Abstract
Objective
Monoclonal antibodies (MAbs) directed against the CD20 and CD52 antigens are used increasingly in patients with multiple sclerosis (MS). Several life-threatening opportunistic infections have been reported in postmarketing case series. The aim of this study was to investigate the incidence of infections and associated prognostic factors during the first year of treatment in patients receiving anti-CD20 (ocrelizumab or rituximab) or anti-CD52 MAbs (alemtuzumab).
Methods
A retrospective study was conducted in patients with MS referring to the Neurodegenerative Diseases Center at the University of Naples Federico II who received MAbs between November 2015 and June 2018.
Results
A total of 163 patients were enrolled. Approximately 40% of patients experienced lymphocytopenia during treatment. Eighty-six infective events were reported in 67 patients (41%). Bacterial infections were significantly more frequent with anti-CD20, whereas viral infections prevailed with alemtuzumab. Cytomegalovirus reactivation rates were significantly higher in the alemtuzumab group than in patients on anti-CD20 (51% vs 6%, P < .001). The overall annualized infection rate was 1.1 per patient-year, higher in patients on anti-CD52 versus those on anti-CD20 regimens (1.5 vs 0.8 per patient-year). Alemtuzumab treatment, prior exposure to ≥2 MS drugs, and iatrogenic immune impairment significantly and independently predicted an infection event (adjusted hazard ratio [aHR], 2.7; P = .013; aHR, 1.7; P = .052; and aHR, 2.9; P = .004; respectively).
Conclusions
Given their considerable infection risk, MS patients receiving MAbs should undergo timely follow up and tailored preventive interventions. Anti-CD52–based treatment, prior exposure to MS drugs, and on-treatment immune impairment are significant predictive factors of infection and their evaluation could help clinicians to stratify a patient’s risk of infection.
Keywords: Alemtuzumab, CMV, infection, monoclonal antibodies, multiple sclerosis
A high incidence of infections was observed in patients with multiple sclerosis (MS) receiving anti-CD20 and -CD52 agents. Cytomegalovirus reactivation rates were particularly high in Alemtuzumab-treated patients. Alemtuzumab treatment, prior exposure to MS drugs, and iatrogenic immune impairment were identified as independent risk factors for infections.
Introduction
The advent of monoclonal antibodies (MAbs)-based regimens was a milestone in the treatment of multiple sclerosis (MS). However, although the efficacy of MAbs is well established, the magnitude of their infective risk remains a matter of debate [1, 2]. Indeed, the risk of infection depends both on the mechanisms of action of drugs, and host-related factors (age, comorbidities, exposure to corticosteroids or to other immune-modulating agents, neurologic dysfunctions, and mobility). As yet, there is no standardized consensus regarding pretreatment testing, vaccinations, patient education or counseling before and during therapy, or infection monitoring strategies [3, 4].
In addition to cases of John Cunningham virus (JCV) infection and progressive multifocal leukoencephalopathy (PML) reported in patients on anti-VLA4 (very late antigen-4) directed agents (ie, natalizumab), major infectious complications, in terms of both frequency and severity, have been observed in patients on MAbs that target lymphoid cell surface antigens, namely anti-CD52 (alemtuzumab [ALM]) and anti-CD20 agents (ocrelizumab [OCR] and rituximab [RTX]) [1, 5]. Despite the relatively low infection rate recorded in randomized controlled trials [6–8], potentially life-threatening infections have been reported in patients receiving these drugs [9–14].
We conducted a retrospective study to evaluate the incidence and predictive factors of infectious adverse events (IAE) in patients affected by MS-related disorders during the first year of treatment with MAbs directed against CD20 and CD52 antigens.
METHODS
Patients
All patients referring to the Centre of Neurodegenerative Diseases of Naples at the University of Naples Federico II who started ALM (Lemtrada®), OCR (Octrevus®) or RTX (Mabthera®, off-label use) from November 1, 2015, to June 1, 2018, were retrospectively recruited. Inclusion criteria were a diagnosis of MS spectrum disorder (relapsing-remitting MS, primary-progressive MS, secondary-progressive MS, or neuromyelitis optica), age ≥18 years, and availability of clinical and laboratory follow-up data. Ongoing infection at enrollment was an exclusion criterion. The MAb schedules are reported in the online supplementary material. Of note, in Italy, ALM was approved for MS treatment in April 2015 while OCR was approved in January 2018. With regard to RTX, this drug was approved for the treatment of neuromyelitis optica in December 2017, while it still warrants off-label administration in MS patients.
Data Collection
Data were collected from paper charts and electronic medical records. All patients consented to anonymous data collection for scientific purposes upon initiation of MAb therapy. Given the retrospective nature of the study, specific informed consent was not required. All data were processed according to current privacy regulations and the standards of good clinical practice. The following data were collected at baseline: age, gender, diagnosis, MAb therapy start date, disease duration, Expanded Disability Status Scale (EDSS) scores, lesion accrual on brain magnetic resonance imaging, previous exposure to disease-modifying therapies (DMTs), comorbidities, total lymphocyte and CD4+ T-cell count, data regarding exposure to infection (namely cytomegalovirus [CMV], hepatitis B virus [HBV], hepatitis C virus [HCV], HIV, and varicella zoster virus [VZV]), JCV serostatus, and Mycobacterium tuberculosis (MTB) exposure (based on Quiagen, ADA s.r.l., Padua, Italy). At the discretion of the physician, patients with resolved HBV infection (HB surface antigen -/HB core antibody +/HBV DNA negative) received prophylaxis with lamivudine (100 mg/day). Patients with latent MTB infection received isoniazid 300 mg/day for 6 months starting at the onset of MAb treatment.
Duration of follow up was defined as the interval between the MAb start date and the last visit in the following year (up to 365 days). In patients who did not experience an infective event, duration of follow up was defined as the observation period. In patients with infective complications, the observation period corresponded to the interval between the MAb start date and the date of the first infective event. In case of subsequent infective events, the date of the first event was considered the observation end-date. Laboratory variables were collected at baseline, +1 month, +3 months, +6 months, +12 months, and in case of infection. New onset of lymphocytopenia (<800 cells/μl) and/or hypogammaglobulinemia (IgG <7 g/dl) and/or neutropenia (<1000 cells/μl) during treatment were considered indicative of iatrogenic immune impairment.
