Abstract
Poplar spiral gall aphid (Pemphigus spyrothecae) forms galls on the petiole in poplars (Populus) and mass infestations are frequent in poplar stands, but how these parasite gall infestations can affect the leaf lamina structure, photosynthetic rate and constitutive and stress volatile emissions is unknown. We investigated how the infestation by the petiole gall aphids affects lamina photosynthetic characteristics (net assimilation rate, stomatal conductance), C and N contents, and constitutive isoprene and induced volatile emissions in Populus × petrovskiana. The dry gall mass per leaf dry mass (Mg/Ml) was used as a quantitative measure of the severity of gall infestation. Very high fraction of leaf biomass was invested in gall formation with Mg/Ml varying between 0.5-2. Over the whole range of the infestation severities, net assimilation rate per area, leaf dry mass per unit area and N content decreased with increasing the severity of infestation. In contrast, stomatal conductance, leaf dry mass per fresh mass, constitutive isoprene emissions, and induced green leaf volatile (GLV), monoterpene, sesquiterpene and benzenoid emissions increased with increasing the severity of gall infestation. The rates of induced emissions were low and these emissions were associated with methyl jasmonate release from leaf laminas. The data demonstrate that petiole gall infestations lead to major changes in leaf lamina sink-source relationships and leaf water relations, thereby significantly altering lamina photosynthesis. Modifications in stress-induced emissions likely indicated systemic signaling triggered by jasmonate transported from the petiole galls to the lamina where jasmonate elicited a cascade of volatile emission responses. Enhance isoprene emissions and induced volatile emissions can play a major role in indirect defense against other herbivores, securing the food source for the gall aphids. In conclusion, a massive infestation by petiole gall aphids can profoundly modify the foliage photosynthetic performance and volatile emission profiles in poplars.
Keywords: petiole gall aphids, photosynthesis, biotic-stressed volatile, methyl jasmonate, quantitative responses
Introduction
Galls are abnormal outgrowths or swellings of plant tissues in a broad range of sizes, shapes, colors, and textures caused by a localized infection of wasps, mites, aphids, nematodes, bacteria, fungi and viruses or by mechanical wounding (Redfern 2011; Shour et al. 2004). The aphid species of the genus Pemphigus induce formation of galls in different parts of the leaves of poplar (Populus) species as the specialized primary host (Halaj and Osiadacz 2013; Osiadacz and Halaj 2009; Shour et al. 2004; Wool 2004). Among the Pemphigus species forming galls on petioles, the sugar beet root aphid (P. betae) commonly forms galls at the junction of the leaf blade and the petiole, while the galls formed by poplar spiral gall aphid (P. spyrothecae) are located near the middle of the petiole (Shour et al. 2004; Wool 2004). The localized gall growth of on the petiole occurs by active cell multiplication in petiole meristematic tissues and is the response of the host plant to the aphid feeding and phytohormones produced by feeding insects (Giron et al. 2016; Richardson et al. 2017; Shour et al. 2004; Takei et al. 2015).
Needle-like stylet mouthparts of the aphids can penetrate plant tissue to reach the vascular tissue, in particular phloem, and suck the plant sap (Chapman 2013; Richardson et al. 2017; Schoonhoven et al. 2005). Swelling and twisting of the petiole by the feeding of aphid nymphs leads to formation of hollow galls on the developing petioles that protect the nymphs from abiotic stress or predators (Halaj and Osiadacz 2013; Shour et al. 2004).
The Pemphigus aphids inhabiting the gall and sucking the leaf sap as well as mechanical restrictions on vascular tissue due to petiole twisting and abnormal outgrowth are suggested to lead to the interference of transport of water, and primary and secondary metabolites, resulting in alterations in morphology, physiology, and biochemistry of their host poplar plant (Compson et al. 2011; Künkler et al. 2013; Larson et al. 1991; Richardson et al. 2017. Infestation by P. spyrothecae increased the phenol content and decreased nitrogen and chlorophyll content in the leaf laminas in P. nigra var. italica (Künkler et al. 2013). Infection by Pemphigus betae feeding on the phloem content led to accumulation of nutritive tissue to support the growing aphid numbers in mature galls (Richardson et al. 2017). In fact, galling results in major changes in the sink-source relationships between host leaf and gall. In P. angustifolia, it was demonstrated by 14C-labeling that P. betae galls not only consumed a large fraction of carbon assimilated by the host leaf, but also sequestered a large amount of carbon from surrounding leaves, especially in aphid-susceptible tree genotypes (Compson et al. 2011; Larson and Whitham 1991). Furthermore, as P. spyrothecae infestation is localized to the petiole, it can compromise the wind-driven rotational lamina movements (elastic leaf torsion that is driven by the elliptical cross-section of the poplar petiole, i.e. fluttering, Roden and Pearcy 1993), and can therefore lead to premature leaf drop during strong winds due to excessive drag (Niinemets and Jiang, unpublished observations). Nevertheless, occasionally the galling-dependent alterations of sink-source relationships may have a positive priming effect on plant development and growth and hence indirectly modify the architecture of the tree. It has been reported that galling by the aphid Baizongia pistaciae of Pistacia palaestina leaflets results in the infected branches to carry more leaves and to gain more biomass after two years since the infestation compared with non-galled branches (Kurzfeld-Zexer et al. 2010).
Although the morphological effects of aphid galling on leaves have been extensively studied (Compson et al. 2011; Künkler et al. 2013; Richardson et al. 2017), it is unknown whether the consumption of photosynthetic products and nutrients by the poplar petiole gall aphids for the gall construction will lead to a reduction of physiological activity of the leaf laminas and changes in leaf biomass. In pedunculate oak (Quercus robur), infestation by different lamina-galling wasps (Neuroterus spp. and Cynips spp.,) resulted in major reductions in lamina dry mass per unit area (without attached gall mass) and lamina photosynthetic rate (Jiang et al. 2017a), but petiolar gall effects on lamina characteristics might be less severe. Furthermore, different lamina infections, including fungal, herbivore and galling arthropod infestations, typically lead to induction of various stress volatiles such as monoterpenes, sesquiterpenes, benzenoids and green leaf volatiles (GLV) (Arimura et al. 2004; Besten et al. 2017; Blande et al. 2007; Copolovici et al. 2014; Copolovici et al. 2017; Hall et al. 2017; Irmisch et al. 2014; Jiang et al. 2016; Jiang et al. 2017a; McCormick et al. 2014; Rand et al. 2017; Yli-Pirilä et al. 2016). In these studies, infecting agents directly impacted the lamina, but how galling arthropod infestations on petioles alter lamina volatile emissions is unknown. Given that herbivore feeding induces volatile emissions in non-infected tissues in hybrid aspen (P. tremula x tremuloides) (Li et al. 2017), and that petiolar galls might also transmit stress signals to the lamina by the transpiration stream, including jasmonic acid and salicylic acid signals as well as water-soluble GLV, induction of stress volatile emissions by petiole gall infestations is likely. If petiole galls do induce volatile emissions from leaf lamina, it is further necessary to know whether the severity of the gall infestation (generally evaluated by the size or number of galls) is quantitatively related to photosynthetic traits and the emission rates of constitutive and induced volatiles. However, to our knowledge, such relationships have not been studied yet for petiole gall infestations.
In addition, in constitutive volatile emitters such as isoprene-emitting poplars and oaks, volatile emissions induced by lamina infestations are typically associated with reductions in isoprene emission (Copolovici et al. 2014; Copolovici et al. 2017; Jiang et al. 2016; Jiang et al. 2017a). This pattern reflects the competition for the same chloroplastic substrate pools for isoprene and monoterpenes and also the circumstance that isoprene emissions are strongly associated with leaf photosynthesis rates (Grote et al. 2013; Monson et al. 2012; Sharkey and Yeh 2001). However, it is unclear how constitutive emissions scale with induced emissions in leaves infected by petiole galls.
To gain an insight into the interactions between petiole gall infestation and alteration of physiological traits of leaf lamina, in this study, lamina structural and photosynthetic characteristics, N and C contents, and constitutive isoprene and induced volatile emissions were examined in leaves of Populus × petrovskiana Dippel (P. deltoides x P. laurifolia) with varying severity of infestation by P. spyrothecae. We aimed at addressing five questions: (1) Does the infestation by P. spyrothecae alter lamina photosynthetic rate? If so, how are changes in photosynthetic characteristics associated with lamina structural characteristics and elemental content? (2) What is the effect of the petiole gall infestation on stomatal conductance and the intrinsic water use efficiency of the leaves? (3) How does the localized infestation modify the constitutive isoprene emission? (4) Does the infestation induce emissions of volatiles characteristic to biotic stresses (GLV, mono- and sesquiterpenes, benzenoids, methyl jasmonate) from the leaf lamina? (5) Are there any quantitative relations between these physiological traits and the petiole gall infestation severity? The results of this study demonstrate significant reduction in lamina photosynthesis rate and enhancement of both induced and constitutive volatile emissions in P. spyrothecae gall-infected leaves and that these physiological modifications occur in infestation severity dependent manner.
Materials and methods
Study sites and plant material
The study was conducted in the vicinity of the campus of the Estonian University of Life Sciences, Tartu, Estonia (58°23′ N, 27°05′ E, elevation 25 m above sea level) in mid-September in 2016 when petiole galls had reached the maximum size. Hybrid poplar leaf yellowing starts at these latitudes in the beginning of October, whereas the onset of intensive leaf fall is in late October, and gall dissections demonstrated that the aphids were still fully active within the galls at the time of sampling. The summer (June to September) of Tartu in 2016 was characterized by a cool and humid weather. The air temperature (mean ± SE) was 16.1 ± 1.2 °C, while the monthly precipitation was 88.6 ± 25.2 mm with the relative air humidity of 76.1 ± 2.9% (data of the Laboratory of Environmental Physics, Institute of Physics, University of Tartu, http://meteo.physic.ut.ee). These humid conditions promote the reproduction of the gall-forming aphids, resulting in enhanced formation of the galls on their hosts. A massive outbreak of poplar spiral gall aphid (P. spyrothecae) galls was observed on all the P. × petrovskiana trees at the study site.
To characterize the quantitative relationships among the degree of infestation by the petiole gall aphid and leaf physiological traits, five about 80-year-old P. × petrovskiana trees (height 25-28 m and diameter of 60-80 cm at breast height) were selected, and leaves with petioles with varying gall sizes and numbers were collected for the measurements. About 20 cm long twigs with multiple leaves with infected petioles were sampled for the physiological measurements in the laboratory. Each twig was excised under water, maintained with the cut end in water and immediately transported to the laboratory where a representative leaf was selected for measurements. As reported in the previous studies with excised branches, branch excision per se did not influence foliage photosynthetic and volatile emission characteristics (Copolovici et al. 2014; Jiang et al. 2016; Jiang et al. 2017a; Portillo-Estrada et al. 2015; Portillo-Estrada et al. 2018). Altogether 16 leaves with different size and number of galls were measured (three leaves with no gall infestation (controls) and 13 leaves with galls). The control leaves with no gall infestation were collected from the twigs carrying both infected and non-infected leaves as branches completely without infestation were not available in the study year.
