Abstract
Atypical anorexia nervosa (AN) usually occurs during adolescence. Patients are often in the normal-weight range at diagnosis; however, they often present with signs of medical complications and severe restraint over eating, body dissatisfaction, and low self-esteem. We investigated functional circuitry underlying the hedonic response in 28 female adolescent patients diagnosed with atypical AN and 33 healthy controls. Participants were shown images of food with high (HC) or low (LC) caloric content in alternating blocks during functional MRI. The HC > LC contrast was calculated. Based on the previous literature on full-threshold AN, we hypothesized that patients would exhibit increased connectivity in areas involved in sensory processing and bottom-up responses, coupled to increased connectivity from areas related to top-down inhibitory control, compared with controls. Patients showed increased connectivity in pathways related to multimodal somatosensory processing and memory retrieval. The connectivity was on the other hand decreased in patients in salience and attentional networks, and in a wide cerebello-occipital network. Our study was the first investigation of food-related neural response in atypical AN. Our findings support higher somatosensory processing in patients in response to HC food images compared with controls, however HC food was less efficient than LC food in engaging patients’ bottom-up salient responses, and was not associated with connectivity increases in inhibitory control regions. These findings suggest that the psychopathological mechanisms underlying food restriction in atypical AN differ from full-threshold AN. Elucidating the mechanisms underlying the development and maintenance of eating behavior in atypical AN might help designing specific treatment strategies.
Subject terms: Psychology, Neuroscience, Pathogenesis, Psychiatric disorders
Introduction
Atypical anorexia nervosa (AN) is a restrictive eating disorder (ED) sharing the same diagnostic criteria of AN, except for the severely low weight1. In fact, patients with atypical AN are typically overweight or even obese before the onset of disorder2,3, usually occurring during adolescence2,3. As a result, they are in the normal-weight range when they come to psychiatric observation, despite often presenting with signs of medical instability2,3.
Neuroimaging research could help shedding light on how the brain circuitry can contribute to the development and maintenance of EDs4. In particular, functional neuroimaging studies in full-threshold AN have led to identify hyperactivity in bottom-up and top-down circuitry as underlying restrictive food choices5. Increased activity in the mesolimbic reward pathways has been consistently reported in patients with full-threshold AN, particularly in adults in response to monetary tasks or food-related visual or gustatory cues5. Few studies performed on adolescents confirmed a hyperactive mesolimbic response to monetary reward5. The top-down frontal circuitry is also hyperactive in full-threshold AN, leading to cognitive restraint over eating. In particular, top-down control mechanisms have been proposed to underlie the persistence of maladaptive behavior and cognitive inflexibility in these patients5.
Research on adolescents is nonetheless scarce. This is an important drawback, as adolescents are most at risk for developing EDs2,3. Moreover, brain plasticity is remarkably dynamic during adolescence6. Therefore, an early intervention targeting specific social and cognitive processes might be of particular importance at this stage, for preventing the chronicization of the disorder. In addition, no research has been undertaken on adolescents with atypical AN. Patients with atypical AN are often regarded as less severely affected than patients with full-threshold AN, but that is actually not the case, as they present the same prevalence of medical complications2,3. Moreover, they tend to exhibit higher restraint over eating and body dissatisfaction, as well as lower self-esteem, compared to patients with full-threshold AN3.
In previous work in our cohort of atypical AN patients, we have reported a reduction in resting-state functional connectivity in areas involved in social cues and face processing7, in the context of preserved gray matter volume8 and white matter microstructure9. In the current exploratory work, we have investigated functional circuitry underlying hedonic responses to food in 28 female adolescent patients diagnosed with atypical AN and 33 healthy controls. Hedonic responses to food are one of the main factors leading personal food choices and food intake10–12. A recent review13 has outlined the main neural alterations observed in adults with full-threshold AN in response to active viewing of food pictures; in particular, participants were required to either imagine eating the food shown, or to rate food cues according to their pleasantness13. Full-threshold AN patients showed lower activity compared with controls during visual processing of food in the inferior parietal lobule and lateral prefrontal cortex14,15; this was hypothesized to be possibly reflective of food-induced weight and body shape concerns16–18. Moreover, the simultaneous hyperactivation of medial prefrontal regions was suggested to indicate top-down control efforts aimed at restraining eating and food avoidance16,17,19,20. In addition, lower activation in the medial orbitofrontal cortex and insula during visual processing of food cues has been linked to lower hedonic reactivity in patients with AN compared with controls14,18. However, a recent study on adolescents with full-threshold AN showed higher bottom-up, as well as top-down activity in patients in response to high calories food compared with controls21. In particular, contrary to what has been reported in adults, high calories food images elicited increased activity in the insula, involved in somatosensory integration and reward attribution, coupled to higher activity in the inferior frontal gyrus (IFG), involved in salience bottom-up responses, and medial prefrontal cortex, involved in top-down control and reappraisal strategies. Based on findings in adolescents, we hypothesized that our patients would exhibit: (a) increased connectivity in areas involved in somatosensory integration, such as the insula21 and the thalamus;22,23 (b) higher connectivity in areas involved in salient bottom-up responses, such as the medial prefrontal cortex, identified in patients with full-threshold AN, and in the orbitofrontal cortex, identified in overweight adolescents24 and dieting individuals;25 (c) a compensatory increase in connectivity from areas related to top-down inhibitory control compared with controls, particularly in the medial prefrontal cortex and in the dorsolateral prefrontal cortex (DLPFC). Indeed, the DLPFC has been reported to be more active in adolescents on a successful diet, compared with overweight and lean controls, in response to food images26. Moreover, the DLPFC is more active in obese children compared with controls when viewing food pictures, with an inverse correlation with self-esteem;27 as atypical AN patients often start out as overweight/obese and subsequently successfully restrain their caloric intake in order to lose weight, we expected higher DLPFC activity in our patients compared with controls when viewing caloric food pictures.
Methods
Participants
The protocol was approved by the regional ethics committee (Etikprövningsnämnden) of Uppsala, Sweden, and complied with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. All participants and their guardians gave written consent to participate in the study.
