Background
With the development of minimally invasive thoracoscopic and anesthesia control techniques, intravenous anesthesia with spontaneous ventilation video-assisted thoracic surgery (SV-VATS) has been increasingly employed in management of pleural effusion, bullectomy for pneumothorax, mediastinal biopsy, thymectomy and thymomectomy, wedge lung resections, anatomical lung resections for radical treatment of lung cancer and even more complex surgical procedures including tracheal resection and sleeve lobectomy (1-7) This changes in surgical strategies have been applied in the believe that SV-VATS can avoid adverse effects of mechanical ventilation and the residual effects of muscle relaxants, can achieve a faster recovery of respiratory muscle function and lower operative morbidity (7,8).
Recently, in addition to the adoption of SV-VATS, avoidance of any invasive tool including urinary catheter, central venous lines and early removal of the chest tube after a thoracic surgery or even removal of the tube at end-procedure, which might be defined as tubeless SV-VATS has been selectively carried out in an attempt to significantly relieve postoperative pain, thus facilitating further patients’ recovery (8-10). As a matter of a fact, in early reports, tubeless SV-VATS has resulted in a reduction of pain and shorter hospital stay than standard VATS (11-21). Watanabe et al. (11) also proposed that indications for tubeless SV-VATS wedge lung resection include a negative air leak test along with a lack of predominant bullous or diffuse emphysema, of fibrous pleural adhesions, and of preoperative pleural effusion.
So far tubeless SV-VATS is being adopted in quite many centers worldwide. Overall, initial results hold promise but warrant further thorough clinical research.
In this consensus statement we summarize the relevant technical points in order to facilitate a safe and wider application of this novel surgical strategy.
Indications
Inclusion criteria
The operation duration is no more than 2 hours.
Aged 16–60 years.
Patients who have undergone simple surgeries such as pulmonary bullous resection, lung biopsy, pulmonary wedge resection, pleural biopsy, thoracic sympathectomy, and/or resection of the posterior mediastinal tumor. Also, patients who are unlikely to develop severe complications such as major bleeding, respiratory obstruction, and severe postoperative pain.
Patients who have an Eastern Cooperative Oncology Group (ECOG) physical performance score of ≤1 point.
Patients who have been measured as having an American Society of Anesthesiologists Standard (ASA) grade of ≤ II.
There is no indications of arrhythmia, such as frequent premature ventricular contractions (PVCs) or atrial fibrillation. For patients with suspicious or positive coronary heart disease and other high-risk conditions, coronary artery CT angiography can be performed; if the coronary artery and its branches have stenosis of 75% or less, coronary angiography is recommended.
Cardiopulmonary function (EF >50% and FEV1% >50% of predicted value) and other vital organ functions are normal. Resting blood gas analysis without oxygen inhaling shows PO2 ≥75 mmHg and PCO2 <45 mmHg.
Exclusion criteria
Exclusion criteria can be separated into patient-related factors: refuses the surgery and anesthesia protocols, has a history of surgery in the ipsilateral thoracic cavity, associated severe acute pulmonary infection and/or tuberculosis, has obesity with a body mass index (BMI) >30, allergy to local anesthesia, coagulopathy, elevated risk of regurgitation (<6 hours of fasting), hypoxemia (PaO2 <60 mmHg) or hypercapnia (PaCO2 >50 mmHg) preoperatively; neurological disorders. Relative contraindications: persistent cough or high airways secretions; spinal deformity or brain edema (if thoracic epidural anesthesia to be used).
Anesthesia-related factors: any contraindications for the use of regional anesthesia technique; difficult airway management.
Surgery-related factors: extensive pleural adhesions; inexperience and poorly cooperative surgical team. Previous ipsilateral surgery.
Preoperative management
Preoperative clinical examination and diagnostic tests
Collection of demographics and accurate patient’s clinical anamnesis including a description of previous and present illnesses.