Identification of the galling aphid species as Pemphigus spyrothecae
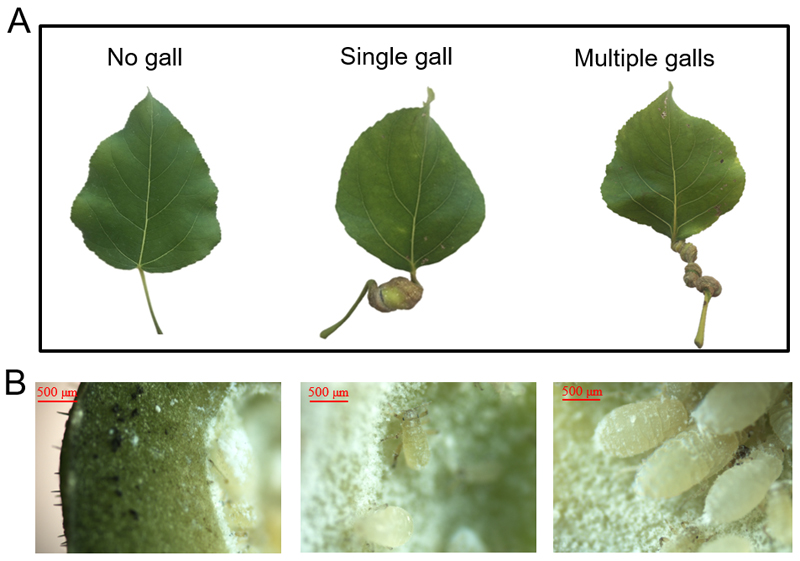
Morphologically, the twisted P. spyrothecae aphid gall consists of swollen petiole with epidermal tissue on the outside and a hollow inside space harboring aphids. The galls are commonly solitary, but occasionally multiple (2-3) galls on the same petiole were found (Fig. 1). The identification of the galling aphid species was based on the Key to European species of Pemphigus (Hałaj and Osiadacz 2013). To identify the organismal group of the possible gall inducer, selected petiole galls were dissected and viewed under a Nikon Eclipse E600 light microscope. The dissected galls and infecting organisms were photographed with a Nikon 5 MP digital microscope camera DS-Fi1 (Nikon Corp., Kyoto, Japan). The parasites were identified as aphids and the key of Blackman & Eastop (2001) was used to narrow down the identification to the species level using the morphological and anatomical characteristics of the galls and the aphids inside the galls (Fig. 1).
Fig. 1.
Representative images of a Populus × petrovskiana control leaf and leaves with different size and number of spiral gall aphid (Pemphigus spyrothecae) galls on the petiole (A) and cross-sections of a representative gall with P. spyrothecae aphids (B).
Foliage gas-exchange measurements
A custom-made gas-exchange system described in detail in Copolovici and Niinemets (2010) was used to investigate photosynthetic characteristics and emissions of volatiles from P. × petrovskiana leaves with varying severity of aphid gall infestation (Figure S1). The system has a temperature-controlled double-walled glass chamber (1.2 L) with a glass and stainless steel bottom. Ambient air with mean ± SE CO2 concentration of 401 ± 18 μmol mol-1 was taken from outside and purified by passing through a custom-made ozone scrubber and a charcoal filter and humidified to the desired humidity by a custom-made humidifier (Copolovici and Niinemets 2010; Copolovici et al. 2014; Jiang et al. 2016). The flow rate through the system was 1.6 L min-1, and high turbulence in the chamber was achieved by a fan inserted at the bottom of the chamber (Niinemets et al. 2011). Chamber air temperature was monitored with a thermocouple and it was within ± 0.2 °C of the water temperature of a thermostatic water bath circulating water between the glass walls of the chamber. Another thermocouple attached to the lower leaf surface was used to monitor leaf temperature. An infra-red two-channel gas-analyzer (CIRAS II, PP-systems, Amesbury, MA, USA) operated in differential mode was used to gauge the concentrations of CO2 and H2O in the chamber in- and outlet.
In this study, the reference environmental conditions used through the measurements were: light intensity at the leaf surface of 1000 μmol m-2 s-1, leaf temperature of 25 °C and relative air humidity of 60%. After the enclosure of the lamina such that the galled petiole was remaining outside the chamber, standard measurement conditions were established and the leaf was conditioned until stomata opened and gas-exchange rates reached a steady state (characteristically in 30 min after enclosure). Thereafter, the steady-state rates of net assimilation and transpiration were recorded and a gas sample was taken for volatile analysis as described in the next section. Calculations of leaf net assimilation and transpiration rates and stomatal conductance were conducted according to von Caemmerer and Farquhar (1981).
Analysis of volatile emissions by gas chromatography-mass spectrometry
Air samples for estimation of volatile emissions were drawn from the gas-exchange chamber by an air sampling pump (210-1003MTX, SKC, Inc., Houston, TX, USA; Niinemets et al. 2011) operated with a flow rate of 0.2 L min-1 for 20 min. Stainless steel cartridges filled with three carbon-based adsorbents with different specific surface area (Carbotrap X, B and C) were used to trap all the volatiles in the C3–C15 range (Kännaste et al. 2014 for details). In all cases, blank chamber samples were taken before and after leaf sampling.
The cartridges were analyzed by a Shimadzu 2010 Plus gas chromatograph with a mass spectrometric sensor (GC–MS) (Shimadzu Corporation, Kyoto, Japan) that was connected to a Shimadzu TD20 automated cartridge desorber. A Zebron ZB-624 fused silica capillary column (0.32 mm i.d.×60 m, 1.8 μm film thickness, Phenomenex, USA) was used and the GC-MS analysis was performed according to the protocol of Kännaste et al (2014). For compound identification, first, authentic standards (Sigma-Aldrich, Finland) were injected into GC-MS to record retention times and mass spectra. Then, the compounds emitted from the leaves were identified by comparing their retention times and mass spectra with those of the authentic standards and with those available in the National Institute of Standards and Technology library (NIST 05) (Copolovici et al. 2009; Kännaste et al. 2014; Jiang et al. 2016). The retention indices were derived using a C8-C20 hydrocarbon standard (Sigma-Aldrich, St. Louis, MO, USA) as in Pazouki et al. (2015) and in Jiang et al. (2016).
Estimation of severity of petiole gall aphid infection
When photosynthesis and volatile emission measurements had been completed, the leaf was harvested and the fresh masses of the gall (aphids inside the gall were removed) and the rest of the leaf were determined. The leaf was further scanned with a resolution of 300 dpi, and the lamina area was estimated with UTHSCSA ImageTool 2.0 (Dental Diagnostic Science, The University of Texas Health Science Center, San Antonio, TX, USA). The number of the galls on each infected leaf, i.e. the number of separated hollow chambers inhabited by aphids (Fig. 1), was estimated. Dry masses of the galls and the leaf were measured after oven-drying at 70 °C for at least 48 h. The total gall number per leaf was not correlated with dry gall mass (data not shown). In addition, unlike the infestation by arthropods forming lamina galls, the formation of P. spyrothecae aphid galls on petioles did not lead to any visible leaf lamina damage (Fig. 1). As leaf lamina damage was missing, and given the differences in gall size, we considered the gall dry mass per leaf dry mass (Mg/Ml) as a more appropriate specific proxy of the severity of infestation than the number of galls per leaf or per unit leaf area (Table 1).
Table 1.
Average (±SE) leaf structural, chemical and gas-exchange traits in non-infected leaves of Populus × petrovskiana and in leaves infected with the spiral gall aphid (Pemphigus spyrothecae)
| Characteristic | Non-infected (n = 3) | Infected (n = 13) | Severely infected (n = 3) |
|---|---|---|---|
| Gall number | 0 | 1.38±0.18** | 1.33±0.33* |
| Gall dry mass per leaf dry mass (Mg/Ml) | 0 | 1.38±0.12** | 1.95±0.12** |
| Leaf dry mass per fresh mass (g g-1) (DF) | 0.283±0.022 | 0.374±0.015** | 0.431±0.033** |
| Leaf dry mass per unit leaf area (g m-2) (MA) | 65.5±1.3 | 55.0±0.8** | 51.2±1.7** |
| Carbon content of leaf lamina (%) | 43.53 ± 0.15 | nd | 43.21± 0.19 |
| Nitrogen content of leaf lamina (%) | 1.652±0.014 | nd | 1.084±0.052 ** |
| Net assimilation rate (μmol m-2 s-1) | 6.6±0.5 | 1.69±0.19** | 1.4±0.8** |
| Stomatal conductance (mmol m-2 s-1) | 112.8±0.8 | 132.3±2.4* | 143.4±2.4* |
| Intercellular CO2 concentration (μmol mol-1) | 164.8±13.2 | 333.6±7.7** | 371.9±16.1** |
Severely infected leaves had the gall to leaf dry mass ratio (Mg/Ml) of about 2.
nd - not determined
The effect of infestation on average trait values compared with controls was tested by ANOVA. The statistical significance is denoted as * - P < 0.05, ** - P < 0.01.
Analysis of N and C contents of the galls and infected leaves
A subset of non-infected leaves (control, n = 3) and leaves with a severe infestation of petiole gall aphids (Mg/Ml of about 2, n = 3) were randomly collected and ground into a powder in liquid nitrogen for chemical analyses. The galls were mechanically separated from the petiole and chemical analyses were conducted separately for the leaf laminas and galls, and the contents of carbon and nitrogen per unit dry mass were estimated by a Vario MAX CNS analyzer (Elementar Analysensysteme GmbH, Hanau, Germany).
Statistical data analyses
One-Way ANOVA was used to compare leaf structural and gas-exchange characteristics and volatile emission rates among control and infected leaves and among control leaves and severely infected leaves (Mg/Ml ≅ 2). The statistical relationships of leaf structural and gas-exchange characteristics and emission rates of volatiles with Mg/Ml were explored by linear and non-linear regression analyses. The statistical functions yielding the greatest degree of explained variance (r 2) were reported. In these analyses, all leaves from different trees were pooled. The statistical analyses were conducted with SPSS 18.0 for Windows (SPSS, Chicago, IL, USA), and all statistical effects were considered significant at P < 0.01.
