Abstract
This study was performed to assess the comparison of the effects amongst butylated hydroxytoluene (BHT), clove extract (CE), and ascorbic acid (AA) as antioxidants on the oxidative stability and color values in fresh beef patties. The adding of BHT, AA, and CE to patties significantly restrained lipid oxidation, lowered hue angle as color value, and expanded redness and chroma values of fresh beef patties in comparison to the control (p<0.05). BHT and AA significantly led to impede the protein oxidation of patties by lowering carbonyl content (p<0.05). CE had no negative effect on protein oxidation. The antioxidant effects of BHT, AA, and CE were obviously manifested. Nonetheless, BHT, AA, and CE appeared to have insignificant difference of each other for lowering the protein oxidation at the end of storage. BHT and CE represented lowered lipid oxidation in comparison to AA. The antioxidant effects of BHT, AA, and CE on lipid oxidation were more marked than the effects on protein oxidation. Furthermore, CE as a natural antioxidant evinced the efficiency in oxidative stability and color stability in fresh beef patties. The study implied that CE could substitute the use of BHT and AA when making beef patties during storage.
Keywords: fresh beef patties, antioxidants, lipid oxidation, protein oxidation, color value
Introduction
Beef patty is one of the most famous meat products that are broadly consuming as fast-food because of the changing style of life for the customer (Mohamed and Mansour, 2012). The quality factors, containing eating quality as taste and smell combination, freshness, appearance, and nutrient contents are employed to assess the beef quality (Cho et al., 2010). Oxidative modifications such as lipid and protein oxidation are involved as the prime causes for reducing the quality and shelf-life of meat and meat products (Dave and Ghaly, 2011; Lund et al., 2011). This type of changes happens more rapidly in mincemeats than in intact meats. It has occurred because of the grinding process disclose the meat surface part to air and the lipid layer to the metal catalytic agent to be oxidized (Devatkal et al., 2010). Many findings have been found in presenting the impacts of lipid oxidation on meat and meat products at refrigerated storage, however; the researches on protein oxidation in treated meat products are restricted (Ganhao et al., 2010). The consequence of protein oxidation leads to alterations in the structures of amino acid and these alterations induce carbonyl generation and reduced thiol content (Bekhit et al., 2013).
The oxidative processes, which can show the adverse impacts on the grade of meat and meat products, induce in broad color changes, flavor deterioration, texture damage and nutrient contents reduction (Shah et al., 2014). Thus, the oxidation level of meat products has to be restrained by adding antioxidants to meat products. Antioxidants are additives type of compounds that can be applied in the processing of meat products to delay or restrain the oxidation process, enhance color stability, and expand shelf-life (Lorenzo et al., 2014).
Butylated hydroxytoluene (BHT) is well known as an artificial antioxidant which may be added to meat and meat products for preventing oxidation and extending the storage life of meat products (Kumar et al., 2015). Nevertheless, synthetic antioxidants, including BHT, BHA, nitrite, and others, have been notified to possess several harmful health impacts. Consequently, this concern induces the using of natural antioxidants in meat products regarding safety, customer acceptance, and expanded shelf-life of meat products (Mokhtar et al., 2014).
Ascorbic acid (AA) is a widely used antioxidant in treated meat products. It is a water-soluble component which was examined to contain no toxic impact on customer (Varvara et al., 2016). AA is ready to lose hydrogen atom to convert into dehydroascorbic acid which exhibits antioxidant activities. As an antioxidant, AA may be incorporated into meat products to restrain oxidation and improve the color-forming (Gadekar et al., 2014).
Cloves, which are the essential plant and aromatic spice, are flower buds of Syzygium aromaticum. Cloves have been broadly employed in the food industries considering their remarkable aroma and useful health characteristics (Ramadan et al., 2013). Clove extracts (CE) obtained from total buds of clove have been broadly investigated to have outstanding antioxidant activities in meat and meat products (Shi et al., 2014). In addition, the principal phenolic components of CE are eugenol and eugenyl acetate, which reveal highly possible antioxidant characteristics (El-Maatia et al., 2016). In this regard, the inclusion of CE can efficiently diminish oxidation level, enhance sensory properties, and prolong the shelf-life of meat products at the refrigerated storage (Radha et al., 2014). However, the comparison of the effects amongst the adding of BHT, CE, and AA to fresh beef patties regarding prevention of lipid and protein oxidation and impacts on color values is not known.
Therefore, the objectives of the current study were to evaluate the comparison of effects amongst BHT, CE, and AA as antioxidants in fresh beef patties, by measuring pH, color values, thiobarbituric acid reactive substances (TBARS), carbonyl content, and thiol content at refrigerated storage. For a better assessment of the impact, beef patties without antioxidants have included in this study.
Materials and Methods
Preparation of clove extract
The clove applied in this study was obtained from a regional market located in Jinju, Korea. The CE powder was procured by employing the process of reflux extraction. The powders were added to distilled water (DW) keeping at 1:5 (w/v) ratio and shifted at 90°C for 6 h to be extraction of clove (part 1). Moreover, the residual extractions were carried out using DW in the proportion of 1:5 at 90°C for 12 h (part 2). These two parts of extraction were merged after cooling at ambient temperature, and the aquatic solution was filtered throughout the Whatman No. 4 paper. Then, the filtered solution was concentrated utilizing a vacuity rotated evaporator at 90°C. The concentrated CE was freeze-dried and stockpiled at -65°C until utilizing.
