Abstract
Wounding is a key plant stress that results in a rapid, within seconds to a few minutes, release of ubiquitous stress volatiles and stored volatiles in species with storage structures. Understanding the timing and extent of wound-dependent volatile elicitation is needed to gain an insight into different emission controls, but real-time monitoring of plant emissions through wounding treatments has been hampered by the need to stop the measurements to perform the wounding, slow stabilization of gas flows upon chamber closure and smearing out the signal by large chambers and long sampling lines. We developed a novel leaf cutter that allows to rapidly perform highly precise leaf cuts within the leaf chamber. The cutter was fitted to the standard Walz GFS-3000 portable gas-exchange system leaf chamber and chamber exhaust air for analysis with a proton transfer reaction time-of-flight mass-spectrometer (PTR-TOF-MS) was taken right at the leaf chamber outlet. Wounding experiments in four species of contrasting leaf structure demonstrated significant species differences in timing, extent and blend of emitted volatiles, and showed unprecedently high emission rates of several stress volatiles and stored monoterpenes. In light of the rapid rise of release of de novo synthesized and stored volatiles, the results of this study suggest that past studies have underestimated the rate of elicitation and maximum emission rates of wound-dependent volatiles.
Keywords: emission measurements, interspecific variability, leaf injury, measurement protocol, lipoxygenase pathway, stress-elicited volatiles, terpene emission
Introduction
Leaf wounding is a frequent stress caused by chewing and piercing herbivores and by mechanical influences such as strong wind and falling debris. Plants respond to wounding by a fast release of a multitude of volatiles, including the release of methanol from cell wall pectins and lipoxygenase pathway volatiles from polyunsaturated fatty acids cleaved from plant membranes by stress-activated phospholipases (Feussner and Wasternack, 2002; Hayward et al., 2017; Liavonchanka and Feussner, 2006). In addition, several species store terpenes in specialized storage structures such as oil glands and resin ducts (Copolovici and Niinemets, 2016), and leaf wounding leads to rapid release of terpenes from these storage structures (Litvak et al., 1999; Loreto et al., 2000).
The wounding-dependent release of volatiles plays important roles in within-plant, plant-to-plant and plant-to-insect communication (Arimura et al., 2009; Arimura et al., 2011; Engelberth et al., 2004; Holopainen et al., 2013; Ton et al., 2007). Thus, understanding the timing, magnitude and composition of wound-elicited emissions is highly relevant to gain an insight into the potency and propagation of the wound-induced signal. Leaf cutting and punching experiments have been widely used to simulate the natural wounding stress and investigate the quantitative relationships between the severity of wounding and release of stress-elicited volatiles (Brilli et al., 2012; Brilli et al., 2011; de Gouw et al., 1999; Fall et al., 1999; Fall et al., 2001; Mithöfer et al., 2005; Portillo-Estrada et al., 2017; Portillo-Estrada et al., 2015). Although there is a broad similarity in the spectrum of volatile compounds released upon wounding by different species, there are also important species variations in magnitude and timing of volatile release and in volatile mixing rations in the emission blends (Ping et al., 2001; Vivaldo et al., 2017). Furthermore, for a given species, the amount and blend of volatiles can vary for leaves of different age (Portillo-Estrada et al., 2017) and for wounds through main veins and through intercostal leaf areas (Portillo-Estrada and Niinemets, 2018). So far, the biochemical basis for these differences in the emission of wound-induced volatiles is still not fully understood.
Understanding the elicitation of different biochemical processes requires continuous measurements through the wounding treatment using fast-response systems. However, many wounding experiments have been conducted with large leaf chambers with inherently slow response time (e. g., Brilli et al., 2012; Brilli et al., 2011; de Gouw et al., 1999; Fall et al., 1999; Fall et al., 2001; Staudt et al., 2010). Furthermore, in most of the experiments, the wounding treatment has been done outside the leaf chamber. This approach is associated with several limitations including missing of a significant fraction of wounding-elicited emissions due to the time needed for leaf reinsertion and for stabilization of gas flows in the leaf chamber. The missing part of the emissions could be gap-filled, but assumptions are needed about the time of elicitation of specific volatiles and time-dependent changes in the emission rates (Portillo-Estrada et al., 2015). A symmetric time-dependent emission change has been used for gap-filling for all volatile species, assuming that the rise of emissions can be predicted on the basis of the decline of emissions at the later stages of the emission response (Portillo-Estrada et al., 2015). However, the shape of the initial rise depends on the relative share of the emission controls between substrate availability and enzymatic activity. In the case of lipoxygenase volatiles, it is typically assumed that there is a large constitutive activity of different lipoxygenases and hydroperoxide lyases (Andreou and Feussner, 2009; Gigot et al., 2010; Hayward et al., 2017). Thus, a rapid increase of the availability of the substrate, the free polyunsaturated fatty acids, can lead to a much faster development of emissions than expected for a symmetric response. This, in turn, implies that we may have strongly underestimated these emissions. Analogously, in the case of terpene-storing species, the initial burst of terpenes when the contents of storage structures become exposed to air can be very high and these emissions cannot be predicted from the declining part of the emission response (e.g. Niinemets et al., 2011 for the initial rise of terpene release after damage of a conifer shoot or multiple shoots in a given tree; Ruuskanen et al., 2005).
The second limitation of temporary chamber opening for leaf cutting is that short-term exposure of the leaf to outside-chamber conditions with slightly different humidity, light, temperature and CO2 concentration can lead to alterations in leaf gas exchange rates. This in turn can alter volatile emissions, e.g. due to direct effects of photosynthesis on isoprene and monoterpene emissions in constitutive de novo emitters (Grote et al., 2013; Grote et al., 2014) and due to effects of stomatal conductance on emissions of water-soluble volatile such as methanol and lipoxygenase pathway volatiles (Harley, 2013; Niinemets et al., 2014).
The aims of this study were to develop a methodology for rapid measurements of volatile emissions through the wounding treatment. We constructed a novel within-chamber leaf cutter that allows making leaf cuts of precisely defined length within the leaf chamber and further modified the sampling of volatile collection protocol to limit the measurement system delays due to tubing connecting the leaf chamber and the gas analyzers. The novel leaf cutting protocol was further used to compare the magnitude, timing and composition of emissions in four representative herbaceous and woody species. The fast measurements with the novel measurement system through wounding treatments in dicot herb Phaseolus vulgaris, monocot herb Zea mays, constitutive isoprene-emitting tree Populus tremula x P. tremuloides and isoprene-emitting and monoterpene-storing tree Eucalyptus globulus suggested that previous measurements of wound-dependent volatile release may have significantly underestimated the rate of elicitation of wound-dependent volatiles, and maximum rate and total amount of volatile release.
Material and methods
Construction of a within-chamber leaf cutter
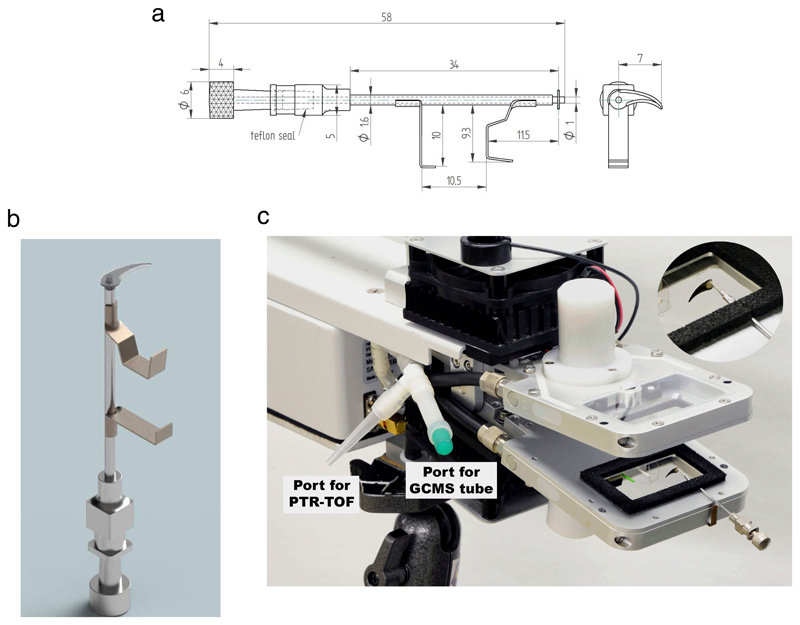
For within-chamber leaf wounding experiments, a novel cutter was developed precisely matching the Walz GFS-3000 gas-exchange system (Walz GmbH, Effeltrich, Germany) standard 4 x 2 cm (inner measures) measurement head 3010-S (Fig. 1). The cutting end of the leaf cutter was made of a razor blade. The blade was silver-soldered to a rod made of stainless steel wire (1 mm diameter). The blade-holding rod was inserted into a stainless steel needle (outer diameter 1.6 mm) of a medical syringe. The ends of the syringe needle were closed with Teflon stoppers, forming a good seal, but also allowing for forceless rotation of the rod with the knife blade around its central axis. The blade-holding rod was finished with a turning knob (Fig. 1a, b).
Fig. 1.
The construction scheme of the novel leaf cutter for leaf wounding stress experiments (a, all measures in mm), image of the cutter (b) and image of the Walz GFS-3000 portable gas-exchange system standard leaf chamber with the leaf cutter attached (c). The leaf cutter was primarily made of stainless steel components (see Material and methods for details of construction) and hermetically attached to the leaf chamber. The novel cutter allows leaf wounding within the chamber without the need to open the chamber for wounding stress application. Panel (c) also shows modifications in standard configuration of the volatile collection that allowed sampling volatiles as close to the leaf chamber as possible (Fig. 2 for differences between the novel setup and standard setup).
To attach the cutter firmly to the leaf chamber, two supports made of springy brass sheet (thickness 0.2 mm) were soldered to the stainless steel syringe needle (Fig. 1a, b). The configuration of the cutter supports allowed for rapid and steady attachment of the cutter onto the lower half of the leaf chamber. To have a good seal and avoid the outer needle surface touching the leaf, a triangular cut was made in the lower gasket of the Walz GFS-3000 leaf chamber (Fig. 1c), and the point of cutter insertion was sealed with a low emission sealing putty. The leaf was cut by turning the knife holder by 180 degrees around its axis. The knife blade length was chosen such that the cut length was 14 mm.
