Abstract
As a result of the research, the possibility of directional control of the operational properties of epoxy composites by the use of small additives of thermally expanded graphite-graphene structures has been proved. The rational content of the structuring additive in the composition of the epoxy composite (0.05 parts by mass.) was selected, which ensured an increase in the studied complex of physico-mechanical properties. The influence of thermally expanded graphite on the process of structure formation of an epoxy composite has been established. The addition of thermally expanded graphite increases thermal, fire and heat resistance as well as the coefficient of heat-conducting epoxy composite.
Subject terms: Chemical engineering, Graphene, Composites, Mechanical properties
Introduction
Currently, polymer composite materials which combine unique properties, high deformation and strength characteristics, and at the same time low specific weight are widely applied in industry1–3. The most common type of a binder in industry is various epoxy oligomers. The most important problem is the fragility of this type of material, so there are various types of system modifications2,3. The use of plasticizers and fillers provides necessary performance characteristics of composite materials. The addition of plasticizers provides the elasticity of polymeric materials and changes their glass transition temperature4. The addition of fillers allows to increase the mechanical and physicochemical characteristics of composites1–7. To provide polymer composite materials and their products with better operational characteristics, various modifying additives and fillers are used1–5. Mineral fine-dispersed fillers such as talc, basalt, and chromite are wide spread, however, the expected useful effect often exceeds the actual one observed in practice, which is due to the low adhesion of mineral materials to polymer matrices and their tendency to agglomerate. As nanofillers, various types of carbon nanotubes, fullerenes, graphenes, astralenes, technical carbons, as well as titanium and silicon dioxide, diamond batch, white carbon black, etc., are widely used8–14. Due to the high specific surface, thermal stability and excellent mechanical characteristics, carbon materials (graphite and graphene oxides, nanotubes, nanofibres) are promising for widespread use in lightweight polymer composites, providing them with enhanced performance characteristics14–21. Despite the enormous number of works devoted to the study of the effects of various modifying systems (including carbon), there are still insufficiently studied issues related to the influence of modifiers on the structure formation processes, the structure and performance characteristics of polymer composite materials, which predetermines the direction of the research of this work.
The main goal of this research is to enhance physicochemical and mechanical properties of epoxy composites using thermally expanded graphite obtained by thermal exfoliation of electrochemically oxidized graphite powder.
Materials and Research Methods
The compositions were developed on the basis of epoxy resin ED-20 (GOST 10587-93) because it has low viscosity, a narrow limit of the epoxy groups content, the stability of physico-chemical properties. As a hardener of epoxy oligomer, an amine type hardener was used - polyethylene polyamine (PEPA) (TS 6-02-594-85), capable of forming a three-dimensional network structure without heating.
For the plasticization of epoxy composites, oligo(resorcinophenyl phosphate) with terminal phenyl groups (ORPP) purity: 99% manufactured by ICL Industrial Products America Inc. (USA), was used in the work. Its chemical formula is shown in Supplementary Fig. S1.
ORPP – oligomeric halogen-free plasticizer with flame retardant properties. The choice of ORPP is due to the presence of combustion inhibitor – phosphorus (10,7%). During thermal decomposition of the composite, the presence of phosphorus provides an increase in the yield of carbonized structures, which are the physical barrier for the inter-diffusion of the oxidant and combustible gases to the combustion zone, which, in general, reduces the flammability of epoxy composite22.
The ratio of epoxy oligomer, plasticizer and hardener was previously determined experimentally: 100 parts by mass of ED-20, 40 parts by mass of ORPP and 15 parts by mass of PEPA22.