Laboratory tests: Routine blood tests; testing for blood type, liver function, kidney function, electrolytes, and coagulation function; arterial blood gas analysis; testing for hepatitis B virus, hepatitis C antibody, and routine urine tests.
Electrocardiogram (ECG) and in selected instances, cardiac color ultrasound is performed.
Chest X-ray (Anterior-posterior/Lateral views), and HRCT scan.
Weight loss
For patients whose preoperative BMI is at the critical level, endocrine therapy, nutrition, and traditional Chinese medicine (TCM)-based acupuncture can be offered for preoperative weight loss treatment, which can optimize body composition by reducing fat content and ensuring protein levels (22,23); it can also reduce abdominal circumference and abdominal fat accumulation, improve diaphragm function, and promote the postoperative recovery of pulmonary function.
Anesthesia
Since the tubeless technique is for simple surgeries, “intravenous anesthesia + local anesthesia at incision + intercostal nerve block + pleural block + thoracic vagus nerve block” is recommended. laryngeal mask airway (LMA) is used to replace double-lumen endotracheal intubation. Also, nasal cannula or facial mask can also be alternatives to LMA in some patients.
Induction of anesthesia
After 15 minutes of dexmedetomidine infusion with a pump at the rate of 1.0 µg/kg/h, propofol (TCI) 2–3.5 µg/mL and sufentanil 0.2 µg/kg are started. When the BIS value is reduced to 60 or lower, a laryngeal mask airway (LMA) is placed and connected with the breathing circuit, and breathing is observed. If there is no spontaneous breathing, manually-assisted ventilation or synchronized intermittent mechanical ventilation (SIMV) (FiO2 1, VT 7–8 mL/kg, RR 10–12 times/min, and oxygen flow 4–5 L/min) can be used. PetCO2 should be monitored. The radial artery is punctured on the non-surgical side to monitor invasive blood pressure (IBP) constantly. Central venipuncture may be performed if necessary.
Maintenance of anesthesia
Before a skin incision is made, propofol (TCI) 1.5–4 µg/mL, remifentanil 0.03–0.08 µg/kg/min, and dexmedetomidine 0.5 µg/kg/h, are used to maintain the BIS value between 45 and 60. If IBP monitoring shows SBP is <90 mmHg, dopamine can be intravenously injected with an initial dose of 3–5 µg/kg/min (maximum dose: <8 µg/kg/min). Dexmedetomidine is stopped immediately after the beginning of closing the pleural cavity, and propofol and remifentanil are withdrawn immediately after surgery. No inhalational anesthetic is used at any time during the procedure.
Induction of the recovery of spontaneous breathing
Recovery of spontaneous breathing may be induced by manually assisted ventilation. If spontaneous breathing cannot be recovered within 10 minutes, the dose of the anesthetic/analgesia can be reduced. After the spontaneous breathing is restored, the oxygen concentration is adjusted to 100% while the oxygen flow rate is adjusted to 4–5 L/min.
Local anesthesia + pleural surface block + thoracic vagus nerve block
First, 5 mL of 1% lidocaine is injected at the lower edge of the intercostal space above the incision and the upper edge of the intercostal space beneath the incision. With the incision as for the center, skin, subcutaneous tissue, intercostal muscle, intercostal nerve, and parietal pleura within 2 cm of the incision are anesthetized by immersion (Figure 1).
Figure 1.
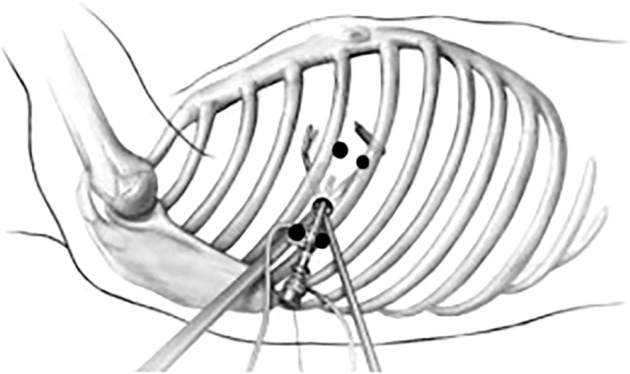
Visceral pleural surface anesthesia with 5 mL of 2% lidocaine (the black spots are the injection sites).