Infective adverse events were defined as new infections that persisted for more than 24 hours. Infection could be either microbiologically or clinically documented. Diagnostic criteria are reported in the online supplementary material. The following bacterial infections were recorded: bacteraemia and sepsis, urinary tract infection (UTI), respiratory tract infection (RTI), endocarditis, acute gastroenteritis, intra-abdominal infection, skin-soft tissues or bone infection, central nervous system infection, and MTB infection [15–18]. Fungal infections were recorded according to the European Organization for Research and Treatment of Cancer/Mycoses Study Group 2008 criteria of possible, probable, or certain invasive fungal infection [19]. Viral infections caused by the following agents were recorded: HSV, VZV, CMV, HBV, and JCV (PML) [20–22]. Cytomegalovirus reactivation was defined as detectable viremia (>85 copies/ml on serum) regardless of signs of CMV disease. Serum CMV viral load was evaluated at +1 month, +3 months, and +6 months after treatment onset.
In case of infection, we evaluated aetiology, severity, outcome, site of infection, and time of infection onset after starting treatment. An infection was considered severe (versus mild or moderate) when it was fatal and/or required hospitalization or intravenous anti-infective drug use. For each patient, we calculated the total number of infective events and infective recurrences (ie, when the same agent caused the same clinical event during follow up). The immunological and virologic tests used are reported in the online supplementary material.
Statistical Analysis
The Gaussian distribution of quantitative variables was evaluated with the Kolmogorov-Smirnov test. Quantitative variables are reported using the mean and standard deviation (SD) in the case of Gaussian distribution and median and interquartile range (IQR) in the case of non-Gaussian distribution. The t test was used for comparisons between parametric quantitative variables. Comparisons between nonparametric and nonpaired continuous variables were assessed with the Mann-Whitney test while paired continuous variables were assessed with the Wilcoxon signed rank test. The χ2 test with Yates correction (or Fisher exact test when appropriate) was used for comparisons between categorical variables. The Kaplan-Meier method was used to evaluate the crude time-to-infection. The effect of the single variables was evaluated using the log rank test. The association between infective events and a variety of potential predictors was investigated with a univariate Cox regression analysis. All results were expressed as adjusted hazard ratios (aHR) with 95% confidence intervals (CI). To evaluate the individual contribution of each independent factor, variables that showed a significant association at univariate analysis were included in a multivariate Cox regression model, together with clinically relevant covariates according to the physician’s judgement. For all tests, P values < .05 were considered statistically significant. Statistical analyses were performed using the software package SPSS version 18.0 (PASW Statistics, Inc., Chicago, IL).
RESULTS
Baseline Characteristics
A total of 163 MS patients were enrolled in the study. Of these, 82 patients (41%) received ALM, 38 patients (23%) received OCR, and 58 patients received RTX (36%). Demographic characteristics are reported in Table 1 and are stratified according to drug class. Patients were equally affected by relapsing-remitting and primary MS phenotypes (48%). The median baseline EDSS score was 5.5 (IQR, 4–6.5). Median lymphocyte and CD4+ T-cell counts were within normal ranges both in patients receiving anti-CD20 and in patients receiving anti-CD52. No patient was HIV or HCV seropositive. No patient had active HBV infection; 20 patients had HBV resolved infection, but only 1 (on RTX-treatment) received lamivudine prophylaxis. No patient had active MTB infection; 4/5 patients with latent MTB infection received isoniazid (2/2 on anti-CD20 drugs and 2/3 on ALM).
