Abstract
Purpose of Review
We review recent research investigating the relationship of hormonal contraceptives and mood with a focus on relevant underlying mechanisms, such as emotion recognition and reactivity, reward processing, and stress response.
Recent Findings
Adverse effects of hormonal contraceptives (HCs) on mood seem most consistent in women with a history of depressive symptoms and/or previous negative experience with HC-intake. Current evidence supports a negativity bias in emotion recognition and reactivity in HC-users, although inconsistent to some extent. Some data, however, do indicate a trend towards a blunted reward response and a potential dysregulation of the stress response in some HC-users.
Summary
HC-effects on psychological and neurophysiological mechanisms underlying mood are likely context-dependent. We provide suggestions on how to address some of the contributing factors to this variability in future studies, such as HC-dose, timing, administration-mode, and individual risk. A better understanding of how and when HCs affect mood is critical to provide adequate contraceptive choices to women worldwide.
Keywords: Hormonal contraceptives, Mood, Depression, Emotion, Reward, Stress
Introduction
With currently more than 100 million users worldwide [1], hormonal contraceptives (HCs) represent one of the most influential discoveries of the twentieth century [2]. HCs provide an effective option for contraception and safe family planning as well as for managing cycle-related physiological symptoms (e.g., ovulation pain, acne, hirsutism). Although this suggests that HC-use is beneficial for many women, there is a subset of women who suffer severe mood-related side effects. Thus, while substantial research has been dedicated to the physiological consequences of HC-use, such as cardiovascular risk, few studies have investigated the effects of HCs on mood and behavior.
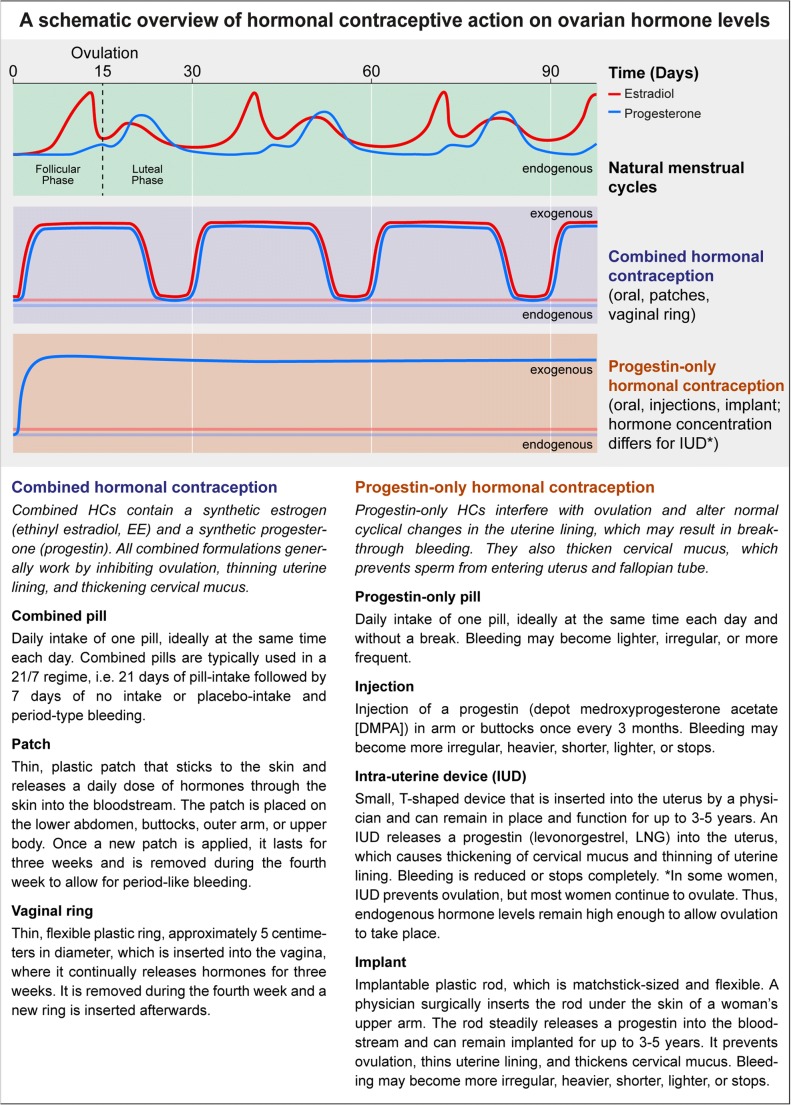
Given that side effects such as depressive symptoms are typically reported as the main reason for discontinuing HC-use [3, 4] and the relative scarcity of neuroimaging studies currently published in this area, additional research efforts to shed light on the neuropsychological side effects of HCs are warranted. With the emerging field of reproductive neuroscience, scientists are beginning to investigate the neural effects of HC-use in humans. A better understanding of how HC-use influences mood may have a critical impact on translational psychiatry, considering that women are approximately twice as likely as men to develop depression [5] and ovarian hormonal fluctuations have been associated with depression susceptibility and prevalence in women [6]. Epidemiological data suggest that hormonal transition periods across the female lifespan, such as puberty, pregnancy and postpartum, and the perimenopause, are windows of heightened risk to develop depression [7•], comprising a possible reproductive subtype of depression [8]. Certain women are particularly susceptible to the subtle hormone fluctuations across the menstrual cycle, which may result in the development of premenstrual dysphoric disorder (PMDD) [9]. Given these reported associations between hormone fluctuations and depression susceptibility, and that HCs introduce synthetic ovarian hormones thereby modulating endogenous ovarian hormone production (for overview, see Fig. 1 and [10, 11, 89, 90]), we review recent research investigating the relationship of HC-use and mood with a focus on relevant underlying mechanisms, such as emotion recognition and reactivity, reward processing, and stress responsivity.
Fig. 1.
Comparison of ovarian hormone profiles across the natural menstrual cycle (top row), and during intake of most common hormonal contraceptives, such as combined hormonal contraception (middle row), and progestin-only hormonal contraception (bottom row). The modes of action as well as intake characteristics of the most common hormonal contraceptives are described below.
Relying on Danish Registry data, Skovlund and colleagues [12••] recently reported a link between antidepressant prescription and HC-use. The authors included data from more than one million women in the age of 15–34 years, who were using combined estradiol/progestin as well as progestin-only HCs in all available forms of administration (see Fig. 1 and [10, 11, 89, 90] for an overview of HC methods). In those women, risk ratios for first diagnoses of depression or first antidepressant-use increased during the first 6 months after initiation of HC-use (1.8-fold relative risk compared with naturally cycling women). Similarly, Zettermark and colleagues [13••] investigated the prescription of psychotropic drugs (anxiolytics, hypnotics, sedatives, or antidepressants) within the first year of HC-use in a sample of 800,000 women from a Swedish health registry. Reported rates for psychotropic drug use indicated an adjusted odds ratio of 1.34 for a first-time psychotropic drug prescription in HC-users. However, both studies [12••, 13••] were correlational in nature and reporting relative risks can be misleading as the incidence of these events is quite low [14]. While causation is not determinable in observational designs, both studies [12••, 13••] investigated impressive sample sizes, providing essential epidemiological evidence to develop hypotheses for potential mechanisms underlying the reported associations of HC-use and depression risk.
Randomized, placebo-controlled trials (RCTs) represent the gold standard in intervention-based studies, in that they can provide the strongest possible evidence for causal effects. Several groups have now successfully applied this study design to investigate HC-effects on mood. Zethraeus and colleagues [15••] included over 300 women in a double-blind RCT, testing the effect of a combined oral contraceptive (OC) versus placebo, on well-being and mood. Over the course of 3 months, women in the OC group reported significantly lower global scores on self-reported well-being compared with placebo, driven by the negative effect of OCs on scales measuring positive well-being, self-control, and vitality. However, mean depression scores did not differ significantly across groups and time points in self-reported Beck Depression Inventory (BDI) scores.
Another Swedish group took a more unconventional approach in their double-blind RCT: they aimed to sample participants more representative of HC-users in the general population, thus deciding not to exclude women with previous or ongoing psychiatric disorders and respective medication, nor any women with a history of OC-use-associated onset of depressed mood [16••, 17••]. In total, over 200 women participated in either a placebo or combined OC group for three treatment cycles. The authors reported small but significant mood-related adverse effects of OCs in self-reported anxiety, irritability, and mood swings. No significant effects of OCs were observed for the Montgomery-Asberg Depression Rating Scale. However, some women in the OC group also reported improvements in mood during the premenstrual phase of the cycle. Women with previous negative OC-associated experiences reported significantly more severe depressed mood after completion of the 3-month trial compared with women with no such history. A further aspect to consider is the effect of HCs on the expression of premenstrual mood symptoms. Here, one study reports no effect of HC-use on premenstrual mood (using a prospective cross-over design; [18]), while another study supports a beneficial association between HC-use and premenstrual mood symptoms (although cross-sectional; [19]).