Thirty-one adolescent female outpatients (mean age 14.8 years) were recruited by the Eating Disorder Unit (EDU) of the Department of Child and Adolescent Psychiatry at the Uppsala University Hospital, Uppsala, Sweden. All patients received a diagnosis of restrictive atypical AN according to the fifth edition of the DSM-51, as they presented with features of AN but were above −2 body mass index (BMI) standard deviations (SDS) for age28. BMI standard deviations (BMI-SDS) per age were calculated, according to the World Health Organization reports (https://www.who.int/growthref/who2007_bmi_for_age/en/). Menstrual status was also recorded. A pediatrician with experience of ED and associated with an ED clinic was responsible for the initial assessment of the patients, consisting of a structured protocol including: the history of the ED, medical history (including medical and psychiatric comorbidities), menstrual status, demographics, and physical examination (including weight and height measurements). The patients were then referred to a psychiatrist at the EDU, who confirmed the diagnoses and further assessed the patients with the diagnostic instruments included in the “Stepwise” data collection system29. The “Stepwise” data collection system was introduced in Sweden since 2014 and is used by all specialized ED services. It comprises semi-structured diagnostic interviews, clinical ratings and self-ratings, automated follow-up schedules and administrative functions29. The MINI-KID interview30 was used to further screen for comorbid diseases. Furthermore, the history of weight and height changes was obtained from the growth charts provided by the school health services. Treatment was started after the diagnosis, and consisted of family-based intervention, aiming at helping the parents to take a leading role against the ED. Parents were advised on regular meals and meal sizes; however, a standardized diet was not provided, to comply with the specific family necessities and situation. Patients had regular visit at the EDU; at each visit, their weight progress was assessed by a nurse, and the ED status were assessed by the psychiatrist. Forty female healthy controls (mean age 15.3 years) were recruited from local schools through advertisement. Inclusion criteria for all participants were age between 13 and 18 years and right-handedness. Patients and controls were excluded from the study if they had past or current history of psychiatric disorders (apart from current ED in patients), past or current pregnancy, comorbid neurological diseases, metallic implants, or claustrophobia. They were also excluded if they made use of psychotropic medication or if they had history of alcohol, drug, or medication abuse. Approximately 50% of new patients were excluded according to these criteria. Controls were further excluded if they had a BMI < −2 BMI-SDS and an EDE-Q total score > 2.0, which has been suggested as the optimal cut-off to distinguish between the clinical and the general population31.
ED-related cognition was assessed via a 38-items self-reported questionnaire, the EDE-Q32, youth version. The EDE-Q comprises four subscales measuring specific features of the ED behavior: Restraint, Eating Concern, Shape Concern, Weight Concern. Depression symptoms were assessed with the MADRS-S33, self-reported.
MRI acquisition and protocol
The participants were instructed to eat before coming to the hospital. Prior to the scanning, they were asked to rate their satiety on a visual scale ranging from 1 to 10. If they indicated a score below 3, they were provided with something to eat, like cookies or snacks. The scanning procedure was carried out within 60 days of the initial visit at the clinic. The diagnosis of atypical AN was still confirmed by the psychiatrist at the time of scanning. Structural and functional brain images were acquired with a Philips 3-T scanner (Achieva, Philips Healthcare, Best, Netherlands) using a standard head coil. Structural images were acquired with a T1-weighted turbo-field-echo (TFE) sequence (TR = 8100 ms; TE = 3.7 ms; flip angle: 8°; slice thickness = 1 mm; slice spacing = 1 mm). 125 volumes were registered during the T2*-weighted echo-planar imaging sequence (TR = 3000 ms; TE = 35 ms; flip angle = 90 °; slice thickness = 3 mm; slice spacing = 1 mm gap; in-plane resolution: (3 mm × 3 mm).
For the fMRI acquisition, images of food were presented in the scanner using MRI-compatible goggles (NordicNeuroLab, Bergen, Norway) attached to the headcoil. Participants were shown images of low calorie (LC) food, high-calorie (HC) food, or a gray screen with crosshair in the center (X) in five cycles of alternating blocks with the following pattern: X, LC, X, HC. Each block contained six images, shown at 3 s intervals. No images were repeated. 54 measurements were acquired for X, 30 for LC and 30 for HC. Participants were instructed to imagine what it felt like to eat the food presented. Food images were selected based on their familiarity according to local palate and on their caloric content as perceived by a focus groups representative of the population to be studied. Food images were controlled for visual features (color, size, etc.). A description of our fMRI paradigm can also be found in Wiemerslage et al.34.
MRI pre-processing
Initial pre-processing was carried out with Statistical Parametric Mapping (SPM) 12 (https://www.fil.ion.ucl.ac.uk/spm/), running on a Matlab 2017a platform. Slice-timing correction was performed on functional images, followed by realignment to correct for head motion. One patient and four controls had moved more than 3 mm; they were thus excluded from further analyses. Functional images were coregistered to structural images. The “old segmentation” was applied to structural images to generate GM, WM and cerebrospinal fluid probability maps using the adolescent tissue probability maps provided by the Imaging Research Center at Cincinnati Children’s Hospital Medical Center (CCHMC; https://irc.cchmc.org/software/pedbrain.php), version 2 (9/2002). In order to better accommodate our adolescent sample, the “old” sample template was used (age range: 13–18 years). Structural and functional images were normalized to Montreal Neurological Institute (MNI) standard brain template35 using the deformation parameters derived from the segmentation procedure, with 3 mm3 voxel size. Results from the normalization procedure were visually inspected, and two patients and two controls were excluded from further analyses due to normalization imperfections. Twenty-eight patients and thirty-three controls were retained. Functional images were smoothed with a four full width at half maximum (FWHM) Gaussian kernel to increase the signal-to-noise ratio and to accommodate for anatomical and functional variability between subjects.
Pre-processed structural and functional images were imported in CONN connectivity toolbox (https://www.nitrc.org/projects/conn). Band-pass filtering was applied, to retain data in the 0.01–0.1 Hz band)36–38. and reduce the interference of physiological noise occurring at higher frequencies39,40. Linear detrending was applied, and the effects of white matter, motion and cerebrospinal fluid were regressed out. First level analysis was performed by including motion parameters as covariates.
Statistical analysis of clinical data
Statistical analyses of clinical measures were performed with Statistical Package for Social Science (SPSS) v. 24 (https://www.ibm.com/analytics/spss-statistics-software). Four separate ANOVA tests were carried out to test for group differences on age, BMI, BMI-SDS per age, EDE-Q and MADRS-S total scores. The threshold for significance was set at p < 0.05. BMI-SDS loss in patients was calculated as the difference between the BMI-SDS per age from the moment of maximum documented weight until the moment the diagnosis of atypical AN was made (Table 1).
Table 1.