Table 1.
Main Characteristics of Patients Receiving Anti-CD20 or Anti-CD52 Agents for Multiple Sclerosis Spectrum Disordersa
| Total (n = 163) n (%) | Anti-CD20 (n = 96) n (%) | Anti-CD52 (n = 67) n (%) | P (χ2 test) | |
|---|---|---|---|---|
| Female sex | 100 (61) | 50 (52) | 50 (75) | .004 |
| Age (years, mean ± SD) | 44.5 ± 11.4 | 48.4 ± 10.3 | 38.9 ± 10.5 | <.001 (t test) |
| Comorbidity burden | ||||
| No comorbidity | 53 (34) | 23 (25) | 30 (48) | .003 |
| 1 comorbidity | 47 (30) | 25 (27) | 22 (35) | .264 |
| 2–3 comorbidities | 31 (20) | 23 (25) | 8 (13) | .069 |
| >3 comorbidities | 26 (17) | 23 (25) | 3 (5) | .001 |
| Median disease duration [years] | 9.8 [4.4–15.8] | 11.1 [5.5–18.1] | 7.5 [4.0–13.5] | .005 (Mann-Whitney test) |
| Lesion accrual on brain MRIb | ||||
| Low | 7 (5) | 4 (5) | 3 (6) | 1.000 |
| Medium | 24 (18) | 12 (15) | 12 (24) | .187 |
| High | 100 (76) | 65 (80) | 35 (70) | .180 |
| EDSS scores | ||||
| <3.5 | 29 (18) | 5 (5) | 24 (36) | <.001 |
| 3.5–5 | 39 (24) | 18 (19) | 21 (31) | .064 |
| 5–7 | 61 (37) | 42 (44) | 19 (28) | .046 |
| ≥7 | 33 (20) | 30 (31) | 3 (5) | <.001 |
| Diagnosis | ||||
| RRMS | 78 (48) | 20 (21) | 58 (87) | <.001 |
| PPMS | 24 (15) | 24 (25) | 0 (0) | <.001 |
| SPMS | 55 (34) | 46 (48) | 9 (13) | <.001 |
| NMO | 5 (3) | 5 (5) | 0 (0) | .079 |
| DMT exposure | ||||
| Naïve | 17 (10) | 10 (10) | 7 (10) | 0.995 |
| Single | 31 (19) | 17 (18) | 14 (21) | 0.610 |
| Two-three lines | 66 (41) | 38 (40) | 28 (42) | 0.778 |
| Four or more lines | 49 (30) | 31 (32) | 18 (27) | 0.457 |
| MAbs-experienced | 61 (38) | 25 (26) | 36 (55) | <0.001 |
| Median wash out time from last DMT [days] | 37 [0–134] | 68 [0–178] | 16 [1–67] | 0.087 (Mann-Whitney test) |
| Infections | ||||
| CMV seropositivity (IgG) | 128 (79) | 78 (81) | 50 (75) | 0.311 |
| VZV seropositivity (IgG) | 155 (95) | 92 (96) | 63 (94) | 0.718 |
| HBV serostatus | ||||
| HBV seronegative | 101 (62) | 68 (71) | 33 (49) | 0.005 |
| Resolved HBV | 20 (12) | 14 (15) | 6 (9) | 0.281 |
| HBV vaccination | 42 (26) | 14 (15) | 28 (42) | <0.001 |
| TBC serostatus | ||||
| LTBI | 5/112 (4) | 2/65 (3) | 3/48 (6) | 0.652 |
| JCV seropositivity (IgG) | 111/132 (84) | 56/72 (78) | 55/60 (92) | 0.030 |
| Baseline immune status | ||||
| Median lymphocyte count [cells/μl] | 1410 [1050–2050] | 1390 [1120–1815] | 1500 [850–2300] | 0.170 (Mann-Whitney test) |
| Lymphocyte count > 800 cells/μl | 133 (82) | 83 (87) | 50 (75) | 0.055 |
| Lymphocyte count 800-500 cells/μl | 22 (14) | 11 (12) | 11(16) | 0.362 |
| Lymphocyte count 500-200 cells/μl | 8 (5) | 2 (2) | 6 (9) | 0.065 |
| Median C4+ T-cell count | 674 [371–1020] | 654 [376–954] | 704 [347–1104] | 0.652 (Mann-Whitney test) |
| C4+ T-cell count < 200 cells/μl | 21/150 (14) | 6/90 (7) | 15/60 (25) | 0.002 |
| Follow-up data | ||||
| Median follow-up days | 226 [96–365] | 133 [64–231] | 365 [345–365] | <0.001 (Mann-Whitney test) |
| Suspension rate | 12 (7) | 4 (4) | 8 (12) | 0.073 |
Abbreviations: CMV, cytomegalovirus; DMT, disease modifying therapies; EDSS, Expanded Disability Status Scale; HBV, hepatitis B virus; Ig, immunoglobulin; JCV, John Cunningham virus; LTBI, latent tuberculosis infection; MAbs, monoclonal antibodies; MRI, magnetic resonance imaging; NMO, neuromyelitis optica, PPMS, primary progressive MS; RRMS, relapsing-remitting MS; SD, standard deviation; SPMS, secondary progressive MS; TBC, tuberculosis; VZV, varicella zoster virus.
aData are expressed as number (percentage) for qualitative variables or median (interquartile range) or mean ± SD for quantitative variables.
bThirty-two records missing.
Treatment Follow up
