Abstract
Magnetic properties of endohedral metallofullerenes with nitride clusters DySc2N and Dy2ScN and different carbon cages are studied by SQUID magnetometry. All molecules behave as single molecule magnets (SMMs) and exhibit magnetic hysteresis. It is found that the blocking temperature of magnetization and relaxation times strongly depend on the fullerene cage, with the C80-Ih isomer offering the best SMM properties.
The ability of fullerenes to stabilize unconventional species inside the protective shell of their carbon cages resulted in a plethora of different classes of endohedral metallofullerenes (EMFs).1 The endohedral metal ions can preserve some of their physical properties (such as spin states) inside the carbon cage, whereas intramolecular interactions with other ions in the cluster as well as the electron transfer to the carbon cage can lead to the development of new properties. In 2012 it was found that DySc2N@C80-Ih exhibits slow relaxation of magnetization in a finite magnetic field of 0.2 T.2 In zero field, the magnetization of DySc2N@C80-Ih relaxes fast via quantum tunnelling of magnetization (QTM), resulting in a characteristic butterfly shape of the magnetic hysteresis typical for many single-ion magnets (SIMs).3 Further studies revealed single molecule magnetism (SMM) in other lanthanide-based EMFs, including clusterfullerenes with nitride,4 sulfide,5 carbide,5,6 or cyanide units,7 as well as in dimetallofullerenes.8 The SMM properties were found to depend substantially on the choice of the metal (Dy gives the longest relaxation times) and the endohedral cluster composition. For instance, QTM at zero field is suppressed in Dy2ScN@C80-Ih due to the ferromagnetic exchange and dipolar interactions of the two Dy ions.4a
Among the various lanthanide clusterfullerenes, DySc2N and Dy2ScN clusterfullerenes showed the best SMM performance presumably due to the strong ligand field created by the nitride ion located at the short distance from the lanthanide.4b,5,9 Studies of the magnetic properties of nitride clusterfullerenes have been limited so far to the C80-Ih cage because the corresponding EMFs are usually produced in much higher yields and constitute ca. 80% of the nitride clusterfullerenes mixtures. Yet, the arc-discharge synthesis of nitride clusterfullerenes affords a variety of other isolable carbon cages, from C68 to C88, as well as another cage isomer of C80, with D5h symmetry (the Gd–Sc and Dy–Sc systems have been studied in most detail).10 Due to the much lower production yield their properties remain largely unexplored. It is not clearly understood if the fullerene cage is merely a container for magnetic species, or if it plays an active role in the relaxation of magnetization. For Dy2S- and TbCN-clusterfullerenes the variation of the carbon cage was found to affect the magnetic properties.5,7b In this Communication, we show that the magnetic properties of several Dy–Sc nitride clusterfullerenes strongly depend on their fullerene cages and that the most symmetric C80-Ih fullerene provides the best SMM properties of the endohedral clusters.