Testing for differences in volatile sampling methods
In the case of portable photosynthesis systems, the volatiles in leaf chamber exhaust air are typically sampled before passing the infrared CO2/H2O gas analyzers. Depending on the system, this can involve relatively long tubing as well as neoprene resin gaskets or O-rings along the air pathway. In the case of the standard sampling method with Walz GFS-3000 system (e.g., Behnke et al., 2013; Heinz Walz GmbH, 2013; Portillo-Estrada et al., 2017; Portillo-Estrada et al., 2015; Portillo-Estrada and Niinemets, 2018), the total tubing length from the leaf chamber to the point of volatile sampling is 2.25 m (inner diameter 3 mm), giving a tubing volume of 15.9 cm3 and tube inner area of 0.212 m2. To minimize the sampling distance from the chamber, we added a Teflon T-piece right next to the chamber for sampling volatiles with a proton transfer reaction mass spectrometer (PTR-MS; Fig. 1c).
To compare the effect of different sampling designs on quantitative detection of volatiles and detection time kinetics, two different tests were conducted. In one, pure standard compounds (isoprene, Z-3-hexen-1-ol and α-pinene, Sigma-Aldrich Chemie GmbH, Munich, Germany) at ppb levels were individually tested, whereas in the other, a characteristic mixture of plant volatiles at ppb levels was used. To generate the plant volatile mixture, leaves of Eucalyptus globulus Labill. were cut into pieces by scissors in an illuminated desiccator. As E. globulus is both an isoprene and monoterpene emitter, protonated masses 69+ (isoprene and pentenol/pentenone fragments) and 137+ (monoterpenes) were studied. In addition, mass 45+ (acetaldehyde) was incorporated as a representative lightweight oxygenated volatile. Pure standard compounds were also individually injected in the desiccator. The desiccator was hermetically sealed, and stabilized for 20 min. A gas sample of 1 ml was drawn with a gas sample syringe from the desiccator gas port and rapidly injected into the empty Walz GFS-3000 leaf chamber through the neoprene gaskets. The flow rate through the system was maintained at 750 μmol s-1 (33.5 cm3 s-1), and thus, this sample volume was small enough to inject rapidly, and the effect of injection to total Walz GFS-3000 air flow was minimal. In these experiments, the environmental conditions inside the leaf chamber were maintained as for the leaf measurements (see the section Set-up of representative leaf wounding experiments).
To compare different sampling methods, we used two PTR-MS instruments, one with a quadrupole mass spectrometer (PTR-QMS, high sensitivity version, Ionicon GmbH, Innsbruck, Austria) and the other with a time-of-flight mass spectrometer (PTR-TOF-MS 8000, Ionicon GmbH, see below for the details of operation of both instruments). First, the two instruments were operated in parallel next to the leaf chamber head and sampled the same gas stream. After the injection of individual volatiles or eucalypt volatile mixture into the leaf chamber, the decay of volatile concentrations was continuously measured by both instruments. These parallel measurements were used to exactly cross-calibrate the two instruments for individual volatile parent ions and mass fragments. Then, for subsequent injections, PTR-TOF-MS was moved to the position of the standard sampling configuration and the injections were repeated again, allowing determination of the changes in the kinetics of emission detection and the total amount of volatiles detected. The data for individual volatiles were ultimately normalized with respect to the maximum (peak) value of the PTR-QMS measurements taken next to the leaf chamber. Three replicate injections with each individual compound and eucalypt leaf volatile mixture were made and averages were calculated. Because the minimum time resolution of PTR-QMS is 0.9 s, the exact sampling delay between the sampling positions cannot be worked out with this setup. We estimated the approximate sampling delay for isoprene by sampling simultaneously with the PTR-TOF-MS instrument from the two sampling positions connected through a T-piece using 10 Hz resolution. Such a setup resulted in a double-peaked volatile signal, and the tubing delay between the two positions was estimated as time difference between the two peaks. The delay in sampling of volatiles due to tubing was estimated to be about 1 s. This is consistent with the theoretical estimate of 0.9 s calculated for given volumetric flow rate and volume of tubing. Given the issues with synchronization and time resolution of the two instruments, and to better show differences among the two setups of sampling, the data of standard sampling were shifted to the right such that the time difference among the peaks was 0.9 s in the figures.
Plant material for leaf wounding experiments
Plants of common bean (Phaseolus vulgaris L. cv. Saxa), hybrid aspen Populus tremula L. × P. tremuloides Michaux. clone H200, Zea mays L. cv. Golden Bantam were grown in a FitoClima 600 growth chamber (Aralab, Rio de Mouro, Portugal) at day/night temperatures of 25/20 °C for 14 h photoperiod and at a quantum flux density of 500 μmol m−2 s−1. The relative humidity was set to 70% and the ambient CO2 concentration was between 390 and 410 μmol mol−1. In experiments, young fully-mature leaves (less than 30 days old) were used.
The plants of Tasmanian blue gum (Eucalyptus globulus Labill.) were grown in a plant growth room at day/night temperatures of 26/23 °C for 12 h photoperiod and at a quantum flux density of 400 μmol m−2 s−1. The relative humidity was between 60 and 70% and the ambient CO2 concentration was between 390 and 410 μmol mol−1. (Kanagendran et al., 2018 for details of plant growth). The plants were three years old at the time of the experiments. The experiments were carried out with non-senescent fully mature leaves.
Set-up of representative leaf wounding experiments
In these measurements, Walz GFS-3000 gas exchange system (Walz GmbH, Effeltrich, Germany) was used simultaneously with a proton transfer reaction time-of-flight mass-spectrometer (PTR-TOF-MS 8000, Ionicon GmbH, Innsbruck, Austria). The Walz gas exchange system was fitted with the Standard Measuring Head 3010-S (4 x 2 cm), modified by including the leaf-cutting knife inside the chamber (Fig. 1). Light was provided by a LED-Array/PAM-Fluorometer 3055-FL (Walz GmbH). The port of PTR-TOF-MS sampling was placed as close to the leaf chamber as possible to minimize the sampling artefacts (Fig. 1c).
The experiments were conducted with attached leaves. The leaf was enclosed in the chamber, and standard environmental conditions - light of 500 μmol m−2 s−1 (growth light), air flow rate of 750 μmol s-1, chamber humidity of 60%, and CO2 concentration of 400 μmol mol−1, and leaf temperature of 25 °C, were established. The leaf was kept under these conditions until stomata opened and steady-state rates of net assimilation and VOC emission were achieved, typically in 20-30 min after leaf enclosure. After stabilization, the cut was performed and leaf gas exchange and emission responses were registered for 30 min. Then the leaf was removed, its area was measured immediately and leaf dry mass after oven-drying at 70 °C for 48 h, and leaf dry mass per unit area (LMA, g m-2) was estimated (average ± SE values obtained were 13.4 ± 1.5 for P. vulgaris, 25.7 ± 0.9 for Z. mays, 75.6 ± 5.6 for P. tremula x P. tremuloides and 73.8 ± 4.5 for E. globulus). Leaf net assimilation rate (A), and leaf conductance to water vapor were computed according to von Caemmerer and Farquhar (1981). Three independent replicate experiments were made with each species.
PTR-TOF-MS and PTR-QMS measurements
The settings of PTR-TOF-MS through the measurements were: the inlet and drift chamber temperature 60 °C, inlet flow 100 ml min-1, water vapor flow 5.0 ml min-1, ion current 4 mA, drift chamber pressure 2.1 mbar, drift tube field density ratio (E/N ratio; E is the electric field strength and N the gas number density) 140 Td, and the pressure of the TOF-MS module was 2.4.10-7 mbar. Source valve and all PTR-TOF-MS internal voltages were optimized for isoprene (mass 69 AMU) detection. To obtain best time/signal sensitivity ratio, the data were recorded with 1 Hz frequency and 400 picosecond sample interval. The settings for PTR-QMS used in comparing different sampling methods were: the inlet and drift chamber temperature 60 °C, inlet flow 100 ml min-1, water vapor flow 5.0 ml min-1, ion current 4 mA, drift chamber pressure 2.1 mbar, the detection pressure 1.6.10-5 mbar, and the data were recorded with 1 Hz.
The PTR-TOF-MS and PTR-QMS instruments were calibrated with a standard gas mixture in N2 that included key representatives of all plant VOC groups (1 ppm for each compound, Ionimed GmbH, Innsbruck, Austria) and a custom-made isoprene standard (3.43 ppm). The data were processed by Ionicon software PTR-MS Viewer 3.2.8 using factory certified mass transmission values, and emission rates were calculated according to Niinemets et al. (2011). Several plant volatiles or their fragments have the same protonated mass, whereas the possible overlap of different volatile parent masses and/or mass fragments is species-dependent. In particular, the protonated mass 69+ corresponds to the parent ion of isoprene that is constitutively emitted in some species. This mass also corresponds to a pentenone and pentenol fragment that can be released upon wounding from any species (Brilli et al., 2012; Jardine et al., 2013; Karl et al., 2001). Analogously, the protonated mass 81+ corresponds to either a monoterpene fragment or a hexenal fragment (de Gouw et al., 2000; Karl et al., 2005). Monoterpenes are constitutively emitted only in some species, and the induction of stress-dependent monoterpene induced emissions is time-consuming, typically taking 10-24 h after stress application (Beauchamp et al., 2005; Jiang et al., 2017), while hexenal emissions are rapidly induced upon wounding (Brilli et al., 2012; Brilli et al., 2011; Portillo-Estrada et al., 2015; Portillo-Estrada and Niinemets, 2018). Given that we were interested in the rapid emission responses right after cutting, but slower GC-MS-based methods with at best 10 min time resolution cannot be effectively used to identify the volatiles during the fast emission phase, we report the rates of emissions of the protonated VOC masses in the text. However, based on previous studies (Beauchamp et al., 2005; Brilli et al., 2012; Brilli et al., 2011; Davison et al., 2008; de Gouw et al., 2000; Fisher et al., 2003; Karl et al., 2005; Portillo-Estrada et al., 2015; Portillo-Estrada and Niinemets, 2018; Staudt et al., 2010; Steeghs et al., 2007), and our past experiments with simultaneous GC-MS and PTR-TOF-MS measurements, the volatile masses were tentatively identified as M33+ - methanol; M45+ - acetaldehyde; M57+ - fragment of hexenals; M69+ - pentenol and pentanone fragment (after water abstraction); M81+ - fragment of hexenals and/or monoterpenes; M83+ - fragments of hexanal and hexenol isomers and 3-hexenyl acetate; M85+ fragment of 1-hexanol, pentenol and pentenone), M95+ - fragment of monoterpenes; M99+ - hexenals; M137+ - monoterpenes.