In summary, the data currently available supports some mood-related side effects of HC-use, most convincingly shown in women with a history of depressive symptoms. However, some women may experience beneficial effects of HC-use, specifically on premenstrual mood symptoms (see [20] for review). As HC-related side effects on mood are not fully understood to date, additional research efforts to shed light on a possible impact of HCs on the mechanisms underlying mood regulation are warranted. Therefore, we review recent research on HC-effects on main psychological and neurophysiological mechanisms underlying mood regulation, such as the behavioral and neural correlates of emotion recognition and reactivity, reward processing, and stress response (Table 1).
Table 1.
Overview of results from studies investigating HC-effects on psychological and neurophysiological mechanisms underlying mood regulation—emotion recognition and reactivity, reward processing, and stress response. Studies are listed in order of appearance in the text
| Research design | Sample size and HC method | Research modality | Task | Results | Main findings in HC-users | ||
|---|---|---|---|---|---|---|---|
| Emotion recognition | |||||||
| Pahnke et al. [21] | Cross-sectional |
42 combined OC 53 NC (35 follicular, 18 luteal) |
Behavioral | RMET | OC-users performed significantly worse in complex face recognition independent of emotional valence or type of OC (androgenic vs antiandrogenic). | ↓ | Impaired emotion recognition |
| Hamstra et al. [22] | Cross-sectional |
49 combined OC 44 NC (21 early follicular, 23 luteal) |
Behavior-genotype interaction | Facial expression recognition task, RMET |
OC-users with MC haplotype 1 and 3 performed generally worse in face recognition task than luteal NC women (trend-level). OC-users with MC haplotype 2 recognized less positive characteristics in the RMET than luteal NC women. |
↓ | Impaired emotion recognition |
| Hamstra et al. [23] | Cross-sectional |
44 combined OC 40 NC (11 follicular, 29 luteal) |
Behavior-genotype interaction | Facial expression recognition task, emotional categorization, and memory task | OC-users showed worse recognition of anger independent of MC haplotype. | ↓ | Impaired emotion recognition of negative emotions |
| OC-users with MC haplotype 1 and 3 recognized fearful and sad images significantly better and recalled more negative characteristics, but also had longer reaction times for detecting these emotions. | ↑ | Attention bias to negative emotions in MC haplotype 1 and 3 carriers | |||||
| Hamstra et al. [24] | Cross-sectional |
26 OC1 14 follicular NC |
Behavioral | Facial expression recognition task | OC-users showed worse recognition of facial expressions depicting anger, as well as a trend for sadness and disgust, compared with NC women. | ↓ | Impaired emotion recognition of negative emotions |
| Hamstra et al. [25] | Longitudinal |
57 combined OC (within subjects active vs. inactive pill phase) 39 NC (within subjects early follicular vs. luteal) |
Behavior-genotype interaction | Facial expression recognition task, RMET |
OC-users showed worse recognition of sadness and happiness (trend-level) and had shorter reaction times for detecting anger and happiness. OC-users recognized more positive characteristics in RMET |
↔ | Mixed findings: both impaired and enhanced emotion recognition |
| Radke et al. [26•] | Cross-sectional |
25 combined OC (inactive phase) 30 combined OC (active phase) 18 NC |
Behavioral | Affective responsiveness task, emotion recognition task, perspective-taking task | No differences in emotion recognition and perspective taking between OC-users and NC women. | ↔ | No differences in emotion recognition |
| Increased accuracy for OC-users in active phase in affective responsiveness compared with OC-users in an inactive phase. | ↑ | Enhanced emotional reactivity in active OC phase | |||||
| Emotional reactivity | |||||||
| Spalek et al. [27•] | Cross-sectional |
1215 HC1 (majority OC) 954 NC |
Behavioral | Picture rating task, picture memory task | HC-users rated emotional pictures (negative and positive) more emotionally intense and neutral images less arousing than NC women. | ↑ | Enhanced emotional reactivity |
| HC-users remembered significantly more emotional pictures (positive and negative) than NC women which were mediated by valence/arousal ratings. | ↑ | Enhanced emotional memory | |||||
| Gingnell et al. [28] | RCT |
17 combined OC-starters 17 placebo-starters (both groups with previous mood-related side effects of OCs) |
Task fMRI | Emotional face-matching task | No differences in face-matching accuracy. | ↔ | Similar ratings |
| OC-starters had significantly more mood swings and depressed mood after 1 month of intake compared with pre-start and to the placebo group. | ↑ | Mood swings and depressed mood | |||||
| OC-starters had reduced BOLD response in the left insula, left MFG, and bilateral IFG compared with placebo and reduced BOLD response in bilateral IFG compared with pre-start. | ↓ | Blunted emotional reactivity | |||||
| Decreased habituation of amygdala in OC-starters compared with placebo between time points. | ↑ | Higher vigilance for negative emotional stimuli | |||||
| Miedl et al. [29•] | Cross-sectional |
23 combined OC 30 NC |
Task fMRI | Traumatic vs neutral video clips | No differences in valence and arousal ratings. | ↔ | Similar ratings |
| Enhanced BOLD responses in OC-users in the insula and dorsal ACC during traumatic vs. neutral clip viewing. | ↑ | Enhanced emotional reactivity for traumatic content | |||||
| Merz et al. [30] | Cross-sectional |
29 combined OC 30 luteal NC 39 men |
Task fMRI, physiology | Fear acquisition and extinction | Slower habituation of SCR rates, correlated with increased BOLD signal in response to fear-evoking stimuli in OC-users compared with NC women in the right amygdala, right ACC, bilateral thalamus, and vmPFC. |
↔ ↑ |
Similar emotional reactivity in the acquisition phase Increased emotional reactivity in the extinction phase |
| Hwang et al. [31] | Cross-sectional |
16 combined OC 32 NC (16 high estradiol, 16 low estradiol) 37 men |
Task fMRI | Fear conditioning, extinction and recall procedures | Reduced BOLD response during fear conditioning in the insular cortex, MCC, amygdala, and hypothalamus in OC-users compared with high estradiol NC women. | ↓ | Blunted emotional reactivity during fear conditioning |
| No differences between OC-users and NC women for unconditioned fear, fear extinction, and recall. | ↔ | Similar emotional reactivity in fear extinction and recall | |||||
| Armbruster et al. [32] | Cross-sectional |
35 combined OC 35 NC (within-subjects early follicular vs. late luteal) |
Physiology (SCR, startle magnitude) | Acoustic startle response task during image presentation | No difference in valence and arousal ratings between OC-users and NC women. | ↔ | Similar ratings |
| OC had blunted startle magnitudes and SCR, especially for negative images. | ↓ | Blunted emotional reactivity | |||||
| Reward processing | |||||||
| Petersen et al. [33] | Cross-sectional |
44 combined OC 46 NC |
Structural MRI | – | OC-use associated with lower cortical thickness in lateral OFC and posterior cingulate cortex. | ↓ | Lower cortical thickness in reward-related region |
| Bonenberger et al. [34] | Cross-sectional |
12 OC1 12 NC |
Task fMRI | Monetary incentive task | Enhanced BOLD response during monetary reward expectation in anterior insula and inferior PFC in OC-users compared with NC women in the follicular phase. | ↑ | Enhanced reward response |
| Arnoni-Bauer et al. [35] | Cross-sectional |
12 combined OC 20 NC |
Task fMRI | Visual food cues | OC-users show similar BOLD response as NC women in the luteal phase but greater BOLD response as NC women in the follicular phase in reward response (amygdala, putamen) and decision-making regions (PFC). | ↔↑ | Similar to enhanced reward response |
| Scheele et al. [36] | Cross-sectional |
21 HC (16 combined OC, 5 IUS) 19 NC |
Task fMRI | Attractiveness rating under single oxytocin nasal dose |
Oxytocin increased attractiveness ratings of the partner’s face in NC women but not in HC-users. Reduced BOLD response in striatal reward regions (NAcc, VTA) in OC-users compared with NC women. |
↓ | Blunted reward response |
| Jakob et al. [37•] | Longitudinal |
38 combined OC (within-subjects active vs. inactive pill phase) 30 NC (within-subjects early vs. late follicular) |
Behavior-hormone-genotype interaction | Probabilistic reinforcement learning task | Decrease in ability to avoid punishment with rising estradiol levels in 9RP carriers NC women, no such behavioral variations in OC-users according to DAT1-genotype differences or intake phase. | ↔ | No genotype interaction for reward responses |
| Stress response | |||||||
| Merz et al. [38] | Cross-sectional |
30 combined OC 60 NC |
Behavioral, physiological | SECPT | Blunted cortisol response in OC-users compared with NC women after stress exposure. | ↓ | Blunted stress response |
| Barel et al. [39] | Cross-sectional |
20 combined OC 17 NC |
Behavioral, physiological | TSST | Blunted cortisol response in OC-users compared with NC women after stress exposure. | ↓ | Blunted stress response |
| Nielsen et al. [40] | Cross-sectional |
49 combined OC 60 NC |