Thermally expanded graphite obtained by thermal exfoliation of electrochemically oxidized and hydrolyzed graphite was used as a structuring additive. Electrochemically oxidized graphite (OG) was obtained by electrochemical oxidation of natural dispersed graphite with a particle size of <160 μm in 58% HNO3 in the galvanostatic mode at the current I = 100 mA/g of graphite, with the capacity Q = 400 mA h/g23. At the next stage, the hydrolysis of the synthesized graphite nitrate was carried out: the reaction mixture was quickly diluted with cold distilled water (t = 15–18 °C) by stirring. Samples were kept in the water for 15 minutes. After dilution, the solid phase was filtered with the help of a Buchner funnel and washed with cold distilled water to pH = 5–7 for wash water. The specific consumption of water for the hydrolysis of graphite nitrate was 100 ml, and for the washing of the obtained oxidized graphite we used 500 ml of H2O per 1 g of the product. The drying of hydrolyzed graphite nitrate to constant weight was carried out in the drying oven at 105 ± 2 °C. The particle size of the solid phase in the aqueous suspension is in the range of 102–106 nm, the fraction of exhaust gas with a dimension of 102–103 nm in the suspension is 5–10%. The weighted fraction of the OG is separated from the main OG fraction by decanting. Thermal reduction and exfoliation of the oxidized graphite was carried out under static conditions for 5 seconds at 250 °C in the SNOL – 1.6.2.5.1/9 – I4 muffle furnace. The schematic of overall synthesis strategy of TEG was shown in Supplementary Fig. S2a.
In plasticized epoxy composition TEG was added as a modifying agent (0.01–1.0 parts by weight). To increase the uniformity of distribution and hinder the aggregation of TEG particles, as well as the activation of its surface and binder, ultrasonic treatment of the composition was used. The parameters of the ultrasound exposure: frequency −22 ± 2 kHz, power - 400 W, duration – 60 minutes4. The mixture was degassed at 25 ± 5 °C for 30 min under vacuum before curing. The preparation process of TEG/epoxy composites was shown in Supplementary Fig. S2b.
The research was carried out using the following methods:
determination of bending stress and flexural modulus [ISO 178: 2010],
determination of strength and modulus of tensile elasticity [ISO 527-2: 2012];
determination of compressive strength [ISO 604: 2002];
determination of impact strength [ISO 179-1: 2010];
determination of Brinell hardness [ISO 2039-1: 2001];
determination of heat resistance according to Vicat [ISO 306: 2004];
change in mass, rate of change in mass and magnitude of thermal effects during the heating of the samples was studied using the method of thermogravimetric analysis with the help of a derivator of the “Paulik – Paulik – Erdei” system of the MOM brand Q-1500D under the experimental conditions: weight - 100 mg, medium – air, heating interval - up to 800 °С, heating rate - 10 °С/min, relative error does not exceed 1%;
the study of the surface morphology of the samples was carried out using a Tescan VEGA 3 SBH scanning electron microscope;
determination of thermal conductivity and thermal resistance was carried out using the ITP-MG4 “100“instrument [ISO 22007-2: 2015];
FT-IR spectroscopy of TEG particles was carried out using the Shimadzu IRTracer-100;
X-ray phase analysis was performed using ARL X’TRA X-ray diffractometer;
determination of the curing kinetics of the epoxy composition was carried out according to the method described in24.
Experimental Results and Discussion
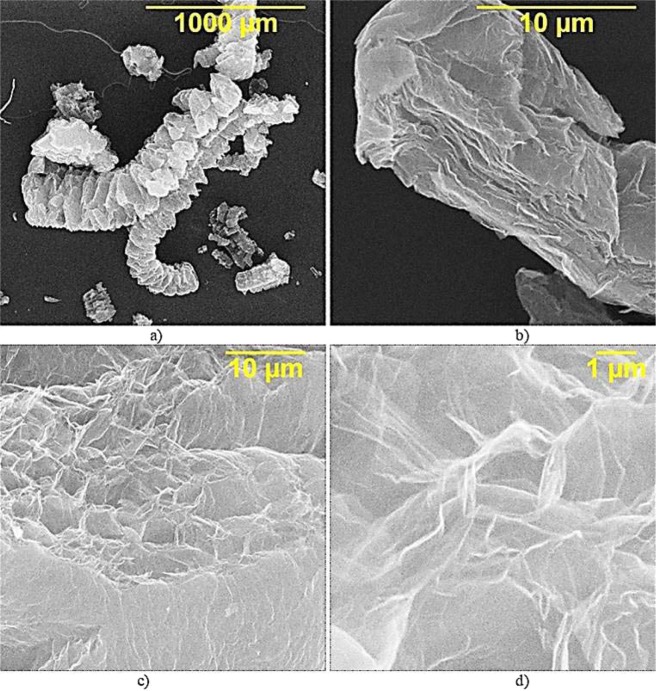
According to the data of scanning electron microscopy, during thermal expansion graphite transforms into a worm-like structure with an increased interlayer distance and a highly active, branching, uneven surface, Fig. 1a.