Chemicals
BHT, AA, 2-thiobarbituric acid (TBA), perchloric acid (PCA), sodium dodecyl sulfate (SDS), tris(hydroxymethyl)amino methane (TRIS) buffer, 5,5′-dithiobis (2-nitrobenzoic acid) (DTNB), sodium chloride (NaCl), phosphate buffer, trichloroacetic acid (TCA), 2,4-dinitrophenylhydrazine (DNPH), ethanol, ethyl acetate, guanidine hydrochloride, and hydrochloric acid (HCl) were procured from Sigma Aldrich (St. Louis, MO, USA). All chemicals applied in this work were of elevated pureness analytical quality.
Preparation of beef patties
Fresh beef loin and beef back fat were received from a native market, and these were cut with knives and crushed applying a grinder (GG-22, German Knife, CA, USA). The grinder, which possessed a plate fixed in 6 mm diameter holes, was applied twice. Four treatments were applied in this study, and each treatment was performed four periods for four batches.
Fresh beef patties were prepared by the application of identical formulates for all patties in the laboratory of meat processing. The beef meat, back fat, and other substances were fully merged in an appropriate proportion using a mixing tool (5K5SS, KitchenAid, MI, USA). The fundamental composition of patties comprised 90.8% beef loin, 8% beef back fat, and 1.2% NaCl. Four treatments for patties were designed: i) no addition of antioxidants (control), ii) addition of 0.02% BHT (T1), iii) addition of 0.05% AA (T2), and iv) addition of 0.1% CE (T3). Hand-held patty maker was used to form beef patty (about 85 g), and the patties were packed in oxygen permeable polyethylene packages. Then, the preservation of fresh beef patties at 4°C in a refrigerator was carried out to continue the analysis of pH, color value, TBARS, carbonyl content, and thiol content.
pH evaluation
The pH was evaluated applying a digitalized pH meter (MP230, Mettler Toledo, Greifensee, Switzerland). Fresh patties (3 g) were added to DW (27 mL), and this mixture was homogenized applying T25 digital Ultra Turrax homogenizer (IKA, Germany). The slurry was retained at ambient temperature to measure pH. The calibration of pH meter was performed by use of two standard buffers of pH 4.0 and 7.0 at 23°C.
Thiobarbituric acid reactive substance (TBARS) value
TBARS level in fresh beef patties was estimated as clarified by Cherian et al. (1996) with few changes. Patties (3 g) were added to 25 mL of 3.86% PCA and homogenized for 20 s through an Ultra Turrax homogenizer. After filtering of homogenates, 2 mL of filtrates were incorporated into 2 mL of 20 mM TBA in DW. These solutions were then conserved for 16 h at ambient temperature to have absorbance. Absorbance was judged at 531 nm employing a UV-Vis spectrophotometer (Cary 60, Agilent Technologies Inc., CA, USA), and the TBARS level was pronounced as mg of malondialdehyde (MDA)/kg of the patties.
Carbonyl content
Carbonyl content was assessed employing DNPH process reported by Levine et al. (1994) with some variations. 2 g of patties was homogenized over 25 mL of 0.6 M NaCl contained phosphate buffer (pH 6.5, 20 mM), and four aliquots of 0.2 mL were received. All aliquots were centrifuged with 1 mL of TCA (10%) at 3,000 g for 25 min, and the supernatant was removed. Then 1 mL of 0.2% 2,4-dinitrophenyl hydrazine (DNPH) dissolved in 2 M HCl was incorporated into two aliquots sample, while 1 mL of 2 M HCl was incorporated into two aliquots as blank samples. The incubation of all samples was executed for 1 h at ambient temperature. Then 0.60 mL of TCA (10%) was incorporated into the samples and centrifuged for 25 min. The supernatant was rejected and the pellet was washed three times using 1 mL 1:1 ethanol: ethyl acetate solution comprising 10 mM HCl. After washing, the pellets were eventually dispersed in 1.5 mL 6 M guanidine hydrochloride in a water-bath at 37°C for 25 min and centrifuged at 9,000×g for 15 min. The absorbance of supernatant acquired was appraised at 280 and 370 nm against 20 mM sodium phosphate 6 M guanidine hydrochloride buffer. Carbonyl content was then computed utilizing coefficient of 21.0/mM/cm and indicated as nmoL carbonyl/mg protein.
Thiol content
Thiol content of control and antioxidants containing beef patties was assessed employing Ellman’s reagent (DTNB) following the process reported by Berardo et al. (2015) with some variations. The patties sample (2 g) was homogenized in 30 mL of 5% SDS in Tris buffer (pH 8.0), followed by incubation at 85°C for 30 min in a water bath. The centrifugation of the homogenates was then carried through at 6,500 g for 20 min. The addition of two mL of 0.1 M Tris buffer and 0.5 mL of Ellman’s reagent (10 mM DTNB in Tris buffer) to the supernatant (0.5 mL) was done to determine the thiol content. A reagent blank considered a solution consisting of two mL of 0.1 M Tris buffer, 0.5 mL of 5% SDS in TRIS buffer (pH 8.0), and 0.5 mL of 10 mM DTNB. Protein content was judged by adding two mL of 0.1 M Tris buffer to the supernatant (0.5 mL). These mixtures were permitted to stay for 30 min in the gloom, and the absorbance was measured at 412 nm through a spectrophotometer. The equating of Lambert-Beer (ε412=14,000 M−1 cm−1) was utilized to compute the thiol content, and the output was explicated in nmoL thiol/mg protein. BSA standard curve was utilized to assess the protein content at 280 nm.