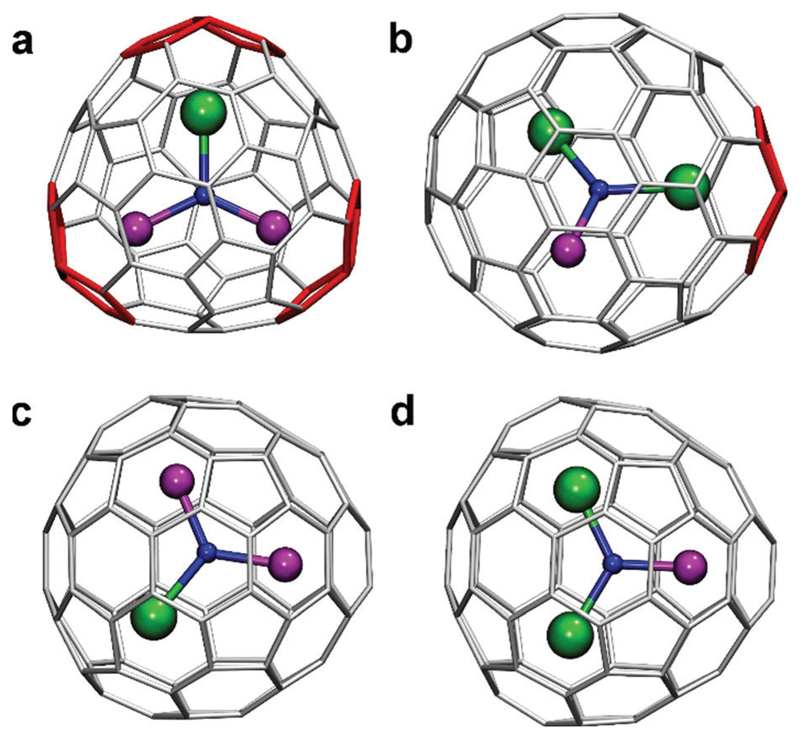
The structures chosen for this study (Fig. 1), DySc2N@C68-D3(6140), Dy2ScN@C84-Cs(51365), DySc2N@C80-D5h(6), and Dy2ScN@C80-D5h(6),‡ have been obtained using arc-discharge synthesis followed by HPLC separation as described in detail earlier.10b,d This set of EMFs allows to compare the influence of the carbon cage isomerism (Ih versus D5h) as well as the size of the fullerene cage (C68 versus C80 versus C84).
Fig. 1.
Molecular structures of Dy–Sc nitride clusterfullerenes studies in this work: (a) DySc2N@C68-D3(6140),the C3 axis of the cage is perpendicular to the paper plane; (b) Dy2ScN@C84-Cs(51365), the symmetry plane of the cage is parallel to the paper plane; (c) DySc2N@C80-D5h(6); (d) Dy2ScN@C80-D5h(6). Dy atoms are green, Sc atoms are magenta, N is blue, carbon cages are grey except for adjacent pentagon pairs shown in red. In (c) and (d), the plane of the cluster is parallel to one of the σh planes of the cage, whereas the C5 axis is oriented vertically and is in the plane of the paper. All structures are based on DFT calculations of Y–Sc analogues.
In DySc2N@C68, the D3-symmetric fullerene cage violates the isolated pentagon rule, as it features three adjacent pentagon pairs coordinated by metal atoms of the endohedral nitride cluster. The cluster is therefore tightly fixed inside the carbon cage. Dy2ScN@C84 with the Cs(51365) fullerene cage has one pentagon adjacency and thus also violates the IPR. Computational studies showed that the structure in which the pentagon pair is coordinated by the bigger metal is more stable. However, the energy of the conformer with a Sc-coordinated pentagon pair is only slightly higher, and thus both structures are likely to be present. The pentagon adjacency fixes the position of the metal cluster, which therefore cannot rotate freely in DySc2N@C68 and Dy2ScN@C84.