Results
Leaf structural characteristics and C and N contents in relation to the degree of infestation by petiole gall aphids
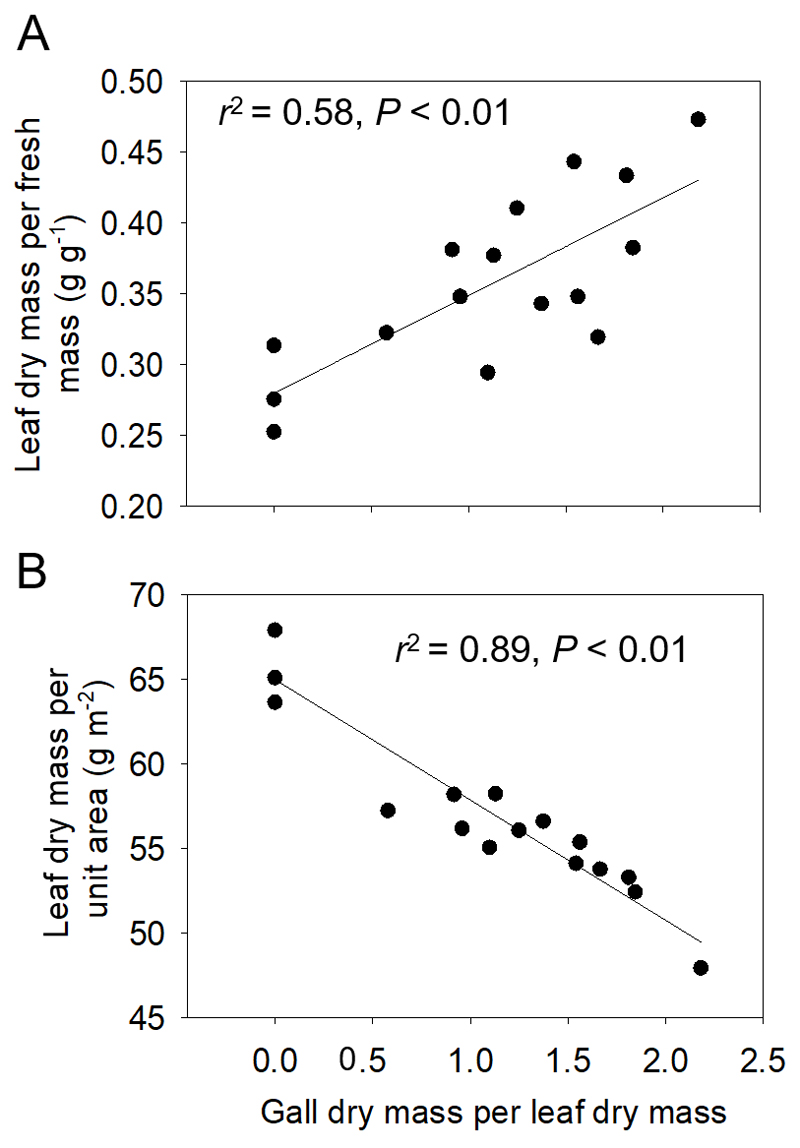
Leaf dry mass per fresh mass increased with increasing severity of aphid gall infestation (Fig. 2A, Table 1), but leaf dry mass per unit area decreased with increasing infection severity (Fig. 2B, Table 1).
Fig. 2.
Leaf dry mass per fresh mass (A) and leaf dry mass per unit leaf area (B) in relation to the severity of infestation by Pemphigus spyrothecae aphid galls in Populus × petrovskiana. The infestation severity was characterized by gall to leaf dry mass ratio (Mg/Ml). Data were fitted by linear regressions as y = 0.280 + 0.0690x (A) and y = 64.6 - 6.89x (C). Altogether 16 leaves with different degrees of infestation were measured (three non-infected control leaves and 13 infected leaves).
Leaf lamina carbon content of non-infected and infected leaves was not significantly different (Table 1, P > 0.1), but N content in aphid-infected leaves was 1.5-fold lower than that in non-infected leaves (Table 1). Carbon content of the galls (mean ± SE = 46.09% ± 0.21%) was 1.1-fold higher than that for leaf laminas in both non-infected and severely infected leaves (P < 0.01 for both mean comparisons). Nitrogen content of the galls (0.69 ± 0.12%) was 2.4-fold lower than that of the non-infected and 1.6-fold lower than that of severely infected leaves (Table 1, P < 0.01 for both mean comparisons).
Photosynthetic characteristics of leaves infected with petiole gall aphids
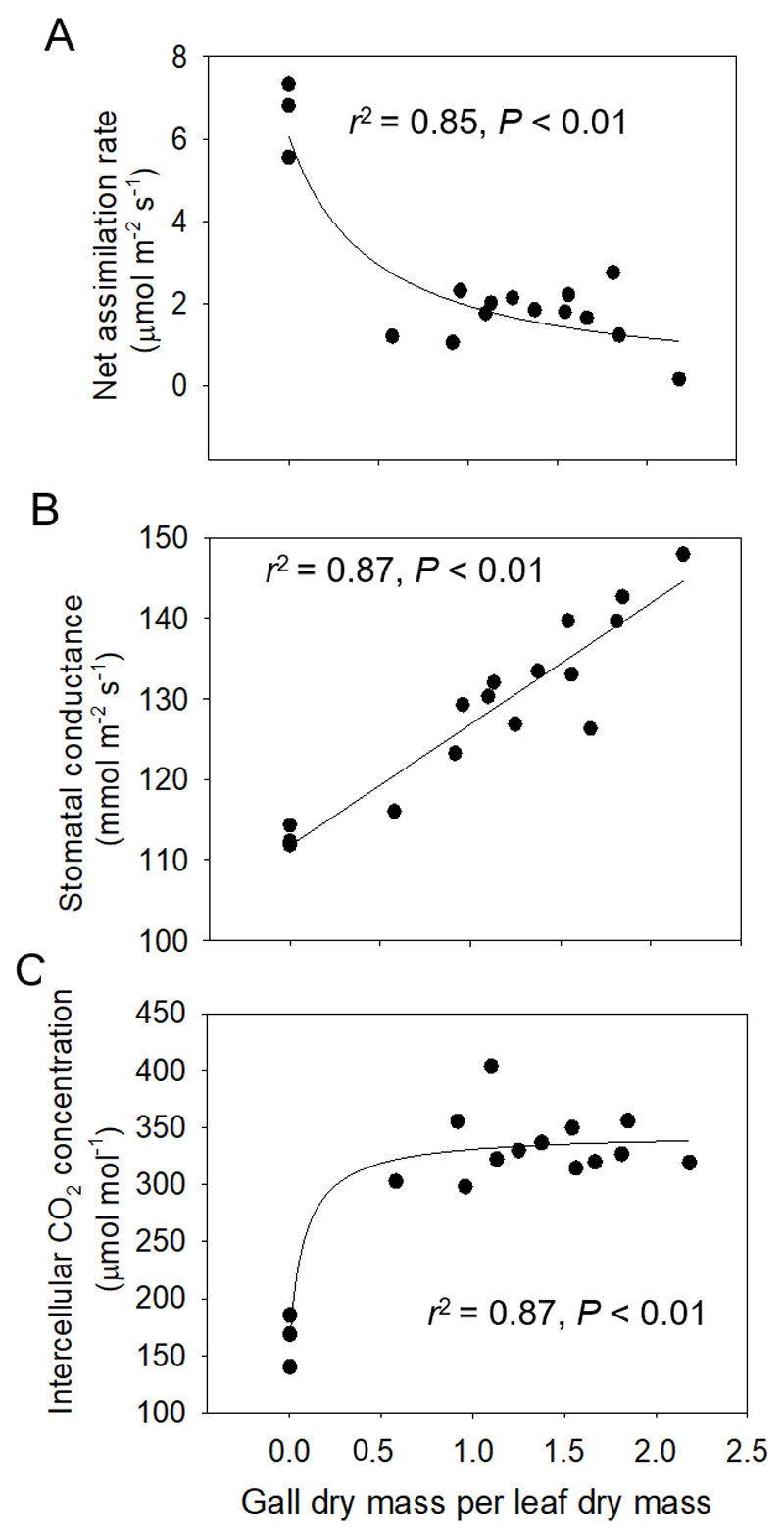
Petiole infestation by aphid galls resulted in an almost four-fold reduction of the lamina net assimilation rate in leaves with the greatest degree of infestation (Table 1). Across all leaves with different infestation severity, there was a negative relationship between the net assimilation rate and petiole infestation severity (Fig. 3A).
Fig. 3.
Relationships of net assimilation rate (A), stomatal conductance to water vapor (B) and intercellular CO2 concentration (C) with the severity of Pemphigus spyrothecae aphid gall infestation in Populus × petrovskiana leaves. Data were fitted by linear (B) or hyperbolic (A, C) regressions, and the corresponding regression equations are: y = 2.758/(0.425 + x) (A), y = 111.8 + 15.1x (B), and y = 164.8 + 180.5x/(0.085+ x) (C). Sample number as in Fig. 2.
Contrarily to net assimilation rate, stomatal conductance increased linearly with the degree of aphid gall infestation (Fig. 3B). Severe infestation by aphid galls on the petiole resulted in 1.3-fold increase of stomatal conductance of P. × petrovskiana leaves (Table 1). As a result of the reduction of net assimilation rate and increase of stomatal conductance, the intercellular CO2 concentration increased non-linearly with increasing the severity of leaf damage (Fig. 3C). In leaves with the greatest degree of infestation, there was a 2.3-fold increase of intercellular CO2 concentration (Table 1).
Effects of petiole gall aphid infestation on constitutive isoprene emissions
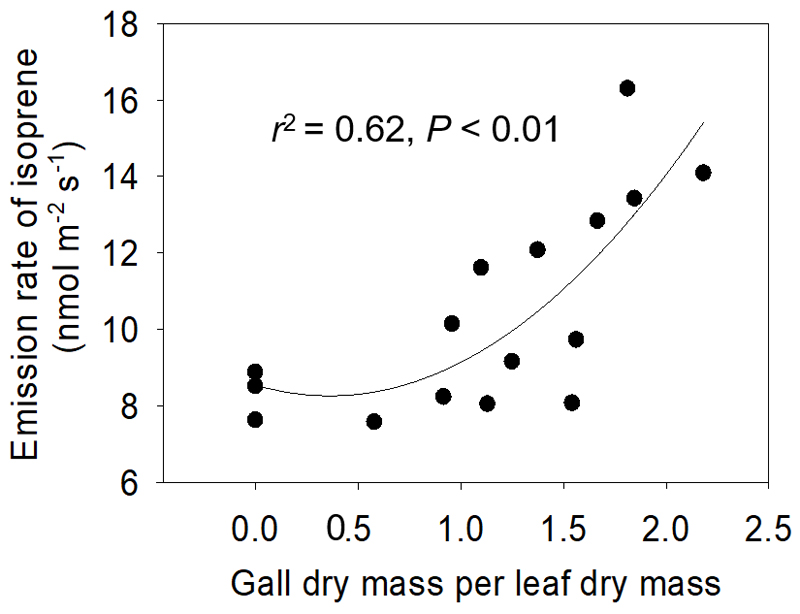
Constitutive isoprene emission rate in non-infected P. × petrovskiana leaves was on average 8.7 nmol m-2 s-1 (Fig. 4, Table 2), and contrarily to the net assimilation rate, infestation by gall-forming aphids led to a non-linear increase of isoprene emission rate with increasing severity of infestation (Fig. 4) such that isoprene emission rate in severely infected leaves was 1.7-fold greater that in control leaves (Table 2). Thus, the percentage of newly assimilated carbon going in isoprene synthesis increased with the increasing severity of infestation.
Fig. 4.
Isoprene emission rate of Populus × petrovskiana leaves in dependence on the degree of infestation by Pemphigus spyrothecae aphid galls. Data were fitted by a second order polynomial regression as y = 8.26 - 1.14x + 2.01x2. Number of measurements as in Fig. 2.
Table 2.