Behavioral, physiological | Emotional recall after CPS | Blunted cortisol after stress exposure, paralleled by weaker performance in emotional recall response in OC-users compared with NC women. | ↓ | Blunted stress response |
| Mordecai et al. [41] | Longitudinal |
39 combined OC (within-subjects active vs. inactive pill phase) 40 NC (within-subjects follicular vs. luteal) |
Behavioral, physiological | Emotional recall after TSST | Blunted cortisol response and worse emotional recall for negative words in OC-users (similar for both active and inactive pill phase) compared with NC women after stress exposure. | ↓ | Blunted stress response |
| Higher baseline cortisol levels in OC-users compared with NC women. | ↑ | Higher baseline cortisol | |||||
| Hertel et al. [42••] | Cross-sectional |
74 OC (70 combined OC, 4 progestin-only) 159 NC |
Structural MRI, physiological | – | Higher baseline cortisol levels and reduced hippocampal gray matter in OC-users compared with NC women. |
↑ ↓ |
Higher baseline cortisol Reduced hippocampal volume |
1No information stated on which specific HC/OC-type used in the study
ACC anterior cingulate cortex, BOLD blood oxygen level-dependent, CPS cold-pressor stress, DAT-1 dopamine transporter, fMRI functional magnetic resonance imaging, HC hormonal contraceptive, IFG inferior frontal gyrus, MC mineralocorticoid, MCC middle cingulate cortex, MFG middle frontal gyrus, NAcc nucleus accumbens, NC naturally cycling, OC oral contraceptive, OFC orbitofrontal cortex, PFC prefrontal cortex, RCT randomized, placebo-controlled trial, RMET Reading the Eyes in the Mind Test, SECPT socially evaluated cold-pressor test, SCR skin conductance response, TSST Trier social stress test, vmPFC ventromedial prefrontal cortex, VTA ventral tegmental area
Influence of HCs on Psychological and Neurophysiological Mechanisms Underlying Mood Regulation
Emotion Recognition and Reactivity
Negativity biases in key facets of emotion processing such as emotion recognition and emotional reactivity are thought to substantially contribute to the development and maintenance of depressed mood [43]. Mitigating negativity biases in emotion recognition and reducing emotional reactivity to negative stimuli can be effective strategies to improve mood [43–45].
The ability to correctly recognize emotional content from faces represents one major component of nonverbal communication [46], and impairments in this ability may play an important role in the development and maintenance of depressive symptoms [47, 48]. Several studies found impaired emotion recognition in OC-users [21, 22], particularly for negative emotions [23–25], compared with naturally cycling women. For example, Pahnke and colleagues [21] report overall facial emotion recognition deficits in OC-users independent of emotional valence during the Reading-the-Mind-in-the-Eyes task, whereas Hamstra and colleagues [23, 24] identified a negativity bias in emotion recognition and emotional memory during a facial expression recognition task and an emotional categorization and memory task, respectively. Here, OC-users had significantly lower recognition accuracies for angry faces compared with naturally cycling women [23, 24]. The authors further suggest that OC-users who are carriers of the mineralocorticoid receptor (MR) haplotype 1 or 3 have a more pronounced negativity bias, as these OC-users (1) had higher accuracy rates for detecting fearful and sad faces (unlike for angry faces), (2) had significantly longer reaction times for detecting these negative emotions, and (3) had better recall of negative characteristics in an emotional memory task, thus implicating an attention bias towards negative emotions [23]. Therefore, MR haplotype 1 or 3 carriers might be more vulnerable to depressogenic side effects of OCs than MR haplotype 2 carriers. Contrary to these findings, Radke and Derntl [26•] did not find evidence for an emotion recognition deficit in OC-users compared with naturally cycling women; however, they used only high-intensity emotional faces.
The current literature seems to confirm a negativity bias in emotion recognition in HC-users, i.e., deficits in recognizing emotions accurately [21, 22, 25] as well as an attentional bias to negative emotions [23–25]. However, emotion recognition abilities in HC-users seem to be affected by the task used in the study [25, 26•] or individual (epi-)genetic characteristics [22, 25, 23].
In addition to emotion recognition, emotional reactivity may also be linked to depressive symptoms. Emotional reactivity is the emotional response to an event, which can occur through multiple systems and differs in intensity and duration between individuals [49]. More intense and labile emotions, often accompanied by physiological arousal [50], have been associated with more depressive and internalizing symptoms [51, 52]. While Radke and Derntl [26•] did not observe any differences in emotion recognition between OC-users and naturally cycling women, they reported that OC-users during the active OC-intake phase performed significantly better in an emotional reactivity task (affective responsiveness task) than OC-users during the pill-free week. Therefore, the active intake of OCs seems to be linked to an enhanced emotional reactivity towards positive as well as negative emotional scenarios. In line with these findings, a large-scale study recently showed that women using HCs showed significantly higher emotional reactivity by rating the valence of emotional stimuli more emotionally intense and recalling these emotional pictures significantly better than did naturally cycling women [27•].
Neuroimaging research sheds further light on the possible modulatory effects of HCs on emotional reactivity. In a double-blind, placebo-controlled, functional magnetic resonance imaging (fMRI) study that only included women who had previously experienced OC-induced depressogenic side effects, Gingnell and colleagues [28] observed no behavioral differences between the OC-assigned group and the placebo-assigned group in an emotional reactivity task (face-matching task with only negative faces) after 1 month of intake. The OC group did, however, show decreased habituation of the amygdala blood oxygenation level-dependent (BOLD) response compared with the placebo group. This finding could point towards a higher continued vigilance for negative emotional stimuli and therefore a biased attention towards negative stimuli in OC-users, possibly explaining adverse effects on mood. The OC group also showed reduced BOLD response of the left insula, the left middle frontal gyrus, and the bilateral inferior frontal gyri compared with the placebo group in response to negative emotional face stimuli [28]. However, these differences in BOLD response occurred in brain regions that are otherwise activated for positive or salient emotional stimuli.
Specifically for fear processing, OC-induced effects on neural activation have been reported, such as enhanced activation of the fear network in OC-users compared with naturally cycling women, particularly in the insula and the dorsal anterior cingulate cortex (ACC) [29•]. These activation differences were independent of the valence and arousal ratings of the presented traumatic videos, which were similar between groups. Consistent with these findings, another study [30] found increased emotional arousal indicated by slower habituation of skin conductance response (SCR) rates, a physiological measure of the autonomic stress response, to be correlated with an increased BOLD signal in response to fear-evoking stimuli in OC-users compared with naturally cycling women. Group differences occurred in the right amygdala, right ACC, bilateral thalamus, and ventromedial prefrontal cortex (vmPFC). Unlike the previous study, Hwang and colleagues [31] did not observe any differences between OC-users and naturally cycling women in fear extinction but reported group differences for fear conditioning, i.e., reduced BOLD response of the fear network in OC-users compared with naturally cycling women. These neural correlates are further supported by physiological data, namely blunted SCR and startle reflex during fear conditioning in OC-users compared with naturally cycling women [32]. While emotional reactivity seems to be enhanced during fear extinction [30], neural [31] as well as physiological responses [32] are reduced during fear conditioning in OC-users.
Overall, the current data suggests a negativity bias in emotional reactivity shown by reduced BOLD responses to negative stimuli in brain regions that are otherwise relevant for processing salient and positive emotions [28], and enhanced BOLD responses in brain regions relevant for processing negative emotions, such as fear [29•, 30]. These neuroimaging results are often not paralleled by behavioral outcomes [26•, 28, 29•, 32] and thus need to be interpreted with caution. However, as emotional reactivity occurs by definition through multiple systems [49], it might as well be that HC-use specifically impacts a very early stage of emotion processing, as reflected by HC-induced modulation of emotional reactivity networks in the brain. On this account, further experimental designs including psychological, physiological, and neuroimaging measures when investigating HC-effects on emotional reactivity are highly encouraged.
