Abstract
Objective: The purpose of this study was to investigate paraoxonase (PON) and arylesterase (ARES) enzyme activity in adults with vitamin B12 deficiency, and specific changes in the activities of these enzymes following vitamin B12 treatment.
Methods: A total of 46 patients with vitamin B12 deficiency (aged 18–82 years) and 45 healthy volunteer controls (aged 19–64 years) participated in this study. Venous blood samples were collected, and serum vitamin B12, homocysteine (HCY), methylmalonic acid, PON1, and ARES levels were measured.
Results: Paired comparison showed that pre- and post-treatment values for PON and ARES were similar between patients and controls (both P > 0.05). There was no statistically significant relationship between patients’ pre-/post-treatment PON or HCY levels and serum vitamin B12 levels, compared with those of the control group (P > 0.05).
Discussion: The results of the present study do not support the hypothesis that the antioxidant enzymes PON and ARES have an underlying role in vitamin B12 deficiency and related hyperhomocysteinemia. Our findings suggest that PON and ARES do not play a role in the systemic effects of vitamin B12 deficiency.
Keywords: Arylesterase, Homocysteine, Paraoxonase, Vitamin B12
Introduction
Vitamin B12 is an essential vitamin that plays a key role as a co-factor in the synthesis of DNA, RNA, and proteins. Functions of vitamin B12 include nucleic acid metabolism, transfer of methyl groups, synthesis and repair of the myelin sheath, and production of erythrocytes.1–4 Vitamin B12 deficiency can result in megaloblastic anemia and/or clinical outcomes that cause neurological dysfunction. Reduction of methionine synthase activity due to vitamin B12 deficiency results in elevated serum homocysteine (HCY) levels. Even in the early stages of low vitamin B12, HCY levels begin progressively increasing.5 Although HCY is a normal metabolite, excessive levels can be toxic.6 A high HCY level is an independent risk factor for many major pathologies, including cardiovascular diseases, osteoporosis, Alzheimer's disease, and birth defects. Most HCY-related pathologies are also linked to oxidative stress.7,8 It has been hypothesized that HCY promotes oxidative stress via generation of reactive oxygen species upon disulfide bond formation; thus, HCY has pro-oxidant activity.9 When the production of reactive oxygen species exceeds the antioxidant defense capacity, oxidative stress damages the structural and functional integrity of tissues.9 The antioxidant capacity of the body plays a major role in determining the level of harmful effects exerted by oxidative conditions.
Paraoxonase 1 (PON1) is a high-density lipoprotein (HDL) cholesterol-bound enzyme with important physiological functions, including detoxification. It has antioxidant properties and is synthesized by the liver.10 PON1 is a multifunctional enzyme with PON, diazoxonase, and arylesterase (ARES) activities.11 These enzymes protect lipids from peroxidation and, consequently, exhibit antioxidant features. A previous study showed that PON1 deficiency predisposes individuals to atherosclerosis and cardiovascular disease.12
One of the characteristics of PON1 is its ability to detoxify HCY-thiolactone. As a HCY metabolite, HCY-thiolactone is a reactive intermediate that is toxic to cells and proteins.13 A previous study reported that the proatherogenic and neurodegenerative effects of HCY might be linked to low PON1 serum activity.6,14,15
To our knowledge, there are no data on the activity of PON and ARES in adult patients with hyperhomocysteinemia due to vitamin B12 deficiency. In a study of children with anemia caused by vitamin B12 deficiency, PON and ARES levels were significantly low and increased after vitamin B12 treatment.16 Knowledge of PON and ARES activity in patients with vitamin B12 deficiency might help to clarify the underlying biochemical mechanisms involved in this type of deficiency. The purpose of this study was to investigate PON and ARES enzyme activities in adults with vitamin B12 deficiency, and to assess specific changes in the activities of these enzymes following vitamin B12 treatment.