Color assessment
The color values such as L* (lightness), a* (redness), and b* (yellowness) of fresh beef patties were judged employing a Minolta colorimeter (Minolta CR 300, Tokyo, Japan). The calibration of colorimeter was implemented considering the white standardizing plate (Y=93.5; x=0.3132; y=0.3198) to measure duplicate for each patty sample. The chroma (C*) value and hue angle (h°) were determined by following the equations:
Statistical analysis
The experimental design of the current study was an entirely randomizing design with four distinct replications. Means with a standard error of means were exhibited from four replications to execute statistical data analysis. The SAS programming as edition 9.3 was utilized for performing the analysis of the data. The analysis of variance (ANOVA) process was performed in this research. In addition, Duncan’s multiple range tests were utilized for identifying the substantial difference of means amongst the treatments.
Results and Discussion
pH values
The effects of BHT, AA, and CE on the pH of fresh beef patties are viewed in Table 1. The pH values of all patties were not shown significant differences among all storage days (p>0.05). The pH values amongst all formulas of patties showed no significant differences during 1 and 10 d of storage. On 5 d, BHT and CE treated beef patties exhibited significantly lower pH values that that of the control (p<0.05), but there was no significant difference between the control and AA added patties (p>0.05). The pH value reduction might be due to the occurrence of fermentation in CE and BHT treated patties. The pH value raising could have been happened because of the release of ammonia in consequence of amino acids usage by proteolytic bacteria during protein deterioration in raw meat (Mokhtar et al., 2012). The outputs are related to Zahid et al. (2018) who viewed that the control and CE treated patties were not substantially different for pH value at storage times. Mokhtar and Youssef (2014) observed that the formulation of beef burger with CE and BHT/BHA exhibited no significant difference in pH value in comparison to the control during storage periods.
Table 1. Effects of various antioxidants on pH of fresh beef patties at refrigerated storage.
| Storage day | C | T1 | T2 | T3 | SEM | |
|---|---|---|---|---|---|---|
| pH | 1 | 5.71 | 5.51 | 5.57 | 5.56 | 0.06 |
| 5 | 5.61a | 5.58b | 5.60ab | 5.58b | 0.01 | |
| 10 | 5.52 | 5.49 | 5.55 | 5.57 | 0.03 | |
| SEM | 0.06 | 0.03 | 0.02 | 0.02 |
Mean values in the same row with different letters showed significant differences (p<0.05).
C, control; T1, added 0.02% BHT; T2, added 0.05% ascobic acid; T3, added 0.1% clove extract; BHT, butylated hydroxytoluene.
Lipid oxidation
The TBARS is the main indicator of lipid oxidation observed in muscle foods. Alterations in MDA concentration (TBARS) in fresh beef patties treated with various antioxidants are displayed in Fig. 1. The impacts of BHT, AA, and CE on lipid oxidation of fresh beef patties at 10 d of refrigerated storage were manifested. TBARS value of the control patties increased substantially for up to storage d 10 (p<0.05), but TBARS value of BHT, AA, and CE processed patties were not substantially altered from initial to final storage day (p>0.05). When compared to the control, beef patties treated with BHT, AA, and CE manifested substantially lower TBARS values at all day of storage (p<0.05), which mentioned that these three antioxidants evinced positive influences on oxidative stability against lipid oxidation in patties. The development of TBARS value of the control was probably due to the formation of MDA, which are considered secondary lipid oxidation product (Zhang et al., 2016). Nevertheless, BHT and CE included patties expressed significantly lowered TBARS values in comparison to AA included patties during 5 and 10 d of storage (p<0.05). The results are identical to Zhang et al. (2016) who documented that CE and BHT substantially delayed the raise of TBA value in chicken meat. Ozer and Saricoban (2010) regarded that TBARS value was significantly lower in AA merged chicken patties in comparison to the control. Kong et al. (2010) recorded that the TBARS origination was substantially inhibited in pork meat patties added with CE. Furthermore, the inclusion of CE significantly diminished the TBARS value of silver carp fillets in comparison to the control (Shi et al., 2014). These reports are in accordance with the existing study and imply that CE may be utilized as a natural antioxidant for improving the shelf-life of meat products. The antioxidant activity of CE is proved owing to its phenolic substances and its hydrogen providing capacity (Wojdylo et al., 2007).
Fig. 1. Effect of various antioxidants on TBARS (mg MDA/kg of sample) value of fresh beef patties during refrigerated storage.