Reward Processing
Recent models from computational psychiatry propose that negative mood may reflect the cumulative impact of differences between reward outcomes and expectations (e.g., [53, 54]). These models suggest a bidirectional interaction between mood and reward processing, which likely plays an important adaptive role in healthy behavior or, if compromised, could contribute to depressive disorders via a blunted hedonic response to rewards, i.e., anhedonia [55].
On a neural level, both endogenous estradiol and progesterone have neuroregulatory effects on the mesolimbic dopaminergic reward system [56–59]. In association with ovarian hormone fluctuations, changes in neural activation occur in the reward system [58], specifically in brain regions relevant for coding reward value and reward-expectancy such as the amygdala, the orbitofrontal cortex (OFC), and the striatum [60].
Literature on HC-related modulations of the reward system is relatively sparse. Petersen and colleagues [33] reported OC-use to be associated with significantly lower cortical thickness in the posterior cingulate cortex and the lateral OFC, with the latter revealing the most pronounced difference in cortical thickness between naturally cycling women and OC-users. This frontal cortex region is critical for the cognitive control of behavior, including response inhibition to stimuli with changing reward value [61]. Post hoc analyses suggest that these differences in cortical thickness were greater comparing OC-users and women in the follicular phase than comparing OC-users and women in the luteal phase. Yet, as this study used a cross-sectional design, we cannot infer causality nor establish a time-dependent association of OC-intake and OFC cortical thickness thus far.
OC-induced changes in brain morphology do not allow direct assumptions about behavioral changes, but task-based fMRI studies can shed light on potential behavioral consequences. In a comparison of naturally cycling women with OC-users during a monetary incentive task, OC-users were more sensitive to monetary rewards and showed enhanced BOLD response during monetary reward expectation in the anterior insula and inferior prefrontal cortex (PFC) relative to naturally cycling women in the follicular phase [34]. Another study observed greater neural activation to visual food stimuli in OC-users than naturally cycling women during the follicular phase, but no group differences between OC-users and naturally cycling women during the luteal phase [35]. This difference in BOLD response during the follicular phase was observed in brain regions of the reward system (amygdala, putamen) as well as executive frontal areas (PFC). The authors proposed that comparable progesterone levels in OC-users and naturally cycling women in the luteal phase may underlie the similar BOLD responses between groups (similar to [33]). However, these studies were limited by their cross-sectional design and small sample sizes [34, 35] or lack of behavioral outcome measures [35]. Another study [36] included behavioral outcome measures and reported enhanced attractiveness ratings of the partner’s face in naturally cycling women but not in HC-users after intranasal administration of oxytocin. The concomitant increased BOLD responses in nucleus accumbens (NAcc) and ventral tegmental area (VTA) were also more pronounced in the naturally cycling group than in the HC group. Taken together, task-based fMRI studies seem to provide rather mixed results, which could be due to the varying tasks used, e.g., investigating primary [35, 36] or secondary rewards [34]. Replication studies, preferably studies comparing performance in both primary and secondary reward tasks, are needed to further elucidate this issue.
Preclinical evidence suggests that endogenous estradiol levels can increase dopamine release in the reward system, specifically in the striatum [62, 63]. Behavioral studies in humans partly support this finding as a positive correlation between endogenous estradiol levels and enhanced reward sensitivity in women, but paradoxically no increase in motivation for higher rewards from the early to the late follicular phase (i.e., with rising endogenous estradiol levels) have been reported [64]. Women have also been shown to be less sensitive for immediate rewards with rising estradiol levels from the early to the late follicular phase, but this effect was mainly driven by women with lower frontal dopamine levels (based on the COMT Met158Val polymorphism) [65]. These results nurtured the hypothesis of a hormone-genotype interaction, suggesting that particularly women with lower dopamine distribution would be affected by endogenous estradiol changes. Jakob and colleagues [37•] tested this hypothesis and investigated how endogenous estradiol levels and polymorphisms of the dopamine transporter (DAT1) interact. In this study, women performed a probabilistic feedback learning task twice: naturally cycling women once during the early (low estradiol) and subsequently during the late follicular phase (high estradiol) in comparison with OC-users once during active and once during inactive pill phase. Results indicated a significant effect of DAT1-genotype on reinforcement learning in naturally cycling women only, i.e., a decrease in the ability to avoid punishment with rising estradiol levels in 9RP carriers. The OC group did not show any such behavioral variations according to DAT1-genotype differences or intake phase. While these results suggest a small, dopamine-agonistic effect of endogenous estradiol on reward and punishment sensitivity (see [66] for an overview), the influence of HC-induced changes in endogenous and exogenous estradiol levels on dopamine neurotransmission needs further research.
Overall, results from studies investigating the impact of HCs on reward processing are mixed (see Table 1): Studies have reported women on HCs to be more sensitive to rewards [34], to show comparable reward responses to naturally cycling women [35], or to experience blunted reward responses than naturally cycling women [33, 36, 37•] as well as lower cortical thickness in brain regions of the reward system [33]. Based on the evidence currently available, the hypothesis of a blunted reward response in HC-users compared with naturally cycling women appears most supported, but remains to be systematically investigated.
Stress Responsivity
In women, high endogenous estradiol levels have been associated with an acutely blunted cortisol response, which is typically viewed as protective against acute psychosocial stress [67•]. A chronically blunted cortisol response, however, might increase the risk for depression. Atypical depression is characterized by hypoactivation of the hypothalamic-pituitary-adrenocortical (HPA) axis and describes a distinct pathophysiological phenotype, which is particularly common in women [6]. Recent work on the role of estradiol in the neural stress circuitry in women revealed increased BOLD response in the amygdala, hippocampus, and hypothalamus after a visual stress challenge in low endogenous estradiol states compared with high endogenous estradiol states (within-subject design, [68]). Notably, only healthy women demonstrated this endocrine regulation, while there was no evidence for this regulatory effect in women with recurrent depression in remission. This suggests a possible endocrine dysregulation associated with an altered stress response in women with depression (see also [69] for review).
HC-studies on stress responsivity using well-validated stress tasks consistently report a blunted cortisol response in OC-using women compared with naturally cycling women [38, 39]. Nielsen and colleagues also found a blunted cortisol response in OC-users compared with naturally cycling women, paralleled by weaker performance for memorizing an emotional story: While naturally cycling women in the stress condition had enhanced recall for gist and detail, OC-users did not show such effects on memory [40]. Another study [41] extended these findings by showing that the blunted cortisol response previously reported in OC-users is similar during both the active and the inactive pill phase, following a psychosocial stress test (Trier social stress test, TSST). Interestingly, the authors also observed that OC-users had higher baseline salivary cortisol levels than naturally cycling women. Another study further substantiated this finding by investigating OC-related alterations in the HPA axis in OC-users compared with naturally cycling women [42••]: The authors found overall elevated cortisol levels in OC-users as well as reduced hippocampal gray matter when investigating structural MRI scans. Given the evidence connecting chronic stress, elevated cortisol levels, and decreased hippocampal volume (e.g., [70]), these findings may indicate a potential protective effect of fluctuating endogenous estradiol levels through the mitigation of neurodegenerative effects of chronic stress on the hippocampus (see [67•] for review). The authors did not, however, find an association between cortisol levels and depressive symptoms (BDI scores) in OC-using women [42••]. Thus, the link between HC-use, chronic stress, and depression susceptibility warrants further investigation [71].
Taken together, HC-intake seems to chronically alter HPA axis regulation, mirrored by (a) blunted cortisol responses after acute psychosocial and physical stressors [38–41] and (b) elevated baseline cortisol levels [41, 42••] (see Table 1). Further research is required to systematically address HC-effects on the response to acute and chronic stress in different states of endogenous and exogenous ovarian hormones in health and disease to conclusively answer the question whether HC-effects on the stress response underlie mood-related HC side effects in women at risk.
Summary and Future Directions
In this review, we provide a summary of the most recent literature on HC-effects on women’s mood, with a specific emphasis on some of the psychological and neurophysiological mechanisms that could underlie mood-related side effects of HCs, which have been reported to occur in subgroups of women. We have reviewed the influence of HCs on emotion and reward processing as well as stress responsivity. We conclude that most of the reported results have yet to be replicated, thus no clear consensus can be reached based on these relatively heterogeneous datasets. From a methodological point of view, it is challenging to draw conclusions from neuroimaging results, which are not always paralleled by behavioral outcomes, and vice versa. Moreover, many neurobiological mechanisms are still not well understood. Given these limitations, most of the reported results have to be interpreted with caution, as the evidence is observational in most studies, and therefore, we cannot infer causality.
