Abstract
Objectives
Some studies have indicated the pathophysiological importance of reactive oxygen species (ROS) in patients with nephrotic syndrome. Myeloperoxidase (MPO) is a leukocyte-derived enzyme-generating ROS that has been proposed to exert a wide array of pro-atherogenic effects throughout all stages of the atherosclerotic process. The aim of this study was to investigate the serum malondialdehyde (MDA) levels, MPO and catalase activities in patients with adult nephrotic syndrome.
Patients and Methods
Twenty-four patients with nephrotic syndrome and 24 healthy controls were enrolled. Serum MPO activity, catalase activity, and MDA levels were assessed.
Results
Serum MPO activity and MDA levels were significantly higher in patients with nephrotic syndrome than controls (both, P < 0.001), while catalase activity was significantly lower (P < 0.001). Serum catalase activity was found to be significantly correlated with MPO activity (r = −0.417, P = 0.003) and MDA levels (r = −0.532, P = 0.007). The serum MDA levels were also found to be significantly correlated with MPO activity (r = 0.419, P = 0.003).
Conclusions
We concluded that serum MPO activity and oxidative stress were increased and that serum catalase activity was decreased in patients with adult nephrotic syndrome. In addition, these results indicate that increased MPO activity is associated with an oxidant–antioxidant imbalance that may contribute to atherosclerosis in patients with adult nephrotic syndrome.
Keywords: Myeloperoxidase activity, Catalase activity, Oxidative stress, Atherosclerosis, Nephrotic syndrome
Introduction
Nephrotic syndrome (NS) is characterized by massive proteinuria, hypoalbuminemia, edema, and hyperlipidemia.1 The molecular mechanisms behind acquired NS remain largely unknown. Many factors may induce proteinuria under experimental conditions.2 It has been reported that NS is a consequence of an oxidant/antioxidant status imbalance. Furthermore, it has been suggested that glomerular capillary wall permeability is possibly influenced by the generation of free radicals, which are strong oxidants.3 NS has also been associated with increased oxidative stress in several studies.4–6 The excessive generation of reactive oxygen species (ROS) is one of the mechanisms that have been identified in the pathogenesis of progressive renal injury.7
Myeloperoxidase (MPO) is an oxidative enzyme present in phagocytes. MPO can be released by activated neutrophils, monocytes, and macrophages and it is an essential part of the anti-microbial system and inflammatory regulation.8 MPO is a heme enzyme that uses the oxidizing potential of superoxide and hydrogen peroxide (H2O2) to convert chloride ions into hypochlorous acid and other ROS.8
Moreover, MPO has emerged as an important mediator of pro-atherogenic changes in the human artery wall.9 MPO and the products of its activity have been observed in atherosclerotic lesions at various stages of severity.10 MPO also promotes the oxidative damage of host tissues at inflammation sites, including atherosclerotic lesions.10 Some authors have suggested that MPO may be involved in the development of coronary artery disease (CAD).11,12 Meuwese et al.13 reported that serum MPO levels are associated with the future risk of CAD in healthy individuals.
Catalase is highly expressed in some tissues, protecting cells against an excess formation of ROS. Catalase prevents the accumulation of H2O2 formed during oxygen transport. Catalase serves as an intracellular antioxidant enzyme, and it is a member of the free radical and ROS scavenging system.14
To the best of our knowledge, serum MPO activity, catalase activity, and oxidative stress have not yet been reported in patients with adult NS. Therefore, the aim of this study was to investigate serum malondialdehyde (MDA) levels, MPO, and catalase activities in patients with adult NS.
Methods
Subjects
The prospective study was conducted in the Departments of Nephrology and Internal Medicine of Medical Faculty of Yuzuncu Yil University.
In this study, 24 patients with NS (12 females and 12 males) and 24 healthy controls (11 females and 13 males) were enrolled.
The NS diagnosis was confirmed according to the following general criteria: proteinuria >3.5 g/1.73 m2/24 h, serum albumin <2.5 g/l, edema, triglyceride (TG), and no current systemic disease.
A renal biopsy was performed in all patients with NS. The etiologys of the NS patients was as follows: membranous glomerulonephritis (n = 10), focal segmental glomerulosclerosis (n = 7), and membranoproliferative glomerulonephritis (n = 7). The average follow-up period for the NS patients was 23.3 ± 13.3 months. All patients were receiving immunosuppressive treatment.