Average (±SE) emission rates of volatile organic compounds of Populus × petrovskiana non-infected leaves and in leaves infected with Pemphigus spyrothecae gall aphids
| Compounds | Retention index | Emission rate (nmol m-2 s-1) |
|
|---|---|---|---|
| Non-infected (n = 3) | Severely infected (n = 3) | ||
| Volatile fatty acid derivatives | |||
| (E)-3-Hexenal | 802 | 0.0213±0.0044 | 0.053±0.008 |
| Heptanal | 899 | 0.0105±0.0010 | 0.0375±0.0008** |
| 6-Methyl-5-hepten-2-one | 985 | 0.0051±0.0011 | 0.110±0.046 |
| 2-Ethyl-hexanal | 989 | 0.0013±0.0007 | 0.0091±0.0009* |
| Octanal | 1001 | 0.0127±0.0001 | 0.090±0.038 |
| 2-Ethyl-1-hexanol | 1015 | 0.019±0.005 | 0.039±0.005 |
| Nonanal | 1098 | 0.0158±0.0016 | 0.23±0.15 |
| Decanal | 1204 | 0.021±0.006 | 0.28±0.15 |
| Isoprenoids | |||
| Isoprene | 507 | 8.70±0.18 | 14.6±1.7 |
| α-Pinene | 932 | 0.0044±0.0014 | 0.0230±0.0029* |
| Camphene | 948 | 0.00072±0.00035 | 0.0026±0.0015 |
| Δ3-Carene | 1009 | 0.00151±0.00024 | 0.01247±0.00044** |
| Limonene | 1029 | 0.00162±0.00010 | 0.00783±0.00071* |
| Eucalyptol | 1033 | ND | 0.00781±0.00093* |
| Linalool | 1098 | 0.0158±0.0016 | 0.0051±0.0012 |
| β-Eudesmol | 1652 | ND | 0.016±0.010 |
| Benzenoids | |||
| Benzaldehyde | 967 | 0.0087±0.0013 | 0.0444±0.0039* |
| Benzothiazole | 1218 | 0.0103±0.0076 | 0.0254±0.0062 |
| Methyl jasmonate | 1647 | ND | 0.030±0.021 |
| Nitrogen containing compounds | |||
| Geranyl nitrile | 1196 | ND | 0.0281 ± 0.0092 |
The volatile concentrations were estimated in air samples collected on cartridges filled with carbon-based adsorbents and analyzed with Shimadzu 2010 Plus GC-MS and thermal desorption system. The limit of detection was 0.5-1 pmol m-2 s-1 for all compounds (see Methods for details).
Data presentation, definition of severe infestation and statistical analysis as in Table 1. For compounds not detected in the control leaves (ND, emission rate below the limit of detection), no statistical comparisons were made.
Induction of stress volatiles by petiole gall aphid infestation
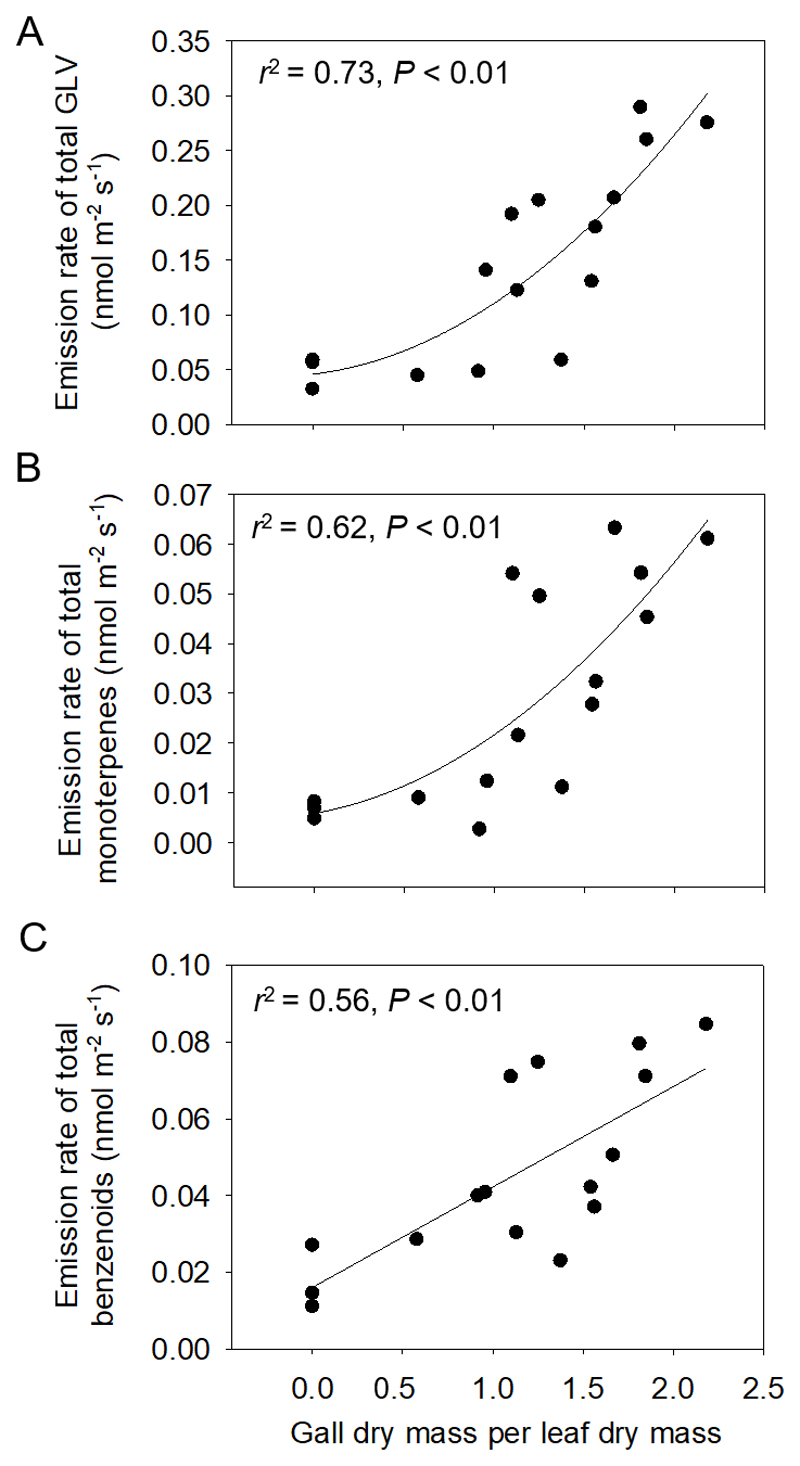
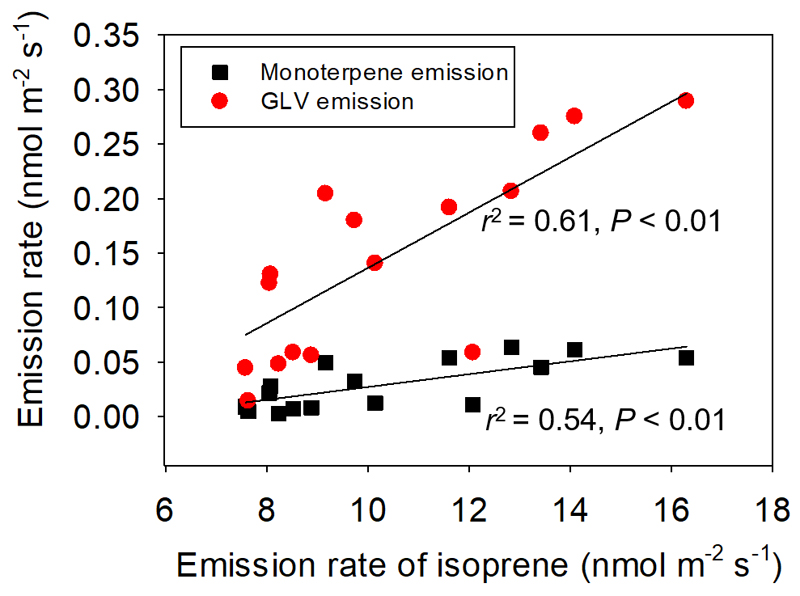
Leaf infestation by gall-forming aphids resulted in profound changes in the volatile emission profiles (Table 2, Fig. 5). Severe infestation by aphid galls (Mg/Ml ≅ 2) induced emissions of specific signaling compounds such as methyl jasmonate and a variety of GLV (heptanal, 2-ethyl hexanal). In addition, emissions of the monoterpene eucalyptol and sesquiterpene β-eudesmol, and a nitrogen-containing compound (geranyl nitrile) were induced and emissions of constitutively released monoterpenes (α-pinene, Δ3-carene, limonene) and benzenoids (benzaldehyde) were significantly enhanced by the petiole gall aphid infestation. Across all leaves, emissions of total GLV (Fig. 5A), monoterpenes (Fig. 5B) and benzenoids (Fig. 5C) increased with increasing the severity of aphid gall infestation, and the constitutive emissions of isoprene were positively correlated with the induced monoterpene emissions and GLV emission (Fig. 6).
Fig. 5.
Emission rates of total green leaf volatiles (GLV, A), total monoterpenes (B) and total benzenoids (C) in P. × petrovskiana leaves in relation to the degree of Pemphigus spyrothecae aphid gall infestation. Data in A and B were fitted by second order polynomial regressions as y = 0.0459 + 0.0197x + 0.0448x2 (A) and y = 0.0056 + 0.0069x + 0.0093x2 (B), and in (C) by a linear regression (y = 0.0161 + 0.0262x). Sample size as in Fig. 2.
Fig. 6.
Correlations of total GLV compounds (red circles) and total induced monoterpene (black squares) emission rates with constitutive isoprene emission rate in Populus × petrovskiana leaves with different degrees of Pemphigus spyrothecae aphid gall infestation (Data from Fig. 4 and Fig. 5A, B). The data were fitted by linear regressions (y = - 0.138+ 0.027x for GLV vs. isoprene emissions and y = - 0.0343 + 0.0061x for monoterpene vs. isoprene emissions). Number of replicates measured as in Fig. 2.
Discussion
Changes in lamina structure and C and N contents upon infestation by petiole galling aphid P. spyrothecae in Populus × petrovskiana
As highlighted in the Introduction, petiole gall aphid infestations result in profound changes in leaf source/sink relationships. In particular, gall-forming aphid infestations can profoundly alter the amount of nutrients transported to the leaf via xylem flow and also determine the extent to which assimilated carbon is transported to the rest of the plant via phloem. Furthermore, growing galls can constitute a stronger carbon sink than the growing lamina and thereby alter lamina structure and biomass accumulation per lamina dry mass. Indeed, very high gall to lamina dry mass ratios (Mg/Ml), even exceeding 2.0 were occasionally observed (Fig. 2), indicating that growing galls on the petiole and feeding aphids inside the galls did constitute a significant carbon sink. This was associated with a reduction of lamina dry mass per unit area (MA; Fig. 2B, Table 1), suggesting that carbon availability could limit the leaf biomass accumulation in growing infected leaves. In contrast, the ratio of leaf dry to fresh mass (DF) increased with increasing Mg/Ml (Fig. 2A). Given that MA is the product of lamina thickness and lamina density, and that lamina density scales positively with DF (Niinemets 1999), we suggest that lamina thickness of poplar leaves was inhibited by petiole gall infestation.