Then, 2.5 mL of 0.5% ropivacaine + 2.5 mL of 2% lidocaine are injected for thoracic vagus nerve block. In the case that anatomical abnormalities such as aortic occlusion and ectopic azygos arch lead to difficulty in vagus nerve block, a vagus nerve block may not be performed.
After the block is satisfactorily achieved, the dose of remifentanil can be decreased to 0.03–0.05 µg/kg/min.
Anesthesia maintenance and respiratory management after the creation of artificial pneumothorax
Mediastinal bulging in artificial pneumothorax: excessive mediastinal bulging will affect the surgical operation. This is mainly because of one-lung breathing and anesthetic drugs (especially remifentanil and propofol) on breathing after the establishment of artificial pneumothorax. The ideal parameters of one-lung spontaneous breathing are VT 3–4 mL/kg and RR 20–25 beats/min. Since dexmedetomidine has a small effect on respiration, the dose of dexmedetomidine remains constant during surgery. The tidal volume and respiratory rate of spontaneous breathing can be changed by adjusting the infusion rates of remifentanil and propofol. The BIS is maintained at 45–60. After local anesthesia + nerve block is achieved, the dose of remifentanil can be gradually decreased to reduce its impact on breathing.
Intraoperative hypercapnia: hypercapnia is common. If PaCO2 is ≥60 mmHg, manually assisted ventilation or synchronized intermittent mechanical ventilation (SIMV) (FiO2 =100%, VT 3–5 mL/kg, RR 12–15 times/min, and oxygen flow 4–5 L/min) can be applied, along with the adjustment of infusion rates of propofol and remifentanil. If the above treatment fails to improve hypercapnia, and the PaCO2 is ≥80 mmHg, conversion of the anesthesia method may be considered (the conversion of anesthesia method is not based on time but depends on the changes in vital signs; see the conversion conditions).
Intraoperative hypoxemia: the incidence of hypoxemia is low. Intraoperative hypoxemia typically occurs after the lung at the surgical side completely collapses. If SpO2 is below 90%, manually assisted ventilation or synchronized intermittent mechanical ventilation (SIMV) (FiO2 =100%, VT 3–5 mL/kg, RR 12–15 times/min, and oxygen flow 4–5 L/min) can be applied. When the operated lung completely collapses, the airway resistance of the operated side is higher than that of the contralateral side. During the low-tidal-volume ventilation, most air will enter the contralateral lung, which does not cause the inflation of the operated lung and has negligible impact on the surgical operation.
-
Intraoperative airway management:
The positional shift of laryngeal mask: the possibility of a positional shift of the laryngeal mask may be considered during surgery if the patient suddenly experiences inspiratory dyspnea, PetCO2 waveform suddenly becomes flat or disappears, and/or VT suddenly drops. The location of the laryngeal mask needs to be properly adjusted under deeper anesthesia.
Air leak test after re-inflation of the operated lung: re-inflation of the operated lung may be difficult if air leaks from the laryngeal mask. Air leakage may be reduced by gently pressing the two sides of the laryngeal prominence.
Intraoperative sputum suctioning: if sputum suctioning is required through the laryngeal mask, the negative pressure should be <10 kPa, and each session shall last for <10 s. Repeated stimulation of the glottis should be avoided, as it may cause cough and laryngospasm.
Timing of LMA withdrawal
The patient is awake and can open eyes when called.
Inhalation of air via the LMA: SpO2 >95% for 5–10 min (or PaO2 >85 mmHg, PaCO2 <50 mmHg).
VT >6–8 mL/kg.
Hemodynamic parameters are stable.