Figure 1.
SEM of TEG particles.
TEG particles have a nano-layered structure, the thickness of the packs of layers is about 100 nm, Fig. 1b, which makes it possible to speak about the formation of particles of a multilayer graphene oxide. The thin leaves of TRG form a complex open cellular microstructure with a pore size of 1–10 microns. In cross section, pores have a polygonal isometric or weakly extended form, Fig. 1c,d.
A series of signals on the IR-spectra confirm the presence of the form of oxidized graphene. This presence of hydroxyl groups between the graphene layers is the band between 2800 cm−1 and 3400 cm−1, Fig. 2. The peak at 1627 cm−1 is due to the presence of sp2-hybridization of C=C in the structure of graphene. The peak at ~2300 cm−1 corresponds to the peak of CO2 molecules absorbed by the TEG. The band between 1106 cm−1 and 1005 cm−1 corresponds to C-O-C (epoxy group). The band at 1384 cm−1 is the deformation vibration of the carboxyl group.
Figure 2.
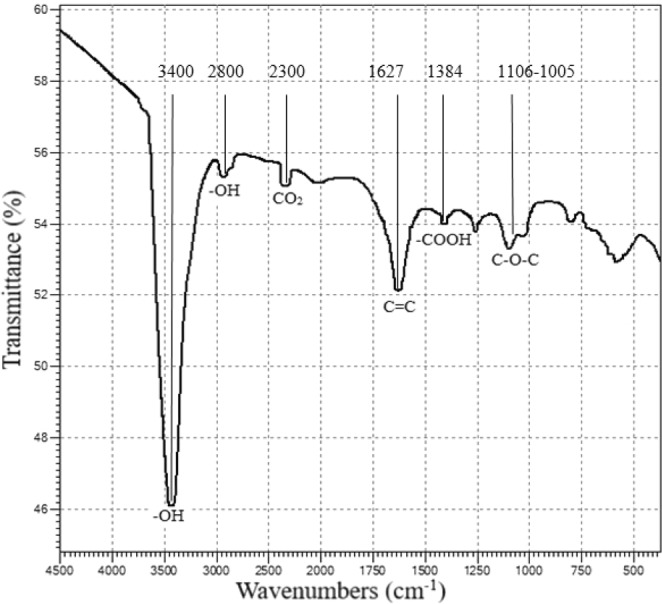
FT-IR spectroscopy of TEG.
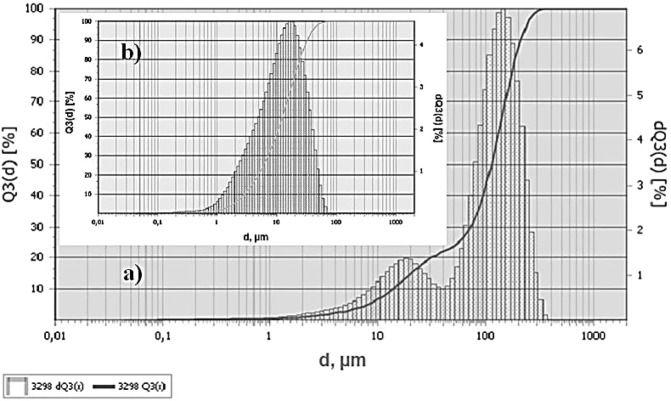
The fractional composition of TEG is characterized by the bimodal distribution of particles and is represented by particles from 1 to 400 μm, with a predominant number of particles with sizes of 15–20 μm and 140–160 μm, Fig. 3a.
Figure 3.
Fractional composition of TEG particles: (a) – without ultrasonic dispersion (b) – after ultrasonic dispersion.
An attempt to use TEG with a particle size of more than 100 µm as a reinforcing filler will lead to the opposite effect – a decrease in the indicators of physico-mechanical properties. Therefore, at the second stage, the method of liquid-phase separation of graphite in the media of the TCPP plasticizer was used to obtain a suspension of graphene particles. Dispersing was carried out using the UZDN-2T ultrasonic disperser with a radiator power of 400 W and a frequency of 22 kHz. Such a combined mechanochemical action led to additional exfoliation and breaking of particles with a large lateral size.
The fractional composition of TEG after ultrasonic dispersion is characterized by a monomodal distribution of particles and is represented by particles from 0.2 to 70 μm, with a predominance of particles with sizes of 10–30 μm, Fig. 3b.