The C80-D5h cage resembles the C80-Ih counterpart. Both isomers can be described as built from identical hemispheres, but rotated with respect to each other at different angles. The NMR studies of Lu–Sc nitride clusterfullerenes showed that the nitride cluster inside the C80-D5h cage rotates freely at room temperature, similar to the rotation inside the C80-Ih cage.11 Thus, disordered positions of the endohedral cluster are to be expected in DySc2N@C80-D5h and Dy2ScN@C80-D5h, and Fig. 1 shows one of the lowest energy configurations determined by DFT calculations.10b
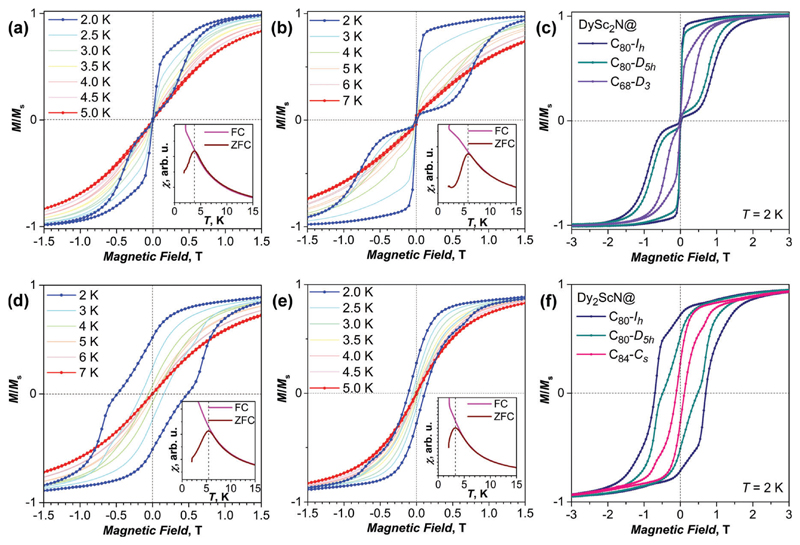
As a measure of the SMM performance we use several parameters (Table 1), which can be obtained from DC SQUID magnetometry (the low yield of these minor structures precludes the isolation of amounts sufficient for AC magnetometry). First of all, magnetic hysteresis is a clear indication that the sample behaves as an SMM (Fig. 2). The width of the hysteresis and the temperature at which it is closing are characteristic parameters of a given SMM. Another important characteristic parameter of the SMM is the blocking temperature of magnetization, TB. Here we adopt the definition of TB based on the divergence of the magnetic susceptibility of the SMM measured during cooling the sample in a field (FC) and for the sample cooled in zero field (ZFC). TB is then defined as the temperature of the maximum of the peak observed in χZFC (measured in a field of 0.2 T with a sweep rate of 5 K min−1 in this work; note that the use of a different DC field or a temperature sweep rate may give somewhat different TB values3b). In these measurement conditions, the magnetic relaxation time at TB is ca. 10 seconds. At higher temperature, the relaxation becomes fast on the time scale of the experiment, and the two curves coincide. In this definition, TB is close to the temperature at which magnetic hysteresis is closing, but the latter also depends on the sweep rate. The temperature at which the relaxation time is equal 100 s is another common parameter denoted here as TB100. Finally, magnetic relaxation times and their evolution with temperature provide information on the SMM performance. Here relaxation times are measured by first magnetizing the sample to saturation, followed by sweeping the field as fast as possible to the desired value (zero field or 0.2 T). Then the decay of the magnetization is recorded, and the resulting decay curves are fitted with a stretched exponential. Due to the finite sweep rates and the time necessary for the stabilization of the field, this procedure gives reliable values for relaxation times longer than 100 s, whereas shorter relaxation times may be somewhat overestimated. A detailed discussion of the relaxation time determination is given in ref. 4d.
Table 1. Selected SMM parameters in Dy–Sc nitride clusterfullerenes.
| EMF | TBa, K | TB100b, K | Hc, T (at 2 K)c |
|---|---|---|---|
| DySc2N@C68-D3(6140) | 3.8 | 2.3 | |
| DySc2N@C80-D5h(6) | 5.9 | 3.6 | |
| DySc2N@C80-Ih(7) | 7.0 | 4.6 | |
| Dy2ScN@C80-D5h(6) | 5.3 | 2.6 | 0.48 |
| Dy2ScN@C80-Ih(7) | 8.0 | 5.0 | 0.70 |
| Dy2ScN@C84-Cs(51365) | 3.3 | ≈1.8 | 0.11 |
TB measured in the field of 0.2 T with a sweep rate of 5 K min−1.
TB100 is estimated from the temperature dependence of relaxation times (in zero field for Dy2ScN-EMFs, and in the field of 0.2 T for DySc2N-EMFs).
Coercive field Hc is measured at 2 K with an average sweep rate of 2.9 mT s−1.
Fig. 2.