Materials and methods
Patients and controls
A total of 46 patients with vitamin B12 deficiency (aged 18–82 years) and 45 healthy volunteer controls (aged 19–64 years) were recruited from the Hematology Department at Yildirim Beyazit University Medical Faculty and included in this study. Venous blood samples (10 ml) were taken from all participants, and serum vitamin B12, HCY, methylmalonic acid (MMA), and baseline and salt-stimulated PON1 and ARES levels were determined. Patients with vitamin B12 deficiency were administered cyanocobalamin for 4–6 weeks, after which the venous sampling was repeated to re-evaluate baseline and salt-stimulated PON1 and ARES levels. Patients with co-morbid systemic disorders and those using any drug(s) were excluded from the study. The control group consisted of healthy adults who were age- and sex-matched with the vitamin B12-deficient participants. Written informed consent was obtained from all participants after they had been provided with a complete description of the study. This study was approved by the ethics committee of Yildirim Beyazit University Medical Faculty.
Blood samples and measurement
To avoid any interference with the study results, participants were advised not to smoke, drink, or eat prior to blood sampling. Venous blood samples (10 ml) were collected from an antecubital vein after a 12-h overnight fast and transferred to biochemistry tubes. The biochemistry tubes were centrifuged at 1800 g for 15 minutes after an incubation period of 30 minutes. Samples were frozen at –80°C and stored before analysis.
Vitamin B12 levels were measured by the electrochemiluminescence method using Roche commercial diagnostics kits and a Cobase 601 immunological test analyzer (Roche, Basel, Switzerland). HCY levels were measured using an IMMULITE 2000 immunoassay analyzer (Siemens Healthcare Diagnostic, Munich, Germany) using the chemiluminescence method. MMA levels were measured using liquid chromatography–mass spectrometry (API 3200 System; AB Sciex, Sao Paulo, Brazil). The activity of ARES was measured using an ARES assay kit, and the activity of PON1 was measured using a fully automated PON activity measurement kit (Rel Assay Diagnostics, Gaziantep, Turkey) with a Cobase 501 automated analyzer (Roche) in the biochemistry laboratory of Yildirim Beyazit University Medical Faculty, Ataturk Training and Research Hospital. Baseline and salt-simulated PON1 were measured.
Statistical analysis
The normality of continuous variables, such as age, serum vitamin B12, and hemoglobin, was tested using the Shapiro–Wilk test. Continuous variables with a normal distribution are reported as mean ± standard deviation, and those with a non-normal distribution as median (interquartile range [IQR]) and minimum − maximum. Sex is expressed as a count (%).
Differences between patient values, such as serum vitamin B12, serum MMA, and HDL cholesterol, measured before and after treatment, and differences between patient and control group values were analyzed using the Student's t test and Mann–Whitney U test according to the normality of the variables. For comparison of pre- and post-treatment values for patients, a paired t test was used for variables that fitted a normal distribution, and Wilcoxon's signed rank test was used for those that did not. Spearman's rho coefficient was calculated to define the correlation between PON and HCY and vitamin B12 levels, and between ARES and HCY and vitamin B12 levels. Variables for which the P-values showed a significant difference are given in bold in the tables.
SPSS version 21.0 (IBM, Armonk, NY, USA) was used for the statistical analyses and calculations in this study. P < 0.05 was considered statistically significant.
Results
The median age of the 46 patients who participated in this study was 44.50 years (IQR 30.00) years, and the median age of the 45 healthy controls was 38.00 (IQR 34.00) (Table 1). Thirteen patients (28.3%) and 19 controls (44.2%) were male. There were no significant differences between the patient and control groups with respect to age or sex (both P > 0.05; Table 1).
Table 1. Demographic features of participants.
| Characteristic | Patients (n = 46) | Controls (n = 45) | P | |
|---|---|---|---|---|
| Median age, years | 44.50 (30.00) | 38.00 (34.00) | 0.057* | |
| (IQR) | ||||
| Age range, years | 18–82 | 19–65 | ||
| Sex, n (%) | ||||
| Male | 13 (28.3) | 19 (42.2) | 0.240† | |
| Female | 33 (71.7) | 26 (57.8) | ||
*Mann–Whitney U test.