Thus, the analysis of the TEG structure showed that it could be used as a structuring additive for epoxy composites, which should provide an increase in their operational properties.
As a polymer matrix we used previously developed composition consisting of 100 parts by mass of ED-20 epoxy resin, 40 parts by mass of ORPP and 15 parts by mass of a hardener – PEPA. ORPP performs simultaneously the functions of both a plasticizer and a flame retardant. The bending stress doubles and toughness increases by 2 times, and the flammability index - the oxygen index (OI) - increases from 19 to 28% by volume, which enables the material to become flame-retardant22.
TEG was added to the epoxy composition in the amount of 0.01–1.0 parts by mass.
The conducted studies have shown that the most rational content of TEG as a structuring additive, providing maximum values of physical and mechanical properties is 0.05 parts by mass, Figs 4–6, at the same time the bending failure stress increases by 48% and the bending elastic modulus increases by 41%, the compression strength increases by 20%, the tensile strength increases by 207% and the tensile elastic modulus increases by 24%, impact resilience increases by 300%.
Figure 5.
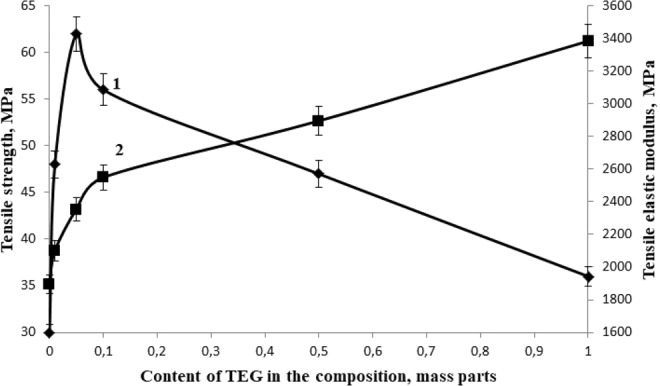
Dependence of tensile strength (1) and tensile elastic modulus (2) of epoxy composite on the TEG content in the composition.
Figure 4.

The dependence of the bending failure stress (1) and the bending elastic modulus (2) of the epoxy composite on the TEG content in the composition.
Figure 6.
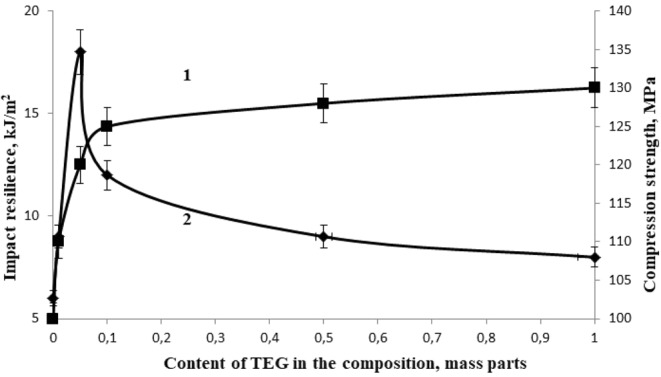
The dependence of compression strength (1) and impact resilience (2) of the epoxy composite on the TEG content in the composition.