As shown in Table 1, median follow up was 226 days (IQR, 96–365 days; 60 patient years). Follow up was longer in patients treated with anti-CD52 than in patients treated with anti-CD20 (365 days; IQR, 345–365 vs 133 days; IQR, 64–231) (P < .001, χ 2 test). Regarding iatrogenic immune impairment, 67 patients (41%) experienced lymphocytopenia, 11 (7%) had concomitant hypogammaglobulinemia, and 2 (1%) developed concurrent neutropenia. The iatrogenic immune impairment rate was significantly higher in patients receiving anti-CD52 regimens than in patients receiving anti-CD20 regimens (87% vs 22%; P ≤ .001, χ 2 test). Nearly all patients (92%) developed an immune impairment during the first month of treatment with no difference between the 2 treatment groups (P = 1.00, χ 2 test).
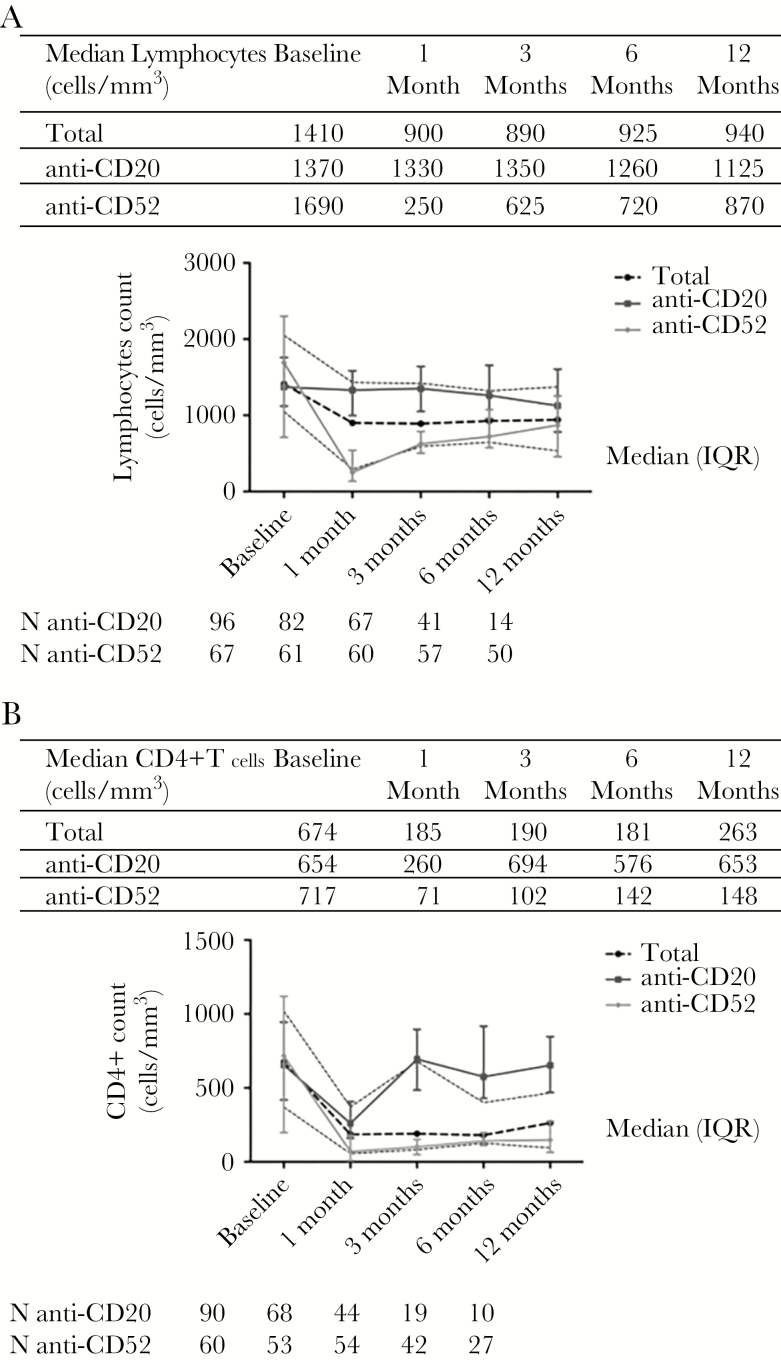
Figure 1 shows the median lymphocyte and CD4+ T-cell counts at baseline and at 1, 3, 6, 12 months, and thereafter, stratified according to drug class. Both total lymphocyte and CD4+ T-cell levels significantly decreased during the first month of treatment (median lymphocytes, 1410 cells/μl; IQR, 1050–2050 at baseline vs 900 cells/μl; IQR, 290–1430 at +1 month, P < .001; median CD4+ T-cells, 674 cells/μl; IQR, 371–1020 at baseline vs 185 cells/μl; IQR, 56–370 at +1 month, P < .001; Wilicoxon signed rank test). At +1 month, the decrease in lymphocyte counts was greater in anti-CD52–treated patients than in anti-CD20–treated patients (Δ median lymphocytes, -505 cells/μl; IQR, -1180–-1945 vs -103 cells/μl; IQR, -60–-230, respectively [P < .001]; Δ median CD4+ T-cells, -544 cells/μl; IQR, -87–-992 vs -379 cells/μl; IQR, -155–-734 cells/μl, respectively [P = .188; Mann-Whitney test]). Almost all patients recovered the total lymphocyte count within +6 months after the first drug infusion, whereas CD4+ T-cell levels remained significantly lower than pretreatment levels in anti-CD52–treated patients even a year later (P < .001, χ 2 test).
Figure 1.
Total lymphocytic (A) and CD4+ T cells (B) counts during treatment.
Infective Adverse Events
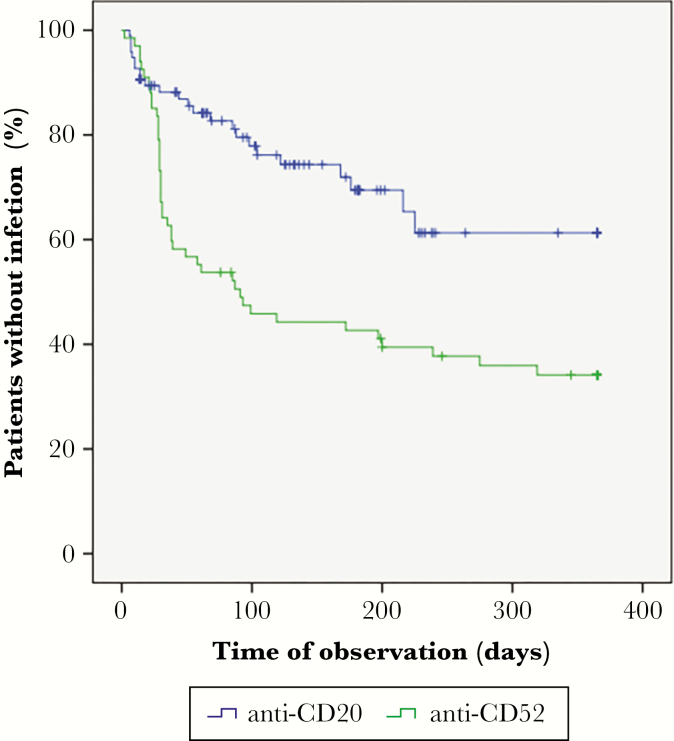
Eighty-six IAEs occurred in 67 (41%) patients. Fifty-one of the 67 patients developed a single infection and 16 patients experienced multiple events (2 events in 14 patients, and 4 and 3 events in 2 patients, respectively). Nineteen infections, which occurred in 15 patients, were considered severe, and 67 infections, which occurred in 52 patients, were considered mild to moderate. Outcome was favorable in all patients, except for a case of fatal aspergillosis. The overall annualized infection rate was 1.1 per patient-year. The rate of IAEs was higher in patients receiving ALM than in those receiving anti-CD20 agents (1.5 vs 0.8 per patient-year). As shown in Figure 2, the mean crude time-to-infection onset was significantly shorter in ALM-treated patients (171 days; 95% CI, 134–208 than in anti-CD20–treated patients (263 days; 95% CI, 229–297; P = .001, log-rank test).
Figure 2.
Crude time-to-infection onset per drug class (P = .001; log-rank test).
The main characteristics of the 86 IAEs observed in the 2 groups of patients (anti-CD52 vs anti-CD20) are reported in Table 2. The IAE rate was significantly higher in the anti-CD52 arm (43/67 patients, 64%) than in the anti-CD20 arm (24 /86, 25%; P < .001, χ 2 test). At the time of infection, anti-CD52–treated patients had lower median lymphocyte counts (550 cells/μl; IQR, 220–910 vs 1205 cells/μl; IQR, 588–1580; P = .007, Mann-Whitney test) and higher rates of lymphocytopenia (71% vs 32%; P = .001, χ 2 test). The median time-to-any-infection did not differ significantly in terms of drug class and infection etiology (median time to bacterial infection onset, 55 days; IQR, 10–179 vs viral infection onset, 30 days; IQR, 28–87; P = .083, χ 2 test). However, infections of any type occurred more frequently during the first posttreatment month, especially in the anti-CD52 arm (32 of 58 infective events, 55%). Bacterial infections were more frequent in patients receiving anti-CD20 treatment than in those receiving anti-CD52 treatment (P < .001, χ 2 test), whereas viral infections were more frequent in anti-CD52–treated patients (P < .001, χ 2 test).