Most patients were receiving angiotensin-converting enzyme inhibitors or angiotensin-II type 1 receptor blockers. None of the patients received non-steroidal anti-inflammatory drugs, albumin or blood transfusions within the 1-month prior to the study. The NS patients were not receiving antioxidant vitamin supplementation, such as vitamins E or C. Three patients were receiving statin treatment.
The control group consisted of 24 age- and sex-matched healthy volunteers from our hospital staffs. These subjects were asymptomatic with an unremarkable medical history and a normal physical examination. No control subjects were receiving supplementation with antioxidant vitamins such as E and C. The control subjects were not receiving any drugs and were not smoking or consuming alcohol.
The study protocol was conducted in accordance with the Helsinki Declaration as revised in 2000 and approved by the Yuzuncuyil University Medical Faculty. All subjects were informed about the study, and written consent was obtained from each subject.
Exclusion criteria
The exclusion criteria included a history of alcohol abuse, habitual smoking, intravenous drug abuse, pregnancy, the use of antioxidant supplements, active infection, diabetes mellitus, liver or pulmonary disease, rheumatoid arthritis, and coronary heart disease.
Blood samples
Blood samples were collected at 9:00 a.m. after an overnight fasting state. The blood samples were collected into empty tubes and immediately stored on ice at 4°C. The serum samples were then separated from the cells by centrifugation at 3000 rpm for 10 minutes. The serum were stored in plastic tubes at −80°C and were used for analyzing MPO activity, catalase activity, and MDA levels.
Measurement of serum lipid peroxidation levels
MDA level of the serum was measured by the following procedure according to Tomotsu et al. 0.5 plasma was shaken with 2.5 ml of 20% trichloroacetic acid in a 10 ml centrifuge tube. One milliliter of 0.6% TBA was added to the mixture, shaken, and warmed for 30 minutes in a boiling water bath followed by rapid cooling. Then it was shaken into a 4 ml of n-butylalcohol layer in a separation tube. The results were expressed as nmol/ml serum.15
Measurement of serum catalase activity
Catalase activity was measured using H2O2 as substrate.16 The disappearance of H2O2 was followed at 240 nm, and enzyme activity was expressed in units per liter of serum (U/l) at 25°C.
Measurement of serum MPO activity
Serum MPO activity was determined by the method of Klebanoff and Clark17 and was based on kinetic measurement of the formation rate of the yellowish-orange product of the oxidation of o-dianisidne with MPO in the presence of H2O2 at 460 nm. One unit of MPO was defined as that degrading 1 µmol of H2O2 per minute at 25°C. A molar extinction coefficient of 1.3 × 104 M−1 cm−1 of oxidized o-dianisidine was used for the calculation. MPO activity was expressed in U/l of serum.
Other parameters
The TG, total cholesterol (TC), high-density lipoprotein-cholesterol (HDL-C), and low-density lipoprotein-cholesterol (LDL-C) levels were determined using commercially available assay kits (Abbott®) with an autoanalyzer (Aeroset®, Abbott®, Abbott Park, IL, USA).
Serum creatinine, uric acid, albumin, and total bilirubin were determined with an autoanalyzer (Aeroset®, Abbott®, Abbott Park, IL, USA) using commercially available assay kits (Abbott®).
Proteinuria levels were measured by turbidimetric methods using an automatic analyzer (Aeroset®, Abbott Park, IL, USA). Proteinuria was defined as a urinary protein excretion rate of >150 mg per 24 hours. Nephrotic proteinuria was defined as >3.5 g per 24 hours.
GFR (glomerular filtration rate) (ml/minutes) was obtained by the following formula: urine creatinine (μmol/l) × urine volume in 24 hours (ml)/serum creatinine (μmol/l) × 1440.
Statistical analysis
The results are expressed as the mean ± standard deviation. Non-parametric continuous variables were compared with the Mann–Whitney U-test. Parametric variables were compared using Student's t-test. Pearson correlation analysis was used to determine the association between MPO activity, catalase activity, and MDA levels. For examining the impact of independent variables on MPO activity, catalase activity, and MDA levels, a linear regression analysis was performed. The results were considered statistically significant when the P value was <0.05. The data were analyzed using the SPSS® for Windows (Version 11.0, SPSS Inc., Chicago, IL, USA).
Results
The demographic characteristics of the NS and control subjects are presented in Table 1. There were no significant differences between the NS patients and controls with respect to age, gender, or body mass index (P > 0.05) (Table 1).