Many studies in this field only include women using OCs or women on different HC methods without stratification for each method. Consequently, the strongest conclusions can be drawn for OC-effects, as most of the available data include this HC method. Studies that did not stratify for different HC methods allow only for limited interpretation, as they contain different compounds and amounts of exogenous ovarian hormones, and also differ in the way of administration (see Fig. 1) and, thus, metabolization. Accumulating evidence [12••, 13••] suggests that different HC methods have divergent effects on mood: Non-oral HC methods (patch, vaginal ring, LNG-IUD) are more strongly associated with depression diagnosis or antidepressive treatment than OCs (combined OC and progestin-only OC). While these findings were correlational, some studies did take an interventional approach: Aleknaviciute and colleagues show that LNG-IUD induced sensitized HPA axis responsivity on both an acute and a chronic stress parameter, compared with women taking combined OCs or naturally cycling women [72•]. However, there was no difference in depression scores 6 months after LNG-IUD insertion, but this study did not include a control group [73]. Vaginal ring contraception did not significantly modulate mood scores after 6 months of use [74, 75], and a systematic review found that vaginal ring users reported less depressive mood, irritability, and emotional liability than combined OC-users [76]. Concerning progestin-only HCs, a recent systematic review found only minimal association between progestin-only methods and validated depression measures [77•]. In summary, a direct comparison of different HC methods, ideally using a RCT, would add critical evidence to the current debate about potential negative side effects of HCs on mood. Such a systematic investigation could also provide insight regarding a more refined neurobiological understanding of how OCs may affect mood compared with other hormonal methods. Finally, this type of research would also have great clinical relevance by informing clinical recommendations for or against a specific HC method for a particular woman (for example, based on previous depression history).
A major methodological aspect that must be addressed but is rarely discussed or oversimplified concerns the way most neuroimaging studies use HC-intake as a control variable for a low ovarian hormone state. This is only partly true. Indeed, the assessment of peripheral plasma levels of endogenous ovarian hormones, i.e., estradiol and progesterone, reveals low hormone levels. However, if a woman continuously takes exogenous ovarian hormones, either in an oral or non-oral route of administration, these exogenous ovarian hormones cross the blood-brain barrier [78, 79], a fact that should be considered in the interpretation of neuroimaging findings. Yet, this is only one aspect to consider: We do not yet know how a high exogenous and a low endogenous hormonal state interact, e.g., via feedback loops and cellular signaling, and how the net-effect of such an interaction can differ from a state of continuously fluctuating endogenous hormonal levels during the menstrual cycle. It is therefore challenging to interpret data from indirect neuroimaging modalities in vivo in the context of neurobiological mechanisms underlying the effects of HCs on behavior and brain function in women.
One technique that could provide essential insight into how HC-induced hormonal states (i.e., low endogenous but high exogenous) may directly influence such neurobiological mechanisms in the brain is positron emission tomography (PET). Radioligand PET studies allow for the visualization and in vivo quantification of a specific neurochemical target at a specific molecular site [80] and thus could clarify the neurochemical changes accompanying HC-use. We still require more tracer development dedicated to ovarian hormone receptors, but there are promising candidates. Ethinyl estradiol, the most commonly used synthetic estrogen in oral contraceptive formulations, is an estrogen receptor alpha (ERα) agonist [81]. The tracer 16α-[18F]fluoroestradiol-17β (FES) can be used to image ERα, although FES is so far mostly used in clinical practice to assess breast cancer [82, 83] and needs further investigation for suitability of ER imaging in the human brain. Two FES-PET studies in female rats [84, 85] only observed specific binding in brain regions with high ER density (i.e., pituitary gland and hypothalamus). One FES-PET study [86] in a small, healthy, post-menopausal sample of women (n = 7) also found significant uptake in the pituitary, as well as in white matter, but administration of an ER antagonist only successfully reduced FES in the pituitary. In a recent review on sex hormones and available PET radiotracers [87], authors conclude that FES could be useful for assessing ER density in ER-dense brain regions but encourage development of novel PET tracers with higher affinity for further research. Progesterone receptor imaging can be done using the tracers 21-[18F]fluoro-16α-ethyl-19-norprogesterone (FENP), 21-[18F]fluoro-16α,17α-[(R)-(1′-α-furylmethylidene)-dioxy]-19-norpregn-4-ene-3,20-dione (FFNP), and the more metabolically stable 4-[18F]Fluoropropyl-Tanaproget (FPTP) [88], although this study [88] was performed in female rats in non-brain areas (e.g., uterus and ovaries) and has yet to be studied in vivo in the human brain. Thus, while these tracers are informative of receptor density and occupancy, there is a critical need for development of radiotracers specifically dedicated to ovarian hormones, which could ultimately clarify how HCs modulate the delicate hormonal balance in the brain and thereby shed light on subsequent consequences for mood and depression susceptibility.
Conclusion
In order to advance our understanding of possible effects of HC-use on mood, we propose the following three perspectives to guide future research endeavors: (1) stratification for HC methods or direct comparison of the effects of different HC methods on mood, (2) initiation and implementation of rigorous RCT designs with adequate samples based on transparent a priori power-analyses, and (3) the development of quantitative methods to differentiate between exogenous and endogenous hormonal effects.
Based on the evidence currently available, it is likely that HC-intake can lead to mood-related side effects, particularly in women with a history of previous depressive episodes. Reported data indicate a trend towards negativity bias in emotion recognition and reactivity, a trend towards a blunted reward response and a potential dysregulation of the stress response in HC-users. Of note and not extensively discussed in this review, however, are the reported positive effects of HC-use on mood in some women, especially for symptoms of PMDD (but see [20] for review). Any HC-effects on mood and the underlying psychological and neurophysiological mechanisms are therefore likely context-dependent.
It is imperative to take any reports on depressed mood as a potential side effect of HC-intake seriously given the recent reports from large cohort studies [12••, 13••] and the reality that discontinuation of HC-intake is most often motivated by such side effects, which can pose subsequent challenges in family planning [89, 90]. In general, possible mood-related HC side effects should be carefully weighed against the profound benefit of HC methods for safe family planning. A better understanding of how and when HCs affect mood is of critical importance to provide adequate contraceptive choices to women worldwide.
Funding Information
Open access funding provided by Max Planck Society. CAL, RGZ, and JS were supported by The Branco Weiss Fellowship – Society in Science, National Association for Research on Schizophrenia and Depression (NARSAD) Young Investigator Grant 25032 from the Brain & Behavior Research Foundation, and by a Minerva Research Group grant from the Max Planck Society (all awarded to Dr. Sacher). ACK and BD were supported by the German Research Foundation, DFG (DE2319/9-1).
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Reproductive Psychiatry and Women’s Health
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.United Nations DoEaSA, Popluation Division. Trends in Contraceptive Use Worldwide 2015 (ST/ESA/SER.A/349). 2015.
- 2.Pletzer BA, Kerschbaum HH. 50 years of hormonal contraception-time to find out, what it does to our brain. Front Neurosci. 2014;8:256. doi: 10.3389/fnins.2014.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Larsson G, Blohm F, Sundell G, Andersch B, Milsom I. A longitudinal study of birth control and pregnancy outcome among women in a Swedish population. Contraception. 1997;56(1):9–16. doi: 10.1016/s0010-7824(97)00068-1. [DOI] [PubMed] [Google Scholar]
- 4.Poromaa IS, Segebladh B. Adverse mood symptoms with oral contraceptives. Acta Obstet Gynecol Scand. 2012;91(4):420–427. doi: 10.1111/j.1600-0412.2011.01333.x. [DOI] [PubMed] [Google Scholar]
- 5.Seedat S, Scott KM, Angermeyer MC, Berglund P, Bromet EJ, Brugha TS, Demyttenaere K, de Girolamo G, Haro JM, Jin R, Karam EG, Kovess-Masfety V, Levinson D, Medina Mora ME, Ono Y, Ormel J, Pennell BE, Posada-Villa J, Sampson NA, Williams D, Kessler RC. Cross-national associations between gender and mental disorders in the World Health Organization World Mental Health Surveys. Arch Gen Psychiatry. 2009;66(7):785–795. doi: 10.1001/archgenpsychiatry.2009.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuehner C. Why is depression more common among women than among men? Lancet Psychiatry. 2017;4(2):146–158. doi: 10.1016/S2215-0366(16)30263-2. [DOI] [PubMed] [Google Scholar]
- 7.Zsido RG, Villringer A, Sacher J. Using positron emission tomography to investigate hormone-mediated neurochemical changes across the female lifespan: implications for depression. Int Rev Psychiatry. 2017;29(6):580–596. doi: 10.1080/09540261.2017.1397607. [DOI] [PubMed] [Google Scholar]
- 8.Baumeister H, Parker G. Meta-review of depressive subtyping models. J Affect Disord. 2012;139(2):126–140. doi: 10.1016/j.jad.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 9.Epperson CN, Steiner M, Hartlage SA, Eriksson E, Schmidt PJ, Jones I, Yonkers KA. Premenstrual dysphoric disorder: evidence for a new category for DSM-5. Am J Psychiatry. 2012;169(5):465–475. doi: 10.1176/appi.ajp.2012.11081302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Health Service. Which method of contraception suits me? 2019, January 3. https://www.nhs.uk/conditions/contraception/which-method-suits-me/.