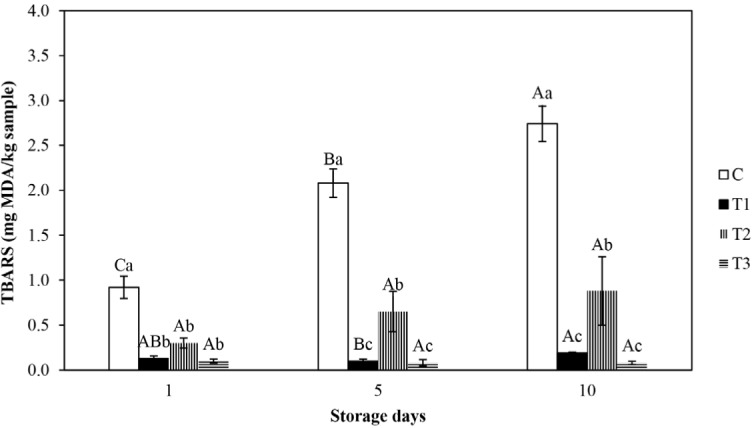
Error bars show standard deviations. Bar charts with different letters show significant differences among the treatments (a-c) at each storage day (p<0.05) or storage days (A-C) in each treatment (p<0.05). C, control; T1, added 0.02% BHT; T2, added 0.05% ascobic acid; T3, added 0.1% clove extract; TBARS, thiobarbituric acid reactive substances; BHT, butylated hydroxytoluene.
Protein oxidation
A widely known observation is that the protein oxidation may affect the meat quality, such as flavor, color, water-holding capacity, texture, and functional characteristics (Lund et al., 2011). Thus, the protein carbonyl and thiol contents were assessed for measuring the protein oxidation level.
Protein carbonyl content
The protein carbonyl content is an important indicator of protein oxidation in meat. Protein carbonyls can be increased by the oxidation of muscle proteins which leads to oxidative degradation of some amino acid side chains such as lysine, proline, arginine, and histidine residues (Stadtman and Levine, 2003). The protein carbonyl content of various treatments of fresh beef patties is presented in Fig. 2. Carbonyl content of the control and AA and CE processed patties were not substantially fluctuated from initial to final storage d (p>0.05), though carbonyl content of BHT included patties varied substantially from d 1 to d 5 and 10 (p<0.05). On all storage days, significantly lower carbonyl content was shown in BHT and AA involved patties than the control (p<0.05). The result suggested that BHT and AA are able to safeguard the patties from protein oxidation. Processing of patties with CE led to significantly lower carbonyl content in comparison to the control on d 5 (p<0.05), nonetheless CE inserted patties evinced no significant variation in carbonyl content in comparison to the control during 1st and 10th d of storage (p>0.05). Furthermore, there were no significant differences in carbonyl content amongst BHT, AA, and CE supplemented patties at last (10th) day of storage (p>0.05). Identically, the control contained the significantly higher volume of protein carbonyl than BHT and pomegranate extract inserted meatballs (Turgut et al., 2016). The protein carbonyl formation may induce protein decomposition, fragmenting or aggregating (Mercier et al., 2004). Zhang et al. (2017) informed that the supplement of CE substantially restrained carbonyl production in pork sausage and this antioxidant activity of CE for restraining the carbonyl production might be due to the presence of phenolic compounds. Like to the ongoing analysis, the decreasing in carbonyl concentration was viewed in vitamin E supplemented microsomal membranes of turkey muscle (Batifoulier et al., 2002) and various plants mixed pork patties (Salminen et al., 2006).
Fig. 2. Effect of various antioxidants on carbonyl contents (nmol carbonyl/mg of protein) of fresh beef patties during refrigerated storage.
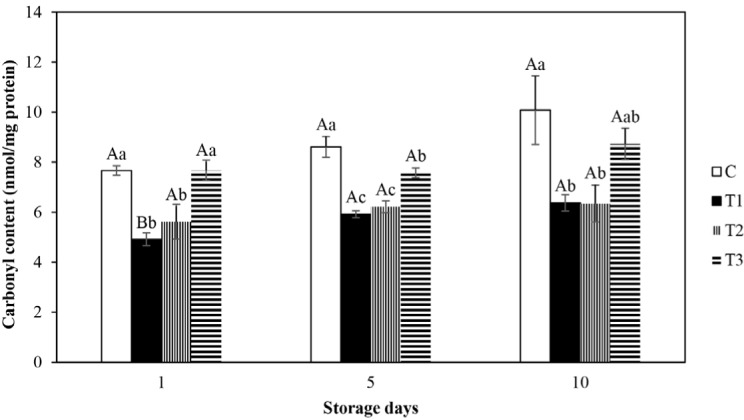
Error bars show standard deviations. Bar charts with different letters show significant differences among the treatments (a-c) at each storage day (p<0.05) or storage days (A,B) in each treatment (p<0.05). C, control; T1, added 0.02% BHT; T2, added 0.05% ascobic acid; T3, added 0.1% clove extract; BHT, butylated hydroxytoluene.
Protein thiol content
The protein thiol content is an important indicator of protein oxidation in muscle foods. The thiol groups concentration, which forms inter and intra molecular disulfide bonds by the result of protein oxidation, decreases with the progress of oxidative reactions (Lund et al., 2011). The protein thiol content of fresh beef patties processed without and with BHT, AA, and CE are manifested in Fig. 3. The significant variations in thiol content for the control and BHT inserted patties were seen from 1–10 d (p<0.05), implied the oxidation of protein with rising of storage time. Nonetheless, there were not seen in significant variations in thiol content for AA and CE inserted patties amongst all storage time (p>0.05), implied that the storage time could not influence on the thiol content. At 1st d, it was no significant variation in thiol content amongst the control and BHT and AA included patties (p>0.05), though significantly lowered thiol content in CE embodied patties in comparison to the control and BHT and AA embodied patties (p<0.05). Protein oxidation generally leads to lowered level of thiol content, which may be ascribed to the creating of disulphide bonds by oxidation (Lara et al., 2011). Within storage time of 5th and 10th d, there were not viewed in substantial variations in thiol content amongst all categories of patties (p>0.05). It can be said that the insertion of BHT, AA, and CE into patties manifested no negative effect on the thiol content as protein oxidation was not intensified. The outputs are correlated with Jongberg et al. (2013) who recited that treatment of sausage with rosemary extract evinced no substantial variation in thiol content in comparison to the control. Shi et al. (2014) accounted that CE implicated fillets significantly impeded the losses of thiol contents.