Table 1.
Demographic characteristics of the two groups in this study
| Parameters | NS (n = 24) | Control (n = 24) | P |
|---|---|---|---|
| Age (years) | 37 ± 9 | 32 ± 8 | ns |
| Sex (female/male) | 12/12 | 11/13 | ns |
| Body mass index (kg/m2) | 23.5 ± 1.3 | 21.4 ± 1.1 | ns |
| Proteinuria (mg/24 hours) | 1215 ± 742 | 120 ± 61 | P < 0.05 |
| Systolic blood pressure (mmHg) | 120 ± 12 | 120 ± 10 | ns |
| Diastolic blood pressure (mmHg) | 80 ± 8 | 82 ± 10 | ns |
| GFR (ml/minute) | 126.9 ± 14.1 | 122.5 ± 12.8 | ns |
| Albumin (g/l) | 38.2 ± 9.1 | 45.1 ± 4.2 | P < 0.05 |
| TG (mmol/l) | 2.46 ± 0.90 | 1.22 ± 0.34 | P < 0.05 |
| TC (mmol/l) | 6.33 ± 1.71 | 4.57 ± 0.57 | P < 0.05 |
| HDL-C (mmol/l) | 1.05 ± 0.24 | 1.19 ± 0.29 | P < 0.05 |
| LDL-C (mmol/l) | 4.14 ± 1.47 | 2.79 ± 0.64 | P < 0.05 |
GFR, glomerular filtration rate; HDL-C, high-density lipoprotein-cholesterol; LDL-C, low-density lipoprotein-cholesterol; NS, nephrotic syndrome; ns, non-significant; TG, triglyceride; TC, total cholesterol.
Values are mean ± SD.
There were also no statistically significant differences between the NS patients and controls with respect to systolic and diastolic blood pressure (P > 0.05). The GFR was higher in the NS patients than controls, but this was not statistically significant (P > 0.05) (Table 1). Furthermore, the average follow-up period for the patients with NS was 23.3 ± 13.3 months.
Proteinuria levels were significantly higher in the NS patients than controls (P < 0.05), whereas the serum albumin levels were significantly lower in the NS patients than controls (P < 0.05). Serum TG, TC, and LDL-C levels were significantly higher in patients with NS compared with controls (P < 0.05 for all three parameters), while HDL-C levels were significantly higher (P < 0.05) (Table 1).
Serum MPO activity and MDA levels were significantly higher in the NS patients than controls (both, P < 0.001) (Figs. 1 and 2), while the catalase levels were significantly lower (P < 0.001) (Table 2) (Fig. 3).
Figure 1.
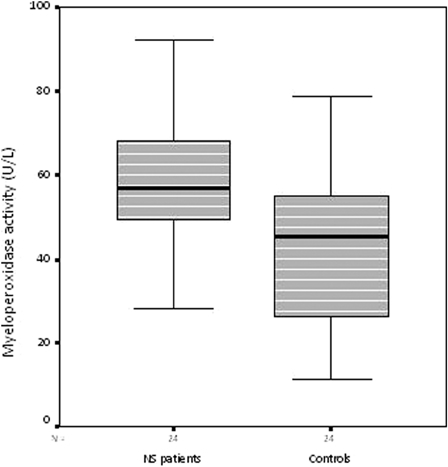
Serum MPO activity in the nephrotic syndrome patients and control subjects
Figure 2.
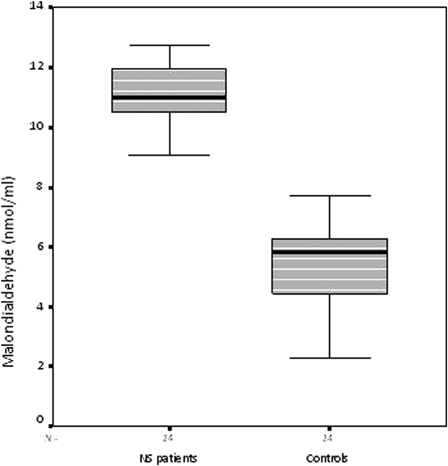
Serum malondialdehyde levels in the nephrotic syndrome patients and control subjects
Table 2.