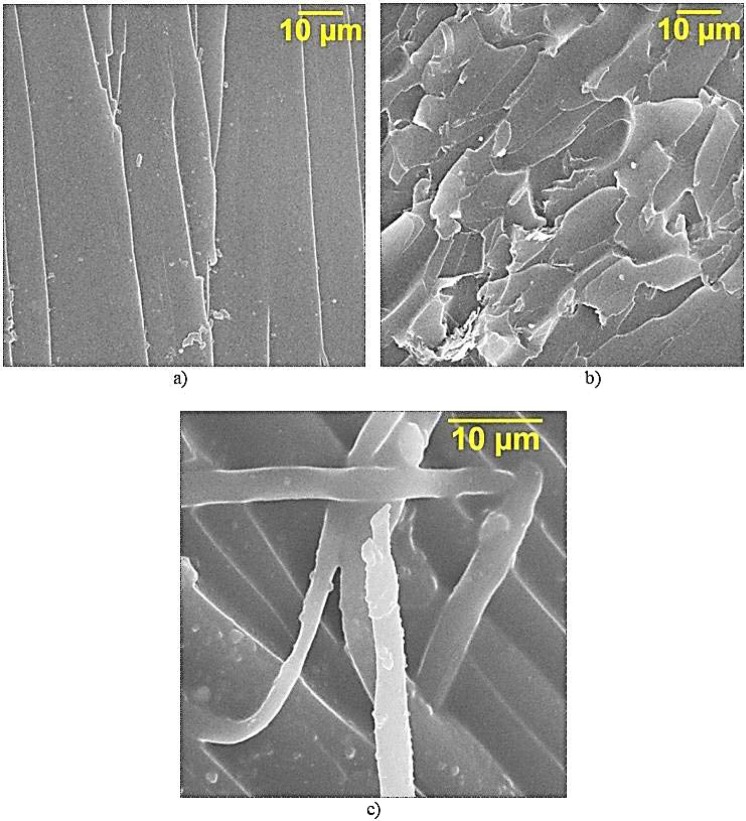
Fractography of the destruction of epoxy composite samples without TEG, Fig. 7a, is characterized by a rather smooth fracture surface, which indicates a low ability to crack resistance. The addition of TEG to the epoxy composition affects the morphology of the matrix — there appear layered structures formed by TEG particles, Fig. 7b. Besides the brittle fracture with the formation of numerous scales, there are local areas in the epoxy composite, indicating the flow of material in the process of its destruction. Moreover, in some places of plastic destruction, pronounced fibrous structures are observed, which are formed as a result of the intensive drawing out of the polymer matrix, Fig. 7c. The increase in plasticity of the epoxy composite can be explained if we consider TEG as a solid phase hardener25. In this case, a smaller number (compared to the volume of the composition) of crosslinks is formed in the border area of TEG and the epoxy composition, and, therefore, this area will have greater mobility.
Figure 7.
SEM of the surface of the destruction of epoxy composites.
When assessing the effect of the modifying additive on network polymers, it is necessary to take into account that the curing process takes place in the presence of a developed surface of the solid material (TEG), which can influence the kinetic characteristics of the polymerization reaction during curing, as well as the formation of the phase structure of the material. The role of the adsorption interaction of the components of the oligomeric composition with the solid surface of the TEG is also great24.
The study of the curing kinetics of epoxy compositions, Fig. 8, showed the inhibitory effect of TEG on the processes of the structure formation of the epoxy composite, which becomes evident in an increase in the duration of gelation processes from 25 to 31–33 minutes and curing from 35 to 45–47 minutes, Table 1.
Figure 8.
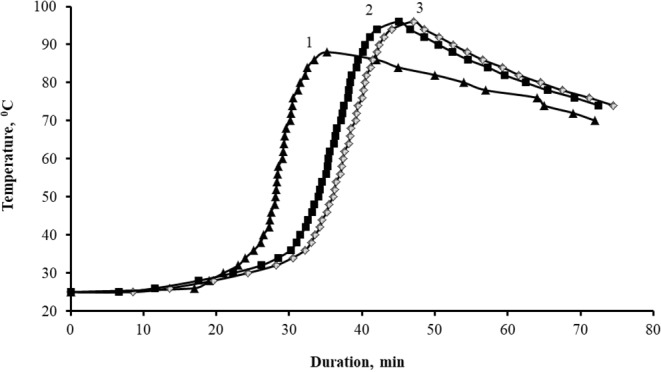
Kinetic curves of the curing process of compositions, parts by mass: 1 – 100ED-20 + 40ORPP + 15PEPA; 2 – 100ED-20 + 40ORPP + 0,05TEG + 15PEPA; 3 – 100ED-20 + 40ORPP + 1,0TEG + 15PEPA.
Table 1.
Values of the curing process of epoxy composites.
| Composition, parts by mass, cured by 15 parts by mass of PEPA | τгел, min | τотв, min | Tmax, °С |
|---|---|---|---|
| 100ED-20 + 40ORPP | 25 | 35 | 88 |
| 100ED-20 + 40ORPP + 0,05TEG | 31 | 45 | 96 |
| 100ED-20 + 40ORPP + 1,0TEG | 33 | 47 | 95 |
Note: τgel is the duration of gelation process, τcur is the duration of curing, Tmax is the maximum temperature of the self-heating of the sample during curing.