Conversion to other anesthesia modes
-
Conversion conditions:
Hypoxemia: SpO2 <85%, which is not improved after SIMV;
-
PaCO2 ≥80 mmHg, which is not improved after ventilation, along with the presence of any of the following criteria:
❖ Change in circulation: HR >100 bpm, or systolic pressure changes by >30% of the baseline value;
❖ Arrhythmia: e.g., frequent atrial or ventricular premature beats ≥6 times/min (not caused by surgical stimulation); and
❖ pH value <7.15 at two sessions of arterial blood gas test (performed at intervals of 15 minutes or more).
Excessive bulging of the surgical field makes it difficult to operate, which is not improved after medical treatment, and the duration is >5 min;
Severe bleeding in the surgical wound and/or thoracic cavity blurs the surgical field;
The tracheal secretions (especially the bloody secretions) remarkably increase, leading to difficulty breathing and increased airway resistance. VT decreases by >30% during spontaneous breathing, and the peak flow is >20 cmH2O during mechanical ventilation;
After thoracotomy for surface anesthesia and vagus nerve block, cough persists (>2 times/min).
-
Selection of an endotracheal tube during conversion to intubated anesthesia:
Single-lumen endotracheal tube + bronchial occlusion device (preferred);
The double-lumen bronchial catheter (single-lumen endotracheal tube + bronchial occlusion device is preferred; if lung isolation is required due to bleeding in the airway, double-lumen endotracheal intubation is recommended).
-
Tracheal intubation at the lateral position:
The head is supported with a small square pillow to make the anterior and inferior sides of the mouth and nose empty;
The head and neck are parallel to the central axis of the body;
A visual laryngoscope is used;
Catheter shaping is applied; and
There is close cooperation between two staff members.
Surgical procedure
Surgical steps
The operation can be performed via a single incision and with a single operation port, or multiple ports. The incision into the chest wall at the surgical site may cause iatrogenic pneumothorax, which leads to lung collapse. Under direct thoracoscopic vision, 2.5 mL of 0.75% ropivacaine + 2.5 mL of 2% lidocaine (a total of 5 mL) should be injected into the thoracic part of the vagus nerve trunk (the right side is located on the surface of the trachea above the azygos arch, and the left side is located on the surface of the ascending aorta above the lung root under the mediastinal pleura).
Postoperative chest tube management: The thoracic cavity should be checked for any bleeding or air leaks. The thoracic surgical incision is first closed, and the drainage port is reserved with a 7# Mersilk suture, accompanied using an indwelling chest tube. After low-vacuum suction, the anesthesiologist assists the inflated lung to exhaust air. The drainage tube is placed below the water level in a container to observe whether there is any bubble overflow. After the air is exhausted, if the water column fluctuates 8–10 cm above the water surface, the negative pressure in the thoracic cavity is good. The drainage tube can then be removed while the reserved suture is tightened to close the drain incision.
Operational precautions
The operator should judge whether an indwelling chest tube should be used according to surgical situations and disease conditions. Any unnecessary use of the chest tube should be avoided.
During the operation (especially before the vagus nerve block), traction of the lung tissue, especially the hilar structures, should be avoided. More gentle maneuvers, such as pushing and pulling, should be used, while rougher actions, such as pressing and lifting, should be avoided. Particular attention should be paid to reduce irritation to the hilum.
If the degree of the mediastinal swing is large during the dissection of hilar structures, a small dose of muscle relaxant can be used.
Postoperative management
Indicators used in routine postoperative monitoring
Respiratory rate, heart rate, blood pressure, and oxygen saturation are measured every 30 minutes within 12 hours after surgery. Subjective symptoms, including pain, chest tightness, and shortness of breath, along with lung signs, are recorded. Chest X-ray is performed within 8 hours after surgery. It is recommended that a chest ultrasound is performed every 8 hours within 24 hours after surgery to observe the pleural effusion.
A second chest X-ray examination and thoracic ultrasound scan for pleural effusion are performed within 48 hours after surgery.
