Abstract
We investigated gait performance utilizing a quantitative gait analysis for 2 groups: (1) idiopathic normal-pressure hydrocephalus (INPH) patients who had a positive response to the cerebrospinal fluid tap test (CSFTT) and (2) healthy controls. The aims of the study were (1) to analyze the characteristics of gait features, (2) to characterize changes in gait parameters before and after the CSFTT, and (3) to determine whether there was any relationship between stride time and stride length variability and Frontal Assessment Battery (FAB) scores in INPH patients. Twenty-three INPH patients and 17 healthy controls were included in this study. Compared with healthy controls, the gait of INPH patients was characterized by lower velocity, shorter stride length, and more broad-based gait. Patients with INPH had a longer stance phase with increased double-limb support. Variability in stride time and stride length was increased in INPH patients. Stride time and stride length variability were correlated with FAB score. After the CSFTT, gait velocity, stride length, and step width significantly improved. There were significant decreases in stride time and stride length variability. These results suggest that the CSFTT for INPH patients might improve the so-called balance-related gait parameter (ie, step width) as well. Stride time and stride length variability also responded to the CSFTT. Association between FAB scores and both stride time and stride length variability suggests involvement of similar circuits producing gait variability and frontal lobe functions in INPH patients.
Subject terms: Diagnostic markers, Hydrocephalus, Neurodegenerative diseases
Introduction
Idiopathic normal-pressure hydrocephalus (INPH) is an uncommon neurological disorder. Of 563 autopsy cases showing neuropathology of dementia, only 9 (1.6%) cases were suspected as INPH1. Despite a low incidence, the diagnosis of INPH is important because INPH is considered a potentially treatable neurological disorder2. INPH is an adult-onset syndrome of uncertain origin that involves nonobstructive enlargement of the cerebral ventricles, and is characterized by symptoms of gait disturbance, cognitive impairment, and urinary dysfunction3. Although INPH patients present with each of these classic clinical symptoms to varying degrees, the most common and important clinical feature of INPH is gait disturbance4.
The cerebrospinal fluid tap test (CSFTT) is considered valuable for predicting shunt effectiveness in INPH patients5. The CSFTT has a high positive predictive value for successful shunt surgery6. In accordance with the Japanese guideline, clinical improvement after the CSFTT increases diagnostic certainty of INPH from possible to probable6.
The GAITRite gait analysis system employs a portable walkway embedded with pressure sensors that detect footfalls as the subject walks the length of the mat7. The software enables the documentation of a wide range of temporo-spatial gait parameters, including walking speed, cadence, stride length, step width, and foot placement angles7. Its validity and reliability have been proved in various patient populations8–10. A few studies on gait changes after the CSFTT have used clinical gait scores rather than quantitative gait analysis procedures11,12, resulting in a limited interpretation of their findings. These studies suggest that equilibrium-related symptoms can be improved11,12. But, there have been 2 quantitative studies of 10 and 11 INPH patients, respectively, which showed balance-related parameters remained unaffected after the CSFTT13,14. However, those were small studies, and further quantitative evidence is needed.
Recent studies have hypothesized an association between INPH and frontal lobe dysfunction. For example, previous reports on INPH brain perfusion patterns have shown diffuse or frontal-focused blood flow reduction in the brain15,16. The pathogenesis of gait disturbance in INPH patients is not well understood11. However, it has been hypothesized that INPH gait may be caused by frontal dysfunction17. Stride time and stride length variability are both parameters that are related to the control of the rhythmic stepping mechanism18–21. Impairment in the ability to maintain a steady gait, with minimal stride-to-stride variations, has been known to be closely related to postural instability and fall risk22. The Frontal Assessment Battery (FAB) has been known as a short bedside cognitive and behavioral test to assess frontal lobe functions23. However, there has been no study to date on any association between FAB and stride time and stride length variability.
In this study, we investigated gait performance utilizing a quantitative gait analysis in 2 groups: (1) INPH patients who had a positive response to the CSFTT and (2) healthy controls. The aims of the study were (1) to analyze the characteristics of gait features, (2) to characterize changes in gait parameters before and after the CSFTT, and (3) to determine whether there was any relationship between stride time and stride length variability and FAB scores in INPH patients.