For each protonated mass measured, we calculated the total amount of induced volatiles emitted during the 30 min measurement period after leaf cutting (integrated emission). The emission rate prior to cutting was used as the baseline. We report in the main text the results of representative individual experiments for each species (Fig. 3–6), and provide the results for the other replicate experiments in the Supplementary Material (Supplementary Figures S2-S9). For all species, average integrated emissions of individual masses were calculated for the three replicate experiments (Table 1).
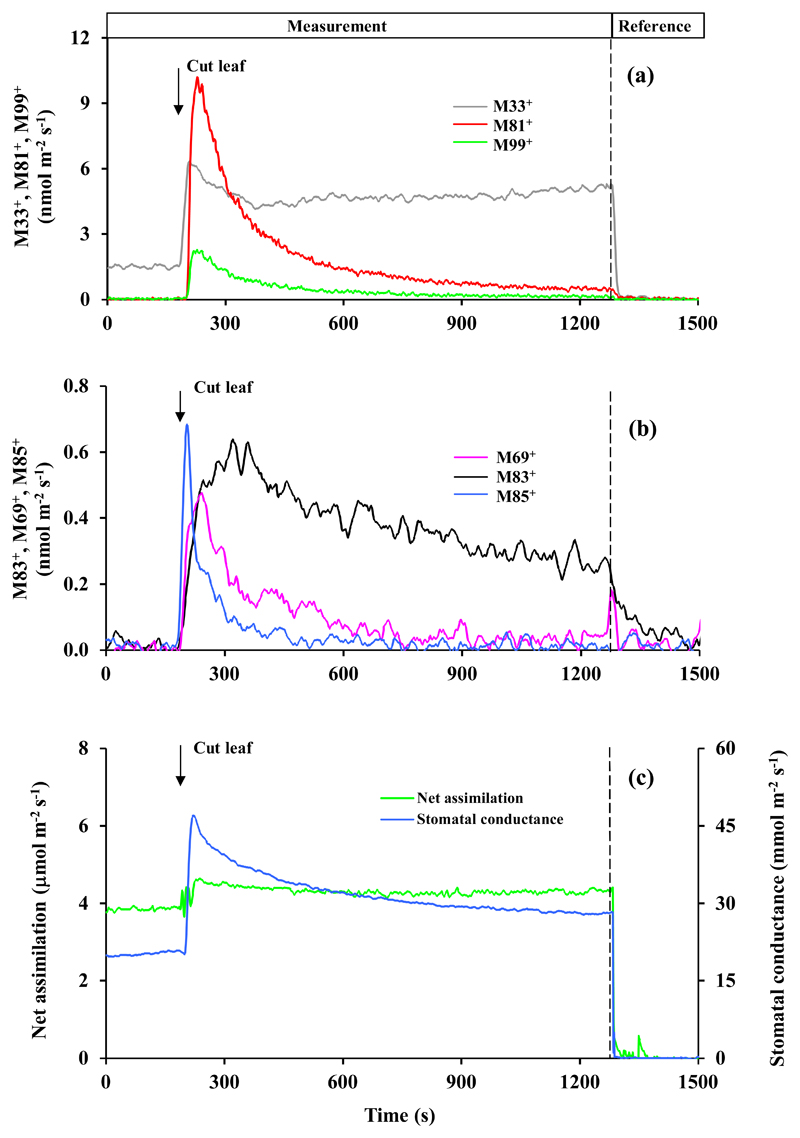
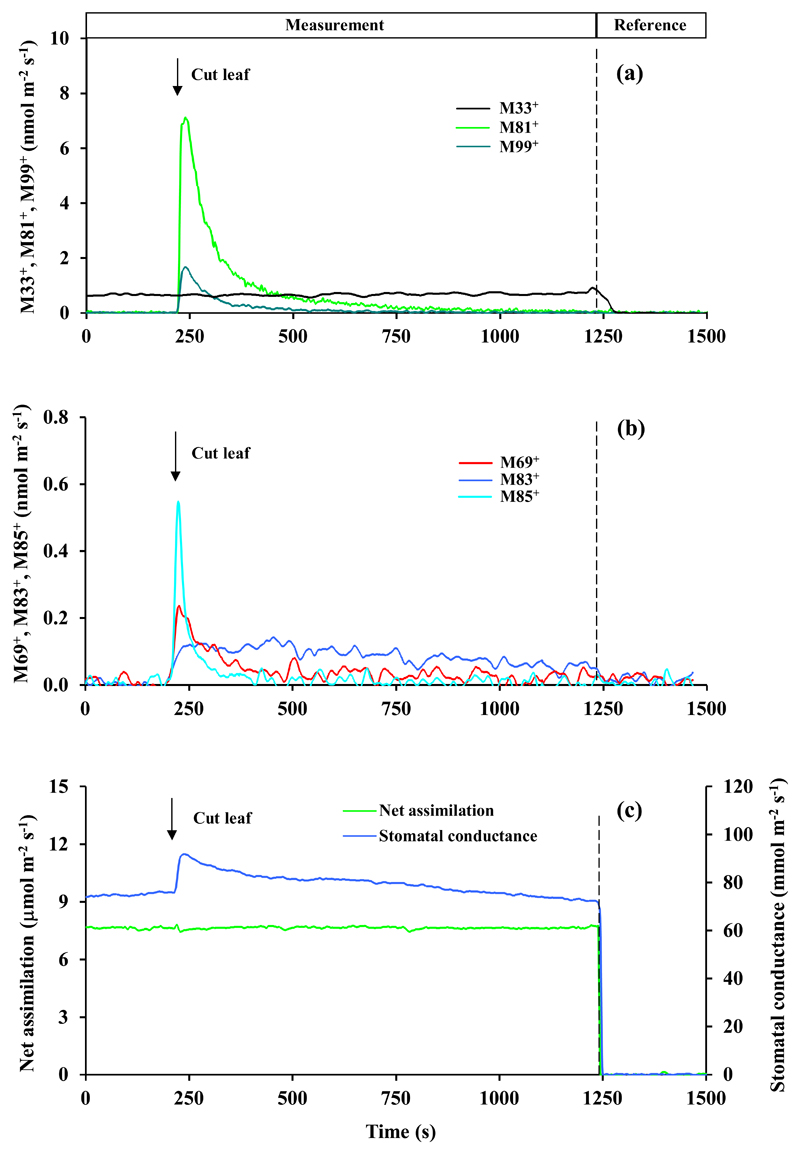
Fig. 3.
Changes in (a) dominant masses M33+, M81+ and M99+ and (b) second more abundant masses M69+, M83+ and M85+ and (c) net assimilation rate and apparent leaf conductance to water vapor following leaf cutting in Phaseolus vulgaris. At time t = 200 s, the leaf was cut with the novel within-chamber leaf cutter (Fig. 1) that produced a cut length of 14 mm. Volatile release by a proton transfer reaction time-of-flight mass-spectrometer (PTR-TOF-MS) and leaf gas exchange rates by Walz GFS-3000 infrared gas analyzers were continuously measured through the experiment. Different detected volatile masses were tentatively identified as: M33+ - methanol; M69+ - pentenol and pentanone fragment (after water abstraction); M81+ - fragment of hexenals, M83+ - fragments of hexanal and hexenol isomers and 3-hexenyl acetate; M85+ - 1-hexanol fragment, pentenol and pentenone; M99+ -hexenals. The mass M69+ is typically attributed to isoprene, but P. vulgaris does not emit isoprene both under non-stressed and stressed conditions. The integrated wound-dependent emissions are reported in Table 1. Data for two additional replicate experiments are shown in Fig. S2 and S3.