†Yates’ Chi-square test.
The median vitamin B12 level was 150.00 pg/ml (IQR 48.50) for patients’ pre-treatment, 706.50 pg/ml (IQR 539.00) for patients’ post-treatment, and 326.70 pg/ml (IQR 243.90) for controls (Table 2). Serum vitamin B12 levels were significantly lower among patients compared with controls pre-treatment (P < 0.001), but significantly higher than those of the control group post-treatment (P < 0.001).
Table 2. Serum vitamin B12, homocysteine, and MMA, urine MMA, and hemoglobin, LDH, and HDL cholesterol values of participants.
| Patients pre-treatment | Controls | P† | Patients post-treatment | P‡ | P | |
|---|---|---|---|---|---|---|
| Serum vitamin B12 (pg/ml) | 150.00 (48.50) (30.00–200.00) | 326.70 (243.90) (141.00–1430.00) | <0.001 | 706.50 (539.00) (193.00–2000.00) | <0.001 | <0.001 |
| Serum homocysteine (µmol/l) | 15.65 (10.85) (5.10–126.00) | 11.70 (3.65) (5.11–49.60) | 0.001 | 8.61 (5.05) (0.56–25.40) | 0.003 | <0.001 |
| Serum MMA (nmol/ml) | 1.32 (1.26) (0.14–3.80) | 0.29 (0.16) (0.08–0.73) | <0.001 | 0.27 (0.19) (0.10–1.24) | 0.363 | <0.001 |
| Urine MMA (nmol/ml) | 2.67 (1.99) (0.06–6.70) | 0.86 (1.11) (0.02–4.44) | <0.001 | 1.02 (1.14) (0.00–12.30) | 0.955 | <0.001 |
| Hemoglobin (g/dl) | 12.38 ± 2.97 (5.70–18.10) | 14.12 ± 2.00 (9.50–17.90) | <0.001* | 13.53 ± 1.76 (9.60–18.00) | 0.139* | <0.001* |
| LDH (U/l) | 190.00 (47.00) (113.00–4781.00) | 180.00 (44.50) (115.00–264.00) | 0.123 | 197.50 (36.75) (125.00–424.00) | 0.006 | 0.269 |
| HDL cholesterol (mg/dl) | 48.50 (22.25) (17.00–79.00) | 63.81 (29.16) (34.00–101.00) | <0.001 | 47.50 (19.50) (25.00–70.00) | 0.001 | 0.655* |
Data are median (interquartile range) or mean ± standard deviation (min. – max.)
HDL: high-density lipoprotein; LDH: lactate dehydrogenase; MMA: methylmalonic acid.
†Patients pre-treatment versus controls, Mann–Whitney U test or *Student's t test.
‡Patients post-treatment versus controls, Mann–Whitney U test or *Student's t test.
Patients pre-treatment versus post-treatment, Wilcoxon signed rank test or *paired samples t test.