Methods
Participants
Patients in the study were prospectively recruited from patients at the Center for Neurodegenerative Diseases of Kyungpook National University Chilgok Hospital, South Korea between August 2017 to July 2018. All study participants gave informed and written consent for study, including information related to clinical data and MRI. Each patient also consented to having a CSFTT. This study protocol was approved by the Institutional Review Board of Kyungpook National University Chilgok Hospital. All methods and procedures were performed in accordance with relevant guidelines and regulations. INPH diagnosis was made using criteria proposed by Relkin et al.24. Patients had to be older than 40 years of age with an insidious progression of 6 months or more of INPH symptoms (gait disturbance plus at least 1 other area of impairment in either cognition, urinary symptoms, or both) and have normal CSF opening pressure. Brain MRI showed widening of the ventricles (Evans’ ratio >0.3) for all patients and no macroscopic obstruction of CSF flow. Exclusion criteria included patients with stroke, a recent history of heavy alcohol use, a history of hospitalization for a major psychiatric disorder, or a history of other neurological, metabolic, or neoplastic disorders that might produce dementia symptoms or parkinsonism. No patient in the study showed evidence of head trauma, intracerebral hemorrhage, meningitis, or another known cause of secondary hydrocephalus.
Criteria for healthy control categorization were as follows: no active neurological, systemic, or psychiatric disorders; normal neurological status in examination; and the ability to function independently. Global cognition of healthy controls was assessed by the Korean-Mini Mental State Examination (K-MMSE). Healthy individuals older than 70 years of age also had cranial MRI to exclude any intracranial abnormalities.
Assessing illness severity
Comprehensive clinical scales for all INPH patients in the study were determined in the following manner. Patients’ dementia severity and general cognition were evaluated with the K-MMSE and Clinical Dementia Rating Scale (CDR)25,26. The FAB was used to assess frontal lobe symptoms23. Total FAB score ranged from 0 to 18, with a higher score meaning a better performance. The INPHGS was used to assess the severity of each main symptom of INPH (cognitive impairment, gait disturbance, and urinary disturbance) following an unstructured interview with patients and caregivers27. The score for each symptom ranges from 0 to 4. Grade 0 indicates normal; grade 1 indicates subjective symptoms but no objective disturbance; grades 2, 3 and 4 indicate mild, moderate, and severe disturbance, respectively. Assessment of gait included timed performance results on the Timed Up and Go (TUG) test and 10 meter walking test27–30. The TUG test measures the time it takes a patient sitting in a chair to stand up, walk forward 3 meters, and return to a seated position. Gait disturbance features related to INPH were determined using the Gait Status Scale (GSS)27. This scale focuses on 8 factors related to gait disturbance: (1) postural stability; (2) independence in walking; (3) wide base gait; (4) lateral sway; (5) petit-pas gait; (6) festinating gait; (7) gait freezing; and (8) disturbed tandem walking. A total GSS score of the 8 items, ranging from 0 to 16, was determined. A higher score reflected more severe symptoms. Most of these scales were not determined for healthy controls, as selection for healthy controls required a normal neurological examination.
Cerebrospinal fluid tap test
A lumbar tap removing 30–50 ml of CSF was done for all INPH patients. After the CSFTT, patients were evaluated again with the INPHGS, which is a validated scale for measuring INPH symptom severity, and the TUG test. Gait changes were evaluated 1 day after the CSFTT, and cognition and urination changes were evaluated at 1 week31. CSFTT responses were determined with these scales. Responders were identified using the following criteria: greater than 10% improvement in time on the TUG test or improvement of 1 point or more on the INPHGS6,31.
Quantitative gait assessment
A computer-based, 5.8-m-long, pressure-sensitive carpet system (GAITRite, CIR System, Havertown, PA) with a sampling rate of 120 Hz was used to asses gait. Temporal and spatial gait cycle parameters related to this study were recorded. All participants were told to walk barefoot at a comfortable and self-selected speed without the use of any walking aid or a cane. The process was repeated 4 times to obtain sufficient data for analysis, and the mean values obtained from walking 4 times were used in the final analysis. To prevent effects related to acceleration and deceleration, participants started walking 1 m before reaching the active area of electronic walkway and completed their walk 1 m beyond it. Each patient was given time to rest when requested between walking trials to avoid fatigue. Patients always had a researcher walking alongside as a safeguard. Spatiotemporal gait parameters were measured using the GAITRite system as follows: gait velocity, cadence, stride length, step width, toe in/out angle, stride time, stance phase (%), and swing phase (%). The coefficient of variation (CV) for stride time and stride length were calculated as follows: SD of parameter × 100/mean of parameter. Each INPH patient was analyzed twice, once before the CSFTT and once 1 day after the CSFTT.