MPO activity, catalase activity and oxidative stress in NS patients and controls
| Parameters | NS (n = 24) | Controls (n = 24) | P |
|---|---|---|---|
| Malondialdehyde (nmol/ml) | 10.84 ± 1.48 | 5.32 ± 1.51 | P < 0.001 |
| MPO activity (U/l) | 59.21 ± 14.76 | 42.61 ± 20.11 | P < 0.002 |
| Catalase (U/l) | 13.49 ± 3.67 | 71.84 ± 30.02 | P < 0.001 |
NS, nephrotic syndrome.
Values are mean ± SD.
Figure 3.
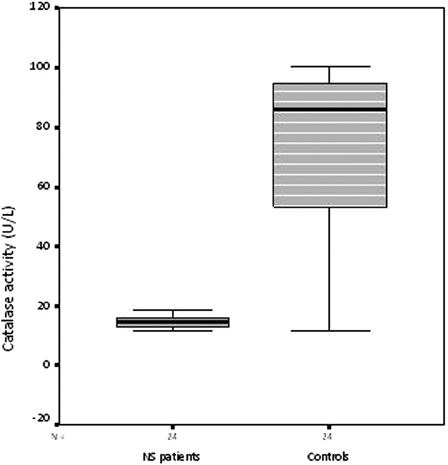
Serum catalase activity in the nephrotic syndrome patients and control subjects
Serum catalase activity was found to be significantly correlated with MPO activity (r = −0.417, P = 0.003) and MDA levels (r = −0.532, P = 0.007). The serum MDA levels were also found to be significantly correlated with MPO activity (r = 0.419, P = 0.03).
Using a Pearson correlated analysis, proteinuria levels were significantly correlated with serum MDA levels (r = 0.388, P = 0.003), MPO (r = 0.338, P = 0.019), and catalase (r = −0.576, P = 0.001) in the NS patients.
In the NS patients, serum TG, TC, LDL-C levels, and HDL-C levels were not correlated with MDA levels, MPO, and catalase activities (P > 0.05).
A linear regression analysis was performed to identify factors that may exert an independent influence on serum MPO activity, catalase activity, and MDA levels. Serum lipid parameters levels, albumin, proteinuria, glomerular filtration rate, age, gender, and body mass index were included as independent variables. No relationship was found between serum MPO activity, catalase activity, and MDA levels, and these parameters in the NS patients or control subjects in a linear regression analysis (P > 0.05).
Discussion
To the best of our knowledge, no previous report concerning serum MDA levels, MPO, and catalase activities in NS patients has been presented in the literature. Therefore, this is the first study to investigate those parameters in patients with adult NS.
In the present study, we investigated this topic for the first time and found that serum MPO enzyme activity was influenced by oxidative stress. We found that NS patients had increased MPO activity and oxidative stress compared with healthy subjects. Moreover, we observed that serum catalase activity was significantly lower in patients with NS than controls. Furthermore, we found a positive correlation between proteinuria levels, and serum MDA levels and MPO activity in patients with NS. Finally, we observed a negative correlation between proteinuria levels and serum catalase activity in patients with NS.
Oxidative damage by free radicals, which are extreme ROS, can lead to renal disease.18 They can damage membranes by lipid peroxidation, increase glomerular permeability to proteins, and alter glomerular hemodynamics.19 ROS can also produce proteinuria by inducing glomerular epithelial cell injury by reducing the electronegative charge of the glomerular filtration barrier or through other unknown mechanisms.20 It has been suggested that the enhanced permeability of the glomerular capillary wall is possibly influenced by the generation of free radicals.21 Zachwieja et al.22 reported the pathophysiological importance of ROS in patients with NS.
Many risk factors, such as hypertension, cigarette smoking, diabetes, hyperlipidemia, and hypercoagulability play a role in the development of atherosclerosis.23 Hyperlipidemia, increased lipid oxidation reactions, and defects in antioxidant status may lead to glomerulosclerosis and glomerular disease progression in NS.24 Recently, we found low serum paraoxonase-1 activity in patients with adult NS.25 In addition, we indicated that lower paraoxonase-1 activity is associated with an oxidant–antioxidant imbalance that may contribute to atherosclerosis in adult NS patients. Finally, we observed higher MDA levels, which is an important biomarker in the pathogenesis of atherosclerosis in patients with adult NS. Outside of these risk factors, we suggest that higher serum MPO levels should be considered ‘novel’ risk factor for atherosclerosis development in NS patients.