In addition to changes in lamina structural characteristics, lamina N content per dry mass was also significantly reduced in gall-infected leaves (Table 1). Clearly enhanced N investment was needed for construction of galls and for the multiplying aphids, and this can provide an explanation for lower N content of lamina in infected leaves. Yet, galls themselves had a low N content, suggesting that it might also be the restriction of xylem flow into growing leaves that was partly responsible for low lamina N contents in infected leaves. Apart from N supply to the growing leaves, lamina N content can also be altered by sink-source relationships (Jeschke et al. 1997; Jordi et al. 2000; Lavigne et al. 2001) and it is plausible that gall infestation can lead to premature leaf senescence and withdrawal of nutrients from leaf lamina.
Reduction of assimilation rate and increasing stomatal conductance in poplar leaves in relation to the severity of petiole gall aphid infestation
The reduction of leaf lamina photosynthesis rate is a universal response to lamina infestation by gall-forming arthropods in a wide range of species (Dorchin et al. 2006; Jiang et al. 2017a; Patankar et al. 2011), but the way petiole infestations can affect lamina photosynthesis has not been studied to our knowledge. As discussed above, both mechanical restrictions due to twisting of the petiole and compression of vascular tissues caused by the gall formation as well as alterations in sink-source relationships can lead to modifications in foliage photosynthesis rate.
The reduction in net assimilation rate per area (AA) was associated with decreases in MA, (cf. Figs. 2B and 3A), but AA decreased 4.7-fold and MA only 1.3-fold, indicating that net assimilation rate per dry mass (AM, AA=AMMA) also scaled negatively with the infestation severity Mg/Ml (data not shown). The reduction in AM in gall-infected leaves was partly explained by reductions in lamina nitrogen content per dry mass (1.5-fold reduction, Table 1), collectively indicating that both limited carbon availability for leaf growth and limited nitrogen availability either due to restricted xylem flow or nitrogen reallocation (see the previous section) explained the reduction in leaf photosynthesis rates.
Differently from net assimilation rate, stomatal conductance increased with increasing the infestation severity (Fig. 3B), indicating a lower intrinsic lamina water use efficiency (rate of photosynthesis per stomatal conductance to water vapor) and an overall increase of leaf water loss caused by aphid infestation of the petiole. This result is counterintuitive given the possible restriction of xylem flow in infected leaves, but it can indicate limited xylem transport of abscisic acid, the hormone that is responsible for stomatal closure (Aasamaa et al. 2002; Comstock 2002). Given the greater water loss, this evidence suggests that the infected leaves operated at lower leaf water potential, which is likely, given their greater leaf dry to fresh mass ratio (Table 2, Fig. 2A) that is a measure of the fractional investment in cell walls (Niinemets 2001; Onoda et al. 2017). As the result of greater stomatal conductance and lower photosynthetic capacity, infected leaves operated at a higher intercellular CO2 concentration (Fig. 3C), and thus, a loss of stomatal control could have partly compensated for the reduced photosynthetic biomass and N content. However, enhanced investment in cell walls is characteristically associated with lower mesophyll diffusion conductance that determines the CO2 drawdown from the intercellular air space to chloroplasts (Flexas et al. 2012; Tomás et al. 2013; Tosens et al. 2016). If so, CO2 concentration in chloroplasts could still have been lower in gall-infected leaves. Overall, these data further emphasize that the reduction in foliage net assimilation rate in gall-infected leaves was non-stomatal. This is consistent with previous studies on lamina gall infestations that demonstrated that the gall-triggered reduction of leaf photosynthesis was caused by an inhibition of photosynthetic electron transport and reductions in the amount or activity of photosynthetic rate-limiting proteins (Jiang et al. 2017), and possibly also by reduced mesophyll diffusion conductance.
We note that the stomatal conductance of leaves (both control and infected poplar leaves) and net assimilation rate of control leaves in our study was about 1.5-fold lower than during the peak growth period (Jiang et al. 2016). Given that the study was conducted at the end of the growing season when the aphid galls had reached their maximum size, about a month before the start of intensive leaf fall, we suggest that overall reduced foliage physiological characteristics can be indicative of the onset of leaf senescence. Albeit the visual signs of leaf senescence were missing during the study, leaf senescence in deciduous trees is a continuous process that starts shortly after leaves have reached the maximum activity (Niinemets et al. 2004; Niinemets et al. 2012; Sun et al. 2012). The reduction in foliage physiological characteristics initially proceeds with a slow rate, and by the time of the study in September, Populus leaf photosynthesis rate typically has been reduced by 20-50% (Sun et al. 2012). However, the rate of reduction in photosynthetic characteristics accelerates as senescence progresses (Niinemets et al. 2012; Sun et al. 2012), and as suggested above, it is plausible the gall infestation induced premature leaf senescence. To gain an insight into the effects of galls on leaf longevity, future studies should monitor the development of foliage photosynthetic activity since the start of gall development through leaf abscission and look into the leaf fall dynamics of non-infected and infected leaves.
Scaling of constitutive isoprene emissions with the severity of petiole gall aphid infestation
infestation of P. × petrovskiana leaves by the petiole gall aphids resulted in an increase of isoprene release in our study in gall infestation severity-dependent manner, altogether by 1.7-fold across the damage severities (Fig. 4). Given further that net assimilation rate decreased with increasing infestation severity, the fraction of photosynthetic carbon going into isoprene emission increased from 0.8% in non-infected leaves to 6.3% in infected leaves. The percentage of carbon use in non-infected leaves is similar to average values in constitutive isoprene emitters, while the maximum values observed in infected leaves have been previously observed in plants under strong abiotic stresses (Sharkey and Yeh 2001).
The evidence of greater isoprene emissions and a greater use of photosynthetic carbon for isoprene emissions is in marked contrast with how biotic infection of leaf lamina alter constitutive isoprene emission, including a reduction of constitutive isoprene emissions upon fungal infection (Copolovici et al. 2014; Jiang et al. 2016; Toome et al. 2010), lamina gall infestations (Jiang et al. 2017a) and lamina herbivory (Copolovici et al. 2017). The reduction of isoprene emission with a synchronous reduction of photosynthesis rate upon biotic infections has been suggested to reflect a strong control of carbon availability for isoprene biosynthesis (Jiang et al. 2017; Jiang et al. 2016). It is unclear why such a control was absent in petiole gall infected leaves. It might be partly associated with relatively low level of induction of other terpenoids competing for the same substrate (see below). In addition, isoprene has been suggested to be involved in preserving plant membrane integrity against a variety of abiotic stresses (Possell and Loreto 2013; Vickers et al. 2009). Given that petiole gall-infected leaves likely operated at a lower leaf water potential, enhanced isoprene production could be associated with protection from enhanced water stress. On the other hand, given that isoprene emissions increase with decreasing CO2 concentration (Loreto and Sharkey 1990; Rasulov et al. 2009; Wilkinson et al. 2009), low chloroplastic CO2 concentration due to limited mesophyll diffusion conductance (see above for the discussion) could have exerted a direct control of isoprene emission.
Induction of stress volatile release from laminas of petiole gall aphid-infected leaves
The release of volatiles including mono- and sesquiterpenes, and GLV, and benzenoids is a common response to different biotic stresses in a wide range of species (Copolovici and Niinemets 2016; Holopainen et al. 2013). In poplar, induction of stress volatiles has been studied in leaf laminas infected by pathogenic fungi (Jiang et al. 2016) and by herbivores (Arimura et al. 2004; Blande et al. 2007; Irmisch et al. 2014; McCormick et al. 2014), but effects of petiole gall infestations on lamina volatile emissions have not been studied. In our study, petiole gall infestation of P. × petrovskiana leaves resulted in major modifications in the blend of emitted volatiles from leaf laminas (Fig. 5, Table 2), including elicitation of GLV, mono- and sesquiterpenes and benzenoids (Fig. 5a-c; Table 2).
Among the compounds exhibiting enhanced emission in infected leaves, the rapid release of GLV is typically the consequence of cellular damage caused by acute abiotic or biotic stresses (Ameye et al.2017; Beauchamp et al. 2005; Brilli et al. 2012; Jiang et al. 2016; Müller et al. 2010; Niinemets et al. 2013; Portillo-Estrada et al. 2015). In our study, emissions of two GLV, heptanal, 2-ethyl hexanal, were increased in poplar leaves by infestation of petiole gall aphids and showed a sustained elevated pattern (Table 2, Fig. 5A). An analogous pattern of sustained GLV emission has been found in rust fungus Melampsora larici-populina infected leaf laminas of balsam poplar (P. balsamifera var. suaveolens) (Jiang et al. 2016) and in Neuroterus and Cynips gall wasp infected pedunculate oak (Q. robur) leaf laminas (Jiang et al. 2017). However, in these two studies, more than an order of magnitude greater GLV emissions were observed (Jiang et al. 2016, 2017). Given that no visual damage was observed in petiole gall-infected poplar leaves (Fig. 1), the question is what is the source of these GLV emissions from leaf laminas? Because the GLV observed are relatively water-soluble, transport from petiole galls to leaf laminas via the transpiration flux can provide an explanation for the emissions of these volatiles from leaf lamina. On the other hand, volatile or non-volatile signals from petiole galls could have induced secondary emissions from leaf laminas. Such volatile signals could be volatile stress hormones such as methyl jasmonate that elicits a blend of volatiles, including GLV (Jiang et al. 2017b) as well as terpenoids (Arimura et al. 2000; Arimura et al. 2001; Fäldt et al. 2003).
Methyl jasmonate was observed in the emission blend of petiole gall infected leaves (Table 2), suggesting that the emissions induced from leaf lamina might reflect jasmonate-dependent signaling. Jasmonic acid (JA) is an important phytohormone in mediating anti-herbivore defenses, and insect feeding damage, especially by chewing herbivores characteristically triggers the jasmonate pathway, (Davies 2004; Erb et al. 2012; Howe and Jander 2008; Wasternack and Hause 2013). This leads to the onset of multiple downstream defensive responses, such as the production of defense enzymes, synthesis of antifeedant chemicals, and synthesis and release of volatile compounds that can attract foraging natural enemies or deter oviposition by herbivores (De Moraes et al. 1998; De Moraes et al. 2001; Howe and Jander 2008; Tooker et al. 2008; Walling 2000; Wasternack and Hause 2013). The methylated derivative of jasmonic acid, methyl jasmonate could play the role as a long-distance airborne signal to initiate defense responses in adjacent non-infected plants and non-infected leaves of the same plant (Cheong et al. 2003; Heil and Ton 2008; Tamogami et al. 2008). Phloem-feeding herbivores like aphids that cause less severe tissue damage, on the other hand, are often perceived by plants similarly to pathogens, and tend to activate the salicylic acid (SA) pathway (Walling 2000). Gall-inducing insects include both chewing herbivores and those with piercing/sucking mouthparts, and there is evidence that JA-, SA-, and ABA-mediated plant defenses are involved in plant responses against these herbivores (Tooker and Helms 2014).