- 11.National Institutes of Health. What are the different types of contraception? 2017, January 31. https://www.nichd.nih.gov/health/topics/contraception/conditioninfo/types.
- 12.Skovlund CW, Morch LS, Kessing LV, Lidegaard O. Association of hormonal contraception with depression. JAMA Psychiatry. 2016;73(11):1154–1162. doi: 10.1001/jamapsychiatry.2016.2387. [DOI] [PubMed] [Google Scholar]
- 13.Zettermark S, Perez Vicente R, Merlo J. Hormonal contraception increases the risk of psychotropic drug use in adolescent girls but not in adults: A pharmacoepidemiological study on 800 000 Swedish women. PLoS One. 2018;13(3):e0194773. doi: 10.1371/journal.pone.0194773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ross RA, Kaiser UB. Reproductive endocrinology: the emotional cost of contraception. Nat Rev Endocrinol. 2016;13(1):7–9. doi: 10.1038/nrendo.2016.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zethraeus Niklas, Dreber Anna, Ranehill Eva, Blomberg Liselott, Labrie Fernand, von Schoultz Bo, Johannesson Magnus, Hirschberg Angelica Lindén. A first-choice combined oral contraceptive influences general well-being in healthy women: a double-blind, randomized, placebo-controlled trial. Fertility and Sterility. 2017;107(5):1238–1245. doi: 10.1016/j.fertnstert.2017.02.120. [DOI] [PubMed] [Google Scholar]
- 16.Bengtsdotter Hanna, Lundin Cecilia, Gemzell Danielsson Kristina, Bixo Marie, Baumgart Juliane, Marions Lena, Brynhildsen Jan, Malmborg Agota, Lindh Ingela, Sundström Poromaa Inger. Ongoing or previous mental disorders predispose to adverse mood reporting during combined oral contraceptive use. The European Journal of Contraception & Reproductive Health Care. 2018;23(1):45–51. doi: 10.1080/13625187.2017.1422239. [DOI] [PubMed] [Google Scholar]
- 17.Lundin C, Danielsson KG, Bixo M, Moby L, Bengtsdotter H, Jawad I, et al. Combined oral contraceptive use is associated with both improvement and worsening of mood in the different phases of the treatment cycle-a double-blind, placebo-controlled randomized trial. Psychoneuroendocrinology. 2017;76:135–143. doi: 10.1016/j.psyneuen.2016.11.033. [DOI] [PubMed] [Google Scholar]
- 18.Ekenros Linda, Bäckström Torbjörn, Hirschberg Angelica Lindén, Fridén Cecilia. Changes in premenstrual symptoms in women starting or discontinuing use of oral contraceptives. Gynecological Endocrinology. 2019;35(5):422–426. doi: 10.1080/09513590.2018.1534097. [DOI] [PubMed] [Google Scholar]
- 19.Yonkers Kimberly A., Cameron Brianna, Gueorguieva Ralitza, Altemus Margaret, Kornstein Susan G. The Influence of Cyclic Hormonal Contraception on Expression of Premenstrual Syndrome. Journal of Women's Health. 2017;26(4):321–328. doi: 10.1089/jwh.2016.5941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robakis T, Williams KE, Nutkiewicz L, Rasgon NL. Hormonal contraceptives and mood: review of the literature and implications for future research. Curr Psychiatry Rep. 2019;21(7):57. 10.1007/s11920-019-1034-z. [DOI] [PubMed]
- 21.Pahnke R, Mau-Moeller A, Junge M, Wendt J, Weymar M, Hamm AO, Lischke A. Oral contraceptives impair complex emotion recognition in healthy women. Front Neurosci. 2018;12:1041. doi: 10.3389/fnins.2018.01041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamstra DA, de Kloet ER, Tollenaar M, Verkuil B, Manai M, Putman P, van der Does W. Mineralocorticoid receptor haplotype moderates the effects of oral contraceptives and menstrual cycle on emotional information processing. J Psychopharmacol. 2016;30(10):1054–1061. doi: 10.1177/0269881116647504. [DOI] [PubMed] [Google Scholar]
- 23.Hamstra D.A., de Kloet E.R., van Hemert A.M., de Rijk R.H., Van der Does A.J.W. Mineralocorticoid receptor haplotype, oral contraceptives and emotional information processing. Neuroscience. 2015;286:412–422. doi: 10.1016/j.neuroscience.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 24.Hamstra Danielle A., De Rover Mischa, De Rijk Roel H., Van der Does Willem. Oral contraceptives may alter the detection of emotions in facial expressions. European Neuropsychopharmacology. 2014;24(11):1855–1859. doi: 10.1016/j.euroneuro.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 25.Hamstra Danielle A., de Kloet E. Ronald, Quataert Ina, Jansen Myrthe, Van der Does Willem. Mineralocorticoid receptor haplotype, estradiol, progesterone and emotional information processing. Psychoneuroendocrinology. 2017;76:162–173. doi: 10.1016/j.psyneuen.2016.11.037. [DOI] [PubMed] [Google Scholar]
- 26.Radke Sina, Derntl Birgit. Affective responsiveness is influenced by intake of oral contraceptives. European Neuropsychopharmacology. 2016;26(6):1014–1019. doi: 10.1016/j.euroneuro.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 27.Spalek Klara, Loos Eva, Schicktanz Nathalie, Hartmann Francina, de Quervain Dominique, Stier Christina, Milnik Annette. Women using hormonal contraceptives show increased valence ratings and memory performance for emotional information. Neuropsychopharmacology. 2019;44(7):1258–1264. doi: 10.1038/s41386-019-0362-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gingnell M, Engman J, Frick A, Moby L, Wikström J, Fredrikson M, Sundström-Poromaa I. Oral contraceptive use changes brain activity and mood in women with previous negative affect on the pill—a double-blinded, placebo-controlled randomized trial of a levonorgestrel-containing combined oral contraceptive. Psychoneuroendocrinology. 2013;38(7):1133–1144. doi: 10.1016/j.psyneuen.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 29.Miedl SF, Wegerer M, Kerschbaum H, Blechert J, Wilhelm FH. Neural activity during traumatic film viewing is linked to endogenous estradiol and hormonal contraception. Psychoneuroendocrinology. 2018;87:20–26. doi: 10.1016/j.psyneuen.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 30.Merz CJ, Tabbert K, Schweckendiek J, Klucken T, Vaitl D, Stark R, Wolf OT. Neuronal correlates of extinction learning are modulated by sex hormones. Soc Cogn Affect Neurosci. 2012;7(7):819–830. doi: 10.1093/scan/nsr063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hwang MJ, Zsido RG, Song H, Pace-Schott EF, Miller KK, Lebron-Milad K, Marin MF, Milad MR. Contribution of estradiol levels and hormonal contraceptives to sex differences within the fear network during fear conditioning and extinction. BMC Psychiatry. 2015;15:295. doi: 10.1186/s12888-015-0673-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Armbruster Diana, Kirschbaum Clemens, Strobel Alexander. The not-so-bitter pill: Effects of combined oral contraceptives on peripheral physiological indicators of emotional reactivity. Hormones and Behavior. 2017;94:97–105. doi: 10.1016/j.yhbeh.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 33.Petersen Nicole, Touroutoglou Alexandra, Andreano Joseph M., Cahill Larry. Oral contraceptive pill use is associated with localized decreases in cortical thickness. Human Brain Mapping. 2015;36(7):2644–2654. doi: 10.1002/hbm.22797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bonenberger Martina, Groschwitz Rebecca C., Kumpfmueller Daniela, Groen Georg, Plener Paul L., Abler Birgit. It’s all about money. NeuroReport. 2013;24(17):951–955. doi: 10.1097/WNR.0000000000000024. [DOI] [PubMed] [Google Scholar]
- 35.Arnoni-Bauer Yardena, Bick Atira, Raz Noa, Imbar Tal, Amos Shoshana, Agmon Orly, Marko Limor, Levin Netta, Weiss Ram. Is It Me or My Hormones? Neuroendocrine Activation Profiles to Visual Food Stimuli Across the Menstrual Cycle. The Journal of Clinical Endocrinology & Metabolism. 2017;102(9):3406–3414. doi: 10.1210/jc.2016-3921. [DOI] [PubMed] [Google Scholar]