Evaluation of lung recruitment
Evaluation method: Chest X-ray is performed within 8 and 48 hours after surgery.
Evaluation criteria: Lung re-inflation is evaluated as good (lung re-inflation ≥70%), medium (70% > lung re-inflation ≥50%), and poor (lung re-inflation <50%) according to the degree of postoperative lung recruitment.
Evaluation of postoperative pleural effusion
Evaluation method: chest ultrasound is performed 8, 16, 24, and 48 hours after surgery.
Evaluation criteria: the volume of pleural effusion is evaluated according to its anteroposterior diameter
Evaluation of whether an indwelling chest tube should be inserted again after the surgery
Evaluation method: an indwelling chest tube should be used again after the surgery if the lung is poorly re-inflated or if thoracentesis is required due to the presence of pleural effusion.
Evaluation criteria: medium or low lung re-inflation, and massive pleural effusion requiring the use of an indwelling chest tube. The indications for the removal of the re-inserted chest tubes are the same as the criteria for routine tube withdrawal.
Indications and methods for removing the chest tube
Removal of the chest tube: The indications for the removal of the chest tube are (including the control group and all patients requiring re-intubation):
Chest X-ray reveals that the remaining lungs are completely re-expanded (there is no residual cavity in the thoracic cavity);
The drainage bottle does not bubble when coughing;
The drainage fluid is not bloody, chylostatic, or purulent;
The volume of chest drainage: the volume of pleural drainage is ≤200 mL/24 h. All drainage tubes are removed with deep inspiration breath-hold, and the open insertion site should be quickly covered with Vaseline® gauze to prevent air from entering the thoracic cavity.
Use of antibiotics
-
Criteria for antibiotic use Typically, only 1 dose of antibiotic is given before surgery. If the operation lasts more than 3 hours or has massive blood loss (>1,500 mL), a second dose of the antimicrobial drug should be applied during the surgery. If the frequency of antibacterial use is bid, 1 dose can be used on the day after surgery. Antibiotic use continues after the surgery until 3 of the following 5 criteria for antibiotic withdrawal are met:
Chest X-ray reveals that the exudation disappears;
Body temperature is lower than 38.5 °C;
There is no purulent secretion in the airway;
White blood cell count (WBC) is <11×109/L;
Procalcitonin level drops to 0.5 ng/mL or less.
-
Criteria for the re-use of antibiotics after drug withdrawal
There is obvious thoracic exudation on chest X-ray;
Body temperature is higher than 38.5 °C;
There is purulent excretion in the airway;
Postoperative WBC count is ≥11×109/L;
Procalcitonin level is higher than 0.5 ng/mL.
If 3 of the above criteria are met after drug withdrawal after surgery, the antibiotics can be re-used until the above criteria for drug withdrawal are met again.
Postoperative complications and their management
Tubeless surgery is simple, and its common complications include the following:
Poor lung re-inflation: this may be caused by air leaks or inadequate postoperative suctioning with negative pressure. If necessary, the chest tube should be re-inserted. With adequate nutritional support, constant low negative-pressure suction (<2 kpa) in the thoracic cavity or biphasic intermittent positive airway pressure (BiPAP)-assisted ventilation (2 h/bid) may promote lung re-inflation.
Pleural effusion: due to the lack of monitoring via the chest tube, patients with pleural effusion should be monitored in a timely fashion with pleural ultrasound to rule out postoperative bleeding inside the thoracic cavity. If necessary, chest CT (and even a second operation) should be performed to evaluate the intrathoracic condition and avoid dire consequences.
Conclusions
It has been previously reported that SV-VATS can accelerate postoperative recovery and reduce the incidences of complications, (4,7,19,24,25).