Sample description
| Patients (mean ± SD) | Controls (mean ± SD) | Sig. | |
|---|---|---|---|
| Age (years) | 14.8 ± 1.6 | 15.3 ± 1.3 | <0.184 |
| BMI (Kg/m2) | 19.4 ± 2.4 | 20.9 ± 2.3 | <0.014a |
| BMI-SDS | −0.36 ± 0.85 | 0.11 ± 0.71 | <0.022a |
| EDE-Q | 3.5 ± 1.7 | 0.6 ± 0.8 | <0.001a |
| MADRS-S | 22.5 ± 11.6 | 5.2 ± 5.4 | <0.001a |
| Disease duration (years) | 0.72 ± 0.45 | – | – |
| BMI-SDS lossb | 1.00 ± 0.72 | – | – |
| Diagnosis-to-scan (days) | 30.9 ± 12 | ||
| BMI gain prior to scanning (Kg/m2)c | 0.79 ± 0.63 | – | – |
aSignificant with p < 0.05
bFrom the moment of maximum documented weight to diagnosis
cFrom clinical assessment to scanning
Functional connectivity analysis
A seed-to-voxel analysis was performed with CONN toolbox. CONN provides a complete brain parcellation including 91 cortical areas and 15 subcortical areas from the FSL Harvard-Oxford Atlas, and 26 cerebellar areas from the AAL atlas, for a total of 132 seeds. Functional connectivity maps from each of these seeds were generated, and tested for differences between patients and controls. Age and BMI-SDS were entered as covariates in the analysis. The primary voxel-level threshold was set at p < 0.00141. A further correction for Family-wise error rate (FWE) at cluster level was applied to voxels surviving the primary threshold41. The final threshold was then set at p < 0.0004 (0.05/132), FWE-corrected, to account for the number of seeds tested.
Moreover, separate regression analyses were carried out separately in patients and controls, to test for correlations of BMI, EDE-Q and MADRS-S scores on connectivity in clusters found to be significantly different between groups. To this purpose, correlation coefficients were extracted and imported in SPSS. Within patients, functional connectivity was also tested for correlations with disease duration and weight loss from the moment of maximum documented weight to the diagnosis, expressed as BMI-SDS loss. All models were corrected for age. The same above-mentioned thresholding was applied. As nine cluster of altered connectivity were identified at the main analysis, the Bonferroni correction for multiple testing led to a final threshold of p < 0.002 in controls (9 clusters * 3 clinical measures) and p < 0.001 in patients (9 clusters * 5 clinical measures).
Results
Clinical measures
Patients had a mean disease duration of 8.6 months, and their BMI-SDS per age had reduced by one unit since the moment of maximum documented weight. Average treatment duration to the time of scanning was 1 month, with an average BMI gain of 0.8 Kg/m2. Nine of our patients had secondary amenorrhea, two were on oral contraceptives, three were in premenarche, and 14 had menstruation. Patients and controls did not differ on age (p < 0.184); however, they were significantly different on BMI (p < 0.014), BMI-SDS per age (p < 0.022), and on the EDE-Q (p < 0.001) and MADRS-S total scores (p < 0.001) (Table 1).
Functional connectivity analysis
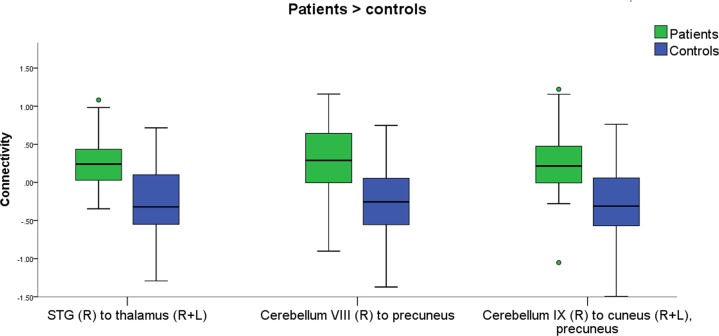
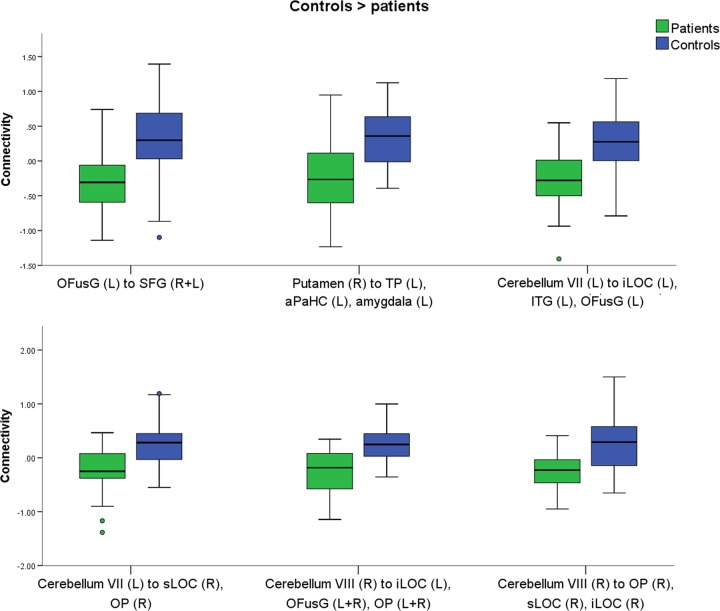
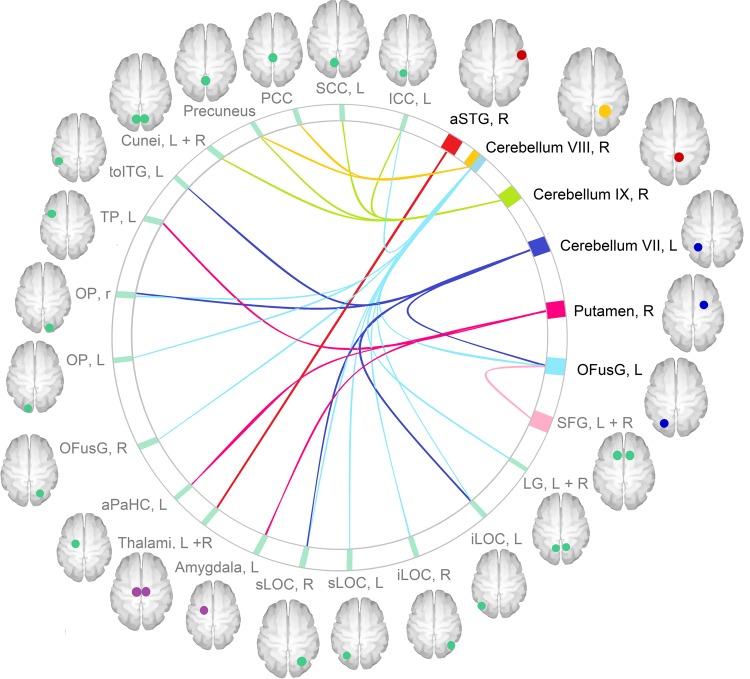
Patients exhibited patterns of increased and reduced connectivity compared with controls. When viewing HC vs. LC food images, connectivity was higher in patients from the right anterior superior temporal gyrus (aSTG) to the thalamus (p < 0.0003, FWE-corrected), from the right cerebellar lobule VIII to the precuneus and posterior cingulate cortex (PCC) (p < 0.00006, FWE-corrected), and from the right cerebellar lobule IX to the cuneus bilaterally, to the precuneus, and to the left intracalcarine cortex (ICC) and supra-calcarine cortex (SCC) (p < 0.00002, FWE-corrected) (Fig. 1). On the other hand, connectivity was lower in patients compared with controls from the left occipital fusiform gyrus (OFusG) to the superior frontal gyri (SFG) bilaterally (p < 0.0002), from the right putamen to the left temporal pole (TP), anterior parahippocampal cortex (aPaHC) and amygdala (p < 0.0002 FWE-corrected), from the left cerebellar lobule VII to the left OFus G, temporo-occipital inferior temporal gyrus (ITG) and occipital areas (p < 0.00003, FWE-corrected), and from the right cerebellar lobule VIII to several occipital and cerebellar areas (p < 0.000001, FWE-corrected) (Fig. 2), when viewing HC vs. LC food images. Results from the connectivity analyses reported in Table 2, and summarized in Fig. 3. No correlations were found between connectivity in the affected functional connections and clinical measures.