Low temperature magnetization of Dy–Sc nitride clusterfullerenes: (a) DySc2N@C68-D3; (b) DySc2N@C80-D5h; (c) comparison of magnetic hysteresis curves of DySc2N@C68, DySc2N@C80-D5h, and DySc2N@C80-Ih measured at 2 K in identical conditions; (d) Dy2ScN@C80-D5h; (e) Dy2ScN@C84-Cs; (f) comparison of magnetic hysteresis curves of Dy2ScN@C84, Dy2ScN@C80-D5h, and Dy2ScN@C80-Ih measured at 2 K in identical conditions. The insets in (a), (b), (d), and (e) show determination of the blocking temperature of magnetization TB from temperature dependence of magnetic susceptibility (FC – field cooled, ZFC – zero-field cooled). Magnetic field sweep rate is 2.9 mT s−1, temperature sweep rate is 5 K min−1.
Fig. 2 shows the magnetization curves of the samples measured at low temperature. All samples exhibit magnetic hysteresis, showing that SMM properties are intrinsic to Dy–Sc nitride clusterfullerenes. However, the width of the magnetic hysteresis and the temperature at which it is closing varies strongly within the series. Both DySc2N@C68 and DySc2N@C80-D5h show a butterfly hysteresis shape with a fast relaxation near zero magnetic field, but the width of the hysteresis is much narrower for the DySc2N@C68. Likewise, the TB value of DySc2N@C68 is only 3.8 K, which is much lower than the TB value of 5.9 K in DySc2N@C80-D5h. For comparison, the blocking temperature of the magnetization of DySc2N@C80-Ih measured in the same conditions is 7 K. Among the studied DySc2N-clusterfullerenes, DySc2N@C80-Ih also shows the broadest hysteresis at 2 K (see Fig. 2c for a comparison).
A similar behavior is found in the series of Dy2ScN-clusterfullerenes. Dy2ScN@C84 has the lowest blocking temperature, TB = 3.3 K, and the narrowest magnetic hysteresis. In Dy2ScN@C80-D5h, the blocking temperature is increased to 5.3 K, and the magnetic hysteresis is considerably broader. But the SMM parameters of the D5h isomer are inferior to those of Dy2ScN@C80-Ih, which exhibits the TB value of 8 K.4b
Thus, both for the DySc2N and Dy2ScN cluster series, we conclude that the C80-Ih cage gives the best SMM properties, the C80-D5h isomer has somewhat lower TB values and narrower hysteresis, and the non-IPR C68-D3 and C84-Cs cages give the softest SMMs with TB values below 4 K and a narrow hysteresis.
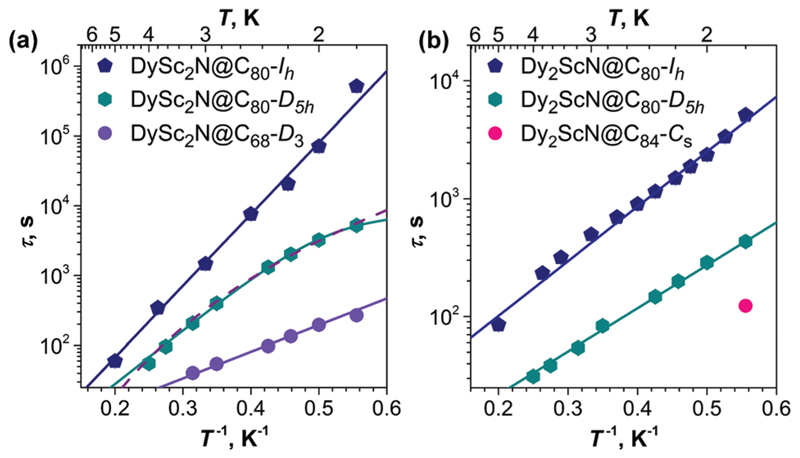
Relaxation times of magnetization (τ) and their temperature dependence provide another tool to follow the SMM behavior at different temperatures (Fig. 3). For DySc2N@C80-Ih our earlier measurements4d revealed a linear dependence of the τ values in Arrhenius coordinates between 1.8 and 5 K (Fig. 3a). Such a dependence corresponds to the Orbach relaxation mechanism:
| (1) |