The new tubeless SV-VATS with maximized avoidance of any auxiliary catheterization and tubes placement, is aimed at reducing further the surgical invasiveness and eventually make ambulatory VATS a reality in the near future (20,21). However, tubeless SV-VATS also places higher demands on the surgical teams, and several measures should be followed in response to this: (I) the anesthesiologists should be able to convert the anesthesia mode during surgery and address a variety of complications such as hypoxemia and hypercapnia; also, they must be able to use the appropriate anesthetics at precise doses during the ambulatory VATS surgery; (II) the operators must accurately evaluate the disease conditions before surgery and establish a complete and systematic access system, to ensure the safety of patients; surgical maneuvers must be performed gently, to reduce postoperative inflammation; due to the lack of a chest drain, postoperative management becomes quite difficult. Finally, (III) as the VATS under intravenous anesthesia with spontaneous breathing has become increasingly mature, it is necessary to establish a complete, standardized, and systematic access policy and to guarantee systems including patient selection, preoperative preparation, surgical methods, postoperative management, discharge standards, and follow-up system to ensure the safety of patients.
Acknowledgments
None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Conflicts of Interests: The authors have no conflicts of interest to declare.
References
- 1.Pompeo E, Rogliani P, Atinkaya C, et al. Nonintubated surgical biopsy of undetermined interstitial lung disease: a multicentre outcome analysis. Interact Cardiovasc Thorac Surg 2019;28:744-50. 10.1093/icvts/ivy320 [DOI] [PubMed] [Google Scholar]
- 2.Liang H, Liu J, Wu S, et al. Nonintubated Spontaneous Ventilation Offers Better Short-term Outcome for Mediastinal Tumor Surgery. Ann Thorac Surg 2019;108:1045-51. 10.1016/j.athoracsur.2019.04.052 [DOI] [PubMed] [Google Scholar]
- 3.Jiang L, Liu J, Gonzalez-Rivas D, et al. Thoracoscopic surgery for tracheal and carinal resection and reconstruction under spontaneous ventilation. J Thorac Cardiovasc Surg 2018;155:2746-54. 10.1016/j.jtcvs.2017.12.153 [DOI] [PubMed] [Google Scholar]
- 4.Elkhouly A, Pompeo E. Nonintubated Subxiphoid Bilateral Redo Lung Volume Reduction Surgery. Ann Thorac Surg 2018;106:e277-e9. 10.1016/j.athoracsur.2018.04.061 [DOI] [PubMed] [Google Scholar]
- 5.Huang J, Qiu Y, Chen L, et al. Nonintubated Spontaneous Respiration Anesthesia for Tracheal Glomus Tumor. Ann Thorac Surg 2017;104:e161-e3. 10.1016/j.athoracsur.2017.02.028 [DOI] [PubMed] [Google Scholar]
- 6.Peng G, Cui F, Ang KL, et al. Non-intubated combined with video-assisted thoracoscopic in carinal reconstruction. J Thorac Dis 2016;8:586-93. 10.21037/jtd.2016.01.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gonzalez-Rivas D, Bonome C, Fieira E, et al. Non-intubated video-assisted thoracoscopic lung resections: the future of thoracic surgery? Eur J Cardiothorac Surg 2016;49:721-31. 10.1093/ejcts/ezv136 [DOI] [PubMed] [Google Scholar]
- 8.Cui F, Liu J, Li S, et al. Tubeless video-assisted thoracoscopic surgery (VATS) under non-intubated, intravenous anesthesia with spontaneous ventilation and no placement of chest tube postoperatively. J Thorac Dis 2016;8:2226-32. 10.21037/jtd.2016.08.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yao F, Wang J, Yao J, et al. Early Chest Tube Removal After Thoracoscopic Esophagectomy with High Output. J Laparoendosc Adv Surg Tech A 2016;26:17-22. 10.1089/lap.2015.0454 [DOI] [PubMed] [Google Scholar]