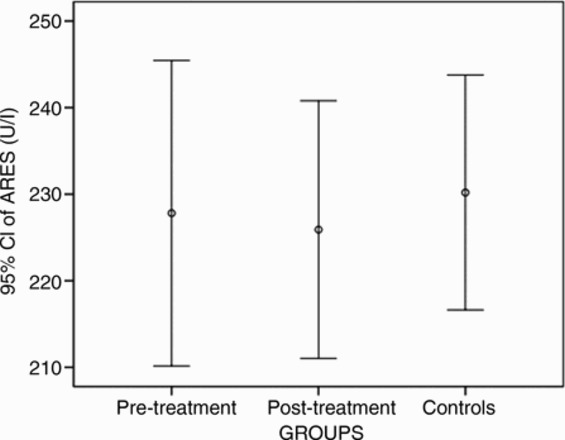
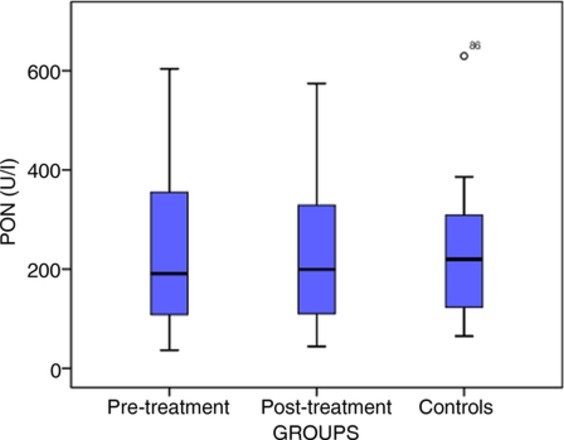
Patients had median PON values of 191.10 U/l (IQR 248.45) pre-treatment and 199.55 U/l (IQR 222.90) post-treatment. The median PON value of the control group was 219.90 U/l (IQR 189.13) (Fig. 1). Paired comparison showed that pre- and post-treatment PON values of patients and those of the control group were similar (P > 0.05). Similarly, ARES values did not differ significantly between the patient and control groups (P > 0.05; Fig. 2).
Figure 1.
Distribution of PON levels. Horizontal lines indicate median values; purple bars indicate the interquartile range; and vertical lines indicate the minimum and maximum. PON, paraoxonase.
Figure 2.
Distribution of ARES levels. Circles indicate the median levels. ARES, arylesterase.
There were no statistically significant relationships between PON and HCY levels for patients’ pre-treatment (ρ = –0.043, P = 0.778; Table 3) or for controls (ρ = 0.097, P = 0.524).
Table 3. Relationships between PON and homocysteine and serum vitamin B12.
| PON (U/l) | ||
|---|---|---|
| Blood measurements | ρ* | P |
| Homocysteine (µmol/l) | ||
| Patients, pre-treatment | –0.043 | 0.778 |
| Patients, post-treatment | –0.123 | 0.416 |
| Controls | 0.097 | 0.524 |
| Vitamin B12 (pg/ml) | ||
| Patients, pre-treatment | –0.042 | 0.782 |
| Patients, post-treatment | –0.183 | 0.222 |
| Controls | 0.075 | 0.624 |
*Spearman's rho correlation coefficient.
Analysis of the relationship between ARES values and HCY and serum vitamin B12 levels showed a low-level positive correlation between serum vitamin B12 and ARES levels among patients before treatment (ρ = 0.349, P = 0.017; Table 4).
Table 4. Relationships between ARES and HCY and serum vitamin B12.
| ARES (U/l) | ||
|---|---|---|
| Blood measurements | ρ* | P |
| HCY (µmol/l) | ||
| Patients, pre-treatment | –0.040 | 0.789 |
| Patients, post-treatment | 0.067 | 0.660 |
| Controls | 0.154 | 0.313 |
| Vitamin B12 (pg/ml) | ||
| Patients, pre-treatment | 0.349 | 0.017 |
| Patients, post-treatment | –0.080 | 0.597 |
| Controls | 0.204 | 0.179 |
*Spearman's rho correlation coefficient.
Discussion
In addition to direct negative effects of vitamin B12 deficiency on the cell cycle, increased HCY levels accompanied by vitamin B12 deficiency exert multiple harmful effects on tissues via biochemical pathways.7 HCY is a metabolite with pro-oxidant activity. Proatherogenic and neurodegenerative effects of HCY have been reported to be possibly related to low serum PON1 activity, which impairs antioxidant function and reduces the degradation capacity of HCY-thiolactone, one of the metabolism pathways of HCY.6,14,15
This study is the first to investigate levels of the antioxidant enzymes PON and ARES in adults with vitamin B12 deficiency and related hyperhomocysteinemia. PON and ARES activity in patients with vitamin B12 deficiency was similar to that of the healthy control group. Accordingly, there were no statistically significant differences between patients’ pre- and post-treatment measurements. We also found no significant correlation between PON and HCY.