Fig. 1. Increased connectivity in patients compared with controls.
The boxplot shows the clusters were increased connectivity was found in patients (green) compared with controls (blue), in response to food with high vs. low caloric content. Circles represent cases lying between 1.5 and 3 times the interquartile range (mild outlier). The results were verified in SPSS by removing the outliers (see methods). Connectivity values were extracted with SPM. L left, R right, STG superior temporal gyrus
Fig. 2. Reduced connectivity in patients compared with controls.
The boxplot shows the clusters were reduced connectivity was found in patients (green) compared with controls (blue), in response to food with high vs. low caloric content. Circles represent cases lying between 1.5 and 3 times the interquartile range (mild outlier). The results were verified in SPSS by removing the outliers (see methods). Connectivity values were extracted with SPM. aPaHC anterior parahippocampal cortex, iLOC = inferior lateral occipital cortex, ITG inferior temporal gyrus, L left, OFusG occipital fusiform gyrus, OP occipital pole, R right, SFG superior frontal gyrus, sLOC superior lateral occipital cortex, TP temporal pole
Table 2.
Functional connectivity differences between patients and controls
| Seed | Target | MNI coordinates | CE | P FWE-corr | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Patients > controls | ||||||
| aSTG (R) | Thalamus (R + L) | 10 | −26 | 20 | 207 | <0.0003 |
| Cerebellum (VIII, R) | Precuneus, PCC | 6 | −58 | 34 | 253 | <0.00006 |
| Cerebellum (IX, R) |
Cuneus (R + L) Precuneus ICC (L) SCC (L) |
−2 | −68 | 16 | 291 | <0.00002 |
| Patients < controls | ||||||
| OFusG (L) | SFG (R + L) | 4 | 56 | 34 | 203 | <0.0002 |
| Putamen (R) |
TP (L) aPaHC (L) Amygdala (L) |
−32 | 10 | −28 | 216 | <0.0002 |
| Cerebellum (VII, L) |
iLOC (L) toITG (L) OFusG (L) |
−48 | −64 | −8 | 404 | <0.000001 |
| Cerebellum (VII, L) |
sLOC (R) OP (R) |
28 | −82 | 34 | 273 | <0.00003 |
| Cerebellum (VIII, R) |
iLOC (L) OFusG (L + R) OP (L + R) sLOC (L) ICC (L) LG (L + R) Cerebellum |
−26 | −70 | −14 | 2558 | <0.000001 |
| Cerebellum (VIII, R) |
OP (R) sLOC (R) iLOC (R) |
28 | −86 | 28 | 570 | <0.000001 |
Fig. 3. Functional connectivity in patients vs controls.
The figure summarizes group differences in seed-to-target functional connectivity when viewing picture of high calories vs low calories food. The seeds with greater connectivity in patients compared with controls are represented in red; the seeds with lower connectivity in patients are represented in blue; the target regions are represented in green. The cerebellar lobule VIII, R is represented in orange as it showed both increased and reduced connectivity to different target regions. The image was created with CONN toolbox. The target regions are depicted as circles merely for representative purposes. The exact peak coordinates and voxel extent of each target cluster are reported in Table 2. aPaHC anterior parahippocampal cortex, aSTG anterior superior temporal gyrus, ICC intracalcarine cortex, iLOC inferior lateral occipital cortex, L left, LG lingual gyri, OFusG occipital fusiform gyrus, OP occipital pole, PCC posterior cingular cortex, R right, SCC supracalcarine cortex, SFG superior frontal gyri, sLOC superior lateral occipital cortex, toITG temporo-occipital inferior temporal gyrus, TP temporal pole
Mild outliers
When inspecting the box plots (Fig. 1, Fig. 2), few individuals were lying between 1.5 and 3 times the interquartile range (mild outliers). When no specific reasons for outliers are detected (e.g. MRI artefacts, excessive movement, normalization issues), mild outliers are likely to represent normal variability in the data at population level. We had inspected the raw and pre-processed data to exclude the presence of artefacts and ensure the good quality of the normalization. Cases with excessive movements were discarded during the first steps of pre-processing. Thus, no instrumental reasons for mild outliers were noticed. Nonetheless, we performed a series of ANCOVAs corrected for age and BMI-SDS in SPSS after the removal of the outliers, to ensure that our results were not driven by mild outliers. All results were confirmed.
Discussion
We investigated neural connectivity underlying the hedonic response to food images in a sample of 28 adolescent females diagnosed with atypical AN, and 33 age- and sex-matched healthy controls. Different connections showed higher or lower functional connectivity in patients compared with controls, when watching images of food with high vs. low caloric content.
Higher connectivity in patients in somatosensory areas
Patients showed higher connectivity from the right cerebellar lobule IX to the cuneal cortices bilaterally, to the precuneus, extending to the primary visual cortex and SCC, in response to food with high vs. low caloric content. These areas are all intercalated on the visual stream. Additionally, the precuneus is involved in visuo-spatial imagery and in episodic memory retrieval42. It has also been related to appetite control, as its activity is elicited when asking subjects to exert cognitive inhibitory control over the urge to eat the food shown in a picture43. Moreover, both the cuneus and precuneus have been related to early imagery processing44. The cerebellar lobule IX is also part of the default-mode network45, and receives sensory inputs from the trigeminal nerve46, which provides motor innervation to the masticatory muscles and tactile and nociceptive innervation to the face and mouth mucosae. It receives also tactile inputs from the hands45. The higher connectivity within this network might underlie an enhanced capacity for visual and tactile imagery of the food shown, in line with our hypothesis of increased somatosensory processing in patients.