Table 2.
Infective Events According to Monoclonal Antibodies Administereda
| Total (n = 86) n (%) | Anti-CD20 (n = 28) n (%) | Anti-CD52 (n = 58) n (%) | P value (χ2 test) | |
|---|---|---|---|---|
| Recurrent IAE | 9 (11) | 4 (14) | 5 (9) | .325 |
| Etiology | ||||
| Bacterial | 31 (36) | 19 (68) | 12 (21) | <.001 |
| Viral | 49 (57) | 7 (25) | 42 (72) | <.001 |
| Fungal | 5 (6) | 2 (7) | 3 (5) | .527 |
| Severity | ||||
| Mild-moderate | 67 (78) | 20 (71) | 47 (81) | .314 |
| Severe | 19 (22) | 8 (29) | 11 (19) | — |
| Type of infection | ||||
| UTI | 23 (27) | 13 (46) | 10 (17) | .004 |
| RTI | 8 (9) | 6 (21) | 2 (3) | .013 |
| CMV reactivation | 42 (49) | 5 (18) | 37 (64) | <.001 |
| HSV or VZV reactivation | 4 (5) | 1 (4) | 3 (5) | .607 |
| Median time of onset (days) [IQR] | 31 [23–92] | 48 [10–103] | 30 [28–91] | .567 (Mann-Whitney test) |
| First month onset | 44 (51) | 12 (43) | 32 (55) | .284 |
| 2–6 months onset | 28 (33) | 10 (36) | 18 (31) | .664 |
| 7–12 months onset | 14 (16) | 6 (21) | 8 (14) | .274 |
| Immune status at infective event | ||||
| Median lymphocyte count [cells/μl, IQR] | 670 [250–1350] | 11205 [588–1580] | 5550 [220–910] | .007 (Mann-Whitney test) |
| Lymphocytopenia (<800 cells/μl) | 50 (58) | 9 (32) | 441 (71) | .001 |
| C4+ T-cell count < 200 cells/μl | 50/62 (81) | 110/13 (77) | 440/49 (82) | .485 |
| Median C4 T-cell countb [cells/μl, IQR] | 84 [22–177] | 1108 [0–427] | 178 [23–174] | .762 (Mann-Whitney test) |
| Median C8 T-cell countb [cells/μl, IQR] | 128 [41–231] | 1149 [0–271] | 1114 [47–235] | .986 (Mann-Whitney test) |
Abbreviations: CMV, cytomegalovirus; DMT, disease-modifying therapies; HSV, herpes simplex virus; IAE, infective adverse events; IQR, interquartile range; NS, not significant; RTI, respiratory tract infections; UTI, urinary tract infections; VZV, varicella zoster virus.
aData are expressed as number (percentage) or median (IQR).
bTwenty-four patient records missing (15 on anti-CD20 and 9 on anti-CD52).
Regarding clinical features, UTI and RTI were more frequent in patients on anti-CD20 therapy than in patients on anti-CD52 therapy (P = .004 and .013, respectively, χ 2 test) (Table 2). Severity and recurrence of IAEs were similar in the 2 groups (Table 2). Eight severe infections requiring hospitalization and/or intravenous anti-infective treatment occurred in RTX-treated patients (1 case of Serratiamarcescens pneumonia, 1 case of pneumonia responsive to cotrimoxazole, 2 complicated UTI, 1 pyelonephritis, 1 acute prostatitis, 1 cellulitis, and 1 probable invasive pulmonary aspergillosis). In the ALM group, 11 patients required hospitalization and/or anti-infective treatment (1 case of Haemophilus influenzae type B pneumonia, 2 cases of complicated urinary tract infection, 1 case of cutaneous HSV infection and 1 of ocular HSV infection, 1 case of cutaneous zoster infection, 3 CMV diseases, 1 proven invasive pulmonary aspergillosis, and 1 case of acute toxoplasmosis during pregnancy). Four infections were reported in patients receiving OCR (4/38[6%], namely, 3 UTI and 1 CMV reactivation), all non-severe. The IAE rate was higher in subjects receiving RTX than in those receiving OCR (34.5% vs 10.5%; P = .008, χ 2 test).
During the first month of treatment, approximately half of the patients receiving ALM experienced CMV reactivation (24/50, 48%). No case of JCV disease (PML) was reported. Furthermore, no patient with resolved HBV infection experienced viral reactivation (mean follow up, 182 days; SD, 156). Similarly, no patient with latent MTB infection developed active disease (median follow up, 61 days; IQR, 21–270). The occurrence of infection caused treatment delay in 9 patients (all on RTX) and suspension of treatment in 6 patients (3 on ALM and 3 on RTX).