Figure 9 shows the data of thermogravimetric analysis of the unfilled composite and composites with the TEG additive. Epoxy composites containing TEG are characterized by better thermal stability in the temperature range of 100–600 °C compared to the unfilled composites, Table 2.
Figure 9.
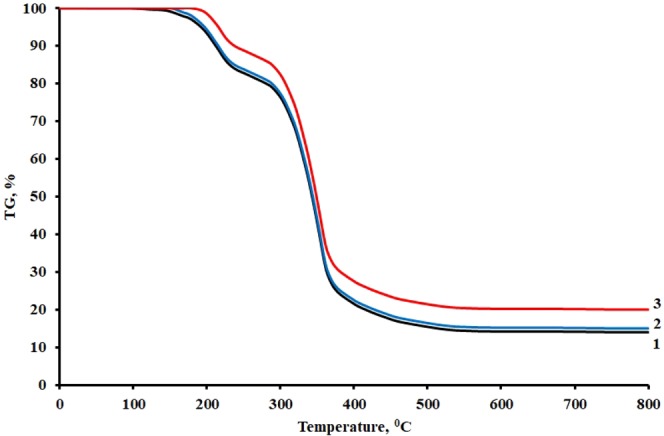
Data of thermogravimetric analysis of samples: 1 – 100ED-20 + 40ORPP + 15PEPA; 2 – 100ED-20 + 40ORPP + 0,05TEG + 15 PEPA; 3 – 100ED-20 + 40ORPP + 1,0TEG + 15 PEPA.
Table 2.
Data of thermogravimetric analysis of epoxy composites.
| Composition, parts by mass, cured by 15 parts by mass of PEPA | Weight loss, % at pyrolysis temperatures, °С | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 100 | 150 | 200 | 250 | 300 | 350 | 400 | 450 | 500 | 550 | 600 | |
| 100ED-20 + 40ORPP | 0 | 1,0 | 6,0 | 17,8 | 24,5 | 59,5 | 79,6 | 83,4 | 85,4 | 85,6 | 85,8 |
| 100ED-20 + 40ORPP + 0,05TEG | 0 | 0 | 5,1 | 16,9 | 23,7 | 57,5 | 78,4 | 82,4 | 84,1 | 84,5 | 86,6 |
| 100ED-20 + 40ORPP + 1,0TEG | 0 | 0 | 1,2 | 12,0 | 18,6 | 53,2 | 73,4 | 77,8 | 79,9 | 79,5 | 79,7 |
The addition of small amounts of TEG to an epoxy composite provides an increase in heat resistance according to Vicat from 132 to 165–182 °C, Table 3. Besides, the addition of TEG to the composition of the epoxy composite provides its increased fire resistance, which becomes evident in the reduction of mass loss during ignition in the air from 4.7 to 2.8%, and an increase in the flammability index - oxygen index from 28 to 33% by volume. The developed compounds, modified by TEG, do not support combustion in the air and belong to the class of flame-resistant materials, Table 3.
Table 3.
Physico-chemical properties of epoxy composites.
| Composition, parts by mass, cured by 15 parts by mass of PEPA | ТV, °С | Δm, % | OI, vol. % |
|---|---|---|---|
| 100ЭД−20 + 40ORPP | 132 | 4,7 | 28,0 |
| 100ЭД−20 + 40ORPP + 0,05TEG | 165 | 4,1 | 28,5 |
| 100ЭД−20 + 40ORPP + 0,5TEG | 171 | 3,6 | 31,0 |
| 100ЭД−20 + 40ORPP + 1,0TEG | 182 | 2,8 | 33,0 |
Note: TV – Vicat heat resistance; Δm – is the mass loss during ignition in the air; OI – oxygen index.
Thermal conductivity of compounds used in electrical and electronic equipment is an important characteristic. In most cases, epoxy resins have a relatively low thermal conductivity of ~0.1 W/m · K. Consequently, if there is local heating, epoxy materials work as thermal insulation, which requires the use of components with higher heat resistance or the use of special heat sinks to dissipate heat, otherwise it can lead to overheating and thermal decomposition of the composite16.
The addition of even small amounts of TEG to the composition of the epoxy composite increases the coefficient of thermal conductivity by 2.6–4.2 times, while thermal resistance decreases, Fig. 10.
Figure 10.