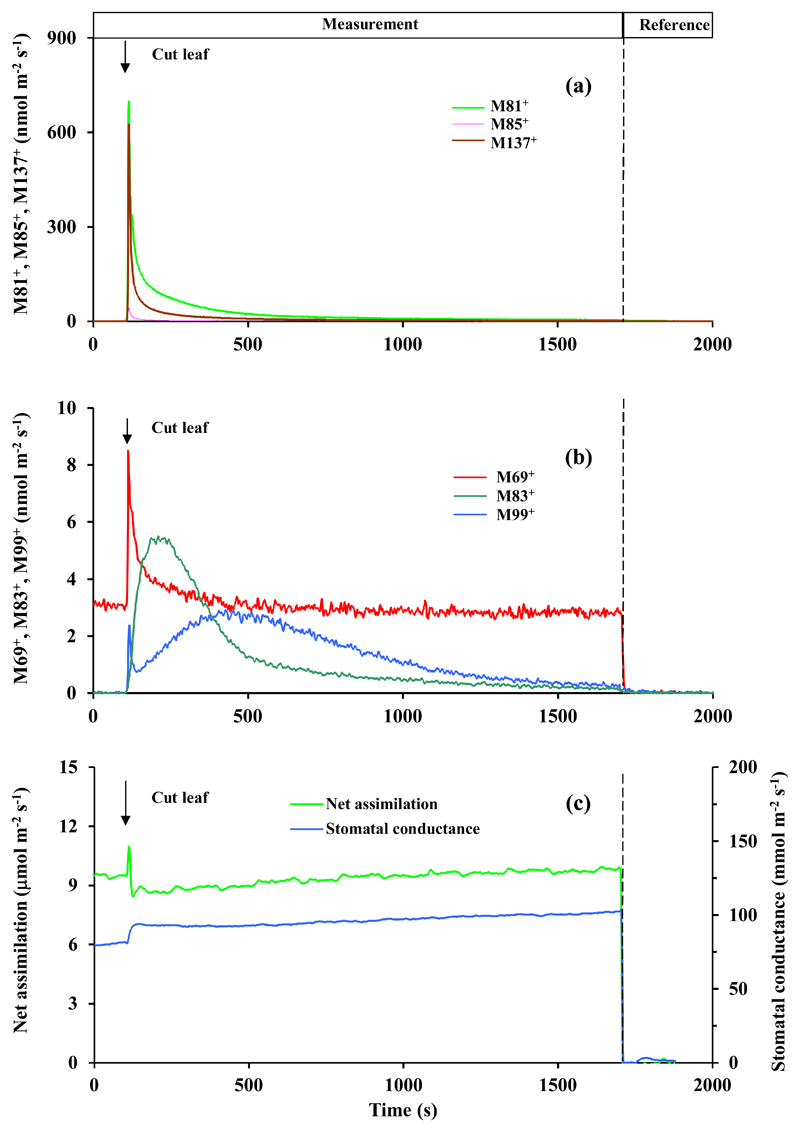
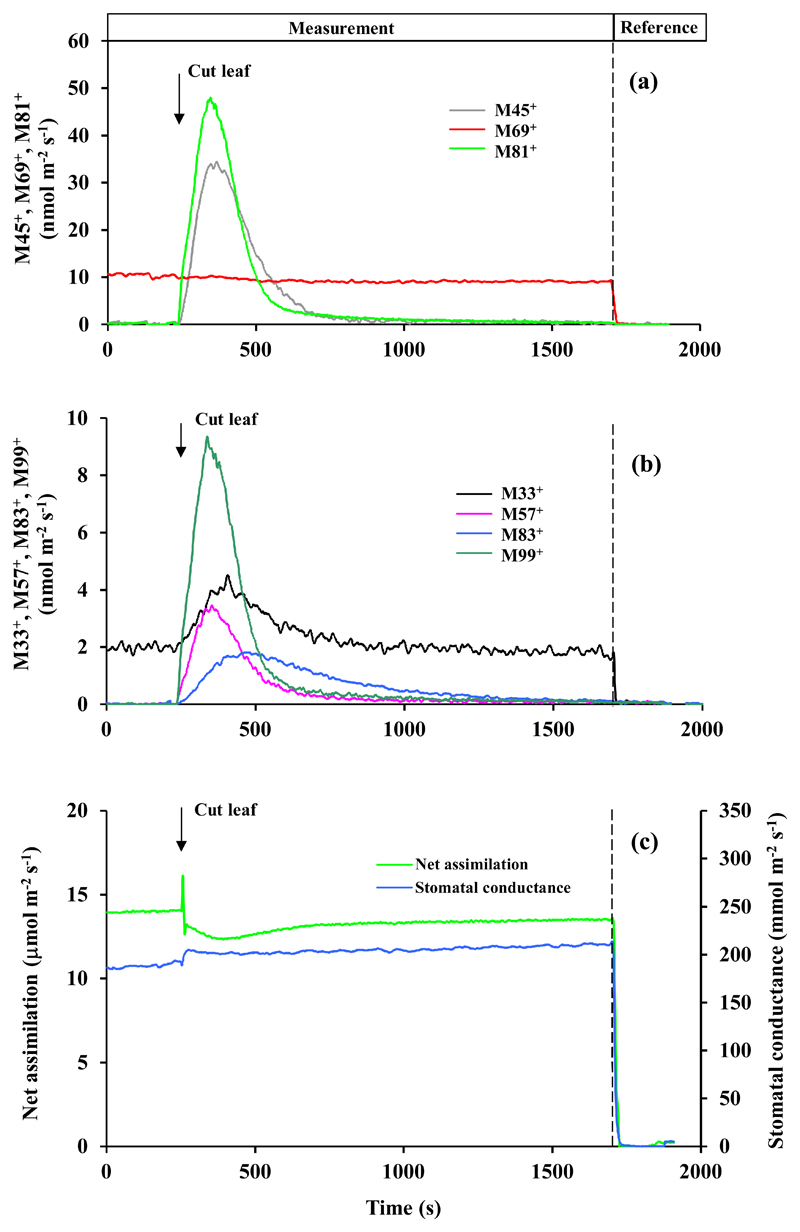
Fig. 6.
Leaf wounding experiment conducted with a representative leaf of Tasmanian blue gum (Eucalyptus globulus). Experimental conditions and data presentation as in Fig. 3. Like P. tremula x P. tremuloides, E. globulus is a moderately strong constitutive isoprene emitter, and the baseline mass M69+ emission was primarily attributed to isoprene. In addition, E. globulus is a low-level monoterpene emitter, but major monoterpene emissions occur when the oil glands are broken. Thus, the mass fragments 81+ and 95+ were primarily attributed to monoterpenes in this species. Table 1 provides the integrated estimate of the emissions following leaf wounding. Figures S8 and S9 demonstrate data obtained in two additional replicate experiments.
Table 1. Average integrated emission bursts of volatiles after leaf cutting in four species with contrasting leaf structure.
| Protonated | Average (± SE) integrated emission bursts after cutting (μmol m-2) | |||
|---|---|---|---|---|
| mass | Phaseolus vulgaris | Zea mays | Populus tremula x P. tremuloides | Eucalyptus globulus |
| M33+ | 4.1 ± 0.7* | 1.03 ± 0.26 | ||
| M45+ | 7.0 ± 1.9 | 2.2 ± 0.7 | ||
| M57+ | 0.352 ± 0.050 | 0.075 ± 0.023 | 0.78 ± 0.20 | 0.71 ± 0.16 |
| ¥M69+ | 0.175 ± 0.045 | 0.0308 ± 0.0049 | 0.46 ± 0.09 | |
| §M81+ | 3.2 ± 0.8 | 0.85 ± 0.25 | 8.9 ± 2.2 | 46 ± 11 |
| M83+ | 0.71 ± 0.16 | 0.128 ± 0.039 | 1.03 ± 0.16 | 1.7 ± 0.6 |
| M85+ | 0.063 ± 0.009 | 0.032 ± 0.012 | 0.033 ± 0.008 | 0.086 ± 0.016 |
| M95+ | 1.75 ± 0.41 | |||
| M99+ | 0.81 ± 0.24 | 0.19 ± 0.06 | 1.75 ± 0.45 | 1.53 ± 0.39 |
| M137+ | 19.7 ± 5.0 | |||
The leaves were cut with a within-chamber leaf cutter (cut length 14 m) and volatiles were measured from elicitation to cessation of the emissions. The wounding experiments were replicated thrice for each species (n = 3). Species-specific time-courses of emissions after wounding are shown in Figs. 3-6 and S2-S9.
The volatile compound parent and their fragment masses were tentatively assigned as: M33+ - methanol; M45+ - acetaldehyde; M57+ - fragment of hexenals; M69+ - pentenol and pentenone fragment (after water abstraction), isoprene; M81+ - fragment of hexenals and/or monoterpenes; M83+ - fragments of hexanal and hexenol isomers and 3-hexenyl acetate; M85+ fragment of 1-hexanol, pentenol and pentenone, M95+ - fragment of monoterpenes; M99+ - hexenals; M137+ - monoterpenes.
as the wounding-dependent rise of methanol had not ceased by the end of the experiment (Fig. 3a, S2a and S3a), the emission above the baseline emission before the cutting (1.77 ± 0.18 nmol m-2 s-1) was integrated for the period of 1100 s since the cutting.
M69+ can come from both isoprene and lipoxygenase (LOX) pathway products pentenol and pentanone. Only P. tremula x P. tremuloides and E. globulus are isoprene-emitters, but the baseline-level M69+ was almost independent of wounding in these species (Fig. 5, 6, S6-S9), suggesting that the wound-dependent M69+ reflected induced C5 LOX emissions.
M81+ can come from both monoterpenes and hexenals, the C6 LOX products. Only E. globulus was emitting monoterpenes in this study (presence of the parent mass 137+ in the emissions, Fig. 6). Thus, in this species mass 81+ primarily reflected monoterpene emissions.
Results
Novel cutter for within-chamber leaf wounding experiments
A within-chamber leaf cutter system consisting of a blade attached to a turnable stainless steel rod within a syringe needle was developed (Fig. 1). Experimentation with cutter prototypes indicated that main problems that could be encountered in the cutting experiments were shifting of the cutter during the cut and imperfect cuts through the leaf, especially in the case of tougher leaves, resulting in uneven cut margins, tearing the leaf or variable cut lengths. Ultimately, we have employed a very sharp and thin razor blade, and use of cutter supports allowed precise fixation of the cutter position in the leaf chamber (Fig. 1). Thus, a highly constant cut length (14 mm) with even margins was achieved across the experiments independent of leaf toughness, allowing standardization of the measurements.