We suggest that the release of methyl jasmonate from laminas of petiole gall infected leaves (Table 2) originates from methylation of JA transferred to the leaf laminas by the xylem stream from the petiole galls. Once transported to the leaf lamina, systemic defense responses were elicited in the leaf lamina, including release of GLV and upregulation of terpenoid and shikimate pathways. This ultimately resulted in elicitation of volatile terpenoids and benzenoids from the leaves (Table 2, Fig. 5B, C). In fact, there was a very low level of constitutive terpene emission in the non-infected leaves P. × petrovskiana, dominated by monoterpenes α-pinene, Δ3-carene and limonene (Table 2), and emissions of these monoterpenes were significantly enhanced in petiole gall-infected leaves (Table 2). In addition, emissions of the monoterpene eucalyptol and sesquiterpene β-eudesmol were induced by aphid gall infestation (Table 2).
Terpene emissions in gall-infected leaves were much lower than the emission rates typically observed in herbivore-attacked leaves (Blande et al. 2007; Frost et al. 2008) or in fungus-infected leaves (Jiang et al. 2016). Nevertheless, the emission rates of all induced volatiles from gall-infected leaves were positively associated with the infestation severity in a dose-dependent manner (Fig. 5A-C). We suggest that these correlations reflect the scaling of the amount of JA produced in the aphid galls and transported to leaf laminas with the severity of petiole gall infestation. Thus, the stress dose-dependence in the case of petiole gall aphid infection is ultimately driven by the JA dose-dependent effects on the expression of a series of downstream genes participating in the isoprenoid biosynthesis.
To our knowledge, positive correlations among constitutive isoprene emission, and induced monoterpene and GLV observed in our study (Fig. 6) have not been observed across different biotic stresses. We argue that these relationships in petiole gall-infected leaves reflect independent gall effects on constitutive isoprene release as mediated by gall effects on leaf water relations and induced volatile emissions as mediated by JA-dependent signaling. Although monoterpenes and isoprene compete for the same carbon intermediates in chloroplasts and the competition is always shifted towards monoterpene production due to lower Km (Michaelis constant) value of geranyl diphosphate synthase than isoprene synthase for their common substrate dimethylallyl diphosphate (Rasulov et al. 2014), monoterpenes were elicited at a low level in our study. Thus, the substrate-level competition did not affect negatively isoprene emissions in gall-infected leaves.
Conclusions
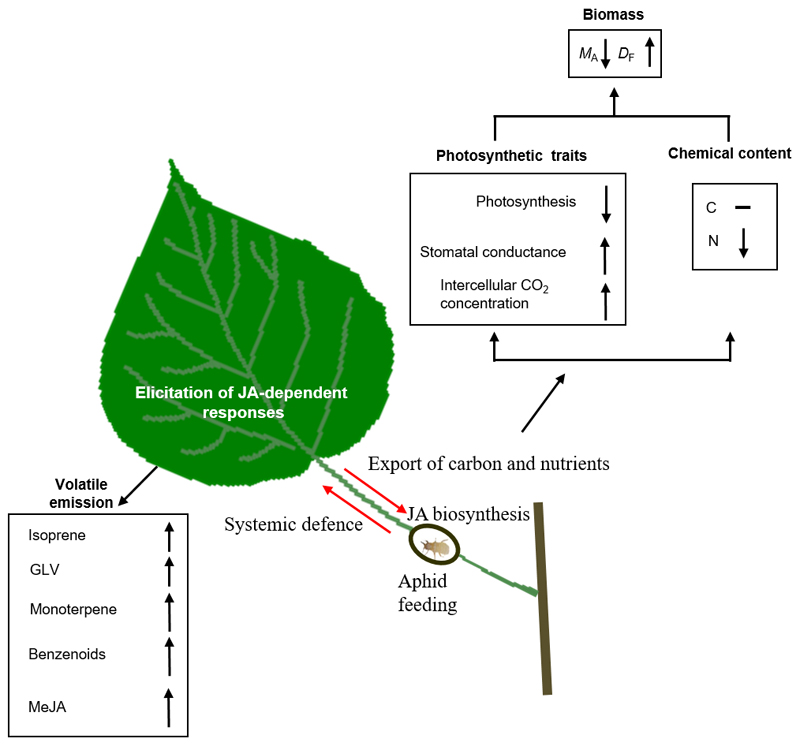
These data demonstrate presence of quantitative relationships between the severity of infestation by petiole gall aphids and leaf lamina photosynthetic characteristics, and constitutive and induced volatile emissions in leaves of Populus × petrovskiana. The interaction between the petiole gall infestation and the leaf lamina is bidirectional (Fig. 7). On the one hand, the localized feeding of the aphids and formation of the galls on the petiole leads to limited carbon and nitrogen availability for leaf lamina construction and for construction of the photosynthetic machinery within the leaf lamina. The loss of carbon and nutrients in leaves leads to a decrease of leaf dry mass per unit area and net assimilation rate per area in dependence on the severity of gall infestation. Aphid feeding further reduces the amount of photosynthetic carbon that the leaf exports to the plant. On the other hand, the feeding on the petiole can trigger a systemic defense in leaf lamina, which might be involved in deterring other herbivores that could reduce the carbon flow to the aphids inside the gall. We propose that the profound influence of petiole galls on volatile emission profiles, including the induction of constitutive isoprene emissions and specialized volatile emission (GLV, monoterpene and benzenoid), results from the systemic defense elicited by JA transported into leaf laminas from petiole galls by the transpiration flux.
Fig. 7.
Schematic summary of the effects of Populus × petrovskiana leaf infestation by Pemphigus spyrothecae aphid galls on leaf structural and photosynthetic traits and volatile emissions. The interaction between the gall infestation and the leaf of the host plant is bidirectional. The localized feeding of the aphids and formation of the galls on the petiole lead to the allocation of carbon and nutrients from the leaf to the galls through the petiole. The loss of the leaf carbon pool and nutrients available for leaf growth leads to a decrease in leaf dry mass per unit area (MA), but greater leaf dry to fresh mass ratio (DF) indicative of increased investment in cell walls (Onoda et al. 2017). On the other hand, gall infestation triggers premature leaf senescence as evident in reduced net assimilation rate per area, albeit stomatal conductance is maintained at a high level. In turn, gall formation and aphid feeding can trigger the systemic defense response in the lamina, thereby enhancing the indirect defenses by volatiles. This includes induction of stress volatile (GLV, monoterpene and benzenoid) emissions from the leaf lamina and enhancement of constitutive isoprene emissions in dependence on the severity of the gall infestation. These systemic defense responses are likely triggered by jasmonate (JA) transported from the galls to the leaf lamina in the transpiration stream. The role of JA pathway in induced volatile emissions is confirmed by observations of the release of methyl jasmonate from leaf laminas (Table 2).
Supplementary Material
Schematic overview of the two-channel custom-designed gas-exchange system applied for measurements of photosynthesis, transpiration and trace gas emissions in Populus × petrovskiana leaf infested by Pemphigus spyrothecae aphid galls.
Key message.
Massive infection of Populus × petrovskiana leaves by petiole gall aphids (Pemphigus spyrothecae) significantly decreased leaf dry mass per unit area, N content per dry mass and net assimilation rate per area, and increased stomatal conductance, leaf dry mass per fresh mass, and constitutive emissions of isoprene. The infection also induced emissions of green leaf volatiles, monoterpenes and benzenoids. The emissions scaled with the infection severity as assessed by dry gall mass per leaf dry mass.
Acknowledgments
This study has been funded by the European Research Council (advanced grant 322603, SIP-VOL+), the Estonian Ministry of Science and Education (institutional grant IUT-8-3) and the European Commission through the European Regional Fund (Center of Excellence EcolChange).
Footnotes
Author contribution statement
JY, YJ and ÜN designed the experiments. JY, YJ and LV performed the experiments and analyzed the data. JY, YJ, LV and ÜN wrote the paper. All authors approved the final version of the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Aasamaa K, Sõber A, Hartung W, Niinemets Ü. Rate of stomatal opening, shoot hydraulic conductance and photosynthesis characteristics in relation to leaf abscisic acid concentration in six temperate deciduous trees. Tree Physiol. 2002;22:267–276. doi: 10.1093/treephys/22.4.267. [DOI] [PubMed] [Google Scholar]
- Ameye M, Allmann S, Verwaeren J, Smagghe G, Haesaert G, Schuurink RC, Audenaert K. Green leaf volatile production by plants: A meta-analysis. New Phytol. 2017 doi: 10.1111/nph.14671. [DOI] [PubMed] [Google Scholar]
- Arimura GI, Ozawa R, Horiuchi J, Nishioka T, Takabayashi J. Plant-plant interactions mediated by volatiles emitted from plants infested by spider mites. Biochem Syst Ecol. 2001;29:1049–1061. [Google Scholar]
- Arimura GI, Ozawa R, Shimoda T, Nishioka T, Boland W, Takabayashi J. Herbivory-induced volatiles elicit defence genes in lima bean leaves. Nature. 2000;406:512–515. doi: 10.1038/35020072. [DOI] [PubMed] [Google Scholar]
- Arimura GI, Huber DPW, Bohlmann J. Forest tent caterpillars (Malacosoma disstria) induce local and systemic diurnal emissions of terpenoid volatiles in hybrid poplar (Populus trichocarpa × deltoides): cDNA cloning, functional characterization, and patterns of gene expression of (–)-germacrene D synthase, PtdTPS1. Plant J. 2004;37:603–616. doi: 10.1111/j.1365-313x.2003.01987.x. [DOI] [PubMed] [Google Scholar]
- Beauchamp J, Wisthaler A, Hansel A, Kleist E, Miebach M, Niinemets Ü, Schurr U, Wildt J. Ozone induced emissions of biogenic VOC from tobacco: relationships between ozone uptake and emission of LOX products. Plant Cell Environ. 2005;28:1334–1343. [Google Scholar]
- Besten MA, Nunes DS, Granato D, Sens SL, Wisniewski A, Jr, Simionatto EL, Riva-Scharf D. Volatile components from galls induced by Baccharopelma dracunculifoliae (Hemiptera: Psyllidae) on leaves of Baccharis dracunculifolia (Asteraceae) Quimica nova. 2015;38:66–70. [Google Scholar]
- Blackman RL, Eastop VF. Aphids on the world’s trees: an identification and information guide. Oriental Insects. 2001;35:104. [Google Scholar]
- Blande JD, Tiiva P, Oksanen E, Holopainen JK. Emission of herbivore-induced volatile terpenoids from two hybrid aspen (Populus tremula × tremuloides) clones under ambient and elevated ozone concentrations in the field. Glob Change Biol. 2007;13:2538–2550. [Google Scholar]
- Brilli F, Ruuskanen TM, Schnitzhofer R, Müller M, Breitenlechner M, Bittner V, Wohlfahrt G, Loreto F, Hansel A. Detection of plant volatiles after leaf wounding and darkening by proton transfer reaction “time-of-flight” mass spectrometry (PTR-TOF) PLoS One. 2011;6:e20419. doi: 10.1371/journal.pone.0020419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caemmerer SV, Farquhar GD. Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta. 1981;153:376–387. doi: 10.1007/BF00384257. [DOI] [PubMed] [Google Scholar]