Fig. 3. Effect of various antioxidants on thiol contents (nmol thiol/mg of protein) of fresh beef patties during refrigerated storage.
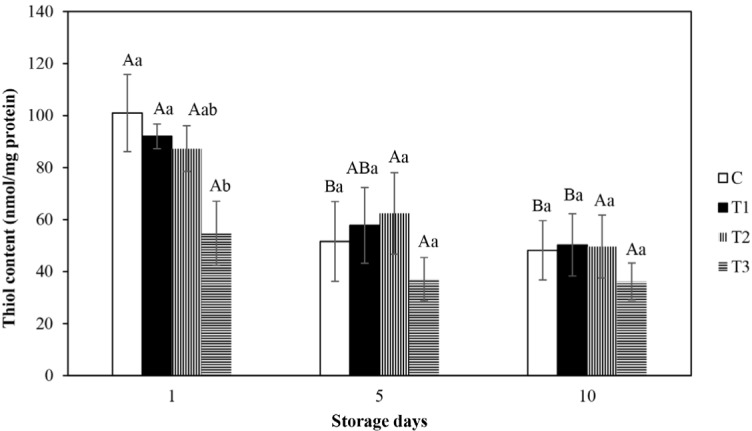
Error bars show standard deviations. Bar charts with different letters show significant differences among the treatments (a,b) at each storage day (p<0.05) or storage days (A,B) in each treatment (p<0.05). C, control; T1, added 0.02% BHT; T2, added 0.05% ascobic acid; T3, added 0.1% clove extract; BHT, butylated hydroxytoluene.
Nonetheless, the outputs (TBARS and thiol contents) for oxidative deterioration evinced a fine similarity; the oxidative deteriorating for the control was intensified significantly from initial to final timing of storage, but the oxidative deteriorating for AA and CE embodied patties were not varied significantly within all storage timings.
Color determination
The color values for fresh beef patties processed with and without BHT, AA, and CE are evinced in Table 2. From 1–10 d, the significant variations in a*, C*, and h° values for the control and AA and CE implicated patties as well as b* values for the control and AA implicated patties were regarded (p<0.05), nonetheless the entire color values for BHT implicated patties did not fluctuate significantly (p>0.05). In comparison to the control, all antioxidants treated patties did not reveal significant variations in L* value on entire storage periods (p>0.05). However, the addition of BHT, AA, and CE to patties resulted in significantly raised a* value in comparison to the control on last (10th) day of storage (p<0.05), and the significantly topmosta* value was revealed in BHT supplemented patties (p<0.05). It is regarded that BHT, AA, and CE might have a preventive effect on the discoloration of beef patties at storage time. This could be linked with the lowering of lipid oxidation in BHT, AA, and CE because lowered lipid oxidation could induce the output of lowered discoloration. Various researchers briefed that lipid oxidation of meat products led to deteriorating of redness (Jia et al., 2012; Jung et al., 2012). The BHT mixed patties had substantially higher b* value than the control and CE mixed patties in the final storing day (p<0.05), though AA mixed patties manifested no substantial variation with all other patties (p>0.05). On the last timing of storing, the control had significantly lower C* value than all antioxidants implicated patties (p<0.05), conversely, the control evinced significantly higher h° value in comparison to all antioxidants implicated beef patties (p<0.05). The outputs are in conformance with Zhang et al. (2017) who narrated that treatment of pork sausage with CE substantially extended a* value in comparison to the control during storage. Falowo et al. (2014) recited that the preventing effects of natural plant sources on the discoloration of meat products were shown owing to the antioxidant performances of phenolic materials. The existing result implies that the lowering of redness in the control might have appeared owing to the oxidation of hemoglobin lipid and the aggregation of brownish methemoglobin (Wetterskog and Undeland, 2004). Ozer and Saricoban (2010) declared that there were no significant variations in L*, a*, b*,C*, and h° values amongst the control and AA and BHA inserted raw chicken patties. In addition, the insertion of CE into fresh beef patties led to significant lowering inh° values and insignificant variations in L* and b* values in comparison to the control within storage (Zahid et al., 2018).