Effects of position of sampling on detection of volatile signals
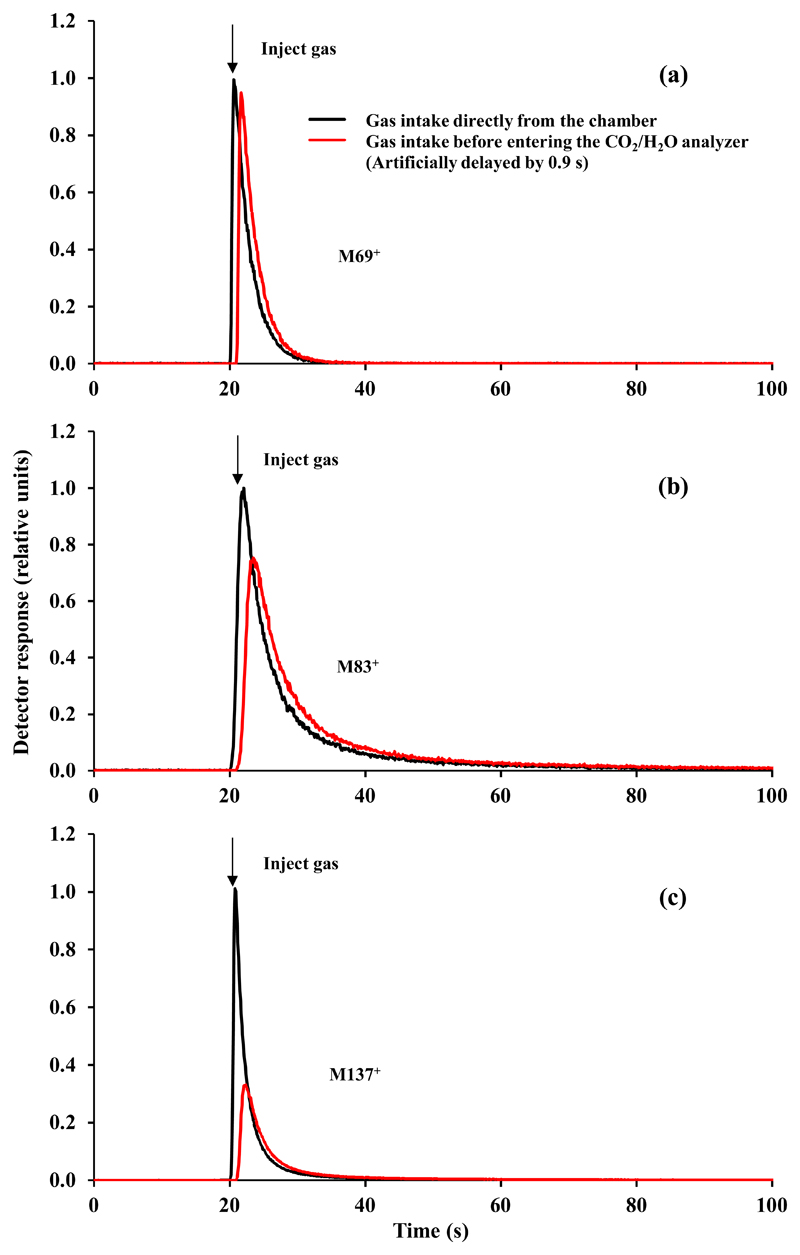
Two different positions of sampling volatiles for PTR-MS measurements were compared by simultaneous measurements with two different PTR-MS instruments (PTR-TOF-MS and PTR-QMS). In the case of the standard sampling, the chamber exhaust air passed the 2.25 m tubing between leaf chamber and site of sampling. In the case of the modified sampling, a T-piece for PTR-MS was added right after the leaf chamber for volatile collection (Fig. 1c). Individual volatiles (isoprene, Z-3-hexen-1-ol and α-pinene) and a mix of E. globulus volatiles obtained after leaf wounding were injected into the leaf chamber to test for differences among the two setups. The delay in sampling of volatiles due to tubing was minor, estimated to be about 1 s for isoprene. However, in all cases, the peak heights were lower for the standard sampling (Fig. 2 and Supplementary Fig. S1). The peak height was reduced only to a minor degree for acetaldehyde (mass 45+, Fig. S1a) and isoprene (mass 69+, Fig. 2a for pure compound injections and S1b for injections of eucalypt volatile mix), but the peak height was reduced by about 25% for pure Z-3-hexen-1-ol (detected as mass fragment 83+, Fig. 2b), and more than 3-fold for pure α-pinene (Fig. 2c) and almost five-fold for the sum of all eucalypt monoterpenes (mass 137+, Fig. S1c). For the standard sampling, the integrated peak area relative to the peak area obtained by sampling right at the chamber outlet was between 0.96-0.98 for acetaldehyde, isoprene and Z-3-hexen-1-ol, but it was only 0.63 for α-pinene and 0.61 for total monoterpenes (eucalypt mix).
Fig. 2.
Comparison of methods for volatile collection using the Walz GFS-3000 portable gas-exchange system. In the standard method, the chamber exhaust air is taken just before entering the infrared CO2 and H2O analyzers and has to pass through 2.25 m (3 mm i.d.) tubing with 0.212 m2 surface area, while in the second method, the sample is taken right after existing the chamber as shown in Fig. 1c. To compare the methods, empty leaf chamber was used and individual pure compounds were injected into the leaf chamber through the leaf chamber gaskets using a gas syringe. The chamber flow rate was maintained at 750 μmol s-1. After injection, the volatiles in the chamber exhaust air were detected continuously with two proton transfer reaction mass spectrometers (PTR-MS). One, a proton transfer reaction quadrupole mass spectrometer (PTR-QMS), was connected next to the leaf chamber, and the other, a proton transfer reaction time-of-flight mass spectrometer (PTR-TOF-MS), was connected at the standard sampling position. Both PTR-MS systems were exactly cross-calibrated for all individual volatiles as explained in the Material and Methods. The compounds injected were (a) isoprene (M69+), (b) Z-3-hexen-1-ol (detected as the main fragment ion M83+) and (c) α-pinene (M137+). The data for individual compounds were normalized with respect to the maximum (peak) value detected By PTR-QMS sampling right next to the chamber. Three replicate injections were made and average time courses are shown. To better show the differences among the curve shapes, the data for the standard sampling were shifted to the right by 0.9 s (time difference between the peaks). Representative experiments showing injections with the mixture of emissions from cut leaves of Eucalyptus globulus leaves are shown in the Supplementary Figure S1.
High-resolution real-time representative wounding responses in different species
Leaf cutting responses were broadly similar, but there were a number of important quantitative and qualitative differences among the four studied species (Fig. 3–6, Supplementary Figures S2-S9, Table 1). In Phaseolus vulgaris, methanol emissions (M33+, Fig. 3a, S2a and S3a) were elicited first, followed by elicitation of lipoxygenase pathway volatile emissions (M81+, M83+, M85+, M99+; Fig. 3a, b, S2a, b and S3a, b). In contrast, methanol emission was absent in Zea mays (Fig. 4, S4 and S5) and Eucalyptus globulus (Fig. 6, S8 and S9), whereas in Populus tremula x P. tremuloides, methanol emissions were induced at a low level after release of most LOX volatiles had been elicited (Fig. 5a, b, S6 and S7, Table 1). In all species (Fig. 3–6, S2-S9, Table 1), the most abundant volatile LOX products were hexenals (characterized by mass 99+ in all species and mass 81+ in all except E. globulus). The rise and decay of hexenals were monophasic and took ca. 300-500 s in P. vulgaris, Z. mays and P. tremula x P. tremuloides (Fig. 3–5, S2-S7), but in E. globulus, the release of mass 99+ was biphasic, starting with a rapid rise, and followed by delayed decay of more than 1500 s (Fig. 6b, S8 and S9b). Phaseolus vulgaris (Fig. 3b, S2b and S3b), Z. mays (Fig. 4b, S4b and S5b) and E. globulus (Fig. 6a, S8a and S9a) were characterized by a rapid rise of mass 85+ that can be the fragment of 1-hexanol, and parent mass of pentenol and pentenone. Indeed the kinetics of M85+ and M69+ (fragment of pentenol and pentenone) were similar in Z. mays (Fig. 4b, S4b, S5b) and E. globulus (Fig. 6b, S8b, S9b), whereas in P. vulgaris, the major rise in M85+ occurred earlier than the rise in M69+ (Fig. 3b). In P. tremula x P. tremuloides, wounding did not have any effect on the release of M69+, suggesting that it was isoprene not LOX (Fig. 5a, S6a and S7a). Compared with other LOX volatiles, the release of M83+ (fragments of hexanal and hexenol isomers and 3-hexenyl acetate) was delayed in all species (Fig. 3–5, S2-S7), except for E. globulus, where M99+ continued for a longer period (Fig. 6b, S8b and S9b). The most conspicuous species-specific effects were the major wounding-dependent acetaldehyde emissions in P. tremula x P. tremuloides (Fig. 5a, S6a and S7a) and major monoterpene emissions in E. globulus (Fig. 6a, S8a and S9a). Wounding-dependent monoterpene emissions exceeded the baseline-level emissions (average ± SE = 0.80 ± 0.37 nmol m-2 s-1 calculated from masses 137+ and 81+ using an experimentally estimated M81+/M137+ ratio of 2.0) by more than 1800 times at the emission peak (calculated from mass M81+ considering the contribution LOX volatiles to M81+, and the estimated M81+/M137+ ratio; Fig. 6a, S8a and S9a).
Fig. 4.
Leaf wounding experiment conducted with a representative leaf of Zea mays. Experimental conditions, mass identification and data presentation as in Fig. 3. The leaves were cut parallel to the major veins of leaves. Table 1 provides the integrated wound-dependent emissions. Data for two additional replicate experiments are demonstrated in Fig. S4 and S5.
Fig. 5.
Leaf wounding experiment conducted with a representative leaf of hybrid aspen (Populus tremula × P. tremuloides). Experimental conditions and data presentation as in Fig. 3. Differently from P. vulgaris and Z. mays, P. tremula x P. tremuloides is a strong constitutive isoprene emitter, and the mass M69+ was primarily attributed to isoprene. In addition, masses M45+ (acetaldehyde) and M57+ (fragment of hexenals) were abundant in hybrid aspen. Integrated emissions after wounding are reported in Table 1. Figures S6 and S7 show data for two additional replicate experiments.