where Ueff is the effective barrier and τ0 is the attempt time, which for DySc2N@C80-Ih equals 23.6 ± 1 K and 0.6 ± 0.2 s, respectively.4d For DySc2N@C80-D5h, a clear deviation from the linear dependence is found in the same temperature range (Fig. 3a). The data can be equally well described either by a combination of an Orbach relaxation (Ueff = 17.7 ± 0.4 K and an attempt time of τ0 = 0.8 ± 0.1 s) with a temperature-independent quantum tunneling (QTM, τQTM = 0.8 ± 0.1 s) denoted by a solid line in Fig. 3a, or by a power function of temperature, τ−1 = ATn, with n = 5.6 ± 0.2 and A = 6.7 ± 0.8 × 10−6 s−1 K−n. The power function with such parameters corresponds to the Raman relaxation mechanism with a contribution of optical phonons.12 Relaxation times of DySc2N@C68 between 1.8 and 3 K can be described by the Orbach mechanism with parameters Ueff = 7.6 ± 0.4 K and τ0 = 4.1 ± 0.9 s. Thus, for all three DySc2N-EMFs the Orbach relaxation with a small barrier appears to be the main relaxation mechanism at low temperatures. Since the barrier is much smaller than expected crystal-field splitting of the Dy 6H15/2 manifold,9a the observed barriers have to be assigned to low-frequency molecular vibrations such as frustrated rotations of the endohedral cluster.4d,5,8
Fig. 3.
Temperature dependence of the relaxation times of magnetization measured by DC magnetometry for (a) DySc2N-EMFs in the field of 0.2 T to avoid QTM, and (b) for Dy2ScN-EMFs in zero field. The lines are fits of the experimental data with the model discussed in the text.
The low-temperature relaxation of dinuclear Dy-EMFs follows the Orbach mechanism via the exchange/dipolar excited state. That is, exchange and dipolar interactions favour the ferromagnetic (FM) coupling of two Dy spins in the magnetic ground state, whereas the first excited state is ascribed to the antiferromagnetic (AFM) coupling of the moments.4a,5 For Dy2ScN@C80-Ih, the energy difference between the FM and AFM states has been estimated as Ueff = 10.7 ± 0.3 K with an attempt time of τ0 = 11.9 ± 1.5 s.4b For Dy2ScN@C80-D5h, this study revealed a somewhat lower barrier of 8.4 ± 0.2 K and an attempt time of 4.1 ± 0.3 s. Thus, while the structure of the Dy–Sc nitride cluster is hardly affected, the D5h isomeric cage reduces both the barrier and the attempt time of the Orbach relaxation via the exchange/dipolar excited state. Even shorter relaxation times of Dy2ScN@C84 preclude the detailed exploration of the temperature dependence. At 1.8 K, the relaxation time of this EMF is 123 s, which is considerably shorter than for both C80 cages.
To conclude, the studies of the low-temperature magnetic properties of a series of Dy–Sc nitride clusterfullerenes showed that the SMM properties of these molecules strongly depend on the fullerene cage. The EMFs with the C80-Ih cage show the best SMM properties, the non-IPR C68-D3 and C84-Cs fullerenes give the lowest blocking temperature and narrow magnetic hysteresis curves, whereas the EMFs with C80-D5h cages exhibit an intermediate behaviour. The size of the fullerene cage does not seem to be as important as its shape. The magnetic relaxation rate can be correlated with the freedom of motion of the endohedral cluster inside the fullerene cage. In the molecules, which exhibit free rotation of the cluster at room temperature, the spin-phonon coupling is presumably weaker than in the molecules with the fixed position of the cluster, which leads to the faster relaxation of magnetization in the latter.