Table 2. Effect of various antioxidants on color of fresh beef patties at refrigerated storage.
| Storage day | C | T1 | T2 | T3 | SEM | |
|---|---|---|---|---|---|---|
| Lightness (L*) | 1 | 40.41ab | 40.57ab | 42.16a | 39.21b | 0.75 |
| 5 | 41.25a | 42.41a | 41.16a | 40.47a | 0.87 | |
| 10 | 41.86ab | 42.70a | 42.33a | 39.82b | 0.63 | |
| SEM | 0.58 | 0.91 | 0.81 | 0.70 | ||
| Redness (a*) | 1 | 21.19Aa | 20.48a | 22.53Aa | 19.66Aa | 0.95 |
| 5 | 14.63Bc | 21.75a | 19.37ABb | 15.86Bc | 0.59 | |
| 10 | 8.89Cc | 20.08a | 15.85Bb | 13.31Cb | 0.79 | |
| SEM | 0.87 | 0.55 | 1.28 | 0.41 | ||
| Yellowness (b*) | 1 | 14.50Aa | 14.27a | 15.54Aa | 14.23a | 0.52 |
| 5 | 12.97Bc | 14.95a | 14.08ABab | 13.30bc | 0.30 | |
| 10 | 13.02Bb | 14.43a | 13.51Bab | 12.90b | 0.28 | |
| SEM | 0.37 | 0.26 | 0.44 | 0.39 | ||
| Chroma (C*) | 1 | 25.94Aa | 24.97a | 27.37Aa | 24.27Aa | 1.11 |
| 5 | 19.57Bc | 26.39a | 23.95ABb | 20.71Bc | 0.63 | |
| 10 | 15.79Cc | 24.76a | 20.91Bb | 18.55Cb | 0.70 | |
| SEM | 0.91 | 0.55 | 1.25 | 0.54 | ||
| Hue angel (h°) | 1 | 34.47Ca | 34.96a | 34.60Ba | 35.90Ca | 0.46 |
| 5 | 41.70Ba | 34.51b | 35.98ABb | 39.99Ba | 0.55 | |
| 10 | 55.88Aa | 35.83c | 41.02Abc | 44.14Ab | 1.60 | |
| SEM | 1.08 | 0.58 | 1.29 | 0.53 |
Mean values in the same row with different letters showed significant differences (p<0.05).
Mean values in the same column with different letters showed significant differences (p<0.05).
C, control; T1, added 0.02% BHT; T2, added 0.05% ascobic acid; T3, added 0.1% clove extract; BHT, butylated hydroxytoluene.
Conclusion
The results evinced the performance of BHT, AA, and CE in significantly restraining lipid oxidation, lowering hue angel as color value, and expanding redness and chroma value of fresh beef patties in comparison to the control. BHT and AA significantly led to impede the protein oxidation of patties by lowering carbonyl content, and there was no significant variation in carbonyl content of CE merged patties related to the control. Furthermore, amongst all patties, the control and BHT, AA, and CE embodied patties viewed no significant variations in thiol content at 5th and 10th days of storage. The antioxidant effects of BHT, AA, and CE were obviously manifested. BHT and CE represented lowered lipid oxidation in comparison to AA. Nonetheless, BHT, AA, and CE appeared to have insignificant difference of each other for lowering the protein oxidation at the end of storage. The antioxidant effects of BHT, AA, and CE on protein oxidation were less marked than the effects on lipid oxidation. In short, the supplement of CE might be used as a secure and synthetic substitute for patties formulation to impede the oxidation and to raise beef patties quality of color value. Therefore, it can be finalized that CE as natural antioxidant can substitute the use of BHT and AA when making beef patties during storage.
Acknowledgements
This research was supported by the Korea Institute of Planning and Evaluation for Technology in Food Agriculture, Forestry and Fisheries, Ministry of Agriculture, Food and Rural Affairs (Project No.316064-02-2-HD030).
Conflict of Interest
The authors declare no potential conflict of interest.
Author Contributions
Conceptualization: Md. Zahid A. Data curation: Seo JK, Md. Zahid A. Formal analysis: Md. Zahid A, Rashida Parvin, Ko JH. Validation: Yang HS, Seo JK. Writing -original draft: Md. Zahid A. Writing -review & editing: Md. Zahid A, Seo JK, Parvin R, Ko J, Yang HS.
Ethics Approval
This article does not require IRB/IACUC approval because there are no human and animal participants.