Within-chamber leaf wounding also allows for direct monitoring of foliage net assimilation rate and stomatal conductance. As right after leaf cutting, cellular contents at the cut surface become exposed to the ambient air, the estimation of stomatal conductance after the cut is biased due to water evaporation from the cut surface. Thus, we suggest that the rise of the estimated conductance in all species after cutting (Fig. 3c–6c and Fig. S2c-S9c) primarily reflects additional evaporation from the cut surface. In most cases, the estimated net assimilation rate was initially unstable, characterized by a rapid increase, followed by a rapid decrease and further stabilization (Fig. 3c, 5c, 6c, S2c, S3c, S6c, S7c, S87, S9c). In a steady-state, net assimilation rate of wounded leaves was typically reduced by 5-15% compared to pre-wounding rate (Fig. 5c, 6c, S2c, S3c, S6c-S9c). However, in one replicate of P. vulgaris, the steady-state rate of net assimilation had even increased somewhat after cutting (Fig. 3c), and in Z. mays, the transient modifications in net assimilation rate were absent and changes in the steady-state net assimilation rate were minor (Fig. 4c, S4c, S5c).
Discussion
Real-time monitoring of volatile emissions and changes in photosynthetic characteristics after wounding
We developed a methodology that allowed for real-time measurement of volatile compound emissions and leaf gas-exchange characteristics through the wounding treatment. The key methodological developments were the construction of a novel within-chamber leaf cutter (Fig. 1) and improved volatile sampling at the chamber air exhaust (Fig. 1c, Fig. 2, Fig. S1). The novel cutter was constructed for the Walz GFS-3000 standard leaf chamber, but analogous cutters can be developed for other portable gas-exchange systems, including for instance, standard leaf chambers of Li-Cor 6400 and 6800 (Li-Cor, Inc.) or CIRAS-2 and CIRAS-3 (PP Systems International, Inc.) series. The only modifications needed are the adjustments of the dimensions of the blade-holding rod, rod housing needle and support positions. In particular, the dimensions should be selected such that the blade does not touch the leaf chamber thermocouple during cutting.
To our knowledge, there are only three studies that have applied mechanical stress within a leaf chamber or within a whole plant chamber. Mithöfer et al. (2005) used an automatic leaf punch to damage the leaves within a whole plant chamber, Brilli et al. (2011) cut leaves within a leaf chamber, and in the experiments described in Niinemets et al. (2011) the whole conifer tree enclosed in the chamber was shaken to induce mechanical stress. The automatic leaf punch used by Mithöfer et al. (2005) could be operated over a long period of time to create damage comparable to sustained herbivory feeding, but the wound edge was ultimately much more heterogeneous than in the case of caterpillar feeding. Brilli et al. (2011) used a knife attached to a Teflon ribbon and the wound was produced by pulling out the Teflon ribbon with attached knife from the chamber. However, the disadvantage of this method is that wounds with different length are produced (Brilli et al., 2011), and our experience in trying to apply their method was also that leaf lamina tended to fold away from the knife. Furthermore, the wounds produced often were not true cuts, but the wounds had ragged or irregular edges, making it difficult to quantitatively characterize the wounding stress. In addition, due to moving the Teflon ribbon between the sealing gaskets, it was difficult to maintain a good seal.
In addition, in all these experiments, relatively large chambers were used. Mithöfer et al. (2005), used a custom-made 500 ml plexiglass chamber, and sampled volatiles for 24 h in a closed-loop mode. Brilli et al. (2011) used the 200 ml conifer chamber of Li-Cor 6400 photosynthesis system. This reflects the circumstance that pulling the knife inside the chamber without touching and eventually damaging the thermocouple of Li-Cor 6400 standard leaf chamber is very difficult. However, the use of large chambers has inherent disadvantages due to slow chamber air turnover time, resulting in a slow rise of the emission peak, and smearing out the signal in time (Niinemets, 2012 for discussion of chamber effects). Indeed, in Brilli et al. (2011), there was ca. 50 s delay in start of the raise of LOX volatile emissions, while no such delay was evident in our study (Fig. 3–6, S2-S9). Furthermore, Fall et al. (1999) used a 3 L Teflon bag in their leaf wounding studies, and no sharp LOX emission peaks were observed and the emissions were smeared out in time for 20-30 min. since the wounding. Thus, appropriate chamber size for given air flow rate and turbulent air mixing within the chamber are needed to avoid artifacts in wounding-elicited volatile emission kinetics.
Comparison of two different approaches for volatile sampling indicated that the differences between the standard sampling through the long tubing and right at the chamber inlet (Fig. 1c) were small for lightweight volatiles acetaldehyde and isoprene (Fig. 2a, S1a, b), but for monoterpenes, both the maximum peak height and peak area were strongly reduced (Fig. 3c,1Sc). Thus, standard sampling significantly altered the kinetics and total amount of detected volatiles. The tubing effect likely reflects adsorption of the compounds on the tube surface, delaying the rate of compound passage through tubing, evening out the high concentration signal and smearing the signal in time. Net loss, however, might be indicative of terpene reactions on tube surface or irreversible adsorption (Niinemets et al., 2011 for a review). This evidence indicates that for accurate high-resolution measurements of terpene release kinetics, air sampling should be as close as possible to the leaf chamber.
Continuous monitoring of leaf gas exchange rates through the wounding treatment also highlighted interesting patterns in net assimilation rate and stomatal conductance right after wounding. The apparent rise of stomatal conductance immediately after wounding (Fig. 3c–6c, S2c-S9c) results to a large degree from evaporation of free water from the wound surface. However, the rise in leaf water loss was also associated with instantaneous rise in net assimilation rate in P. vulgaris (Fig. 3c, S2c and S3c), P. tremula x P. tremuloides (Fig. 5c, S6c and S7c) and E. globulus (Fig. 6c, S8c and S9c). This rapid rise suggests that stomatal openness did increase right after wounding, resulting in enhanced entry of CO2 into the leaf. Such a rapid response can be explained by a reduction in turgor of epidermal cells after wounding, leading to stomatal opening without any changes of guard cell osmotic relations (so-called Ivanov effect, Moldau et al., 1993). Alternatively, solubilization of chamber CO2 in the water released at the cut surface, especially from veins, could result in apparent artificial decrease in CO2 concentration, interpreted as changes in net assimilation rate. As the released water evaporates, CO2 is released again, leading to a rapid reduction in apparent net assimilation rate as was observed in our experiments (Fig. 3c, 5c, 6c, S2c, S3c, S6c-9c). This alternative explanation is plausible as in the parallel-veined Z. mays, where the cuts were performed parallel to the major veins and the release of water from the wound surface was likely less than in the other species, the transient changes in net assimilation rate were absent. However, due to lack of vein damage, anomalous behavior of Z. mays might, however, also reflect different behavior of turgor pressure after leaf cutting.
Capacity to detect rapid changes in water release and evaporation is relevant as it could also partly explain some of the kinetics of volatile release after wounding. In particular, water-soluble volatiles such as short-chained oxygenated compounds can accumulate in leaf liquid phase and increases in stomatal conductance will lead to a rapid release of these volatiles from the aqueous pools (Niinemets and Reichstein, 2003a; Niinemets and Reichstein, 2003b). Analogous changes are expected upon evaporation of dissolved volatiles from the water released at the cut surface. In our study, part of the rapid rise of M69+ and M99+ (the initial rapid rise) in E. globulus (Fig. 6b) could have resulted from changed stomatal conductance after wounding.
Volatile release in wounding experiments: we have underestimated the maximum emissions as well as the rate of induction
Most previous studies on the induction of leaf volatiles by wounding have first made the cut outside the leaf chamber and then enclosed the cut leaf in the chamber to measure leaf volatile emissions. Due to time needed to make the cut, leaf insertion and stabilization of the gas flows after leaf enclosure, this results in partial loss of the signal, typically for the first 5-10 min. (Portillo-Estrada et al., 2015). As our results indicate (Fig. 3–6, S2-S9), this is a critical period during which there are major changes in the emission rate of most key emitted volatiles.
The high-resolution wounding experiments in representative herbaceous dicot (P. vulgaris, Fig. 3, S2 and S3) and monocot (Z. mays, Fig. 4, S4 and S5) species, in woody isoprene-emitting species P. tremula x P. tremuloides (Fig. 5, S6 and S7) and in isoprene-emitting and monoterpene-storing species E. globulus (Fig. 6, S7 and S8) indicated broad similarities in induced emission responses as well as multiple species-specific differences in kinetics of emission of induced volatiles and differences in the emission blend. As the key features of the emissions in all species, we observed the start of the immediate rise of LOX volatiles, in particular, hexenals (masses 99+ and 81+ in non-monoterpene emitting species; Fig. 3–6, S2-S9) right after cutting. In addition, rapid emissions of masses 85+ and 69+ putatively attributed to C5 LOX volatiles were observed in herbaceous species P. vulgaris (Fig. 3, S2 and S3) and Z. mays (Fig. 4, S4 and S5), indicating a large constitutive activity of lipoxygenases (Andreou and Feussner, 2009; Feussner and Wasternack, 2002). Furthermore, methanol emissions started even earlier than LOX emissions in P. vulgaris (Fig. 3a, S2s and S3a), whereas methanol emissions were induced after induction of LOX emissions in P. tremula x P. tremuloides (Fig. 5b, S6b and S7b), and were not induced in Zea mays (Fig. 4a, S4a and S5a) and E. globulus (data not shown). Typically, abiotic and biotic stresses first impact cell walls and lead to activation of pectin methylesterases and release of methanol, and this is followed by the activation of membrane-level processes, and release of LOX products (Beauchamp et al., 2005; Jiang et al., 2017; Li et al., 2017) as was observed for P. vulgaris in this study (Fig. 3a, S2a and S3a). The reason why the cell wall specific response was delayed or absent in the other species studied is unclear. As cuts though major veins lead to much greater release of stress-volatiles than cuts through intercostal areas (Portillo-Estrada and Niinemets, 2018), lack of induction of methanol emission in the case of Z. mays might reflect the circumstance that no veins were severed in these experiments. Nevertheless, this explanation does not clarify why enhancement of methanol emissions was not observed in the other two species. Identification of such species differences highlights the importance of kinetic studies spanning the whole wounding treatment from cutting to cessation of induced emissions.