Moreover, the connectivity from the right aSTG to the thalamus, bilaterally, was greater in patients compared with controls when viewing (and imaging to eat) HC vs. LC foods. The STG’s main function has been traditionally related to the auditory pathway, however its function seems to be more complex, and more broadly related to the multimodal integration of sensorial stimuli through its rich connections with the thalamus47,48. Indeed, the thalamus acts as a sensory relay structure and as a hub on sensory pathways23. Interestingly, the thalamus is strongly involved in gustatory processing49,50 and food intake regulation22, while the STG has been previously found to be more active in response to the presentation of food cues compared with non-food matched stimuli of different modalities43,51,52. In particular, the STG was more responsive to high calories food irrespective of the drive to eat53. Thus, when imaging eating a food with higher caloric content, the connectivity between associative and gustatory areas is actually increased in patients compared with controls, supporting our hypothesis of an increased somatosensory elaboration of food stimuli.
Finally, the connectivity from the right cerebellar lobule VIII to the precuneus, extending to the PCC, was also higher. The cerebellar lobule VIII is part of the sensorimotor cerebellum, active during sensorimotor and somatosensory tasks45,54. The activity of the PCC in response to food images is modulated by the caloric content of the food displayed55. Interestingly, the PCC has been largely implicated in self-referential processing during mind-wandering or judgemental tasks, and it has been proposed to be specifically involved in cognitive processing of self-reference56, defined by Brewer et al.56. as “getting caught up in” one’s experience, such as in drug craving or in a particular viewpoint56. The precuneus, on the other hand, is involved in self-centered thinking and episodic memory retrieval42. The higher connectivity between these regions might therefore support an enhanced brain response to the imaginary eating of food with higher caloric content. However, this interpretation has to be considered speculative, and more research has to be undertaken to specifically investigate food-related self-referential processing in these patients.
Lower connectivity in patients in areas related to salience attribution and inhibitory control
The connectivity was lower in patients compared with controls when watching images of food with high, compared to low, caloric content, from the left OFusG to the SFG bilaterally. The fusiform gyrus is involved in semantic memory57,58. Meta-analyses, however, have also demonstrated the involvement of the fusiform gyrus in visual processing of food images in both adolescents and adults52,59. Interestingly, connectivity of the fusiform gyrus has been reported to be decreased in AN patients60. The SFG, on the other hand, is involved in introspection and in self-related processing61, as well as in exerting inhibitory control over appetite in response to visual food cues43. This finding seems to be in contrast with our hypothesis of an increase top-down inhibitory control over appetite and food-related visual stimulation. However, this might be due to an overall reduced rewarding value for the caloric content of food. In fact, patients also showed reduced connectivity from the right putamen to the left TP, aPaHC and amygdala in response to high calories compared with low calories food. These areas are part of the reward system62 and salience network63. The putamen has been reported to be more active in response to low calories rather than high calories food in satiated females64. Moreover, the amygdala-striatal circuitry underlies impulsive behaviors64, and activity in the amygdala and in the right para-hippocampal gyrus are elicited by food cues in obese individuals64. Thus, in our atypical AN patients, imaging to eat the HC foods shown is associated to a weaker connectivity in the salience network compared to controls, and this might explain the subsequent lack of recruitment of inhibitory control regions in response to visual processing of food cues.
The connectivity was also lower in patients compared with controls from the left cerebellar lobule VII to the left OFus G, left temporo-occipital ITG and left occipital areas. The cerebellar lobule VII is part of the posterior cerebellum, also known as the cognitive cerebellum45. Activity in this lobule is elicited by executive, working memory and affective tasks45. In particular, the cerebellar lobule VII seems to be consistently active when viewing emotional vs. neutral stimuli45,54. The OFusG and the ITG are part of the ventral visual stream, involved in objects recognition65. As mentioned previously, the fusiform gyrus is also involved in visual processing of food images52,59, and of meaningful stimuli in general66. Indeed, the left visual stream in particular appears to be more involved in the analytic, attention-dependent processing of objects65. The LOC, on the other hand, beside its role in object recognition, is also important in tactile perception, serving as a hub for recognition of tactile imagery67. Interestingly, LOC activation seems to be independent of object familiarity68. Thus, the lower connectivity between the cerebellar lobule VII and the left ventral visual stream is in line with a failure in engaging patients’ attention with foods with high vs. low caloric content.
Finally, the connectivity was lower in patients when viewing high calories food images from the right cerebellar lobule VIII to several occipital and cerebellar areas. As mentioned above, the cerebellar lobule VIII is part of the sensorimotor cerebellum, active during sensorimotor and somatosensory tasks45,54. The cerebellar lobule VIII is particularly engaged during tactile tasks, as it stores a representation of the hand54. Most of the target areas with decreased connectivity are primarily involved in visual processing, although recently some of them have been also involved in association processing67. The LOC in particular is a hub for multimodal integration of visual stimuli, and is particularly involved in tactile imagery and in decoding of objects’ shapes, forms, and orientations in a multimodal perspective67. The finding of a reduced connectivity between these areas is of difficult interpretation, and will need to be further explored by future studies specifically targeting the cerebellum and involving different visual stimuli.
Implications for treatment
In full-threshold AN, the aberrant eating behavior is likely to be facilitated by increased cognitive control and reappraisal strategies toward salient food stimuli21, and sustained by lack of cognitive flexibility69,70, leading to difficulties in changing the adopted behavioral patterns despite adverse consequences. In our patients, however, the neurobiological mechanisms underlying food restriction seems to differ from full-threshold AN, as HC food images were not associated to increased bottom-up response nor to increased top-down response. The specific neuro-behavioral mechanisms underpinning restraint over eating in atypical AN needs further investigation, as it can have relevant implications for treatment. Indeed, though Family-based treatment (FBT) is currently the leading therapy for adolescents with full-threshold AN71, the risk for relapse, particularly in the first 16 months after treatment, is nonetheless still high72–74. Enhanced cognitive behavioral therapy (CBT-E), targeting the specific psychopathological mechanisms sustaining the ED in each individual, seems to be a promising alternative treatment. Thus, elucidating the mechanisms underlying the development and maintenance of unhealthy eating habits in atypical AN might help designing and improving specific treatment strategies. However, it has to be noted that none of the clinical scores tested were significantly associated with the functional connectivity coefficients in the detected seed-to-target pairs, as would have been expected if they contributed to within-group variability in symptoms severity. Thus, their potential prognostic value needs further investigation.