The effect of TEG on the thermal conductivity and trermal resistance of epoxy compositions.
Conclusion
As a result of the conducted studies, the possibility of directional control of the operational properties of epoxy composites with the introduction of small TEG additives into the composition, ensuring the creation of high performance epoxy composites, has been proved.
The rational content of TEG was selected as a structuring additive in the composition of the epoxy composite (0.05 parts by mass), which provides an increase in the studied complex of physical and mechanical properties. The influence of thermally expanded graphite on the process of structure formation of an epoxy composite has been established. The addition of thermally expanded graphite increases thermal, fire and heat resistance as well as the coefficient of heat-conducting epoxy composite. The developed compounds, modified by TEG, do not support combustion in the air and belong to the class of flame-resistant materials.
Supplementary information
Acknowledgements
The reported study was funded by RFBR according to the research project No. 18-29-19048.
Author contributions
Yakovlev A.V. synthesized of thermally expanded graphite-graphene structures and investigated their properties. Mostovoy A.S. developed of epoxy composites compositions modified with thermally expanded graphite-graphene structures, and conducted a study of their physicochemical and mechanical characteristics. All authors participated in the discussion of the results and the writing of the text of the article.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
A. S. Mostovoy, Email: Mostovoy19@rambler.ru
A. V. Yakovlev, Email: aw_71@mail.ru
Supplementary information
is available for this paper at 10.1038/s41598-019-52751-z.
References
- 1.Burmistrov I, et al. Mechanical and electrical properties of ethylene-1-octene and polypropylene composites filled with carbon nanotubes. Compos. Sci. Technol. 2017;147:71–77. doi: 10.1016/j.compscitech.2017.05.005. [DOI] [Google Scholar]
- 2.Amirbeygi H, Khosravi H, Tohidlou E. Reinforcing effects of aminosilane-functionalized graphene on the tribological and mechanical behaviors of epoxy nanocomposites. J. Appl. Polym. Sci. 2019;136:47410. doi: 10.1002/app.47410. [DOI] [Google Scholar]
- 3.Radoman TS, et al. Improvement of epoxy resin properties by incorporation of TiO2 nanoparticles surface modified with gallic acid esters. Mater. Des. 2014;62:158–167. doi: 10.1016/j.matdes.2014.05.015. [DOI] [Google Scholar]
- 4.Mostovoi AS, Yakovlev EA, Burmistrov IN, Panova LG. Use of Modified Nanoparticles of Potassium Polytitanate and Physical Methods of Modification of Epoxy Compositions for Improving Their Operational Properties. Russ. J. Appl. Chem. 2015;88:129–137. doi: 10.1134/S107042721501019X. [DOI] [Google Scholar]
- 5.Hu JH, et al. Flame retardant, mechanical properties and curing kinetics of DOPO-based epoxy resins. Polym. Degrad. Stab. 2014;109:218–225. doi: 10.1016/j.polymdegradstab.2014.07.026. [DOI] [Google Scholar]
- 6.Mukbaniani O, et al. Effect of modification of bentonite by tetraethoxysilane on the properties of composites based on epoxy resin. Oxid. Commun. 2010;33:555–560. [Google Scholar]
- 7.Mostovoy AS, Kadykova YA, Bekeshev AZ, Tastanova LK. Epoxy composites modified with microfibers of potassium polytitanates. J. Appl. Polym. Sci. 2018;135:46651. doi: 10.1002/app.46651. [DOI] [Google Scholar]
- 8.Vahedi H, Pasbakhsh P. Instrumented impact properties and fracture behaviour of epoxy/modified halloysite nanocomposites. Polym. Test. 2014;39:101–114. doi: 10.1016/j.polymertesting.2014.07.017. [DOI] [Google Scholar]