An earlier study of PON and ARES activity found significantly lower pre-treatment values of these enzymes in children with anemia caused by iron and vitamin B12 deficiency compared with controls.16 The same study reported that there was no significant difference in PON and ARES activity in the pre- and post-treatment periods in the iron-deficiency anemia group. However, in vitamin B12-deficiency group, the activity of PON and ARES had significantly increased when evaluated 1 month after vitamin B12 treatment. These findings are in contrast to those of our study. The increase in post-treatment PON and ARES levels in children with vitamin B12-deficiency anemia might have been related to lower pre-treatment levels than control values; it might also have been due to antioxidant enzymes responding more quickly to treatment in childhood. In the study of Koç et al.,16 the children with vitamin B12-deficiency anemia had lower levels of hemoglobin (7.69 ± 1.74 g/dl) and vitamin B12 (101 ± 55 pg/ml) than found in our study group; therefore, it is possible that a more severe deficiency might be associated with more pronounced decreases in the activity of PON and ARES.
Karikas et al. have reported a negative relationship between high HCY levels and PON1 activity in healthy pre-school children.17 Likewise, various studies reporting a correlation between reduced PON and ARES activity and atherosclerosis have linked this reduction to an increase in HCY.15,18 Koç et al. found a reduction in PON and ARES levels in children with vitamin B12 deficiency, but serum HCY levels were not measured and the relationship of these enzymes to HCY could therefore not be evaluated.16
The results of the present study do not support the hypothesis that, in adults with vitamin B12 deficiency and related hyperhomocysteinemia, the antioxidant enzymes PON and ARES have a role in the underlying biochemical mechanisms of vitamin B12 deficiency. Our findings suggest that PON and ARES do not play a role in the systemic effects of vitamin B12 deficiency. On the other hand, the absence of any additional major systemic findings (e.g., neurological dysfunction) due to vitamin B12 deficiency, apart from anemia, might be considered to be related to the fact that there were no significant changes in PON and ARES levels. The lack of any significant difference in PON and ARES levels of the pre-treatment patient group compared with controls might have masked possible biochemical changes in enzyme activities that occurred after vitamin B12 treatment. In addition, the post-treatment evaluation in the patient group took place at 4–6 weeks. Changes at the cellular level may occur in the longer term, and it is possible that changes in PON and ARES activity could be difficult to detect at this early time point.
The main limitation of this study is the relatively small sample size. Nevertheless, the reliability of our study is increased by the exclusion of participants with co-morbid systemic disorders in the vitamin B12-deficiency population and the evaluation of plasma HCY levels.
In conclusion, this study found no specific changes in the activities of the antioxidant enzymes PON and ARES in adults with vitamin B12 deficiency and related hyperhomocysteinemia compared with controls, and that vitamin B12 replacement treatment did not have any significant effect on the activities of these enzymes. Further measurements of PON and ARES after a longer duration of treatment with a larger study group are needed.
Disclaimer statements
Contributors None.
Funding None.
Conflict of interest No author has a financial or other conflict of interest regarding this work.
Ethics approval This study was approved by the ethics committee of the Yildirim Beyazit University Medical Faculty.