Limitations
Our study has some limitations according to the recent guidelines for neuroimaging studies in EDs suggested by4, and has thus to be considered of exploratory nature; nonetheless, it laid the foundations for future research on atypical AN. The sample size was adequate for fMRI studies75; however, future studies on larger sample will be needed to verify our findings, and ideally complemented with food ratings by the participants. Moreover, there was a delay of up to 60 days between the diagnosis and the scanning procedure, and patients had undergone treatment during that period. This delay was due to the informed consent protocol and hospital procedures. In fact, patients and their guardians were provided with information regarding the study, and given one week to decide whether they wanted to participate. Then, the MRI scan had to be booked according to the patients’ and hospital necessities, leading to a certain delay. Patients did, however, start the treatment right away, due to ethical reasons. It must be noticed that all patients were still diagnosed with atypical AN at the time of scanning; however, whether early-onset effects of the treatment might have affected our results needs to be clarified. The meal schedule was not standardized between patients; however, all patients were satiated before scanning. We did not have information pertaining the menstrual status of the controls, thus we could not control our findings for menstrual status. Moreover, though the treatment protocol required patients not to exercise, non-compliance cannot be ruled out. Finally, the controls did not undergo a complete psychological examination, and their mental health history was self-reported. However, using the subclinical cut-off on the EDE-Q as exclusion criterion ensured the exclusion of controls with possible underdiagnosed EDs.
Conclusions
We investigated neural connectivity underlying hedonic response to food images in a sample of 28 adolescent females diagnosed with atypical AN, and 33 age- and sex-matched healthy controls. Based on previous literature on adolescents with full-threshold AN, we hypothesized that viewing food with high caloric content would be associated to increased connectivity in patients in areas involved in somatosensory integration and bottom-up responses, possibly coupled to a compensatory increase in connectivity from areas related to top-down inhibitory control, compared with controls. In accordance with our hypothesis, our results showed a stronger connectivity in patients in pathways related to the integration of multimodal somatosensory input and memory retrieval, in response to food with high vs. low caloric content. This, however, was coupled to a lower connectivity in salience and attentional networks, and reduced connectivity between areas involved in visual food cues processing and appetite regulatory regions, contrary to our hypothesis. Thus, food with high caloric content is associated to increased processing of somatosensory information in patients; however, it is attributed less salience and engage patients’ attention less than food with low caloric content. Consequently, no compensatory increases in inhibitory control regions are observed. Elucidating the mechanisms underlying the development and maintenance of unhealthy eating habits in atypical AN might help designing and improving specific treatment strategies. Thus, future studies will be needed to further elucidate the role of cerebellar-occipital connectivity and the role of visual areas in the pathogenesis of atypical AN, possibly using different visual stimuli or specifically targeting cerebellar circuitry, and complementing the fMRI protocol with food ratings by the participants.
Acknowledgements
The authors would like to acknowledge the fundamental contribution of Dr. Ingemar Swenne, who has recently passed away. He participated in the conception and design of the study and had a key role in recruiting and treating patients. The studies were supported by Formas, the Swedish Research Council, the Swedish Brain Research foundation, Gunvoroch Josef Anérs foundation, A Karlssons foundation, LR Åkerhams foundation, Kempe-Carlgrenska Foundation, Tore Nilsons foundation and the Gillbergska foundation.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders 5th edn (American Psychiatric Association, Washington, DC, 2015).
- 2.Moskowitz L, Weiselberg E. Anorexia nervosa/atypical anorexia nervosa. Curr. Probl. Pediatr. Adolesc. Health Care. 2017;47:70–84. doi: 10.1016/j.cppeds.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Sawyer S. M., Whitelaw M., Le Grange D., Yeo M., Hughes E. K. Physical and psychological morbidity in adolescents with atypical anorexia nervosa. Pediatrics137, pii: e20154080 (2016). [DOI] [PubMed]
- 4.Frank GKW, Favaro A, Marsh R, Ehrlich S, Lawson EA. Toward valid and reliable brain imaging results in eating disorders. Int J. Eat. Disord. 2018;51:250–261. doi: 10.1002/eat.22829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steinglass JE, Walsh BT. Neurobiological model of the persistence of anorexia nervosa. J. Eat. Disord. 2016;4:19. doi: 10.1186/s40337-016-0106-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Selemon LD. A role for synaptic plasticity in the adolescent development of executive function. Transl. Psychiatry. 2013;3:e238. doi: 10.1038/tp.2013.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olivo G, et al. Reduced resting-state connectivity in areas involved in processing of face-related social cues in female adolescents with atypical anorexia nervosa. Transl. Psychiatry. 2018;8:275. doi: 10.1038/s41398-018-0333-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olivo G, et al. Atypical anorexia nervosa is not related to brain structural changes in newly diagnosed adolescent patients. Int J. Eat. Disord. 2018;51:39–45. doi: 10.1002/eat.22805. [DOI] [PubMed] [Google Scholar]
- 9.Olivo G, et al. Preserved white matter microstructure in adolescent patients with atypical anorexia nervosa. Int J. Eat. Disord. 2019;52:166–174. doi: 10.1002/eat.23012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson SL, Boles RE, Burger KS. Using participant hedonic ratings of food images to construct data driven food groupings. Appetite. 2014;79:189–196. doi: 10.1016/j.appet.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brockmeyer T, et al. Inhibitory control and hedonic response towards food interactively predict success in a weight loss programme for adults with obesity. Obes. Facts. 2016;9:299–309. doi: 10.1159/000447492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hofmann W, van Koningsbruggen GM, Stroebe W, Ramanathan S, Aarts H. As pleasure unfolds. Hedonic responses to tempting food. Psychol. Sci. 2010;21:1863–1870. doi: 10.1177/0956797610389186. [DOI] [PubMed] [Google Scholar]
- 13.Simon Joe J., Stopyra Marion A., Friederich Hans-Christoph. Neural Processing of Disorder-Related Stimuli in Patients with Anorexia Nervosa: A Narrative Review of Brain Imaging Studies. Journal of Clinical Medicine. 2019;8(7):1047. doi: 10.3390/jcm8071047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia-Garcia I, et al. Neural responses to visual food cues: insights from functional magnetic resonance imaging. Eur. Eat. Disord. Rev. 2013;21:89–98. doi: 10.1002/erv.2216. [DOI] [PubMed] [Google Scholar]