Although serum MPO levels have been well documented in several studies9–12,26–38, to the best of our knowledge, no previous report concerning serum MPO activity in NS patients exists in the literature. MPO is a major glycoprotein present in the azurophilic granules of polymorphonuclear neutrophils39 and certain tissue macrophages, such as in atherosclerotic plaques.26 MPO, which is an independent risk factor for cardiovascular disease, has also been shown to be a potential risk marker for atherosclerosis.27,28 Moreover, MPO has been implicated in the initiation and propagation of atherosclerosis.29 MPO is a potent oxidative contributor to atherosclerosis that has the ability to produce a large group of oxidative compounds.30 The role of MPO, an enzyme secreted from activated neutrophils and monocytes, has attracted scientific interest as a source of free radicals and diffusible oxidants.31 MPO has been detected in atherosclerotic lesions, and high blood MPO concentrations have been correlated with CAD incidence.32
Some studies have emphasized the importance of MPO in cardiovascular disease.11,27,33 MPO levels are higher in patients with CAD.11 The association between increased MPO concentrations and the progression of atherosclerotic disease indicates that MPO may be a prognostic marker for unstable plaques.34 Increased MPO has emerged as an important pro-atherogenic mediator in both animal models35 and observational studies in patients with advanced cardiovascular disease.28,36 MPO has also been shown to be a strong predictor of future CAD in a healthy population.13 MPO was shown to contribute to adverse remodeling and left ventricular dilatation after acute myocardial infarction in an animal model and may thus contribute to the development of heart failure.37,38
Endothelial dysfunction is an early atherosclerotic change, manifested by the abnormal reactivity and expression of local pro-inflammatory and prothrombotic factors.40 MPO has potent pro-inflammatory properties that directly contribute to endothelial injury.27 In humans, MPO levels were found to be associated with endothelial dysfunction.41 MPO levels have been shown to reflect endothelial dysfunction, inflammation, atherosclerosis, and oxidative stress.42
Our study has several limitations. First, it is cross-sectional design. Second, the number of study patients was small, and these observations must be confirmed in a larger patient sample. Thirty, we investigated MPO and catalase activities and MDA levels in adult NS patients. A cause–effect relationship between microvascular damage and oxidative stress and MPO activity cannot be established in this type of study. Furthermore, glomerular hemodynamics was not evaluated. Thus, the current study did not allow testing the hypothesis whether increased oxidative stress and MPO levels might cause microvascular damage and glomerular hemodynamic changes.
In conclusion, we found that serum MPO activity and oxidative stress were increased and serum catalase activity was decreased in patients with adult NS. In addition, these results indicate that increased MPO activity is associated with an oxidant–antioxidant imbalance that may contribute to atherosclerosis in patients with adult nephrotic syndrome. Further studies enrolling a larger number of patients are required to clarify the results obtained here.
Acknowledgements
The authors thank the staff at the Harran University Clinical Biochemistry for their generous and friendly assistance in every step of this study.
References
- 1.Dogra G, Ward N, Croft KD, Mori TA, Barrett PH, Herrmann SE, et al.. Oxidant stress in nephrotic syndrome: comparison of F (2)-isoprostanes and plasma antioxidant potential. Nephrol Dial Transplant 2001;16:1626–30. [DOI] [PubMed] [Google Scholar]
- 2.Savin VJ. Mechanisms of proteinuria in noninflammatory glomerular diseases. Am J Kidney Dis 1993;21:347–62. [DOI] [PubMed] [Google Scholar]