References
- Batifoulier F, Mercier Y, Gatellier P, Renerre M. Influence of vitamin E on lipid and protein oxidation induced by H2O2-activated MetMb in microsomal membranes from turkey muscle. Meat Sci. 2002;61:389–389. doi: 10.1016/S0309-1740(01)00209-1. [DOI] [PubMed] [Google Scholar]
- Bekhit AEDA, Hopkins DL, Fahri FT, Ponnampalam EN. Oxidative processes in muscle systems and fresh meat: Sources, markers, and remedies. Compre Rev Food Sci Food Saf. 2013;12:565–597. doi: 10.1111/1541-4337.12027. [DOI] [PubMed] [Google Scholar]
- Berardo A, Claeys E, Vossen E, Leroy F, De Smet S. Protein oxidation affects proteolysis in a meat model system. Meat Sci. 2015;106:78–78. doi: 10.1016/j.meatsci.2015.04.002. [DOI] [PubMed] [Google Scholar]
- Cherian G, Wolfe FW, Sim JS. Dietary oils with added tocopherols: Effects on egg or tissue tocopherols, fatty acids, and oxidative stability. Poult Sci. 1996;75:423–423. doi: 10.3382/ps.0750423. [DOI] [PubMed] [Google Scholar]
- Cho SH, Kim J, Park BY, Seong PN, Kang GH, Kim JH, Jung SG, Im SK, Kim DH. Assessment of meat quality properties and development of a palatability prediction model for Korean Hanwoo steer beef. Meat Sci. 2010;86:236–236. doi: 10.1016/j.meatsci.2010.05.011. [DOI] [PubMed] [Google Scholar]
- Dave D, Ghaly AE. Meat spoilage mechanisms and preservation techniques: A critical review. Am J Agri Biol Sci. 2011;6:486–486. doi: 10.3844/ajabssp.2011.486.510. [DOI] [Google Scholar]
- Devatkal SK, Narsaiah K, Borah A. Anti-oxidant effect of extracts of kinnow rind, pomegranate rind and seed powders in cooked goat meat patties. Meat Sci. 2010;85:155–155. doi: 10.1016/j.meatsci.2009.12.019. [DOI] [PubMed] [Google Scholar]
- El-Maatia MFA, Mahgoubb SA, Labiba SM, Al-Gabya AMA, Ramadan MF. Phenolic extracts of clove (Syzygium aromaticum) with novel antioxidant and antibacterial activities. Eur J Integr Med. 2016;8:494–504. doi: 10.1016/j.eujim.2016.02.006. [DOI] [Google Scholar]
- Gadekar YP, Sharma BD, Shinde AK, Verma AK, Mendiratta SK. Effect of natural antioxidants on the quality of cured, restructured goat meat product during refrigerated storage (4±1°C) Small Rumin Res. 2014;119:72–72. doi: 10.1016/j.smallrumres.2014.03.005. [DOI] [Google Scholar]
- Ganhao R, Morcuende D, Estevez M. Protein oxidation in emulsified cooked burger patties with added fruit extracts: Influence on colour and texture deterioration during chill storage. Meat Sci. 2010;85:402–402. doi: 10.1016/j.meatsci.2010.02.008. [DOI] [PubMed] [Google Scholar]
- Gok V, Akkaya L, Obuz E, Bulut S. Effect of ground poppy seed as a fat replacer on meat burgers. Meat Sci. 2011;89:400–400. doi: 10.1016/j.meatsci.2011.04.032. [DOI] [PubMed] [Google Scholar]
- Jia N, Kong B, Liu Q, Diao X, Xia X. Antioxidant activity of black currant (Ribes nigrum L.) extract and its inhibitory effect on lipid and protein oxidation of pork patties during chilled storage. Meat Sci. 2012;91:533–533. doi: 10.1016/j.meatsci.2012.03.010. [DOI] [PubMed] [Google Scholar]
- Jongberg S, Torngren MA, Gunvig A, Skibsted LH, Lund MN. Effect of green tea or rosemary extract on protein oxidation in Bologna type sausages prepared from oxidatively stressed pork. Meat Sci. 2013;93:538–538. doi: 10.1016/j.meatsci.2012.11.005. [DOI] [PubMed] [Google Scholar]
- Jung EY, Yun IR, Go GW, Kim GD, Seo HW, Joo ST, Yang HS. Effects of radix puerariae extracts on physicochemical and sensory quality of precooked pork sausage during cold storage. LWT-Food Sci Technol. 2012;46:556–556. doi: 10.1016/j.lwt.2011.11.007. [DOI] [Google Scholar]
- Kong BH, Zhang HY, Xiong YL. Antioxidant activity of spice extracts in a liposome system and in cooked pork patties and the possible mode of action. Meat Sci. 2010;85:772–772. doi: 10.1016/j.meatsci.2010.04.003. [DOI] [PubMed] [Google Scholar]
- Kumar Y, Yadav DN, Ahmad T, Narsaiah K. Recent trends in the use of natural antioxidants for meat and meat products. Compre Rev Food Sci Food Saf. 2015;14:796–796. doi: 10.1111/1541-4337.12156. [DOI] [Google Scholar]
- Lara MS, Gutierrez JI, Timon M, Andres AI. Evaluation of two natural extracts (Rosmarinus officinalis L. and Melissa officinalis L.) as antioxidants in cooked pork patties packed in MAP. Meat Sci. 2011;88:481–481. doi: 10.1016/j.meatsci.2011.01.030. [DOI] [PubMed] [Google Scholar]
- Levine RL, Williams JA, Stadtman EP, Shacter E. Carbonyl assays for determination of oxidatively modified proteins. Methods Enzymol. 1994;233:346–357. doi: 10.1016/S0076-6879(94)33040-9. [DOI] [PubMed] [Google Scholar]
- Lorenzo JM, Sineiro J, Amado IR, Franco D. Influence of natural extracts on the shelf life of modified atmosphere-packaged pork patties. Meat Sci. 2014;96:526–526. doi: 10.1016/j.meatsci.2013.08.007. [DOI] [PubMed] [Google Scholar]
- Lund MN, Heinonen M, Baron CP, Estevez M. Protein oxidation in muscle foods: A review. Mol Nutr Food Res. 2011;55:83–83. doi: 10.1002/mnfr.201000453. [DOI] [PubMed] [Google Scholar]