References
- 1.Tefferi A, Pruthi RK. The biochemical basis of cobalamin deficiency. Mayo Clin Proc 1994;69(2):181–6. doi: 10.1016/S0025-6196(12)61046-5 [DOI] [PubMed] [Google Scholar]
- 2.Allen RH, Stabler SP, Savage DG, Lindenbaum J. Metabolic abnormalities in cobalamin (vitamin B12) and folate deficiency. FASEB J 1993;7(14):1344–53. [DOI] [PubMed] [Google Scholar]
- 3.Scalabrino G, Veber D, Mutti E. New pathogenesis of the cobalamin-deficient neuropathy. Med Secoli 2007;19(1):9–18. [PubMed] [Google Scholar]
- 4.Scalabrino G, Peracchi M. New insights into the pathophysiology of cobalamin deficiency. Trends Mol Med 2006;12(6):247–54. doi: 10.1016/j.molmed.2006.04.008 [DOI] [PubMed] [Google Scholar]
- 5.Antony AC. Megaloblastic anemias. In: Hoffman R, Benz E, Silberstein L, Heslop H, Weitz J, Anastasi J, (eds.) Hematology basic principles and practice, 6th ed. Canada: Elsevier Saunders; 2013. pp. 473–504. [Google Scholar]
- 6.Jakubowski H. Pathophysiological consequences of homocysteine excess. J Nutr 2006;136(6 Suppl):1741S–9S. [DOI] [PubMed] [Google Scholar]
- 7.Yilmaz N. Relationship between paraoxonase and homocysteine: crossroads of oxidative diseases. Arch Med Sci 2012;8(1):138–53. doi: 10.5114/aoms.2012.27294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suszynska J, Tisonczyk J, Lee HG, Smith MA, Jakubowski H. Reduced homocysteine-thiolactonase activity in Alzheimer's disease. J Alzheimers Dis 2010;19:1177. [DOI] [PubMed] [Google Scholar]
- 9.Jakubowski H. The pathophysiological hypothesis of homocysteine thiolactone-mediated vascular disease. J Physiol Pharmacol 2008;59(Suppl. 9):155–67. [PubMed] [Google Scholar]
- 10.Blatter MC, James RW, Messmer S, Barja F, Pometta D. Identification of a distinct human high-density lipoprotein subspecies defined by a lipoprotein-associated protein, K-45: identity of K-45 with paraoxonase. Eur J Biochem 1993;211:871–9. doi: 10.1111/j.1432-1033.1993.tb17620.x [DOI] [PubMed] [Google Scholar]
- 11.Mackness B, Durrington PN, Mackness MI. Human serum paraoxonase. Gen Pharmacol 1998;31:329–36. doi: 10.1016/S0306-3623(98)00028-7 [DOI] [PubMed] [Google Scholar]
- 12.Canales A, Sanchez-Muniz FJ. Paraoxonase, something more than an enyzme? Med Clin (Barc) 2003;121:537–48. doi: 10.1016/S0025-7753(03)74011-1 [DOI] [PubMed] [Google Scholar]
- 13.Jakubowski H. Homocysteine is a protein amino acid in humans. Implications for homocysteine-linked disease. J Biol Chem 2002;277:30425–8. doi: 10.1074/jbc.C200267200 [DOI] [PubMed] [Google Scholar]
- 14.Perla-Kaján J, Jakubowski H. Paraoxonase 1 protects against protein N-homocysteinylation in humans. FASEB J 2010;24(3):931–6. doi: 10.1096/fj.09-144410 [DOI] [PubMed] [Google Scholar]
- 15.Perla-Kaján J, Jakubowski H. Paraoxonase 1 and homocysteine metabolism. Amino Acids 2012;43(4):1405–17. doi: 10.1007/s00726-012-1321-z [DOI] [PubMed] [Google Scholar]
- 16.Koç A, Cengiz M, Ozdemir ZC, Celik H. Paraoxonase and arylesterase activities in children with iron deficiency anemia and vitamin B12 deficiency anemia. Pediatr Hematol Oncol 2012;29(4):345–53. doi: 10.3109/08880018.2011.645185 [DOI] [PubMed] [Google Scholar]
- 17.Karikas GA, Kriebardis A, Samara I, Papachristodoulou M, Fytou-Pallikari A. Serum homocysteine levels and paraoxonase 1 activity in preschool aged children in Greece. Clin Chem Lab Med 2006;44:623–7. doi: 10.1515/CCLM.2006.110 [DOI] [PubMed] [Google Scholar]
- 18.Bhattacharyya T, Nicholls SJ, Topol EJ, Zhang R, Yang X, Schmitt D. Relationship of paraoxonase 1 (PON1) gene polymorphism and functional activity with systemic oxidative stress and cardiovascular risk. JAMA 2008;299:1265–76. doi: 10.1001/jama.299.11.1265 [DOI] [PMC free article] [PubMed] [Google Scholar]