The most conspicuous wounding response in this study was the massive release of monoterpenes in monoterpene-storing species E. globulus (Fig. 6a, S8a, S9a). The baseline-level monoterpene emissions in this species on the order of 1 nmol m-2 s-1 were similar to the emissions observed in other studies (He et al., 2000; Kanagendran et al., 2018), reflecting slow diffusion of monoterpenes out of the terpene-storing oil glands (Niinemets, 2018). These low baseline emissions were hugely enhanced upon wounding, resulting in peak emission rates as high as 1500-4000 nmol m-2 s-1 (Fig. 6a, S8a and S9a; calculated monoterpene emission rate considering the fragmentation of parent mass 137+ during PTR-TOF-MS analysis). To our knowledge, such high emission rates have never been experimentally observed before. In fact, much of this initial terpene emission burst would have been left unnoticed by using larger chambers, long tubing and opening the leaf chamber for making the wound.
In addition to timing of emissions and maximum emissions, another important aspect to consider is the shape of the emission response after wounding. A slow rise of emissions after wounding could be indicative of gradual increase of substrate availability and/or enzyme activity as well as transport of systemically-induced compounds from other parts of the leaf (e.g. Matsui et al., 2012 for transport of lipoxygenase volatiles within the leaf). A fast initial rise can occur when there is a large constitutive enzymatic activity and a rapid release of substrate after wounding. For non-enzymatic volatile release, a rapid release of volatiles is expected from the storage pools, expected to result in a strongly asymmetric emission response. Such a release from the storage pools is expected to be particularly prominent for species with specialized storage structures such as resin ducts and oil glands, destruction of which leads to major terpene emissions (Loreto et al., 2000), but as noted above, plants can also store non-specifically water-soluble compounds such as methanol, potentially leading to a significant rise of the emissions once the leaf is cut (Harley et al., 2007; Hüve et al., 2007). Large-chamber measurements have suggested that the rise and decline of emissions are symmetrical (Fall et al., 1999), and in experiments where the wounding was done outside the chamber, the initial missing part of the emission when the leaf was outside the chamber has been gap-filled assuming a symmetric emission response (Portillo-Estrada et al., 2015). However, the rapid measurements through the wounding treatment demonstrated that the wounding-dependent emissions are often strongly asymmetric (Fig. 3–6, S2-S9). In particular, the release of hexenals (M81+ and M99+) rises very rapidly, indicating large constitutive activity of lipoxygenases, hydroperoxide lyases and rapid release of substrates (Fig. 3a–5a, 6b, S2a-S7a, S8b and S9b). Also, methanol emission in P. vulgaris was elicited very rapidly (Fig. 3a, S2a and S3a), suggesting fast activation of pectin methylesterases. In contrast, methanol emissions raised slowly in P. tremula x P. tremuloides (Fig. 5b, S6b and S7b), indicating slower enzymatic activation. Among LOX volatiles, the raise of mass 83+ was also slow in all species (Fig. 3b–5b and S2b-S7b, except for a fast initial raise in E. globulus, Fig. 6b, S8b and S9b). The mass fragment 83+ can either come from 1-hexenols and hexenyl acetate downstream of hexenals (synthesized from linolenic acid) or from hexanal (synthesized from linoleic acid). In the first case, this slow raise can be indicative of a slower activation of corresponding aldehyde dehydrogenase and alcohol acyltransferase (Gigot et al., 2010; Hayward et al., 2017; Salas et al., 2013). In the second case, the slow rise might be indicative of a slower release of linoleic acid from membranes. Clearly these differences in the shape of the kinetics of emission response provide additional insight into the underlying biochemical controls, further underscoring the importance of obtaining the full time series of emissions after leaf wounding.
Conclusions
Leaf wounding has been a difficult stress to study due to lack of tools to perform precisely defined leaf damage treatments within the leaf chamber. A novel cutter and improved volatile sampling approach developed in this study make it possible to monitor the release of wounding-induced emissions in real time. Experiments with representative herbaceous and woody species demonstrated that with the developed protocol, the timing of different emissions, the maximum emission rates and the shape of the emission response through the elicitation to cessation of different compounds can be precisely estimated, allowing gaining an insight into biochemical controls on wounding stress response. The study demonstrates that past measurements of wounding-elicited emissions have likely underestimated the rate of emission development and maximum emissions due to use of slower-response systems that smear the volatile signal in time, and in case the wounding was performed outside the chamber, due to missing the initial emission burst. A particularly high emission burst of monoterpenes was observed upon cutting the leaves of monoterpene-storing eucalypt. The huge peaks of monoterpene emission resulting from breakage of oil glands would be missed when the chamber had to be opened for performing the leaf cuts.
Our analysis further demonstrated a plethora of species-specific differences in timing and composition of wounding emissions. We observed that the wounding-dependent emissions are often highly asymmetric in time, contrary to the kinetics observed in experiments using slower-response systems where the peak emissions are not accurately recorded. The shape of time-dependent emissions provides important insight into the enzyme activity vs. substrate-level controls of emissions. The evidence obtained in this study indicates that there are broad similarities in the share of different emission controls across species, but also that emissions of several key wound-induced volatiles are controlled differently in different species.
Supplementary Material
Supplementary Figures S1-S9 are available online at www…
Acknowledgements
Funding for this study was provided by the Estonian Ministry of Science and Education (team grant PRG537), the European Research Council (advanced grant 322603, SIP-VOL+) and the European Commission through European Regional Development Fund (Center of Excellence EcolChange).
References
- Andreou A, Feussner I. Lipoxygenases - structure and reaction mechanism. Phytochemistry. 2009;70:1504–1510. doi: 10.1016/j.phytochem.2009.05.008. [DOI] [PubMed] [Google Scholar]
- Arimura G-I, Matsui K, Takabayashi J. Chemical and molecular ecology of herbivore-induced plant volatiles: proximate factors and their ultimate functions. Plant and Cell Physiology. 2009;50:911–923. doi: 10.1093/pcp/pcp030. [DOI] [PubMed] [Google Scholar]
- Arimura G-I, Ozawa R, Maffei ME. Recent advances in plant early signaling in response to herbivory. International Journal of Molecular Sciences. 2011;12:3723–3739. doi: 10.3390/ijms12063723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp J, Wisthaler A, Hansel A, Kleist E, Miebach M, Niinemets Ü, Schurr U, Wildt J. Ozone induced emissions of biogenic VOC from tobacco: relations between ozone uptake and emission of LOX products. Plant, Cell and Environment. 2005;28:1334–1343. [Google Scholar]
- Behnke K, Ghirardo A, Janz D, Kanawati B, Esperschütz J, Zimmer I, Schmitt-Kopplin P, Niinemets Ü, Polle A, Schnitzler J-P, Rosenkranz M. Isoprene function in two contrasting poplars under salt and sunflecks. Tree Physiology. 2013;33:562–578. doi: 10.1093/treephys/tpt018. [DOI] [PubMed] [Google Scholar]
- Brilli F, Hörtnagl L, Bamberger I, Schnitzhofer R, Ruuskanen TM, Hansel A, Loreto F, Wohlfahrt G. Qualitative and quantitative characterization of volatile organic compound emissions from cut grass. Environmental Science & Technology. 2012;46:3859–3665. doi: 10.1021/es204025y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brilli F, Ruuskanen TM, Schnitzhofer R, Müller M, Breitenlechner M, Bittner V, Wohlfahrt G, Loreto F, Hansel A. Detection of plant volatiles after leaf wounding and darkening by proton transfer reaction "time-of-flight" mass spectrometry (PTR-TOF) PloS ONE. 2011;6:e20419. doi: 10.1371/journal.pone.0020419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copolovici L, Niinemets Ü. Environmental impacts on plant volatile emission. In: Blande J, Glinwood R, editors. Deciphering chemical language of plant communication. Springer International Publishing; Berlin: 2016. pp. 35–59. [Google Scholar]
- Davison B, Brunner A, Ammann C, Spirig C, Jocher M, Neftel A. Cut-induced VOC emissions from agricultural grasslands. Plant Biology. 2008;10:76–85. doi: 10.1055/s-2007-965043. [DOI] [PubMed] [Google Scholar]
- de Gouw JA, Howard CJ, Custer TG, Baker BM, Fall R. Proton-transfer chemical-ionization mass spectrometry allows real-time analysis of volatile organic compounds released from cutting and drying of crops. Environmental Science & Technology. 2000;34:2640–2648. [Google Scholar]
- de Gouw JA, Howard CJ, Custer TG, Fall R. Emissions of volatile organic compounds from cut grass and clover are enhanced during the drying process. Geophysical Research Letters. 1999;26:811–814. [Google Scholar]
- Engelberth J, Alborn HT, Schmelz EA, Tumlinson JH. Airborne signals prime plants against insect herbivore attack. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:1781–1785. doi: 10.1073/pnas.0308037100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fall R, Karl T, Hansel A, Jordan A, Lindinger W. Volatile organic compounds emitted after leaf wounding: on-line analysis by proton-transfer-reaction mass spectrometry. Journal of Geophysical Research. 1999;104:15963–15974. [Google Scholar]
- Fall R, Karl T, Jordan A, Lindinger W. Biogenic C5 VOCs: release from leaves after freeze-thaw wounding and occurrence in air at a high mountain observatory. Atmospheric Environment. 2001;35:3905–3916. [Google Scholar]
- Feussner I, Wasternack C. The lipoxygenase pathway. Annual Review of Plant Biology. 2002;53:275–297. doi: 10.1146/annurev.arplant.53.100301.135248. [DOI] [PubMed] [Google Scholar]