Supplementary Material
† Electronic supplementary information (ESI) available: Mass-spectra and determination of relaxation times. See DOI: 10.1039/c8cc05029e
Acknowledgments
The authors acknowledge funding by the European Research Council under the European Union’s Horizon 2020 research and innovation programme (grant No 648295 “GraM3”), the Swiss National Science Foundation (proposals 206021_150784 and 200021L_147201), the Swedish Research Council (Grant No. 2015-00455), and Sklodowska Curie Actions co-founding project INCA 600398. We thank Denis Krylov for the help in determination of relaxation times.
Footnotes
Ariane Brandenburg: 0000-0002-1848-0431
Shangfeng Yang: 0000-0002-6931-9613
Thomas Greber: 0000-0002-5234-1937
Alexey A. Popov: 0000-0002-7596-0378
Conflicts of interest
There are no conflicts to declare.
Fullerene cages are denoted following the spiral algorithm.13 For the cages with adjacent pentagon pairs (non-IPR), the full numbering system is used. For IPR cages, more common abridged system counting only IPR isomer is employed.
References
- 1.(a) Popov AA, Yang S, Dunsch L. Chem Rev. 2013;113:5989. doi: 10.1021/cr300297r. [DOI] [PubMed] [Google Scholar]; (b) Yang S, Wei T, Jin F. Chem Soc Rev. 2017;46:5005. doi: 10.1039/c6cs00498a. [DOI] [PubMed] [Google Scholar]; (c) Lu X, Feng L, Akasaka T, Nagase S. Chem Soc Rev. 2012;41:7723. doi: 10.1039/c2cs35214a. [DOI] [PubMed] [Google Scholar]; (d) Rodriguez-Fortea A, Balch AL, Poblet JM. Chem Soc Rev. 2011;40:3551. doi: 10.1039/c0cs00225a. [DOI] [PubMed] [Google Scholar]
- 2.Westerström R, Dreiser J, Piamonteze C, Muntwiler M, Weyeneth S, Brune H, Rusponi S, Nolting F, Popov A, Yang S, Dunsch L, et al. J Am Chem Soc. 2012;134:9840. doi: 10.1021/ja301044p. [DOI] [PubMed] [Google Scholar]
- 3.(a) Woodruff DN, Winpenny REP, Layfield RA. Chem Rev. 2013;113:5110. doi: 10.1021/cr400018q. [DOI] [PubMed] [Google Scholar]; (b) Liu J-L, Chen Y-C, Tong M-L. Chem Soc Rev. 2018;47:2431. doi: 10.1039/c7cs00266a. [DOI] [PubMed] [Google Scholar]; (c) Dreiser J. J Phys: Condens Matter. 2015;27 doi: 10.1088/0953-8984/27/18/183203. 183203. [DOI] [PubMed] [Google Scholar]
- 4.(a) Westerström R, Dreiser J, Piamonteze C, Muntwiler M, Weyeneth S, Krämer K, Liu S-X, Decurtins S, Popov A, Yang S, Dunsch L, et al. Phys Rev B: Condens Matter Mater Phys. 2014;89 060406. [Google Scholar]; (b) Krylov DS, Liu F, Avdoshenko SM, Spree L, Weise B, Waske A, Wolter AUB, Büchner B, Popov AA. Chem Commun. 2017;53:7901. doi: 10.1039/c7cc03580b. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Dreiser J, Westerström R, Zhang Y, Popov AA, Dunsch L, Krämer K, Liu S-X, Decurtins S, Greber T. Chem – Eur J. 2014;20:13536. doi: 10.1002/chem.201403042. [DOI] [PubMed] [Google Scholar]; (d) Krylov D, Liu F, Brandenburg A, Spree L, Bon V, Kaskel S, Wolter A, Buchner B, Avdoshenko S, Popov AA. Phys Chem Chem Phys. 2018;20:11656. doi: 10.1039/c8cp01608a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen C-H, Krylov DS, Avdoshenko SM, Liu F, Spree L, Yadav R, Alvertis A, Hozoi L, Nenkov K, Kostanyan A, Greber T, et al. Chem Sci. 2017;8:6451. doi: 10.1039/c7sc02395b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Junghans K, Schlesier C, Kostanyan A, Samoylova NA, Deng Q, Rosenkranz M, Schiemenz S, Westerström R, Greber T, Büchner B, Popov AA. Angew Chem, Int Ed. 2015;54:13411. doi: 10.1002/anie.201505870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.(a) Liu F, Wang S, Gao C-L, Deng Q, Zhu X, Kostanyan A, Westerström R, Jin F, Xie S-Y, Popov AA, Greber T, et al. Angew Chem, Int Ed. 2017;56:1830. doi: 10.1002/anie.201611345. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Liu F, Gao C-L, Deng Q, Zhu X, Kostanyan A, Westerström R, Wang S, Tan Y-Z, Tao J, Xie S-Y, Popov AA, et al. J Am Chem Soc. 2016;138:14764. doi: 10.1021/jacs.6b09329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu F, Krylov DS, Spree L, Avdoshenko SM, Samoylova NA, Rosenkranz M, Kostanyan A, Greber T, Wolter AUB, Büchner B, Popov AA. Nat Commun. 2017;8 doi: 10.1038/ncomms16098. 16098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.(a) Vieru V, Ungur L, Chibotaru LF. J Phys Chem Lett. 2013;4:3565. doi: 10.1021/jz5006626. [DOI] [PubMed] [Google Scholar]; (b) Zhang Y, Krylov D, Rosenkranz M, Schiemenz S, Popov AA. Chem Sci. 2015;6:2328. doi: 10.1039/c5sc00154d. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Cimpoesu F, Dragoe N, Ramanantoanina H, Urland W, Daul C. Phys Chem Chem Phys. 2014;16:11337. doi: 10.1039/c4cp00953c. [DOI] [PubMed] [Google Scholar]
- 10.(a) Yang SF, Dunsch L. J Phys Chem B. 2005;109:12320. doi: 10.1021/jp051597d. [DOI] [PubMed] [Google Scholar]; (b) Wei T, Liu F, Wang S, Zhu X, Popov AA, Yang S. Chem – Eur J. 2015;21:5750. doi: 10.1002/chem.201406265. [DOI] [PubMed] [Google Scholar]; (c) Svitova AL, Popov AA, Dunsch L. Inorg Chem. 2013;52:3368. doi: 10.1021/ic400049k. [DOI] [PubMed] [Google Scholar]; (d) Yang SF, Popov AA, Dunsch L. Chem Commun. 2008:2885. doi: 10.1039/b803200a. [DOI] [PubMed] [Google Scholar]; (e) Yang S, Popov AA, Dunsch L. J Phys Chem B. 2007;111:13659. doi: 10.1021/jp709650d. [DOI] [PubMed] [Google Scholar]; (f) Olmstead MM, Lee HM, Duchamp JC, Stevenson S, Marciu D, Dorn HC, Balch AL. Angew Chem, Int Ed. 2003;42:900. doi: 10.1002/anie.200390237. [DOI] [PubMed] [Google Scholar]; (g) Mercado BQ, Beavers CM, Olmstead MM, Chaur MN, Walker K, Holloway BC, Echegoyen L, Balch AL. J Am Chem Soc. 2008;130:7854. doi: 10.1021/ja8032263. [DOI] [PubMed] [Google Scholar]
- 11.Yang S, Popov AA, Chen C, Dunsch L. J Phys Chem C. 2009;113:7616. [Google Scholar]
- 12.Shrivastava KN. Phys Status Solidi B. 1983;117:437. [Google Scholar]
- 13.Fowler P, Manolopoulos DE. An Atlas of Fullerenes. Clarendon Press; Oxford, UK: 1995. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.