- 36.Scheele Dirk, Plota Jessica, Stoffel-Wagner Birgit, Maier Wolfgang, Hurlemann René. Hormonal contraceptives suppress oxytocin-induced brain reward responses to the partner’s face. Social Cognitive and Affective Neuroscience. 2015;11(5):767–774. doi: 10.1093/scan/nsv157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.• Jakob K, Ehrentreich H, Holtfrerich SKC, Reimers L, Diekhof EK. DAT1-Genotype and menstrual cycle, but not hormonal contraception, modulate reinforcement learning: preliminary evidence. Front Endocrinol (Lausanne). 2018;9:60. 10.3389/fendo.2018.00060. The authors show a possible underlying neurobiological mechanism of how estradiol may impact reward processing in women using a longitudinal study design. Although the influence of HC-induced changes in endogenous and exogenous estradiol levels on dopamine neurotransmission remains to be investigated, this study provides first hypotheses. [DOI] [PMC free article] [PubMed]
- 38.Merz Christian J. Contribution of stress and sex hormones to memory encoding. Psychoneuroendocrinology. 2017;82:51–58. doi: 10.1016/j.psyneuen.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 39.Barel Efrat, Abu-Shkara Randa, Colodner Raul, Masalha Refaat, Mahagna Lila, Zemel Or Chen, Cohen Ami. Gonadal hormones modulate the HPA-axis and the SNS in response to psychosocial stress. Journal of Neuroscience Research. 2018;96(8):1388–1397. doi: 10.1002/jnr.24259. [DOI] [PubMed] [Google Scholar]
- 40.Nielsen Shawn E., Ahmed Imran, Cahill Larry. Postlearning stress differentially affects memory for emotional gist and detail in naturally cycling women and women on hormonal contraceptives. Behavioral Neuroscience. 2014;128(4):482–493. doi: 10.1037/a0036687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mordecai Kristen L., Rubin Leah H., Eatough Erin, Sundermann Erin, Drogos Lauren, Savarese Antonia, Maki Pauline M. Cortisol reactivity and emotional memory after psychosocial stress in oral contraceptive users. Journal of Neuroscience Research. 2016;95(1-2):126–135. doi: 10.1002/jnr.23904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hertel J, Konig J, Homuth G, Van der Auwera S, Wittfeld K, Pietzner M, et al. Evidence for stress-like alterations in the hpa-axis in women taking oral contraceptives. Sci Rep. 2017;7(1):14111. doi: 10.1038/s41598-017-13927-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harmer Catherine J. Behavioral Neurobiology of Depression and Its Treatment. Berlin, Heidelberg: Springer Berlin Heidelberg; 2012. Emotional Processing and Antidepressant Action; pp. 209–222. [Google Scholar]
- 44.Warren Matthew B., Pringle Abbie, Harmer Catherine J. A neurocognitive model for understanding treatment action in depression. Philosophical Transactions of the Royal Society B: Biological Sciences. 2015;370(1677):20140213. doi: 10.1098/rstb.2014.0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Godlewska BR, Browning M, Norbury R, Cowen PJ, Harmer CJ. Early changes in emotional processing as a marker of clinical response to SSRI treatment in depression. Transl Psychiatry. 2016;6(11):e957. doi: 10.1038/tp.2016.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Adolphs Ralph. The neurobiology of social cognition. Current Opinion in Neurobiology. 2001;11(2):231–239. doi: 10.1016/s0959-4388(00)00202-6. [DOI] [PubMed] [Google Scholar]
- 47.Joiner TE, Timmons KA. Depression in its interpersonal context. In: Gotlib IH, Hammen CL, editors. Hanbook of depression. New York: Guilford Press; 2009. p. 322–39.
- 48.Fieker Martina, Moritz Steffen, Köther Ulf, Jelinek Lena. Emotion recognition in depression: An investigation of performance and response confidence in adult female patients with depression. Psychiatry Research. 2016;242:226–232. doi: 10.1016/j.psychres.2016.05.037. [DOI] [PubMed] [Google Scholar]
- 49.Davidson Richard J. Affective Style and Affective Disorders: Perspectives from Affective Neuroscience. Cognition & Emotion. 1998;12(3):307–330. [Google Scholar]
- 50.Sontag LM, Graber JA. Coping with perceived peer stress: gender-specific and common pathways to symptoms of psychopathology. Dev Psychol. 2010;46(6):1605–1620. doi: 10.1037/a0020617. [DOI] [PubMed] [Google Scholar]
- 51.Shapero Benjamin G., Abramson Lyn Y., Alloy Lauren B. Emotional Reactivity and Internalizing Symptoms: Moderating Role of Emotion Regulation. Cognitive Therapy and Research. 2015;40(3):328–340. doi: 10.1007/s10608-015-9722-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McLaughlin Katie A., Kubzansky Laura D., Dunn Erin C., Waldinger Robert, Vaillant George, Koenen Karestan C. Childhood social environment, emotional reactivity to stress, and mood and anxiety disorders across the life course. Depression and Anxiety. 2010;27(12):1087–1094. doi: 10.1002/da.20762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Montague P. Read, Dolan Raymond J., Friston Karl J., Dayan Peter. Computational psychiatry. Trends in Cognitive Sciences. 2012;16(1):72–80. doi: 10.1016/j.tics.2011.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huys Quentin J.M., Daw Nathaniel D., Dayan Peter. Depression: A Decision-Theoretic Analysis. Annual Review of Neuroscience. 2015;38(1):1–23. doi: 10.1146/annurev-neuro-071714-033928. [DOI] [PubMed] [Google Scholar]
- 55.Eldar Eran, Rutledge Robb B., Dolan Raymond J., Niv Yael. Mood as Representation of Momentum. Trends in Cognitive Sciences. 2016;20(1):15–24. doi: 10.1016/j.tics.2015.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Diekhof Esther K., Ratnayake Melanie. Menstrual cycle phase modulates reward sensitivity and performance monitoring in young women: Preliminary fMRI evidence. Neuropsychologia. 2016;84:70–80. doi: 10.1016/j.neuropsychologia.2015.10.016. [DOI] [PubMed] [Google Scholar]
- 57.Reimers L, Buchel C, Diekhof EK. How to be patient. The ability to wait for a reward depends on menstrual cycle phase and feedback-related activity. Front Neurosci. 2014;8:401. doi:10.3389/fnins.2014.00401. [DOI] [PMC free article] [PubMed]
- 58.Dreher J.-C., Schmidt P. J., Kohn P., Furman D., Rubinow D., Berman K. F. Menstrual cycle phase modulates reward-related neural function in women. Proceedings of the National Academy of Sciences. 2007;104(7):2465–2470. doi: 10.1073/pnas.0605569104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ossewaarde Lindsey, van Wingen Guido A., Kooijman Sabine C., Bäckström Torbjörn, Fernández Guillén, Hermans Erno J. Changes in functioning of mesolimbic incentive processing circuits during the premenstrual phase. Social Cognitive and Affective Neuroscience. 2010;6(5):612–620. doi: 10.1093/scan/nsq071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.O’Doherty John P. Reward representations and reward-related learning in the human brain: insights from neuroimaging. Current Opinion in Neurobiology. 2004;14(6):769–776. doi: 10.1016/j.conb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 61.Elliott R. Dissociable Functions in the Medial and Lateral Orbitofrontal Cortex: Evidence from Human Neuroimaging Studies. Cerebral Cortex. 2000;10(3):308–317. doi: 10.1093/cercor/10.3.308. [DOI] [PubMed] [Google Scholar]
- 62.Shams Waqqas M., Cossette Marie-Pierre, Shizgal Peter, Brake Wayne G. 17β-estradiol locally increases phasic dopamine release in the dorsal striatum. Neuroscience Letters. 2018;665:29–32. doi: 10.1016/j.neulet.2017.11.039. [DOI] [PubMed] [Google Scholar]