- 15.Lloyd EC, Steinglass JE. What can food-image tasks teach us about anorexia nervosa? A systematic review. J. Eat. Disord. 2018;6:31. doi: 10.1186/s40337-018-0217-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uher R, et al. Medial prefrontal cortex activity associated with symptom provocation in eating disorders. Am. J. Psychiatry. 2004;161:1238–1246. doi: 10.1176/appi.ajp.161.7.1238. [DOI] [PubMed] [Google Scholar]
- 17.Kim KR, Ku J, Lee JH, Lee H, Jung YC. Functional and effective connectivity of anterior insula in anorexia nervosa and bulimia nervosa. Neurosci. Lett. 2012;521:152–157. doi: 10.1016/j.neulet.2012.05.075. [DOI] [PubMed] [Google Scholar]
- 18.Uher R, et al. Recovery and chronicity in anorexia nervosa: brain activity associated with differential outcomes. Biol. Psychiatry. 2003;54:934–942. doi: 10.1016/S0006-3223(03)00172-0. [DOI] [PubMed] [Google Scholar]
- 19.Cowdrey FA, Park RJ, Harmer CJ, McCabe C. Increased neural processing of rewarding and aversive food stimuli in recovered anorexia nervosa. Biol. Psychiatry. 2011;70:736–743. doi: 10.1016/j.biopsych.2011.05.028. [DOI] [PubMed] [Google Scholar]
- 20.Rothemund Y, et al. Compulsivity predicts fronto striatal activation in severely anorectic individuals. Neuroscience. 2011;197:242–250. doi: 10.1016/j.neuroscience.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 21.Horndasch S, et al. Neural processing of food and emotional stimuli in adolescent and adult anorexia nervosa patients. PLoS ONE. 2018;13:e0191059. doi: 10.1371/journal.pone.0191059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ong ZY, Liu JJ, Pang ZP, Grill HJ. Paraventricular thalamic control of food intake and reward: role of glucagon-like peptide-1 receptor signaling. Neuropsychopharmacology. 2017;42:2387–2397. doi: 10.1038/npp.2017.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tyll S, Budinger E, Noesselt T. Thalamic influences on multisensory integration. Commun. Integr. Biol. 2011;4:378–381. doi: 10.4161/cib.15222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bruce AS, et al. Obese children show hyperactivation to food pictures in brain networks linked to motivation, reward and cognitive control. Int J. Obes. (Lond.). 2010;34:1494–1500. doi: 10.1038/ijo.2010.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Courtney AL, PeConga EK, Wagner DD, Rapuano KM. Calorie information and dieting status modulate reward and control activation during the evaluation of food images. PLoS ONE. 2018;13:e0204744. doi: 10.1371/journal.pone.0204744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jensen CD, Kirwan CB. Functional brain response to food images in successful adolescent weight losers compared with normal-weight and overweight controls. Obesity (Silver Spring). 2015;23:630–636. doi: 10.1002/oby.21004. [DOI] [PubMed] [Google Scholar]
- 27.Davids S, et al. Increased dorsolateral prefrontal cortex activation in obese children during observation of food stimuli. Int J. Obes. (Lond.). 2010;34:94–104. doi: 10.1038/ijo.2009.193. [DOI] [PubMed] [Google Scholar]
- 28.Lindgren G, Strandell A, Cole T, Healy M, Tanner J. Swedish population reference standards for height, weight and body mass index attained at 6 to 16 years (girls) or 19 years (boys) Acta Paediatr. 1995;84:1019–1028. doi: 10.1111/j.1651-2227.1995.tb13819.x. [DOI] [PubMed] [Google Scholar]
- 29.Birgegard A, Bjorck C, Clinton D. Quality assurance of specialised treatment of eating disorders using large-scale Internet-based collection systems: methods, results and lessons learned from designing the Stepwise database. Eur. Eat. Disord. Rev. 2010;18:251–259. doi: 10.1002/erv.1003. [DOI] [PubMed] [Google Scholar]
- 30.Sheehan DV, et al. Reliability and validity of the mini international neuropsychiatric interview for children and adolescents (MINI-KID) J. Clin. Psychiatry. 2010;71:313–326. doi: 10.4088/JCP.09m05305whi. [DOI] [PubMed] [Google Scholar]
- 31.Ekeroth K, Birgegard A. Evaluating reliable and clinically significant change in eating disorders: comparisons to changes in DSM-IV diagnoses. Psychiatry Res. 2014;216:248–254. doi: 10.1016/j.psychres.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 32.Fairburn CG, Beglin SJ. Assessment of eating disorders: interview or self-report questionnaire? Int J. Eat. Disord. 1994;16:363–370. [PubMed] [Google Scholar]
- 33.Svanborg P, Asberg M. A new self-rating scale for depression and anxiety states based on the Comprehensive Psychopathological Rating Scale. Acta Psychiatr. Scand. 1994;89:21–28. doi: 10.1111/j.1600-0447.1994.tb01480.x. [DOI] [PubMed] [Google Scholar]
- 34.Wiemerslage L, et al. An obesity-associated risk allele within the FTO gene affects human brain activity for areas important for emotion, impulse control and reward in response to food images. Eur. J. Neurosci. 2016;43:1173–1180. doi: 10.1111/ejn.13177. [DOI] [PubMed] [Google Scholar]
- 35.Mazziotta J, et al. A probabilistic atlas and reference system for the human brain: International Consortium for Brain Mapping (ICBM) Philos. Trans. R. Soc. Lond. B Biol. Sci. 2001;356:1293–1322. doi: 10.1098/rstb.2001.0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn. Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 37.Cordes D, et al. Mapping functionally related regions of brain with functional connectivity MR imaging. AJNR Am. J. Neuroradiol. 2000;21:1636–1644. [PMC free article] [PubMed] [Google Scholar]
- 38.De Luca M, Beckmann CF, De Stefano N, Matthews PM, Smith SM. fMRI resting state networks define distinct modes of long-distance interactions in the human brain. Neuroimage. 2006;29:1359–1367. doi: 10.1016/j.neuroimage.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 39.Birn RM, Diamond JB, Smith MA, Bandettini PA. Separating respiratory-variation-related fluctuations from neuronal-activity-related fluctuations in fMRI. Neuroimage. 2006;31:1536–1548. doi: 10.1016/j.neuroimage.2006.02.048. [DOI] [PubMed] [Google Scholar]
- 40.Van Dijk KR, et al. Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. J. Neurophysiol. 2010;103:297–321. doi: 10.1152/jn.00783.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Woo CW, Krishnan A, Wager TD. Cluster-extent based thresholding in fMRI analyses: pitfalls and recommendations. Neuroimage. 2014;91:412–419. doi: 10.1016/j.neuroimage.2013.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129(Pt 3):564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- 43.Tuulari JJ, et al. Neural circuits for cognitive appetite control in healthy and obese individuals: an fMRI study. PLoS ONE. 2015;10:e0116640. doi: 10.1371/journal.pone.0116640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee JS, et al. The effect of word imagery on priming effect under a preconscious condition: an fMRI study. Hum. Brain Mapp. 2014;35:4795–4804. doi: 10.1002/hbm.22512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stoodley CJ, Schmahmann JD. Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex. 2010;46:831–844. doi: 10.1016/j.cortex.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rondi-Reig L, Paradis AL, Lefort JM, Babayan BM, Tobin C. How the cerebellum may monitor sensory information for spatial representation. Front Syst. Neurosci. 2014;8:205. doi: 10.3389/fnsys.2014.00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kosmal A, Malinowska M, Kowalska DM. Thalamic and amygdaloid connections of the auditory association cortex of the superior temporal gyrus in rhesus monkey (Macaca mulatta) Acta Neurobiol. Exp. (Wars.). 1997;57:165–188. doi: 10.55782/ane-1997-1224. [DOI] [PubMed] [Google Scholar]