- 3.Zima T, Tesar V, Stipek S. The influence of cyclosporine on lipid peroxidation and superoxide dismutase in adriamycin nephropathy in rats. Nephron 1997;75:464–9. [DOI] [PubMed] [Google Scholar]
- 4.Ueda N, Baliga R, Shah SV. Role of ‘catalytic’ iron in an animal model of minimal change nephrotic syndrome. Kidney Int 1996;49:370–3. [DOI] [PubMed] [Google Scholar]
- 5.Yoshioka T, Ichikawa J, Fogo A. Reactive oxygen metabolites cause massive, reversible proteinuria and glomerular sieving defect without apparent ultrastructural abnormality. J Am Soc Nephrol 1991;2:902–12. [DOI] [PubMed] [Google Scholar]
- 6.Clemens MR, Burza-Zanetti Z. Lipid abnormalities and peroxidation of erythrocytes in nephrotic syndrome. Nephron 1989;53:325–9. [DOI] [PubMed] [Google Scholar]
- 7.Bulucu F, Vural A, Aydin A, Sayal A. Oxidative stress status in adults with nephrotic syndrome. Clin Nephrol 2000;53:169–73. [PubMed] [Google Scholar]
- 8.Winterbourn CC, Vissers MC, Kettle AJ. Myeloperoxidase. Curr Opin Hematol 2000;7:53–8. [DOI] [PubMed] [Google Scholar]
- 9.Shao B, Oda MN, Oram JF, Heinecke JW. Myeloperoxidase: an oxidative pathway for generating dysfunctional HDL. Chem Res Toxicol 2010;23:447–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Podrez EA, Abu-Soud HM, Hazen SL. Myeloperoxidase-generated oxidants and atherosclerosis. Free Radic Biol Med 2000;28:1717–25. [DOI] [PubMed] [Google Scholar]
- 11.Zhang R, Brennan ML, Fu X, Aviles RJ, Pearce GL, Penn MS, et al.. Association between myeloperoxidase levels and risk of coronary artery disease. JAMA 2001;286:2136–42. [DOI] [PubMed] [Google Scholar]
- 12.Daugherty A, Rateri DL, Dunn JL, Heinecke JW. Myeloperoxidase, a catalyst for lipoprotein oxidation, is expressed in human atherosclerotic lesions. J Clin Invest 1994;94:437–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meuwese MC, Stroes ES, Hazen SL, van Miert JN, Kuivenhoven JA, Schaub RG, et al.. Serum myeloperoxidase levels are associated with the future risk of coronary artery disease in apparently healthy individuals: the EPIC-Norfolk Prospective Population Study. J Am Coll Cardiol 2007;50:159–65. [DOI] [PubMed] [Google Scholar]
- 14.Ece A, Gurkan F, Kervancıoglu M, Kocamaz H, Güneş A, Atamer Y, et al.. Oxidative stress, inflammation and early cardiovascular damage in children with chronic renal failure. Pediatr Nephrol 2006;21:545–52. [DOI] [PubMed] [Google Scholar]
- 15.Yoshioka T, Kawada K, Shimada T, Mori M. Lipid peroxidation in maternal and cord blood and protective mechanisms against activated oxygen toxicity in the blood. Am J Obster Gynecol 1979;135:372–6. [DOI] [PubMed] [Google Scholar]
- 16.Aebi H. Catalase. In: , Bergmeyer HU, (ed.). Methods of enzymatic analysis. New York and London: Academic Pres Inc.; 1974. p. 673–7. [Google Scholar]
- 17.Klebanoff SJ, Clark FA. The neutrophil: function and clinical disorders, 1. Amsterdam, The Netherlands: Elsevier/North Holland Biomedical Press; 1978. p. 810. [Google Scholar]
- 18.El-Far MA, Bakr MA, Farahat SE, Abd El-Fattah EA. Glutathione peroxidase activity in patients with renal disorders. Clin Exp Nephrol 2005;9:127–31. [DOI] [PubMed] [Google Scholar]
- 19.Cheesman KH, Slatter TF. An introduction to free radical biochemistry. Br Med Bull 1993;49:481–93. [DOI] [PubMed] [Google Scholar]
- 20.Alfrey AC. Role of iron and oxygen radicals in the progression of chronic renal failure. Am J Kidney Dis 1994;23:183–7. [DOI] [PubMed] [Google Scholar]
- 21.Diamond JR. The role of reactive oxygen species in animal models of glomerular disease. Am J Kidney Dis 1992;19:292–300. [DOI] [PubMed] [Google Scholar]
- 22.Zachwieja J, Bobkowski W, Dobrowolska-Zachwieja A, Zaniew M, Maciejewski J. Decreased antioxidant activity in hypercholesterolemic children with nephrotic syndrome. Med Sci Monit 2003;9:235–9. [PubMed] [Google Scholar]
- 23.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature 1993;632:801–9. [DOI] [PubMed] [Google Scholar]
- 24.Guijarro C, Keane WF. Lipid-induced glomerular injury. Nephron 1994;67:1–6. [DOI] [PubMed] [Google Scholar]