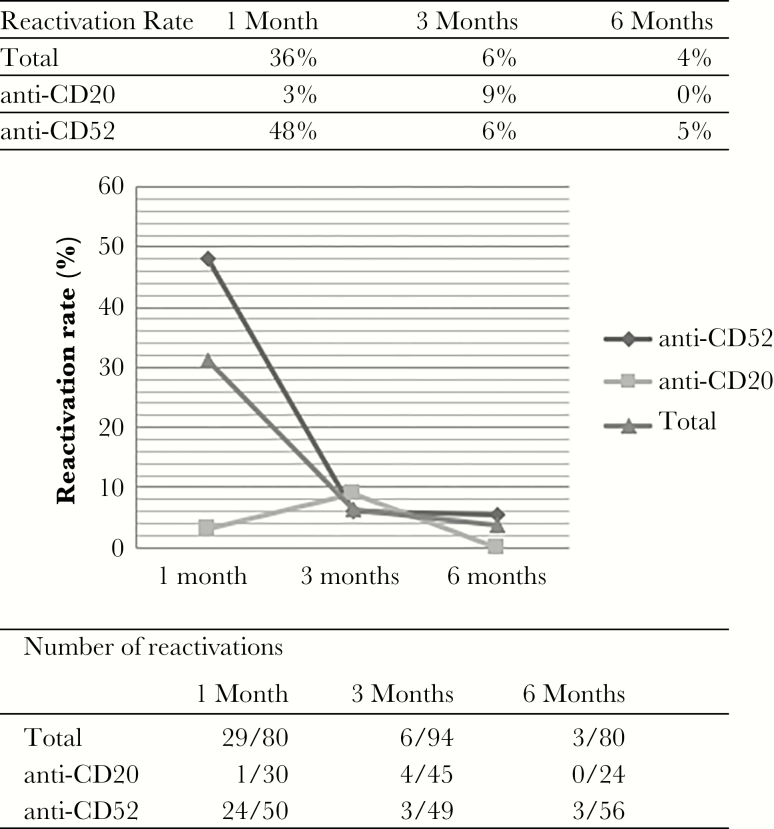
CMV Reactivation
As shown in Figure 3, 80 patients were screened for CMV viral load at the end of the first month (30/96 patients on anti-CD20 and 50/67 patients on anti-CD52), 94 patients were screened at the end of the third month (45/96 patients on anti-CD20 and 49/67 patients on anti-CD52), and 80 patients were screened at the end of the sixth month (24/96 patients on anti-CD20 and 56/67 patients on anti-CD52). Cytomegalovirus reactivation rates fluctuated in both groups during follow up (Figure 3). Episodes of CMV reactivation were significantly more frequent in patients receiving anti-CD52 MAbs than in those receiving anti-CD20 MAbs (34 /67, 50.7% vs 5/96, 5.2%; P < .001, χ 2 test). No patient in the anti-CD20 group had a viral load >160 copies/ml or experienced active disease during reactivation. In the ALM-treated group, 3 patients experienced 2 reactivation events, 10/34 patients (29%) had a CMV viral load >1000 copies/ml (8 patients with CMV DNA > 3000 copies/ml), and 4/34 (12%) developed active disease requiring antiviral treatment (1 with pneumonia, 2 patients with fever and thrombocytopenia, and 1 reactivation during pregnancy, which caused congenital CMV infection and sensorineural hearing loss in the newborn).
Figure 3.
CMV reactivation rates during treatment according to drug class.
Predictors of Infection
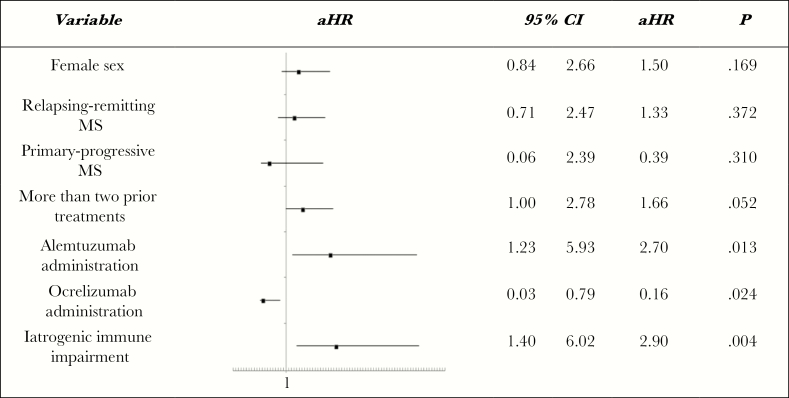
As shown in Table 3, at univariate Cox analysis, women and subjects affected by relapsing-remitting MS or with a history of more than 2 DMTs were significantly more prone to experience an IAE, whereas patients with primary-progressive MS were less likely to have an IAE. Administration of ALM was associated with an increased risk of subsequent infections, whereas the rate of infections was significantly lower in OCR-treated patients. Consequently, statistically significant covariates and the development of immune impairment were included in the multivariate Cox regression model. As shown in Figure 4, ALM and prior MS treatment were predictive of infection (aHR, 2.7; CI, 1.2–5.9; P = .012 and aHR, 1.7; CI, 1.0–2.8; P = .052, respectively, Cox test), while IAEs were significantly less common in OCR-treated patients (aHR, 0.2; CI, 0.0–0.8; P = .024, Cox test). Furthermore, iatrogenic immune impairment had an additional effect on IAE rate (aHR, 2.9; CI, 1.4–6.0; P = .004, Cox test). We also evaluated risk factors for IAEs not including cases of CMV reactivation with spontaneous resolution. At multivariate Cox analysis, only female sex was a significant predictive factor of infection (aHR, 2.447; CI, 1.173–5.104; P = .017), whereas patients receiving OCR were significantly less likely to develop infective complications (aHR, 0.183; CI, 0.044- 0.763; P = .020).
Table 3.
Risk Factors for the Development of Infective Adverse Events (n = 86)
| Patients without IAE (n = 96) n (%) | Patients with IAE (n = 67) n (%) | HR | 95% CI | P (Cox-Mantel test) | ||
|---|---|---|---|---|---|---|
| Female sex | 51 (53) | 49 (73) | 1.93 | 1.12 | 3.31 | .017 |
| Age > 40 years | 64 (67) | 38 (57) | 0.95 | 0.58 | 1.55 | .835 |
| Disease features | ||||||
| Disease duration > 10 years | 49 (51) | 32 (48) | 0.96 | 0.60 | 1.56 | .877 |
| EDSS > 5 | 59 (62) | 36 (54) | 0.72 | 0.36 | 1.38 | .304 |
| Presence of comorbidities | 63/93 (68) | 41/64 (64) | 1.18 | 0.71 | 1.96 | .529 |
| Diagnosis | ||||||
| RRMS | 36 (38) | 42 (63) | 1.83 | 1.11 | 3.01 | .018 |
| PPMS | 21 (22) | 3 (5) | 0.29 | 0.09 | 0.91 | .035 |
| SPMS | 34 (35) | 21 (31) | 0.85 | 0.51 | 1.42 | .535 |
| NMO | 4 (4) | 1 (2) | 0.53 | 0.07 | 3.87 | .528 |
| Prior DMT | ||||||
| More than two prior DMT | 36 (38) | 38 (57) | 1.67 | 1.03 | 2.70 | .039 |
| MAbs exposure | 30 (31) | 31 (46) | 1.30 | 0.80 | 2.12 | .285 |
| Less than 4 weeks wash out time | 34/80 (43) | 33/57 (58) | 1.13 | 0.66 | 1.92 | .656 |
| MAbs | ||||||
| Alemtuzumab-based regimen | 24 (25) | 43 (64) | 2.24 | 1.35 | 3.72 | .002 |
| Ocrelizumab-based regimen | 34 (35) | 4 (6) | 0.20 | 0.07 | 0.55 | .002 |
| Rituximab-based regimen | 38 (40) | 20 (30) | 0.93 | 0.55 | 1.58 | .792 |
| Immune status | ||||||
| BL lymphocytopenia (<800 cells/μl) | 14 (15) | 16 (24) | 1.13 | 0.64 | 1.98 | .677 |
| BL CD4+ T-cell count < 200 cells/μl | 9/88 (10) | 12/62 (19) | 1.25 | 0.66 | 2.35 | .491 |
| Iatrogenic immune impairmenta | 38 (40) | 41 (61) | 1.21 | 0.73 | 2.00 | .463 |
Abbreviations: BL, baseline; CI, confidence intervals; DMT, disease-modifying therapies; EDSS, Expanded Disability Status Scale; HR, hazard ratio; IAE, infective adverse events; MAbs, monoclonal antibodies; NMO, neuromyelitis optica; PPMS, primary progressive multiple sclerosis; RRMS, relapsing-remitting multiple sclerosis; SPMS, secondary progressive multiple sclerosis.