- 48.Mastropasqua C, Bozzali M, Spano B, Koch G, Cercignani M. Functional anatomy of the thalamus as a model of integrated structural and functional connectivity of the human brain in vivo. Brain Topogr. 2015;28:548–558. doi: 10.1007/s10548-014-0422-2. [DOI] [PubMed] [Google Scholar]
- 49.Reilly S. The role of the gustatory thalamus in taste-guided behavior. Neurosci. Biobehav Rev. 1998;22:883–901. doi: 10.1016/S0149-7634(98)00015-3. [DOI] [PubMed] [Google Scholar]
- 50.Arthurs J, Reilly S. Role of the gustatory thalamus in taste learning. Behav. Brain Res. 2013;250:9–17. doi: 10.1016/j.bbr.2013.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.St-Onge MP, Sy M, Heymsfield SB, Hirsch J. Human cortical specialization for food: a functional magnetic resonance imaging investigation. J. Nutr. 2005;135:1014–1018. doi: 10.1093/jn/135.5.1014. [DOI] [PubMed] [Google Scholar]
- 52.Huerta CI, Sarkar PR, Duong TQ, Laird AR, Fox PT. Neural bases of food perception: coordinate-based meta-analyses of neuroimaging studies in multiple modalities. Obes. (Silver Spring). 2014;22:1439–1446. doi: 10.1002/oby.20659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Charbonnier L, van der Laan LN, Viergever MA, Smeets PA. Functional MRI of challenging food choices: forced choice between equally liked high- and low-calorie foods in the absence of hunger. PLoS ONE. 2015;10:e0131727. doi: 10.1371/journal.pone.0131727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stoodley CJ, Schmahmann JD. Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. Neuroimage. 2009;44:489–501. doi: 10.1016/j.neuroimage.2008.08.039. [DOI] [PubMed] [Google Scholar]
- 55.Siep N, et al. Hunger is the best spice: an fMRI study of the effects of attention, hunger and calorie content on food reward processing in the amygdala and orbitofrontal cortex. Behav. Brain Res. 2009;198:149–158. doi: 10.1016/j.bbr.2008.10.035. [DOI] [PubMed] [Google Scholar]
- 56.Brewer JA, Garrison KA, Whitfield-Gabrieli S. What about the “self” is processed in the posterior cingulate cortex? Front Hum. Neurosci. 2013;7:647. doi: 10.3389/fnhum.2013.00647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mion M, et al. What the left and right anterior fusiform gyri tell us about semantic memory. Brain. 2010;133:3256–3268. doi: 10.1093/brain/awq272. [DOI] [PubMed] [Google Scholar]
- 58.Rice GE, Lambon Ralph MA, Hoffman P. The roles of left versus right anterior temporal lobes in conceptual knowledge: an ALE meta-analysis of 97 functional neuroimaging studies. Cereb. Cortex. 2015;25:4374–4391. doi: 10.1093/cercor/bhv024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van Meer F, van der Laan LN, Adan RA, Viergever MA, Smeets PA. What you see is what you eat: an ALE meta-analysis of the neural correlates of food viewing in children and adolescents. Neuroimage. 2015;104:35–43. doi: 10.1016/j.neuroimage.2014.09.069. [DOI] [PubMed] [Google Scholar]
- 60.Ehrlich S, et al. Reduced functional connectivity in the thalamo-insular subnetwork in patients with acute anorexia nervosa. Hum. Brain Mapp. 2015;36:1772–1781. doi: 10.1002/hbm.22736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Goldberg II, Harel M, Malach R. When the brain loses its self: prefrontal inactivation during sensorimotor processing. Neuron. 2006;50:329–339. doi: 10.1016/j.neuron.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 62.Schultz W. Reward functions of the basal ganglia. J. Neural Transm. (Vienna). 2016;123:679–693. doi: 10.1007/s00702-016-1510-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Seeley WW, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen R, et al. Decision making deficits in relation to food cues influence obesity: a triadic neural model of problematic eating. Front Psychiatry. 2018;9:264. doi: 10.3389/fpsyt.2018.00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thoma V, Henson RN. Object representations in ventral and dorsal visual streams: fMRI repetition effects depend on attention and part-whole configuration. Neuroimage. 2011;57:513–525. doi: 10.1016/j.neuroimage.2011.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Devlin JT, Jamison HL, Gonnerman LM, Matthews PM. The role of the posterior fusiform gyrus in reading. J. Cogn. Neurosci. 2006;18:911–922. doi: 10.1162/jocn.2006.18.6.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pundik S, Scoco A, Skelly M, McCabe JP, Daly JJ. Greater cortical thickness is associated with enhanced sensory function after arm rehabilitation in chronic stroke. Neurorehabil Neural Repair. 2018;32:590–601. doi: 10.1177/1545968318778810. [DOI] [PubMed] [Google Scholar]
- 68.Margalit E, et al. The lateral occipital complex shows no net response to object familiarity. J. Vis. 2016;16:3. doi: 10.1167/16.11.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Firk C, et al. Implicit sequence learning in juvenile anorexia nervosa: neural mechanisms and the impact of starvation. J. Child Psychol. Psychiatry. 2015;56:1168–1176. doi: 10.1111/jcpp.12384. [DOI] [PubMed] [Google Scholar]
- 70.Hildebrandt T, et al. Evidence of prefrontal hyperactivation to food-cue reversal learning in adolescents with anorexia nervosa. Behav. Res Ther. 2018;111:36–43. doi: 10.1016/j.brat.2018.08.006. [DOI] [PubMed] [Google Scholar]
- 71.Gorrell S, Loeb K, Le Grange D. Family-based treatment of eating disorders: a narrative review. Psychiatr. Clin. North Am. 2019;42:193–204. doi: 10.1016/j.psc.2019.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Khalsa SS, Portnoff LC, McCurdy-McKinnon D, Feusner JD. What happens after treatment? a systematic review of relapse, remission, and recovery in anorexia nervosa. J. Eat. Disord. 2017;5:20. doi: 10.1186/s40337-017-0145-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Berends T, et al. Rate, timing and predictors of relapse in patients with anorexia nervosa following a relapse prevention program: a cohort study. BMC Psychiatry. 2016;16:316. doi: 10.1186/s12888-016-1019-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Legenbauer TM, Meule A. Challenges in the treatment of adolescent anorexia nervosa—is enhanced cognitive behavior therapy the answer? Front Psychiatry. 2015;6:148. doi: 10.3389/fpsyt.2015.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Thirion B, et al. Analysis of a large fMRI cohort: Statistical and methodological issues for group analyses. Neuroimage. 2007;35:105–120. doi: 10.1016/j.neuroimage.2006.11.054. [DOI] [PubMed] [Google Scholar]