- 10.Nakanishi R, Fujino Y, Kato M, et al. Early chest tube removal after thoracoscopic lobectomy with the aid of an additional thin tube: a prospective multi-institutional study. Gen Thorac Cardiovasc Surg 2018;66:723-30. 10.1007/s11748-018-0993-z [DOI] [PubMed] [Google Scholar]
- 11.Nakashima S, Watanabe A, Mishina T, et al. Feasibility and safety of postoperative management without chest tube placement after thoracoscopic wedge resection of the lung. Surg Today 2011;41:774-9. 10.1007/s00595-010-4346-5 [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez-Rivas D, Yang Y, Guido W, et al. Non-intubated (tubeless) uniportal video-assisted thoracoscopic lobectomy. Ann Cardiothorac Surg 2016;5:151-3. 10.21037/acs.2016.03.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mineo TC, Tamburrini A, Perroni G, et al. 1000 cases of tubeless video-assisted thoracic surgery at the Rome Tor Vergata University. Future Oncol 2016;12:13-8. 10.2217/fon-2016-0348 [DOI] [PubMed] [Google Scholar]
- 14.Yang SM, Wang ML, Hung MH, et al. Tubeless Uniportal Thoracoscopic Wedge Resection for Peripheral Lung Nodules. Ann Thorac Surg 2017;103:462-8. 10.1016/j.athoracsur.2016.09.006 [DOI] [PubMed] [Google Scholar]
- 15.Li S, Jiang L, Ang KL, et al. New tubeless video-assisted thoracoscopic surgery for small pulmonary nodules. Eur J Cardiothorac Surg 2017;51:689-93. [DOI] [PubMed] [Google Scholar]
- 16.Petersen RH, Holbek BL, Hansen HJ, et al. Video-assisted thoracoscopic surgery-taking a step into the future. Eur J Cardiothorac Surg 2017;51:694-5. [DOI] [PubMed] [Google Scholar]
- 17.Zhao ZR, Lau RWH, Ng CSH. Anaesthesiology for uniportal VATS: double lumen, single lumen and tubeless. J Vis Surg 2017;3:108. 10.21037/jovs.2017.07.05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xia Z, Qiao K, He J. Recent advances in the management of pulmonary tuberculoma with focus on the use of tubeless video-assisted thoracoscopic surgery. J Thorac Dis 2017;9:3307-12. 10.21037/jtd.2017.08.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peng G, Liu M, Luo Q, et al. Spontaneous ventilation anesthesia combined with uniportal and tubeless thoracoscopic lung biopsy in selected patients with interstitial lung diseases. J Thorac Dis 2017;9:4494-501. 10.21037/jtd.2017.10.76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu CY, Hsu PK, Chien HC, et al. Tubeless single-port thoracoscopic sublobar resection: indication and safety. J Thorac Dis 2018;10:3729-37. 10.21037/jtd.2018.05.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lirio F, Galvez C, Bolufer S, et al. Tubeless major pulmonary resections. J Thorac Dis 2018;10:S2664-S70. 10.21037/jtd.2018.06.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang YL, Zhou C, Li XF, et al. Beinaglutide showed significant weight-loss benefit and effective glycaemic control for the treatment of type 2 diabetes in a real-world setting: a 3-month, multicentre, observational, retrospective, open-label study. Obes Sci Pract 2019;5:366-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yao J, He Z, Chen Y, et al. Acupuncture and weight loss in Asians: A PRISMA-compliant systematic review and meta-analysis. Medicine (Baltimore) 2019;98:e16815. 10.1097/MD.0000000000016815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hung WT, Hung MH, Wang ML, et al. Nonintubated Thoracoscopic Surgery for Lung Tumor: Seven Years' Experience With 1,025 Patients. Ann Thorac Surg 2019;107:1607-12. 10.1016/j.athoracsur.2019.01.013 [DOI] [PubMed] [Google Scholar]
- 25.Pompeo E, Sorge R, Akopov A, et al. Non-intubated thoracic surgery-A survey from the European Society of Thoracic Surgeons. Ann Transl Med 2015;3:37. [DOI] [PMC free article] [PubMed] [Google Scholar]