- 25.Soyoral YU, Aslan M, Emre H, Begenik H, Erdur FM, Turkel A, et al.. Serum paraoxonase activity and oxidative stress in patients with adult nephrotic syndrome. Atherosclerosis 2011;218:243–6. [DOI] [PubMed] [Google Scholar]
- 26.Sugiyama S, Okada Y, Sukhova GK, Virmani R, Heinecke JW, Libby P. Macrophage myeloperoxidase regulation by granulocyte macrophage colony-stimulating factor in human atherosclerosis and implications in acute coronary syndromes. Am J Pathol 2001;158:879–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baldus S, Heeschen C, Meinertz T, Zeiher AM, Eiserich JP, Munzel T, et al.. Myeloperoxidase serum levels predict risk in patiens with acute coronary syndrome. Circulation 2003;108:1440–5. [DOI] [PubMed] [Google Scholar]
- 28.Brennan ML, Penn MS, VanLente F, Nambi V, Shishehbor MH, Aviles RJ, et al.. Prognostic value of myeloperoxidase in patients with chest pain. New Engl J Med 2003;349:1595–604. [DOI] [PubMed] [Google Scholar]
- 29.Schindhelm RK, van der Zwan LP, Teerlink T, Scheffer PG. Myeloperoxidase: a useful biomarker for cardiovascular disease risk stratification? Clin Chem 2009;55:1462–70. [DOI] [PubMed] [Google Scholar]
- 30.Hazen SL, Hsu FF, Gaut JP. Modification of proteins and lipids by myeloperoxidase. Methods Enzymol 1999;300:88–105. [DOI] [PubMed] [Google Scholar]
- 31.Eiserich JP, Baldus S, Brennan ML, Ma W, Zhang C, Tousson A, Castro L, et al.. Myeloperoxidase, a leukocyte derived vascular NO oxidase. Science 2002;296:2391–4. [DOI] [PubMed] [Google Scholar]
- 32.Hazell LJ, Arnold L, Flowers D, Waeg G, Malle E, Stocker R. Presence of hypochlorite-modified proteins in human atherosclerotic lesions. J Clin Invest 1996;97:1535–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brennan ML, Hazen SL. Emerging role of myeloperoxidase and oxidant stress markers in cardiovascular risk assessment. Curr Opin Lipidol 2003;14:353–9. [DOI] [PubMed] [Google Scholar]
- 34.Pawlus J, Hołub M, Kozuch M, Dabrowska M, Dobrzyckı S. Serum myeloperoxidase levels and platelet activation parameters as diagnostic and prognostic markers in the course of coronary disease. Int Jnl Lab Hem 2010;32:320–8. [DOI] [PubMed] [Google Scholar]
- 35.McMillen TS, Heinecke JW, LeBoeuf RC. Expression of human myeloperoxidase by macrophages promotes atherosclerosis in mice. Circulation 2005;111:2798–804. [DOI] [PubMed] [Google Scholar]
- 36.Tang WH, Brennan ML, Philip K, Tong W, Mann S, Van Lente F, et al.. Plasma myeloperoxidase levels in patients with chronic heart failure. Am J Cardiol 2006;98:796–9. [DOI] [PubMed] [Google Scholar]
- 37.Askari AT, Brennan ML, Zhou X, Drinko J, Morehead A, Thomas JD, et al.. Myeloperoxidase and plasminogen activator inhibitor 1 play a central role in ventricular remodeling after myocardial infarction. J Exp Med 2003;197:615–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vasilyev N, Williams T, Brennan ML, Unzek S, Zhou X, Heinecke JW, et al.. Myeloperoxidase generated oxidants modulate left ventricular remodeling but not infarct size after myocardial infarction. Circulation 2005;112:2812–20. [DOI] [PubMed] [Google Scholar]
- 39.Hoy A, Treagoueet D, Leininger-Muller B, Poirier O, Maurice M, Sass C, et al.. Serum myeloperoxidase concentration in a healthy population: biological variations, familial resemblance and new genetic polymorphisms. Eur J Hum Genet 2001;9:780–6. [DOI] [PubMed] [Google Scholar]
- 40.Hamm C. A classification of unstable angina revisited. Circulation 2000;102:118–22. [DOI] [PubMed] [Google Scholar]
- 41.Vita JA, Brennan ML, Gokce N, Mann SA, Goormastic M, Shishehbor MH, et al.. Serum myeloperoxidase levels independently predict endothelial dysfunction in humans. Circulation 2004;110:1134–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Michowitz Y, Kisil S, Guzner-Gur H, Rubinstein A, Wexler D, Sheps D. Usefulness of serum myeloperoxidase in prediction of mortality in patients with severe heart. IMAJ 2008;10:884–8. [PubMed] [Google Scholar]