Statistical analyses
The IBM SPSS Statistics for Windows version 25.0.0 was used for analyses of data. The demographic data were compared between the INPH and control groups. Fisher’s exact and chi-square tests were used to compare categorical variables, while the Student t tests and Mann-Whitney U tests were used to compare continuous variables. The changes in quantitative gait parameters before and after the CSFTT were analyzed using the repeated-measures analysis of variance. Pearson’s or Spearman’s correlations were employed to investigate the relationship between stride time and stride length variability and FAB scores in INPH patients. Statistical significance was set at P < 0.05.
Results
Table 1 lists the demographic and clinical features for INPH and control subjects. There were no significant differences in the distributions of age and gender between the 2 groups. Patients with INPH had significantly lower K-MMSE scores than the control subjects.
Table 1.
Demographic data and clinical characteristics of INPH patients and controls at baseline.
| Characteristics | INPH (n = 23) | Healthy control (n = 17) | P value |
|---|---|---|---|
| Gender, male | 11 (47.8) | 4 (23.5) | 0.117 |
| Age (year) | 73.0 ± 7.0 | 69.0 ± 5.1 | 0.052 |
| Education (year) | 7.9 ± 4.7 | 11.3 ± 4.4 | 0.025 |
| Duration of symptoms (year) | 2.4 ± 1.1 | ||
| K-MMSE | 19.5 ± 5.0 | 29.2 ± 1.1 | <0.001 |
| CDR (0:0.5:1:2:3) | 0:10:10:3:0 | ||
| INPHGS | |||
| GS-Gait | 1.0 ± 0.0 | ||
| GS-Cogn | 2.1 ± 0.5 | ||
| GS-Urin | 1.9 ± 0.7 | ||
| TUG | 22.2 ± 25.2 | ||
| 10-meter walking test | 18.4 ± 15.7 | ||
| GSS | 6.9 ± 3.6 | ||
| FAB | 9.0 ± 3.3 | ||
| Drainage volume of CSF | 33.3 ± 3.5 | ||
| CSF opening pressure (cm H2O) | 10.0 ± 3.3 | ||
| Evans’ ratio | 0.32 ± 0.02 | ||
For INPH patients, data were collected before the CSFTT. Values denote number (%) or mean ± standard deviation. INPH = idiopathic normal-pressure hydrocephalus; CSFTT = cerebrospinal fluid tap test; K-MMSE = Korean version of Mini-Mental State Examination; CDR = Clinical Dementia Rating Scale; INPHGS = Idiopathic Normal-Pressure Hydrocephalus Grading Scale; GS-Gait = INPHGS for gait; GS-Cogn = INPHGS for cognition; GS-Urin = INPHGS for urinary function; TUG = Timed Up-and-Go test; GSS = Gait Status Scale; FAB = Frontal Assessment Battery.
Differences in gait parameters between patients with INPH and healthy controls
Most gait parameters differed significantly between healthy controls and patients with INPH (Table 2). Compared with healthy controls, the gait of patients with INPH was characterized by a lower velocity (P < 0.001) and shorter stride length (P < 0.001). INPH patients had significantly higher step width than the control subjects (P < 0.001). The toe-out angle was also increased in the INPH group relative to the control group (P < 0.01). And, patients with INPH showed a longer stance phase with decreased swing phase than control subjects (P < 0.001). Variability in stride time and stride length was increased in INPH patients compared to control subjects (P < 0.05 for the CV of stride time and P < 0.001 for the CV of stride length).
Table 2.
