To the Editor
Single-dose azithromycin is the first-choice antibiotic for the treatment of trachoma. The World Health Organization (WHO) currently recommends annual mass azithromycin treatment for 3 years in communities in which the prevalence of the clinical sign “trachomatous inflammation–follicular” in children between 1 and 9 years of age is 10% or more.
We previously reported the effect of high-coverage, single-dose mass azithromycin treatment on ocular Chlamydia trachomatis infection in Kahe Mpya, Tanzania.1 In all, 97.6% of residents were treated; the prevalence of ocular C. trachomatis fell from 9.5% at baseline to 0.1% 24 months later. We subsequently carried out a second round of mass treatment at 24 months, examined residents at 42 and 60 months, and took conjunctival swabs for testing for C. trachomatis by means of a polymerase-chain-reaction assay at 60 months. Just as at 6, 12, and 18 months, at 42 months, persons with active disease (21 of the 821 residents examined at 42 months) were offered tetracycline eye ointment. Field, laboratory, and statistical methods have been described previously,1,2 although because we expected the prevalence of infection to be low after the two mass treatments, we combined aliquots from each of five eluted swabs for each assay, intending to retest individual samples if results were positive or equivocal.3 Approval for the study was obtained from ethics committees at the London School of Hygiene and Tropical Medicine and Tumaini University.
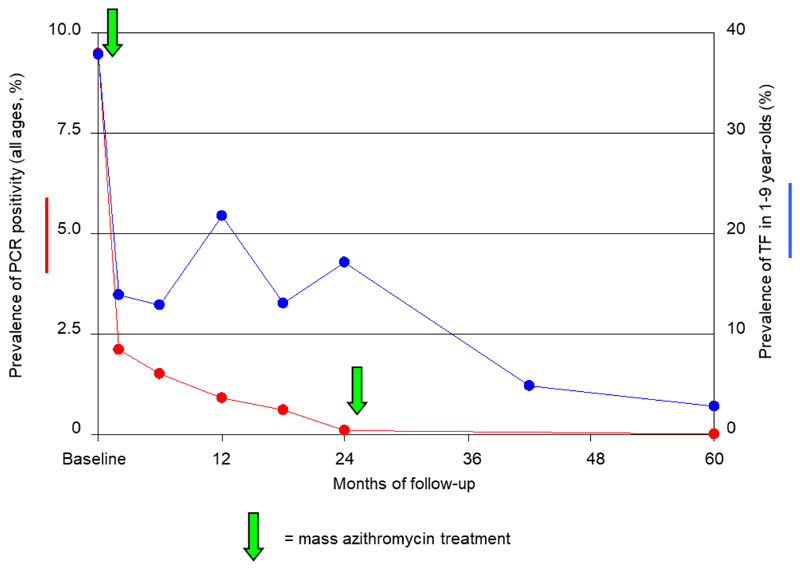
At 24 months (when the prevalence of infection was 0.1%1), the rate of antibiotic coverage was 93.1% (917 of 985 persons). At 42 months, 821 of 975 residents (84.2%) were examined, as were 859 of 964 (89.1%) at 60 months. The prevalence of trachomatous inflammation–follicular in children between 1 and 9 years of age fell from 16.3% at 24 months to 4.6% at 42 months and 2.6% at 60 months. At 60 months (3 years after the second mass treatment), C. trachomatis DNA was not detected in the conjunctiva of any of the 859 residents from whom swab specimens were obtained, suggesting that infection may have been eliminated.
One or two rounds of high-coverage mass treatment with azithromycin may be sufficient to eliminate ocular C. trachomatis in communities with moderate levels of infection. In this Tanzanian community, the fall in the prevalence of trachomatous inflammation–follicular lagged considerably behind the fall in the prevalence of infection (Fig. 1). Had WHO recommendations on antibiotic use been followed, three or possibly five annual rounds of mass treatment would have been offered, whereas our data suggest that one round was sufficient. A field-based assay for estimating the prevalence of infection4 is needed to guide treatment decisions in the 3 to 5 years after the first distribution of antibiotics for trachoma control.
Figure 1. Prevalences of Ocular Chlamydia trachomatis Infection (Positive Result on Polymerase-Chain-Reaction Assay [PCR]) and Disease (Trachomatous Inflammation–Follicular) during Follow-up in a Tanzanian Community.
The prevalence data for the first 24 months are from our previous report.1 The arrows indicate the timing of mass azithromycin treatment.
Acknowledgments
Supported by grants from Pfizer (2005-0812) and the Wellcome Trust–Burroughs Wellcome Fund (059134).
Drs. Solomon, Bailey, and Mabey report receiving grant support from the International Trachoma Initiative, which is partly funded by Pfizer, the manufacturers of azithromycin; and Drs. Solomon and Mabey, support from Pfizer to attend an international meeting on trachoma research.
Footnotes
No other potential conflict of interest relevant to this letter was reported.
References
- 1.Solomon AW, Holland MJ, Alexander ND, et al. Mass treatment with single-dose azithromycin for trachoma. N Engl J Med. 2004;351:1962–71. doi: 10.1056/NEJMoa040979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Solomon AW, Holland MJ, Burton MJ, et al. Strategies for control of trachoma: observational study with quantitative PCR. Lancet. 2003;362:198–204. doi: 10.1016/S0140-6736(03)13909-8. [DOI] [PubMed] [Google Scholar]
- 3.Diamant J, Benis R, Schachter J, et al. Pooling of Chlamydia laboratory tests to determine the prevalence of ocular Chlamydia trachomatis infection. Ophthalmic Epidemiol. 2001;8:109–17. doi: 10.1076/opep.8.2.109.4156. [DOI] [PubMed] [Google Scholar]
- 4.Michel CE, Solomon AW, Magbanua JP, et al. Field evaluation of a rapid point-of-care assay for targeting antibiotic treatment for trachoma control: a comparative study. Lancet. 2006;367:1585–90. doi: 10.1016/S0140-6736(06)68695-9. [DOI] [PubMed] [Google Scholar]