aDefined as new onset of lymphocytopenia and/or hypogammaglobulinemia and/or neutropenia during treatment.
Figure 4.
Predictive risk factors of infections at multivariant Cox regression analysis.
Discussion
In this retrospective study, we investigated the incidence and main predictors of infection in patients with MS treated with biological agents that target lymphocyte cell surface antigens. To the best of our knowledge, this is the first study to compare the infective risk of the various classes of MAbs in patients affected by MS. We recruited 163 patients who started targeted therapy within a 28-month period (86 on anti-CD20 MAb and 67 on anti-CD52 MAb). Median follow up was longer in patients receiving ALM (365 days) than in patients receiving anti-CD20 agents (133 days; P < .001, Mann-Whitney test), probably because ALM was approved for MS almost 3 years before anti-CD20 drugs. Approximately 40% of patients experienced 1 or more IAE, irrespective of demographic and neurological clinical features at baseline. Severe infections were mainly of bacterial origin (53%). Outcome was favorable in all patients, except in a young woman who developed severe neutropenia and lymphopenia 1 month after ALM infusion and died from fulminant necrotizing pulmonary aspergillosis. Several opportunistic infections were reported, namely, mucocutaneous candidiasis (3 cases), invasive pulmonary infection caused by Aspergillus fumigatus (2 cases), primary Toxoplasma gondii infection (1 case), Herpes simplex reactivation (3 cases), VZV reactivation (1 case), and CMV reactivation (41 cases). Most of these complications occurred soon after drug infusion and could be favored by the considerable decrease of lymphocytes (total and CD4+ T-cells) and premedication with high doses of corticosteroids. Although we did not observe any case of PML, HBV, or MTB reactivation, the relatively small sample size and the limited follow up preclude any firm conclusion regarding these infections.
Notably, the infective risk was significantly higher in patients receiving anti-CD52 regimens who had a history of DMT or who developed iatrogenic immune impairment (namely, lymphocytopenia) during therapy. Patients treated with ALM experienced mainly viral infections (42 out of 58 IAEs). Remarkably, the CMV reactivation rate was 55% in patients treated with ALM (37/67). This finding is in agreement with the results of studies conducted in hematologic settings. In fact, Laurenti et al observed a comparable rate of CMV reactivation (66%) in a small cohort of patients receiving ALM for chronic lymphocytic leukaemia [23]. Similarly, Vallejo and colleagues found a CMV reactivation rate of about 40% in patients treated with ALM for lymphoproliferative disorders, and the reactivation rate was even higher (46%) when corticosteroids were co-administered [24]. Such high reactivation rates may be biased by missing samples or differences in viral monitoring strategies (eg, timing or type of assays). Nevertheless, given recent postmarketing reports of CMV disease in MS patients [10–12, 25], this complication should invariably be considered in case of suggestive clinical features in the MS setting.
Most of the infections in the anti-CD20–treated patients were of bacterial origin, which coincides with the finding of Trivin et al that 79% of all infections were bacterial in a cohort of patients receiving RTX for autoimmune renal syndromes [26]. Moreover, among CD20-treated patients, the infective rate was significantly lower in patients receiving OCR than in those receiving RTX, which further supports the concept that safety profiles differ between the 2 agents in terms of IAEs.
As in most retrospective studies, missing data constitute a possible limitation of our study. Mild infections may have been underreported because they did not require treatment or a medical consultation. The lack of a control group, which was due to different screening and follow-up protocols in historical cohorts of our institute, is another potential limitation of our work. The strengths of the study are the real-life profile of the examined population, irrespective of potential differences in disease activity, comorbidities and prior treatments, and the collection of data regarding all types of infection. In addition, the finding of an elevated IAE rate in MAb-experienced patients, who are generally excluded from randomized clinical trials, sheds new light on drug safety and tolerability in this difficult-to-treat subset of patients.
Finally, the evidence that MAbs therapy is associated with a significant infective risk in patients affected by MS, particularly during the early months after infusion, reinforces the need to set-up specific management protocols. Because on-treatment immune impairment is a more effective predictor of IAEs than is baseline immune status, follow up should be customized for each patient. Moreover, given the high CMV reactivation rate reported in ALM-treated patients, serial serum CMV DNA monitoring may be advisable shortly after drug infusion. In fact, viral load monitoring could encourage timely reporting of CMV reactivation in these patients, helping to better estimate the probability of such events.
In conclusion, our findings provide new insights into infectious safety issues of anti-CD20 and -CD52 agents in an MS setting. These results can facilitate the assessment of infective risk in this population, thereby helping clinicians to stratify patients and optimize preventive interventions.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We thank Jean Ann Gilder (Scientific Communication srl., Naples, Italy) for language assistance.
Financial support. None reported.