- Mercier Y, Gatellier P, Renerre M. Lipid and protein oxidation in vitro, and antioxidant potential in meat from Charolais cows finished on pasture or mixed diet. Meat Sci. 2004;66:467–467. doi: 10.1016/S0309-1740(03)00135-9. [DOI] [PubMed] [Google Scholar]
- Mohamed HMH, Mansour HA. Incorporating essential oils of marjoram and rosemary in the formulation of beef patties manufactured with mechanically deboned poultry meat to improve the lipid stability and sensory attributes. LWT-Food Sci Technol. 2012;45:79–79. doi: 10.1016/j.lwt.2011.07.031. [DOI] [Google Scholar]
- Mokhtar S, Mostafa G, Taha R, Eldeep GSS. Effect of different starter cultures on the biogenic amines production as a critical control point in fresh fermented sausages. Eur Food Res Technol. 2012;235:527–527. doi: 10.1007/s00217-012-1777-9. [DOI] [Google Scholar]
- Mokhtar SM, Youssef KM, Morsy NE. The effects of natural antioxidants on colour, lipid stability and sensory evaluation of fresh beef patties stored at 4°C. J Agroaliment Proc Technol. 2014;20:282–282. [Google Scholar]
- Mokhtar SM, Youssef KM. Antioxidant effect of some plant extracts as compared with BHA/BHT on lipid oxidation and some quality properties of fresh beef burgers stored at 4°C. Suez Canal Univ J Food Sci. 2014;2:19–19. doi: 10.21608/scuj.2014.6668. [DOI] [Google Scholar]
- Ozer O, Saricoban C. The effects of butylated hydroxyanisole, ascorbic acid, and α-tocopherol on some quality characteristics of mechanically deboned chicken patty during freeze storage. Czech J Food Sci. 2010;28:150–150. doi: 10.17221/160/2009-CJFS. [DOI] [Google Scholar]
- Radha Krishnan K, Babuskin S, Azhagu Saravana Babu P, Sasikala M, Sabina K, Archana G, Sivarajan M, Sukumar M. Antimicrobial and antioxidant effects of spice extracts on the shelf life extension of raw chicken meat. Int J Food Microbiol. 2014;171:32–32. doi: 10.1016/j.ijfoodmicro.2013.11.011. [DOI] [PubMed] [Google Scholar]
- Ramadan MF, Asker MMS, Tadros M. Lipid profile, antiradical power and antimicrobial properties of Syzygium aromaticum oil. Grasas y Aceites. 2013;64:509–509. doi: 10.3989/gya.011713. [DOI] [Google Scholar]
- Salminen H, Estevez M, Kivikari R, Heinonen M. Inhibition of protein and lipid oxidation by rapeseed, camelina and soy meal in cooked pork meat patties. Eur Food Res Technol. 2006;223:461–461. doi: 10.1007/s00217-005-0225-5. [DOI] [Google Scholar]
- Shah MA, Bosco SJD, Mir SA. Plant extracts as natural antioxidants in meat and meat products. Meat Sci. 2014;98:21–21. doi: 10.1016/j.meatsci.2014.03.020. [DOI] [PubMed] [Google Scholar]
- Shi C, Cui J, Yin X, Luo Y, Zhou Z. Grape seed and clove bud extracts as natural antioxidants in silver carp (Hypophthalmichthys molitrix) fillets during chilled storage: Effect on lipid and protein oxidation. Food Control. 2014;40:134–139. doi: 10.1016/j.foodcont.2013.12.001. [DOI] [Google Scholar]
- Stadtman ER, Levine RL. Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids. 2003;25:207–207. doi: 10.1007/s00726-003-0011-2. [DOI] [PubMed] [Google Scholar]
- Talukder S, Sharma DP. Development of dietary fiber rich chicken meat patties using wheat and oat bran. J Food Sci Technol. 2010;47:224–224. doi: 10.1007/s13197-010-0027-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turgut SS, Soyer A, Isikci F. Effect of pomegranate peel extract on lipid and protein oxidation in beef meatballs during refrigerated storage. Meat Sci. 2016;116:126–126. doi: 10.1016/j.meatsci.2016.02.011. [DOI] [PubMed] [Google Scholar]
- Varvara M, Bozzo G, Celano G, Disanto C, Pagliarone CN, Celano GV. The use of ascorbic acid as a food additive: Technical-legal issues. Ital J Food Safe. 2016;5:4313. doi: 10.4081/ijfs.2016.4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetterskog D, Undeland I. Loss of redness (a*) as a tool to follow hemoglobin-mediated lipid oxidation in washed cod mince. J Agri Food Chem. 2004;52:7214–7214. doi: 10.1021/jf0307907. [DOI] [PubMed] [Google Scholar]
- Wojdylo A, Oszmianski J, Czemerys R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007;105:940–940. doi: 10.1016/j.foodchem.2007.04.038. [DOI] [Google Scholar]
- Zahid MA, Seo JK, Park JY, Jeong JY, Jin SK, Park TS, Yang HS. The effects of natural antioxidants on protein oxidation, lipid oxidation, color, and sensory attributes of beef patties during cold storage at 4°C. Korean J Food Sci Anim Resour. 2018;38:1029–1029. doi: 10.5851/kosfa.2018.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Peng X, Li X, Wu J, Guo X. The application of clove extract protects Chinese-style sausages against oxidation and quality deterioration. Korean J Food Sci Anim Resour. 2017;37:114–114. doi: 10.5851/kosfa.2017.37.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Wu J, Guo X. Effects of antimicrobial and antioxidant activities of spice extracts on raw chicken meat quality. Food Sci Hum Wellness. 2016;5:39–39. doi: 10.1016/j.fshw.2015.11.003. [DOI] [Google Scholar]