- Fisher AJ, Grimes HD, Fall R. The biochemical origin of pentenol emissions from wounded leaves. Phytochemistry. 2003;62:159–163. doi: 10.1016/s0031-9422(02)00521-6. [DOI] [PubMed] [Google Scholar]
- Gigot C, Ongena M, Fauconnier M-L, Wathelet J-P, Du Jardin P, Thonart P. The lipoxygenase metabolic pathway in plants: potential for industrial production of natural green leaf volatiles. Biotechnologie, Agronomie, Société et Environnement. 2010;14:451–460. [Google Scholar]
- Grote R, Monson RK, Niinemets Ü. Leaf-level models of constitutive and stress-driven volatile organic compound emissions. In: Niinemets Ü, Monson RK, editors. Biology, controls and models of tree volatile organic compound emissions. Springer; Berlin: 2013. pp. 315–355. [Google Scholar]
- Grote R, Morfopoulos C, Niinemets Ü, Sun Z, Keenan TF, Pacifico F, Butler T. A fully integrated isoprenoid emission model coupling emissions to photosynthetic characteristics. Plant, Cell and Environment. 2014;37:1965–1980. doi: 10.1111/pce.12326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley P, Greenberg J, Niinemets Ü, Guenther A. Environmental controls over methanol emission from leaves. Biogeosciences. 2007;4:1083–1099. [Google Scholar]
- Harley PC. The roles of stomatal conductance and compound volatility in controlling the emission of volatile organic compounds from leaves. In: Niinemets Ü, Monson RK, editors. Biology, controls and models of tree volatile organic compound emissions. Springer; Berlin: 2013. pp. 181–208. [Google Scholar]
- Hayward S, Cilliers T, Swart P. Lipoxygenases: from isolation to application. Comprehensive Reviews in Food Science and Food Safety. 2017;16:199–211. doi: 10.1111/1541-4337.12239. [DOI] [PubMed] [Google Scholar]
- He C, Murray F, Lyons T. Monoterpene and isoprene emissions from 15 Eucalyptus species in Australia. Atmospheric Environment. 2000;34:645–655. [Google Scholar]
- Heinz Walz GmbH. Handbook of operation. 7th Edition. Heinz Walz GmbH; Effeltrich: 2013. Portable gas exchange fluorescence system GFS-3000. [Google Scholar]
- Holopainen JK, Nerg A-M, Blande JD. Multitrophic signalling in polluted atmospheres. In: Niinemets Ü, Monson RK, editors. Biology, controls and models of tree volatile organic compound emissions. Springer; Berlin: 2013. pp. 285–314. [Google Scholar]
- Hüve K, Christ MM, Kleist E, Uerlings R, Niinemets Ü, Walter A, Wildt J. Simultaneous growth and emission measurements demonstrate an interactive control of methanol release by leaf expansion and stomata. Journal of Experimental Botany. 2007;58:1783–1793. doi: 10.1093/jxb/erm038. [DOI] [PubMed] [Google Scholar]
- Jardine KJ, Meyers K, Abrell L, Alves EG, Serrano AMY, Kesselmeier J, Karl T, Guenther A, Vickers C, Chambers JQ. Emissions of putative isoprene oxidation products from mango branches under abiotic stress. Journal of Experimental Botany. 2013;64:3669–3679. doi: 10.1093/jxb/ert202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Ye J, Li S, Niinemets Ü. Methyl jasmonate-induced emission of biogenic volatiles is biphasic in cucumber: a high-resolution analysis of dose dependence. Journal of Experimental Botany. 2017;68:4679–4694. doi: 10.1093/jxb/erx244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanagendran A, Pazouki L, Niinemets Ü. Differential regulation of volatile emission from Eucalyptus globulus leaves upon single and combined ozone and wounding treatments through recovery and relationships with ozone uptake. Environmental and Experimental Botany. 2018;145:21–38. doi: 10.1016/j.envexpbot.2017.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karl T, Fall R, Crutzen PJ, Jordan A, Lindinger W. High concentrations of reactive biogenic VOCs at a high altitude site in late autumn. Geophysical Research Letters. 2001;28:507–510. [Google Scholar]
- Karl T, Harren F, Warneke C, de Gouw J, Grayless C, Fall R. Senescing grass crops as regional sources of reactive volatile organic compounds. Journal of Geophysical Research. 2005;110:D15302. [Google Scholar]
- Li S, Harley PC, Niinemets Ü. Ozone-induced foliar damage and release of stress volatiles is highly dependent on stomatal openness and priming by low-level ozone exposure in Phaseolus vulgaris. Plant, Cell & Environment. 2017;40:1984–2003. doi: 10.1111/pce.13003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liavonchanka A, Feussner N. Lipoxygenases: occurrence, functions and catalysis. Journal of Plant Physiology. 2006;163:348–357. doi: 10.1016/j.jplph.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Litvak ME, Madronich S, Monson RK. Herbivore-induced monoterpene emissions from coniferous forests: potential impact on local tropospheric chemistry. Ecological Applications. 1999;9:1147–1159. [Google Scholar]
- Loreto F, Nascetti P, Graverini A, Mannozzi M. Emission and content of monoterpenes in intact and wounded needles of the Mediterranean pine, Pinus pinea. Functional Ecology. 2000;14:589–595. [Google Scholar]
- Matsui K, Sugimoto K, Mano Ji, Ozawa R, Takabayashi J. Differential metabolisms of green leaf volatiles in injured and intact parts of a wounded leaf meet distinct ecophysiological requirements. PloS ONE. 2012;7:e36433. doi: 10.1371/journal.pone.0036433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mithöfer A, Wanner G, Boland W. Effects of feeding Spodoptera littoralis on lima bean leaves. II. Continuous mechanical wounding resembling insect feeding is sufficient to elicit herbivory-related volatile emission. Plant Physiology. 2005;137:1160–1168. doi: 10.1104/pp.104.054460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldau H, Wong S-C, Osmond CB. Transient depression of photosynthesis in bean leaves during rapid water loss. Australian Journal of Plant Physiology. 1993;20:45–54. [Google Scholar]
- Niinemets Ü. Whole plant photosynthesis. In: Flexas J, Loreto F, Medrano H, editors. Terrestrial photosynthesis in a changing environment. A molecular, physiological and ecological approach. Cambridge University Press; Cambridge: 2012. pp. 399–423. [Google Scholar]
- Niinemets Ü. Storage of defense metabolites in the leaves of Myrtaceae: news of the eggs in different baskets. Tree Physiology. 2018;38:1445–1450. doi: 10.1093/treephys/tpy115. [DOI] [PubMed] [Google Scholar]
- Niinemets Ü, Fares S, Harley P, Jardine KJ. Bidirectional exchange of biogenic volatiles with vegetation: emission sources, reactions, breakdown and deposition. Plant, Cell and Environment. 2014;37:1790–1809. doi: 10.1111/pce.12322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niinemets Ü, Kuhn U, Harley PC, Staudt M, Arneth A, Cescatti A, Ciccioli P, Copolovici L, Geron C, Guenther AB, Kesselmeier J, et al. Estimations of isoprenoid emission capacity from enclosure studies: measurements, data processing, quality and standardized measurement protocols. Biogeosciences. 2011;8:2209–2246. [Google Scholar]
- Niinemets Ü, Reichstein M. Controls on the emission of plant volatiles through stomata: a sensitivity analysis. Journal of Geophysical Research - Atmospheres. 2003a;108:4211. 4210.1029/2002JD002626. [Google Scholar]
- Niinemets Ü, Reichstein M. Controls on the emission of plant volatiles through stomata: sensitivity or insensitivity of the emission rates to stomatal closure explained. Journal of Geophysical Research - Atmospheres. 2003b;108:4208. 4210.1029/2002JD002620. [Google Scholar]
- Ping L, Shen Y-B, Jin Y-J, Hao J-H. Leaf volatiles induced by mechanical damage from diverse taxonomic tree species. Journal of Integrative Plant Biology. 2001;43:261–266. [Google Scholar]
- Portillo-Estrada M, Kazantsev T, Niinemets Ü. Fading of wound-induced volatile release during Populus tremula leaf expansion. Journal of Plant Research. 2017;130:157–165. doi: 10.1007/s10265-016-0880-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portillo-Estrada M, Kazantsev T, Talts E, Tosens T, Niinemets Ü. Emission timetable and quantitative patterns of wound-induced volatiles across different damage treatments in aspen (Populus tremula) Journal of Chemical Ecology. 2015;41:1105–1117. doi: 10.1007/s10886-015-0646-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portillo-Estrada M, Niinemets Ü. Massive release of volatile organic compounds due to leaf midrib wounding in Populus tremula. Plant Ecology. 2018;219:1021–1028. doi: 10.1007/s11258-018-0854-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruuskanen TM, Kolari P, Bäck J, Kulmala M, Rinne J, Hakola H, Taipale R, Raivonen M, Altimir N, Hari P. On-line field measurements of monoterpene emissions from Scots pine by proton-transfer-reaction mass spectrometry. Boreal Environment Research. 2005;10:553–567. [Google Scholar]
- Salas JJ, Harwood JL, Martínez-Force E. Lipid metabolism in olive: biosynthesis of triacylglycerols and aroma components. In: Aparicio R, Harwood J, editors. Handbook of olive oil. 2nd ed. Springer; New York, Heidelberg, Dordrecht, London: 2013. pp. 97–127. [Google Scholar]
- Staudt M, Jackson B, El-Aouni H, Buatois B, Lacroze J-P, Poëssel J-L, Sauge M-H. Volatile organic compound emissions induced by the aphid Myzus persicae differ among resistant and susceptible peach cultivars and a wild relative. Tree Physiology. 2010;10:1320–1334. doi: 10.1093/treephys/tpq072. [DOI] [PubMed] [Google Scholar]
- Steeghs MML, Crespo E, Harren FJM. Collision induced dissociation study of 10 monoterpenes for identification in trace gas measurements using the newly developed proton-transfer reaction ion trap mass spectrometer. International Journal of Mass Spectrometry. 2007;263:204–212. [Google Scholar]
- Ton J, D'Allesandro M, Jourdie V, Jakab G, Karlen D, Held M, Mauch-Mani B, Turlings TCJ. Priming by airborne signals boosts direct and indirect resistance in maize. The Plant Journal. 2007;49:16–26. doi: 10.1111/j.1365-313X.2006.02935.x. [DOI] [PubMed] [Google Scholar]
- Vivaldo G, Masi E, Taiti C, Caldarelli G, Mancuso S. The network of plants volatile organic compounds. Scientific Reports. 2017;7:11050. doi: 10.1038/s41598-017-10975-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Caemmerer S, Farquhar GD. Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta. 1981;153:376–387. doi: 10.1007/BF00384257. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.