- Chapman RF. The insects: Structure and function. Cambridge University Press; 2013. Chapter 2: Mouthparts and feeding; p. 961. [Google Scholar]
- Cheong JJ, Choi YD. Methyl jasmonate as a vital substance in plants. Trends Genet. 2003;19:409–413. doi: 10.1016/S0168-9525(03)00138-0. [DOI] [PubMed] [Google Scholar]
- Compson ZG, Larson KC, Zinkgraf MS, Whitham TG. A genetic basis for the manipulation of sink-source relationships by the galling aphid Pemphigus betae . Oecologia. 2011;167:711–721. doi: 10.1007/s00442-011-2033-x. [DOI] [PubMed] [Google Scholar]
- Comstock JP. Hydraulic and chemical signalling in the control of stomatal conductance and transpiration. J Exp Bot. 2002;53:195–200. doi: 10.1093/jexbot/53.367.195. [DOI] [PubMed] [Google Scholar]
- Copolovici L, Kännaste A, Niinemets Ü. Gas chromatography-mass spectrometry method for determination of monoterpene and sesquiterpene emissions from stressed plants. Stud Univ Babes-Bolyai Chem. 2009;54:329–339. [Google Scholar]
- Copolovici L, Niinemets Ü. Flooding induced emissions of volatile signaling compounds in three tree species with differing waterlogging tolerance. Plant Cell Environ. 2010;33:1582–1594. doi: 10.1111/j.1365-3040.2010.02166.x. [DOI] [PubMed] [Google Scholar]
- Copolovici L, Niinemets Ü. Environmental impacts on plant volatile emission. In: Blande J, Glinwood R, editors. Deciphering chemical language of plant communication. Springer International Publishing; Berlin: 2016. pp. 35–59. [Google Scholar]
- Copolovici L, Pag A, Kännaste A, Bodescu A, Tomescu D, Copolovici D, Soran ML, Niinemets Ü. Disproportionate photosynthetic decline and inverse relationship between constitutive and induced volatile emissions upon feeding of Quercus robur leaves by large larvae of gypsy moth (Lymantria dispar) Environ Exp Bot. 2017;138:184–192. doi: 10.1016/j.envexpbot.2017.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copolovici L, Väärtnõu F, Portillo Estrada M, Niinemets Ü. Oak powdery mildew (Erysiphe alphitoides)-induced volatile emissions scale with the degree of infection in Quercus robur. Tree Physiol. 2014;34:1399–1410. doi: 10.1093/treephys/tpu091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies PJ. The plant hormones: their nature, occurrence, and functions. In: Davies PJ, editor. Plant hormones: biosynthesis, signal transduction, action! Academic Publishers; Dordrecht, The Netherlands: 2004. pp. 1–15. [Google Scholar]
- De Moraes CM, Lewis WJ, Pare PW, Alborn HT, Tumlinson JH. Herbivore-infested plants selectively attract parasitoids. Nature. 1998;393:570–573. [Google Scholar]
- De Moraes CM, Mescher MC, Tumlinson JH. Caterpillar-induced nocturnal plant volatiles repel nonspecific females. Nature. 2001;410:577–580. doi: 10.1038/35069058. [DOI] [PubMed] [Google Scholar]
- Dorchin N, Cramer MD, Hoffmann JH. Photosynthesis and sink activity of wasp-induced galls in Acacia pycnantha. Ecology. 2006;87:1781–1791. doi: 10.1890/0012-9658(2006)87[1781:pasaow]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Erb M, Meldau S, Howe GA. Role of phytohormones in insectspecific plant reactions. Trends Plant Sci. 2012;17:250–259. doi: 10.1016/j.tplants.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fäldt J, Arimura GI, Gershenzon J, Takabayashi J, Böhlmann J. Functional identification of AtTPS03 as (E)-ß-ocimene synthase: a monoterpene synthase catalyzing jasmonate- and wound-induced volatile formation in Arabidopsis thaliana. Planta. 2003;216:745–751. doi: 10.1007/s00425-002-0924-0. [DOI] [PubMed] [Google Scholar]
- Flexas J, Barbour MM, Brendel O, Cabrera HM, Carriquí M, Díaz-Espejo A, Douthe C, Dreyer E, Ferrio JP, Gago J, Gallé A, et al. Mesophyll diffusion conductance to CO2: an unappreciated central player in photosynthesis. Plant Science. 2012;193–194:70–84. doi: 10.1016/j.plantsci.2012.05.009. [DOI] [PubMed] [Google Scholar]
- Frost CJ, Mescher MC, Dervinis C, Davis JM, Carlson JE, De Moraes CM. Priming defense genes and metabolites in hybrid poplar by the green leaf volatile cis-3-hexenyl acetate. New Phytol. 2008;180:722–734. doi: 10.1111/j.1469-8137.2008.02599.x. [DOI] [PubMed] [Google Scholar]
- Giron D, Huguet E, Stone GN, Body M. Insect-induced effects on plants and possible effectors used by galling and leaf-mining insects to manipulate their host-plant. J Insect Physiol. 2016;84:70–89. doi: 10.1016/j.jinsphys.2015.12.009. [DOI] [PubMed] [Google Scholar]
- Grote R, Monson RK, Niinemets Ü. Leaf-level models of constitutive and stress-driven volatile organic compound emissions. In: Niinemets Ü, Monson RK, editors. Biology, controls and models of tree volatile organic compound emissions. Springer; Berlin: 2013. pp. 315–355. [Google Scholar]
- Hałaj R, Osiadacz B. European gall-forming Pemphigus (Aphidoidea: Eriosomatidae) Zool Anz. 2013;252:417–423. [Google Scholar]
- Hall CR, Carroll AR, Kitching RL. A meta-analysis of the effects of galling insects on host plant secondary metabolites. Arthropod Plant Interactions. 2017;11:463–473. [Google Scholar]
- Heil M, Ton J. Long-distance signaling in plant defense. Trends Plant Sci. 2008;13:264–272. doi: 10.1016/j.tplants.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Holopainen JK, Nerg AM, Blande JD. Multitrophic signalling in polluted atmospheres. In: Niinemets Ü, Monson RK, editors. Biology, controls and models of tree volatile organic compound emissions. Springer; Berlin: 2013. pp. 285–314. [Google Scholar]
- Howe GA, Jander G. Plant Immunity to Insect Herbivores. Annu Rev Plant Biol. 2008;59(1):41. doi: 10.1146/annurev.arplant.59.032607.092825. [DOI] [PubMed] [Google Scholar]
- Irmisch S, Jiang YF, Chen F, Gershenzon J, Köllner GT. Terpene synthases and their contribution to herbivore-induced volatile emission in western balsam poplar (Populus trichocarpa) BMC Plant Biol. 2014;14:1–16. doi: 10.1186/s12870-014-0270-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeschke WD, Baig A, Hilpert A. Sink-stimulated photosynthesis, increased transpiration and increased demand-dependent stimulation of nitrate uptake: nitrogen and carbon relations in the parasitic association Cuscuta reflexa-Coleus blumei. J Exp Bot. 1997;48:915–925. [Google Scholar]
- Jiang YF, Ye JY, Veromann L-L, Niinemets Ü. Scaling of photosynthesis and constitutive and induced volatile emissions with severity of leaf infection by rust fungus (Melampsora larici-populina) in Populus balsamifera var. suaveolens. Tree Physiol. 2016;36(7):856–872. doi: 10.1093/treephys/tpw035. [DOI] [PubMed] [Google Scholar]
- Jiang YF, Veromann L-L, Ye JY, Niinemets Ü. Oak gall wasp infections of Quercus robur leaves lead to profound modifications in foliage photosynthetic and volatile emission characteristics. Plant Cell Environ. 2017a;41(1):160–175. doi: 10.1111/pce.13050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang YF, Ye JY, Li S, Niinemets Ü. Methyl jasmonate-induced emission of biogenic volatiles is biphasic in cucumber: a high-resolution analysis of dose dependence. J Exp Bot. 2017b;68:4679–4694. doi: 10.1093/jxb/erx244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordi W, Schapendonk A, Davelaar E, Stoopen GM, Pot CS, De Visser R, Van Rhijn JA, Gan S, Amasino RM. Increased cytokinin levels in transgenic P-SAG12-IPT tobacco plants have large direct and indirect effects on leaf senescence, photosynthesis and N partitioning. Plant Cell Environ. 2000;23:279–289. [Google Scholar]
- Kännaste A, Copolovici L, Niinemets Ü. Gas chromatography mass-spectrometry method for determination of biogenic volatile organic compounds emitted by plants. In: Rodríguez-Concepción M, editor. Plant isoprenoids: methods and protocols. Humana Press; New York, NY: 2014. pp. 161–169. [DOI] [PubMed] [Google Scholar]
- Künkler N, Brandl R, Brändle M. Changes in clonal poplar leaf chemistry caused by stem galls alter herbivory and leaf litter decomposition. PLOS One. 2013;8:e79994. doi: 10.1371/journal.pone.0079994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurzfeld-Zexer L, Wool D, Inbar M. Modification of tree architecture by a gallforming aphid. Trees-Struct Funct. 2010;24:13–18. [Google Scholar]
- Larson KC, Whitham TG. Manipulation of food resources by a gall-forming aphid: the physiology of sink-source interactions. Oecologia. 1991;88:15–21. doi: 10.1007/BF00328398. [DOI] [PubMed] [Google Scholar]
- Lavigne MB, Little CHA, Major JE. Increasing the sink: source balance enhances photosynthetic rate of 1-year-old balsam fir foliage by increasing allocation of mineral nutrients. Tree Physiol. 2001;21:417–426. doi: 10.1093/treephys/21.7.417. [DOI] [PubMed] [Google Scholar]
- Li T, Blande JD. Volatile-Mediated within-Plant Signaling in Hybrid Aspen: Required for Systemic Responses. J Chem Ecol. 2017;43(4):1–12. doi: 10.1007/s10886-017-0826-z. [DOI] [PubMed] [Google Scholar]
- Loreto F, Sharkey TD. A gas-exchange study of photosynthesis and isoprene emission in Quercus rubraL. Planta. 1990;182:523–531. doi: 10.1007/BF02341027. [DOI] [PubMed] [Google Scholar]
- McCormick AC, Boeckler GA, Köllner TG, Gershenzon J, Unsicker SB. The timing of herbivore-induced volatile emission in black poplar (Populus nigra) and the influence of herbivore age and identity affect the value of individual volatiles as cues for herbivore enemies. BMC Plant Biol. 2014;14:304. doi: 10.1186/s12870-014-0304-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monson RK, Grote R, Niinemets Ü, Schnitzler JP. Tansley review. Modeling the isoprene emission rate from leaves. New Phytol. 2012;195:541–559. doi: 10.1111/j.1469-8137.2012.04204.x. [DOI] [PubMed] [Google Scholar]
- Müller M, Graus M, Ruuskanen TM, Schnitzhofer R, Bamberger I, Kaser L, Titzmann T, Hörtnagl L, Wohlfahrt G, Karl T, Hansel A. First eddy covariance flux measurements by PTR-TOF. Atmos Meas Tech. 2010;3:387–395. doi: 10.5194/amt-3-387-2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabity PD, Haus MJ, Berenbaum MR, DeLucia EH. Leaf-galling phylloxera on grapes reprograms host metabolism and morphology. PNAS. 2013;110:16663–16668. doi: 10.1073/pnas.1220219110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niinemets Ü. Components of leaf dry mass per area-thickness and density-alter leaf photosynthetic capacity in reverse directions in woody plants. New Phytol. 1999;144:35–47. [Google Scholar]