Potential conflicts of interest. I.G. acted as a consultant for MSD, Abbvie, and Correvio. He received grants from Gilead Science in the framework of a fellowship program. R.L. received grants or honoraria for public speaking from Biogen, Roche, Novartis, Merck, Genzyme, and Teva. F.S. received compensations for public speaking, advisory boards, and travel grants from Almirall, Argenx, Biogen, Forward Pharma, Merck, Novartis, Pomona, Roche, Sanofi, and Teva. All other authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Grebenciucova E, Pruitt A. Infections in patients receiving multiple sclerosis disease-modifying therapies. Curr Neurol Neurosci Rep 2017; 17:88. [DOI] [PubMed] [Google Scholar]
- 2. Celius EG. Infections in patients with multiple sclerosis: implications for disease-modifying therapy. Acta Neurol Scand 2017; 136(Suppl 201):34–6. [DOI] [PubMed] [Google Scholar]
- 3. Epstein DJ, Dunn J, Deresinski S. Infectious complications of multiple sclerosis therapies: implications for screening, prophylaxis, and management. Open Forum Infect Dis 2018; 5:ofy174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Buonomo AR, Zappulo E, Viceconte G, et al. . Risk of opportunistic infections in patients treated with alemtuzumab for multiple sclerosis. Expert Opin Drug Saf 2018; 17:709–17. [DOI] [PubMed] [Google Scholar]
- 5.Ho PR, Koendgen H, Campbell N, Haddock B, Richman S, Chang I. Risk of natalizumab-associated progressive multifocal leukoencephalopathy in patients with multiple sclerosis: a retrospective analysis of data from four clinical studies. Lancet Neurol 2017; 16:925–33. [DOI] [PubMed] [Google Scholar]
- 6. Coles AJ, Twyman CL, Arnold DL, et al. ; CARE-MS II investigators Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomised controlled phase 3 trial. Lancet 2012; 380:1829–39. [DOI] [PubMed] [Google Scholar]
- 7. Montalban X, Hauser SL, Kappos L, et al. ; ORATORIO Clinical Investigators Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med 2017; 376:209–20. [DOI] [PubMed] [Google Scholar]
- 8. Hawker K, O’Connor P, Freedman MS, et al. ; OLYMPUS trial group Rituximab in patients with primary progressive multiple sclerosis: results of a randomized double-blind placebo-controlled multicenter trial. Ann Neurol 2009; 66:460–71. [DOI] [PubMed] [Google Scholar]
- 9. Holmøy T, von der Lippe H, Leegaard TM. Listeria monocytogenes infection associated with alemtuzumab - - a case for better preventive strategies. BMC Neurol 2017; 17:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Buonomo AR, Saccà F, Zappulo E, et al. . Bacterial and CMV pneumonia in a patient treated with alemtuzumab for multiple sclerosis. Mult Scler Relat Disord 2019; 27:44–5. [DOI] [PubMed] [Google Scholar]
- 11. Clerico M, De Mercanti S, Artusi CA, et al. . Active CMV infection in two patients with multiple sclerosis treated with alemtuzumab. Mult Scler 2017; 23:874–6. [DOI] [PubMed] [Google Scholar]
- 12. Pappolla A, Midaglia L, Boix Rodríguez CP, et al. . Simultaneous CMV and Listeria infection following alemtuzumab treatment for multiple sclerosis. Neurology 2019; 92:296–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Penkert H, Delbridge C, Wantia N, et al. . Fulminant central nervous system nocardiosis in a patient treated with alemtuzumab for relapsing-remitting multiple sclerosis. JAMA Neurol 2016; 73:757–9. [DOI] [PubMed] [Google Scholar]
- 14. Rissanen E, Remes K, Airas L. Severe neutropenia after rituximab-treatment of multiple sclerosis. Mult Scler Relat Disord 2018; 20:3–5. [DOI] [PubMed] [Google Scholar]
- 15. Singer M, Deutschman CS, Seymour CW, et al. . The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016; 315:801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mermel LA, Allon M, Bouza E, et al. . Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis 2009; 49:1–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Habib G, Lancellotti P, Antunes MJ, et al. ; ESC Scientific Document Group 2015 ESC Guidelines for the management of infective endocarditis: the Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J 2015; 36:3075–128. [DOI] [PubMed] [Google Scholar]
- 18. World Health Organization. Treatment of tuberculosis: guidelines. 4th edition. Geneva, Switzerland: WHO Press; 2009. [Google Scholar]
- 19. De Pauw B, Walsh TJ, Donnelly JP, et al. ; European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group; National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis 2008; 46:1813–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. European Association for the Study of the Liver. Clinical practice guidelines on the management of hepatitis B virus infection. J Hepatol 2017; 67:370–98. [DOI] [PubMed] [Google Scholar]
- 21. Pawlotsky J-M, Negro F, Aghemo A, et al. . EASL recommendations on treatment of hepatitis C 2018. J Hepatol 2018;69:461– 511. [DOI] [PubMed] [Google Scholar]
- 22. Berger JR, Aksamit AJ, Clifford DB, et al. . PML diagnostic criteria: consensus statement from the AAN neuroinfectious disease section. Neurology 2013; 80:1430–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Laurenti L, Piccioni P, Cattani P, et al. . Cytomegalovirus reactivation during alemtuzumab therapy for chronic lymphocytic leukemia: incidence and treatment with oral ganciclovir. Haematologica 2004; 89:1248–52. [PubMed] [Google Scholar]
- 24. Vallejo C, Ríos E, de la Serna J, et al. . Incidence of cytomegalovirus infection and disease in patients with lymphoproliferative disorders treated with alemtuzumab. Expert Rev Hematol 2011; 4:9–16. [DOI] [PubMed] [Google Scholar]
- 25. Eichau S, Lopez Ruiz R, Caston Osorio JJ, Ramírez E, Domínguez-Mayoral A, Izquierdo G. Primary cytomegalovirus infection in a patient with relapsing-remitting multiple sclerosis under treatment with alemtuzumab. Neurologia (Barcelona, Spain) 2018; 10.1016/j.nrl.2018.03.015. [DOI] [PubMed] [Google Scholar]
- 26. Trivin C, Tran A, Moulin B, et al. . Infectious complications of a rituximab-based immunosuppressive regimen in patients with glomerular disease. Clin Kidney J 2017; 10:461–9. [DOI] [PMC free article] [PubMed] [Google Scholar]