Xue CC, et al.. Anti-oxidant and anti-hypercholesterolemic activities of Wasabia japonica. Evid Based Complement Alternat Med 2010;7:459–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cardellina JH, Fuller RW, Gamble WR, Westergaard C, Boswell J, Munro MHG, et al.. Evolving strategies for the selection, dereplication and prioritization of antitumor and HIV inhibitory natural products extracts. In: , Bohlin L, Bruhn JG (eds.) Bioassay methods in natural product research and development. Kluwer Academic Publishers, Dordrecht; 1999. p. 25–36. [Google Scholar]
- 33.Lin HH, Chen JH, Kuo WH, Wang CJ. Chemopreventive properties of Hibiscus sabdariffa L. on human gastric carcinoma cells through apoptosis induction and JNK/p38 MAPK signaling activation. Chem Biol Interact 2007;165:59–75. [DOI] [PubMed] [Google Scholar]
- 34.Srivastava JK, Gupta S. Antiproliferative and apoptotic effects of chamomile extract in various human cancer cells. J Agric Food Chem 2007;55:9470–8. [DOI] [PubMed] [Google Scholar]
- 35.Ju EM, Lee SE, Hwang HJ, Kim JH. Antioxidant and anticancer activity of extract from Betula platyphylla var. japonica. Life Sci 2004;74:1013–26. [DOI] [PubMed] [Google Scholar]
- 36.Vladislav VG, Galina K, Olga VG, Valeri VM, Thomas PM, James RT, et al.. Synthetic galectin-3 inhibitor increases metastatic cancer cell sensitivity to taxol-induced apoptosis In vitro and In vivo. Neoplasia 2009;11:901–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baskar AA, Ignacimuthu S, Paulraj GM, Al Numair KS. Chemopreventive potential of beta-sitosterol in experimental colon cancer model – an in vitro and in vivo study. BMC Complement Altern Med 2010;10:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baskar AA, Ignacimuthu S, Paulraj GM, Al Numair KS. Cancer chemopreventive potential of luteolin-7-O-glucoside isolated from Ophiorrhiza mungos Linn. Nutr Cancer 2011;63:130–8. [DOI] [PubMed] [Google Scholar]