- 9.Syugaev AV, Maratkanova AN, Shakov AA, Nelyubov AV, Lomayeva SF. Surface modification of iron particles with polystyrene and surfactants under high-energy ball milling. Surf. Coat. Technol. 2013;236:429–437. doi: 10.1016/j.surfcoat.2013.10.030. [DOI] [Google Scholar]
- 10.Wong Т-Т, et al. Effects of silane surfactant on Nano-ZnO and rheology properties of nano-ZnO/epoxy on the UV absorbability of nano-ZnO/epoxy/micron-HGF composite. Composites Part B. 2016;90:378–385. doi: 10.1016/j.compositesb.2016.01.005. [DOI] [Google Scholar]
- 11.Huang M-H, et al. Self-transformation of ultra-small gold nanoclusters to gold nanocrystals toward boosted photoreduction catalysis. Chem. Commun. 2019;55:10591–10594. doi: 10.1039/C9CC04562G. [DOI] [PubMed] [Google Scholar]
- 12.Zhang J, Xiao F-X. Modulation of interfacial charge transfer by self-assembly of single-layer graphene enwrapped one-dimensional semiconductors toward photoredox catalysis. J. Mater. Chem. A. 2017;5:23681–23693. doi: 10.1039/C7TA08415C. [DOI] [Google Scholar]
- 13.Han C, Zhang N, Xu Y-J. Structural diversity of graphene materials and their multifarious roles in heterogeneous photocatalysis. Nano Today. 2016;11:351–372. doi: 10.1016/j.nantod.2016.05.008. [DOI] [Google Scholar]
- 14.Zhang N, et al. Waltzing with the Versatile Platform of Graphene to Synthesize Composite Photocatalysts. Chem. Rev. 2015;115:10307–10377. doi: 10.1021/acs.chemrev.5b00267. [DOI] [PubMed] [Google Scholar]
- 15.Wang X, Song L, Pornwannchai W, Hu Y, Kandola B. Composites Part A. 2013;53:88–96. doi: 10.1016/j.compositesa.2013.05.017. [DOI] [Google Scholar]
- 16.Kharitonov AP, et al. Reinforcement of Bisphenol-F epoxy resin composites with fluorinated carbon nanotubes. Composites Science and Technology. Compos. Sci. Technol. 2016;134:161–167. doi: 10.1016/j.compscitech.2016.08.017. [DOI] [Google Scholar]
- 17.Buketov АV, et al. Impact toughness of nanocomposite materials filled with fullerene С60 particles. Composites: Mechanics, Computations, Applications. 2018;9:141–161. [Google Scholar]
- 18.Li S, Yang Z, Xu J, Xie J. Montmorillonite–Graphene Oxide Hybrids and Montmorillonite–Graphene Oxide/Epoxy Composite: Synthesis, Characterization, and Properties. Polym. Compos. 2018;39:E2084–E2095. doi: 10.1002/pc.24461. [DOI] [Google Scholar]
- 19.Edwards RS, Coleman KS. Graphene Synthesis: Relationship to Applications. Nanoscale. 2013;5:38–51. doi: 10.1039/C2NR32629A. [DOI] [PubMed] [Google Scholar]
- 20.Avouris P, Dimitrakopoulos C. Graphene: Synthesis and Applications. Mater. Today. 2012;15:86–97. doi: 10.1016/S1369-7021(12)70044-5. [DOI] [Google Scholar]
- 21.Ghorbani M, Abdizadeh H, Golobostanfard MR. Reduction of Graphene Oxide via Modified Hydrothermal Method. Procedia Materials Science. 2015;11:326–330. doi: 10.1016/j.mspro.2015.11.104. [DOI] [Google Scholar]
- 22.Mostovoy AS, Nurtazina AS, Kadykova YA, Bekeshev AZ. Highly Efficient Plasticizers-Antipirenes for Epoxy Polymers. Inorganic Materials: Applied Research. 2019;10:1135–1139. doi: 10.1134/S2075113319050228. [DOI] [Google Scholar]
- 23.Yakovlev AV, Zabud’kov SL, Yakovleva EV, Finaenov AI. A study of the possibility of anodic oxidation of suspensions formed by dispersed graphite and nitric acid. Russ. J. Appl. Chem. 2006;79:1600–1604. doi: 10.1134/S1070427206100077. [DOI] [Google Scholar]
- 24.Mostovoy AS, Nurtazina AS, Burmistrov IN, Kadykova YA. Effect of Finely Dispersed Chromite on the Physicochemical and Mechanical Properties of Modifi ed Epoxy Composites. Russ. J. Appl. Chem. 2018;91:1758–1766. doi: 10.1134/S1070427218110046. [DOI] [Google Scholar]
- 25.Kablov EN, Kondrashov SV, Yurkov G. Yu. Prospects for the use of carbon-containing nanoparticles in binders for polymer composites. Russian nanotechnology. 2012;8:24–42. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.