- 63.Yoest KE, Cummings JA, Becker JB. Estradiol, dopamine and motivation. Cent Nerv Syst Agents Med Chem. 2014;14(2):83–9. [DOI] [PMC free article] [PubMed]
- 64.Diekhof Esther K. Be quick about it. Endogenous estradiol level, menstrual cycle phase and trait impulsiveness predict impulsive choice in the context of reward acquisition. Hormones and Behavior. 2015;74:186–193. doi: 10.1016/j.yhbeh.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 65.Smith C. T., Sierra Y., Oppler S. H., Boettiger C. A. Ovarian Cycle Effects on Immediate Reward Selection Bias in Humans: A Role for Estradiol. Journal of Neuroscience. 2014;34(16):5468–5476. doi: 10.1523/JNEUROSCI.0014-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Diekhof Esther K. Estradiol and the reward system in humans. Current Opinion in Behavioral Sciences. 2018;23:58–64. [Google Scholar]
- 67.Albert Kimberly M., Newhouse Paul A. Estrogen, Stress, and Depression: Cognitive and Biological Interactions. Annual Review of Clinical Psychology. 2019;15(1):399–423. doi: 10.1146/annurev-clinpsy-050718-095557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jacobs Emily G, Holsen Laura M, Lancaster Katie, Makris Nikos, Whitfield-Gabrieli Sue, Remington Anne, Weiss Blair, Buka Stephen, Klibanski Anne, Goldstein Jill M. 17β-Estradiol Differentially Regulates Stress Circuitry Activity in Healthy and Depressed Women. Neuropsychopharmacology. 2014;40(3):566–576. doi: 10.1038/npp.2014.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hodes Georgia E., Epperson C. Neill. Sex Differences in Vulnerability and Resilience to Stress Across the Life Span. Biological Psychiatry. 2019;86(6):421–432. doi: 10.1016/j.biopsych.2019.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Snyder Jason S., Soumier Amélie, Brewer Michelle, Pickel James, Cameron Heather A. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature. 2011;476(7361):458–461. doi: 10.1038/nature10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Holsboer F. The Corticosteroid Receptor Hypothesis of Depression. Neuropsychopharmacology. 2000;23(5):477–501. doi: 10.1016/S0893-133X(00)00159-7. [DOI] [PubMed] [Google Scholar]
- 72.Aleknaviciute Jurate, Tulen Joke H.M., De Rijke Yolanda B., Bouwkamp Christian G., van der Kroeg Mark, Timmermans Mirjam, Wester Vincent L., Bergink Veerle, Hoogendijk Witte J.G., Tiemeier Henning, van Rossum Elisabeth F.C., Kooiman Cornelis G., Kushner Steven A. The levonorgestrel-releasing intrauterine device potentiates stress reactivity. Psychoneuroendocrinology. 2017;80:39–45. doi: 10.1016/j.psyneuen.2017.02.025. [DOI] [PubMed] [Google Scholar]
- 73.Tazegül Pekin Aybike, Seçilmiş Kerimoğlu Özlem, Kebapcılar Ayşe Gül, Yılmaz Setenay Arzu, Benzer Nilgün, Çelik Çetin. Depressive symptomatology and quality of life assessment among women using the levonorgestrel-releasing intrauterine system: an observational study. Archives of Gynecology and Obstetrics. 2014;290(3):507–511. doi: 10.1007/s00404-014-3237-1. [DOI] [PubMed] [Google Scholar]
- 74.Morotti Elena, Casadio Paolo, Guasina Francesca, Battaglia Bruno, Mattioli Mara, Battaglia Cesare. Weight gain, body image and sexual function in young patients treated with contraceptive vaginal ring. A prospective pilot study. Gynecological Endocrinology. 2017;33(8):660–664. doi: 10.1080/09513590.2017.1306850. [DOI] [PubMed] [Google Scholar]
- 75.Jain S, Vaid NB, Narang Y, Suneja A, Guleria K. A randomised controlled trial comparing the efficacy and side-effects of intravaginal ring (Nuvaring((R))) with combined oral hormonal preparation in dysfunctional uterine bleeding. J Clin Diagn Res. 2016;10(3):QC21–4. 10.7860/JCDR/2016/16545.7516. [DOI] [PMC free article] [PubMed]
- 76.Lopez LM, Grimes DA, Gallo MF, Stockton LL, Schulz KF. Skin patch and vaginal ring versus combined oral contraceptives for contraception. Cochrane Database Syst Rev. 2013;4:CD003552. 10.1002/14651858.CD003552.pub4. [DOI] [PMC free article] [PubMed]
- 77.Worly Brett L., Gur Tamar L., Schaffir Jonathan. The relationship between progestin hormonal contraception and depression: a systematic review. Contraception. 2018;97(6):478–489. doi: 10.1016/j.contraception.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 78.Pardridge William M., Mietus Lawrence J. Transport of Steroid Hormones through the Rat Blood-Brain Barrier. Journal of Clinical Investigation. 1979;64(1):145–154. doi: 10.1172/JCI109433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kim Harrison, Yu Tao, Cam-Etoz Betul, van Groen Thomas, Hubbard William J., Chaudry Irshad H. Treatment of traumatic brain injury with 17α-ethinylestradiol-3-sulfate in a rat model. Journal of Neurosurgery. 2017;127(1):23–31. doi: 10.3171/2016.7.JNS161263. [DOI] [PubMed] [Google Scholar]
- 80.Pike Victor W. PET radiotracers: crossing the blood–brain barrier and surviving metabolism. Trends in Pharmacological Sciences. 2009;30(8):431–440. doi: 10.1016/j.tips.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Barkhem T, Carlsson B, Nilsson Y, Enmark E, Gustafsson J-Å, Nilsson SJMp. Differential response of estrogen receptor α and estrogen receptor β to partial estrogen agonists/antagonists 1998;54(1):105–112. [DOI] [PubMed]
- 82.Jones EF, Ray KM, Li W, Chien AJ, Mukhtar RA, Esserman LJ et al. Initial experience of dedicated breast PET imaging of ER+ breast cancers using [F-18] fluoroestradiol. 2019;5(1):12. [DOI] [PMC free article] [PubMed]
- 83.van Kruchten M, de Vries EG, Brown M, de Vries EF, Glaudemans AW, Dierckx RA et al. PET imaging of oestrogen receptors in patients with breast cancer. 2013;14(11):e465-ee75. [DOI] [PubMed]
- 84.Moresco R, Casati R, Lucignani G, Carpinelli A, Schmidt K, Todde S, et al. Systemic and cerebral kinetics of 16α [18F] Fluoro-17β-estradiol: a ligand for the in vivo assessment of estrogen receptor binding. Parameters. 1995;15(2):301–311. doi: 10.1038/jcbfm.1995.35. [DOI] [PubMed] [Google Scholar]
- 85.Khayum MA, de Vries EF, Glaudemans AW, Dierckx RA, Doorduin JJJoNN. In vivo imaging of brain estrogen receptors in rats: a 16α-18F-fluoro-17β-estradiol PET study. 2014;55(3):481–487. [DOI] [PubMed]
- 86.Hattersley G, David F, Harris A, Clarkin M, Banks K, Glaudemans AWJM, et al. A phase 1 dose escalation study of RAD1901, an oral selective estrogen receptor degrader, in healthy postmenopausal women. Cancer Res. 2016;76. 10.1158/1538-7445.Sabcs15-P6-13-02.
- 87.Moraga-Amaro R, Van Waarde A, Doorduin J, De Vries EJJon. Sex steroid hormones and brain function: PET imaging as a tool for research 2018;30(2):e12565. [DOI] [PMC free article] [PubMed]
- 88.Lee JH, Zhou H-b, Dence CS, Carlson KE, Welch MJ, Katzenellenbogen JAJBc. Development of [F-18] fluorine-substituted tanaproget as a progesterone receptor imaging agent for positron emission tomography. 2010;21(6):1096–104. [DOI] [PubMed]
- 89.Sanders Stephanie A, Graham Cynthia A, Bass Jennifer L, Bancroft John. A prospective study of the effects of oral contraceptives on sexuality and well-being and their relationship to discontinuation. Contraception. 2001;64(1):51–58. doi: 10.1016/s0010-7824(01)00218-9. [DOI] [PubMed] [Google Scholar]
- 90.Lindh Ingela, Blohm Febe, Andersson-Ellström Agneta, Milsom Ian. Contraceptive use and pregnancy outcome in three generations of Swedish female teenagers from the same urban population. Contraception. 2009;80(2):163–169. doi: 10.1016/j.contraception.2009.01.019. [DOI] [PubMed] [Google Scholar]