- Niinemets Ü. Global-scale climatic controls of leaf dry mass per area, density, and thickness in trees and shrubs. Ecology. 2001;82:453–469. [Google Scholar]
- Niinemets Ü, Kull O, Tenhunen JD. Within canopy variation in the rate of development of photosynthetic capacity is proportional to integrated quantum flux density in temperate deciduous trees. Plant Cell Environ. 2004;27:293–313. [Google Scholar]
- Niinemets Ü, García-Plazaola JI, Tosens T. Photosynthesis during leaf development and ageing. In: Flexas J, Loreto F, Medrano H, editors. Terrestrial photosynthesis in a changing environment. A molecular, physiological and ecological approach. Cambridge University Press; Cambridge: 2012. pp. 353–372. [Google Scholar]
- Niinemets Ü, Kuhn U, Harley PC, Staudt M, Arneth A, Cescatti A, Ciccioli P, Copolovici L, Geron C, Guenther A, Kesselmeier J, et al. Estimations of isoprenoid emission capacity from enclosure studies: measurements, data processing, quality and standardized measurement protocols. Biogeosciences. 2011;8:2209–2246. [Google Scholar]
- Niinemets Ü, Kännaste A, Copolovici L. Quantitative patterns between plant volatile emissions induced by biotic stresses and the degree of damage. Front Plant Sci. 2013;4:262. doi: 10.3389/fpls.2013.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onoda Y, Wright IJ, Evans JR, Hikosaka K, Kitajima K, Niinemets Ü, Poorter H, Tosens T, Westoby M. Physiological and structural tradeoffs underlying the leaf economics spectrum. New Phytol. 2017;214:1447–1463. doi: 10.1111/nph.14496. [DOI] [PubMed] [Google Scholar]
- Osiadacz B, Hałaj R. The aphids (Hemiptera: Sternorrhyncha: Aphidinea) of Poland. A distributional checklist. Polish Entomological Monographs. 2009;6:1–96. [Google Scholar]
- Pazouki L, Memari HR, Kännaste A, Bichele R, Niinemets Ü. Germacrene A synthase in yarrow (Achillea millefolium) is an enzyme with mixed substrate specificity: gene cloning, functional characterization and expression analysis. Front Plant Sci. 2015;6:111. doi: 10.3389/fpls.2015.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patankar R, Thomas SC, Smith SM. A gall-inducing arthropod drives declines in canopy tree photosynthesis. Oecologia. 2011;167:701–709. doi: 10.1007/s00442-011-2019-8. [DOI] [PubMed] [Google Scholar]
- Pincebourde S, Frak E, Sinoquet H, Regnard JL, Casas J. Herbivory mitigation through increased water use efficiency in a leaf mining moth-apple tree relationship. Plant Cell Environ. 2006;29:2238–2247. doi: 10.1111/j.1365-3040.2006.01598.x. [DOI] [PubMed] [Google Scholar]
- Portillo-Estrada M, Kazantsev T, Talts E, Tosens T, Niinemets Ü. Emission timetable and quantitative patterns of wound-induced volatiles across different leaf damage treatments in aspen (Populus tremula) J Chem Ecol. 2015;41:1105–1117. doi: 10.1007/s10886-015-0646-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portillo-Estrada M, Kazantsev T, Niinemets Ü. Massive release of volatile organic compounds due to leaf midrib wounding in Populus tremula. 2018 doi: 10.1007/s11258-018-0854-y. Vegetatio xx: In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Possell M, Loreto F. The role of volatile organic compounds in plant resistance to abiotic stresses: responses and mechanisms. In: Niinemets Ü, Monson RK, editors. Biology, controls and models of tree volatile organic compound emissions. Springer; Berlin: 2013. pp. 209–235. [Google Scholar]
- Rand K, Bar E, Ben Ari M, Davidovich-Rikanati R, Dudareva N, Inbar M, Lewinsohn E. Differences in Monoterpene Biosynthesis and Accumulation in Pistacia palaestina Leaves and Aphid-Induced Galls. J Chem Ecol. 2017;43:143–152. doi: 10.1007/s10886-016-0817-5. [DOI] [PubMed] [Google Scholar]
- Rasulov B, Bichele I, Laisk A, Niinemets Ü. Competition between isoprene emission and pigment synthesis during leaf development in aspen. Plant Cell Environ. 2014;37:724–741. doi: 10.1111/pce.12190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasulov B, Hüve K, Välbe M, Laisk A, Niinemets Ü. Evidence that light, carbon dioxide and oxygen dependencies of leaf isoprene emission are driven by energy status in hybrid aspen. Plant Physiol. 2009;151:448–460. doi: 10.1104/pp.109.141978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redfern M. The new naturalist library. Harper Collins Publishers; London: 2011. Plant galls; p. 562. [Google Scholar]
- Richardson RA, Body M, Warmund MR, Schultz JC, Appel HM. Morphometric analysis of young petiole galls on the narrow-leaf cottonwood, Populus angustifolia, by the sugarbeet root aphid, Pemphigus betae. Protoplasma. 2016;254:203–216. doi: 10.1007/s00709-015-0937-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roden JS, Pearcy RW. Effect of leaf flutter on the light environment of poplars. Oecologia. 1993;93:201–207. doi: 10.1007/BF00317672. [DOI] [PubMed] [Google Scholar]
- Sharkey TD, Yeh S. Isoprene emission from plants. Annu Rev Plant Biol. 2001;52:407–436. doi: 10.1146/annurev.arplant.52.1.407. [DOI] [PubMed] [Google Scholar]
- Shour M, Jesse L, Lewis D. Insect galls on trees and shrubs. 2004 http://www.oakgov.com/msu/Documents/publication/ic417_insect_galls.pdf.
- Schoonhoven LM, Van Loon JJA, Dicke M. Insect-plant biology. Oxford University Press; 2005. p. 440. [Google Scholar]
- Sun Z, Copolovici L, Niinemets Ü. Can the capacity for isoprene emission acclimate to environmental modifications during autumn senescence in temperate deciduous tree species Populus tremula? J Plant Res. 2012;125:263–274. doi: 10.1007/s10265-011-0429-7. [DOI] [PubMed] [Google Scholar]
- Sun Z, Niinemets Ü, Copolovici L. Foliar isoprene emission during autumn senescence in aspen (Populus tremula) Geochmica et Cosmochimica Acta. 2009;73(13) [Google Scholar]
- Takei M, Yoshida S, Kawai T, Hasegawa M, Suzuki Y. Adaptive significance of gall formation for a gall-inducing aphids on Japanese elm trees. J Insect Physiol. 2015;72:43–51. doi: 10.1016/j.jinsphys.2014.11.006. [DOI] [PubMed] [Google Scholar]
- Tamogami S, Rakwal R, Agrawal GK. Interplant communication: airborne methyl jasmonate is essentially converted into JA and JA-Ile activating jasmonate signaling pathway and VOCs emission. Biochem Bioph Res Co. 2008;376:723–727. doi: 10.1016/j.bbrc.2008.09.069. [DOI] [PubMed] [Google Scholar]
- Tomás M, Flexas J, Copolovici L, Galmés J, Hallik L, Medrano H, Tosens T, Vislap V, Niinemets Ü. Importance of leaf anatomy in determining mesophyll diffusion conductance to CO2 across species: quantitative limitations and scaling up by models. J Exp Bot. 2013;64:2269–2281. doi: 10.1093/jxb/ert086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooker JF, De Moraes CM. A gall-inducing caterpillar species increases essential fatty acid content of its host plant without concomitant increases in phytohormone levels. Mol Plant-Microbe Interact. 2009;22:551–559. doi: 10.1094/MPMI-22-5-0551. [DOI] [PubMed] [Google Scholar]
- Tooker JF, Rohr JR, Abrahamson WG, De Moraes CM. Gall insects can avoid and alter indirect plant defenses. New Phytol. 2008;178:657–671. doi: 10.1111/j.1469-8137.2008.02392.x. [DOI] [PubMed] [Google Scholar]
- Tooker JF, Helms AM. Phytohormone dynamics associated with gall insects, and their potential role in the evolution of the gall-inducing habit. J Chem Ecol. 2014;40:742–753. doi: 10.1007/s10886-014-0457-6. [DOI] [PubMed] [Google Scholar]
- Toome M, Randjärv P, Copolovici L, Niinemets Ü, Heinsoo K, Luik A, Noe SM. Leaf rust induced volatile organic compounds signalling in willow during the infection. Planta. 2010;232:235–243. doi: 10.1007/s00425-010-1169-y. [DOI] [PubMed] [Google Scholar]
- Tosens T, Nishida K, Gago J, Coopman R, Cabrera HM, Carriquí M, Laanisto L, Morales L, Nadal M, Rojas R, Talts E, et al. The photosynthetic capacity in 35 ferns and fern allies: mesophyll CO2 diffusion as a key trait. New Phytol. 2016;209:1576–1590. doi: 10.1111/nph.13719. [DOI] [PubMed] [Google Scholar]
- Vickers CE, Gershenzon J, Lerdau MT, Loreto F. A unified mechanism of action for volatile isoprenoids in plant abiotic stress. Nature Chemical Biology. 2009;5:283–291. doi: 10.1038/nchembio.158. [DOI] [PubMed] [Google Scholar]
- Walling L. The myriad of plant responses to herbivores. J Plant Growth Regul. 2000;19:195–216. doi: 10.1007/s003440000026. [DOI] [PubMed] [Google Scholar]
- Wasternack C, Hause B. Jasmonates: biosynthesis, perception, signal transduction and action in plant stress response, growth and development. Ann Bot. 2013;111:1021–1058. doi: 10.1093/aob/mct067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson MJ, Monson RK, Trahan N, Lee S, Brown E, Jackson RB, Polley HW, Fay PA, Fall R. Leaf isoprene emission rate as a function of atmospheric CO2 concentration. Global Change Biology. 2009;15:1189–1200. [Google Scholar]
- Wool D. Galling aphids: specialization, biological complexity, and variation. Annu Rev Entomol. 2004;49:175. doi: 10.1146/annurev.ento.49.061802.123236. [DOI] [PubMed] [Google Scholar]
- Yli-Pirilä P, Copolovici L, Kännaste A, Noe S, Blande JD, Mikkonen S, Klemola T, Pulkkinen J, Virtanen A, Laaksonen A, Joutsensaari J, et al. Herbivory by an outbreaking moth increases emissions of biogenic volatiles and leads to enhanced secondary organic aerosol formation capacity. Environ Sci Techno. 2016;50:11501–11510. doi: 10.1021/acs.est.6b02800. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Schematic overview of the two-channel custom-designed gas-exchange system applied for measurements of photosynthesis, transpiration and trace gas emissions in Populus × petrovskiana leaf infested by Pemphigus spyrothecae aphid galls.