Gait parameters in healthy controls and patients with INPH.
| Healthy controls | Patients with INPH | ||
|---|---|---|---|
| Before CSF tap | 24 hours after tap | ||
| Velocity, cm/s | 99.12 ± 10.37 | 55.12 ± 4.81c | 67.84 ± 5.01e |
| Cadence, steps/min | 110.16 ± 8.81 | 105.33 ± 3.56 | 112.99 ± 3.40d |
| Stride length, cm | 109.12 ± 4.74 | 62.76 ± 5.14c | 72.38 ± 5.11d |
| Step width, cm | 7.99 ± 0.75 | 13.57 ± 0.57c | 12.90 ± 0.60d |
| Toe in/out, ° | 8.69 ± 1.61 | 15.28 ± 1.65b | 14.57 ± 1.51 |
| Stride time, s | 1.09 ± 0.04 | 1.17 ± 0.04 | 1.09 ± 0.04d |
| Stance phase, % | 62.79 ± 0.69 | 67.81 ± 0.59c | 67.28 ± 0.93 |
| Swing phase, % | 37.25 ± 0.69 | 32.19 ± 0.59c | 32.71 ± 0.93 |
| Double-limb support phase, % | 25.61 ± 1.56 | 36.49 ± 1.34c | 34.81 ± 1.90 |
| CV of stride time, % | 4.34 ± 2.04 | 10.47 ± 2.27a | 6.05 ± 0.79d |
| CV of stride length, % | 5.10 ± 1.78 | 14.35 ± 1.98c | 9.44 ± 0.82e |
Values denote mean ± standard deviation. INPH = idiopathic normal-pressure hydrocephalus; CSF = cerebrospinal fluid; CV = coefficient of variability.
aMean differs from healthy controls, p < 0.05.
bMean differs from healthy controls, p < 0.01.
cMean differs from healthy controls, p < 0.001.
dMean differs from baseline, p < 0.05.
eMean differs from baseline, p < 0.01.
Gait parameters in patients with INPH before and after the CSFTT
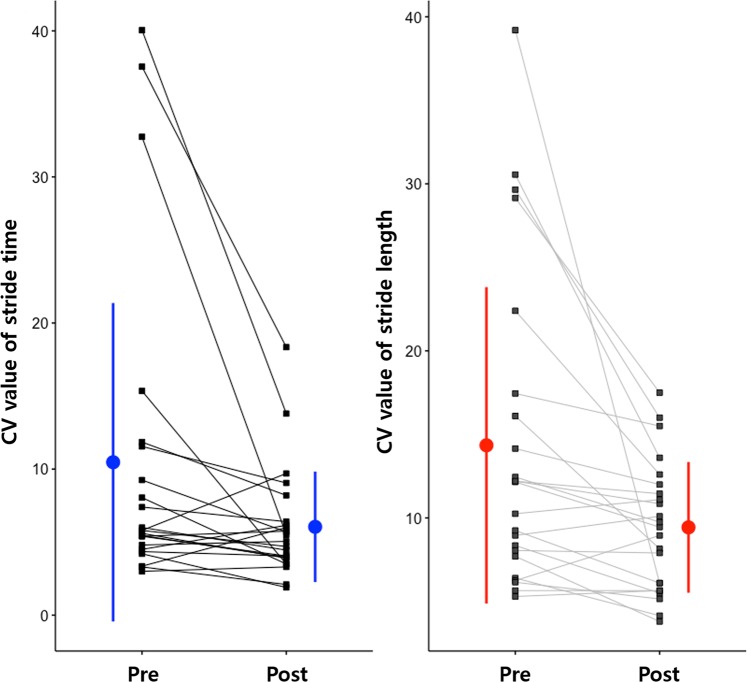
Differences in the gait parameters before and 24 hours after the CSFTT are shown in Table 2. Gait velocity and stride length improved significantly (P < 0.01 for the gait velocity and P < 0.05 for the stride length). Step width also improved significantly (P < 0.05). Cadence increased significantly (P < 0.05). Stride time decreased significantly (P < 0.05). Variability in stride time and stride length was improved significantly (P < 0.05 for the CV of stride time and P < 0.01 for the CV of stride length) (Fig. 1).
Figure 1.
Differences in CV values of stride time and stride length before and after the CSFTT for INPH patients. The mean CV values for each individual before and after the CSFTT are depicted. Filled circles show means and standard deviations.
Correlations between FAB Scores and gait variability in INPH
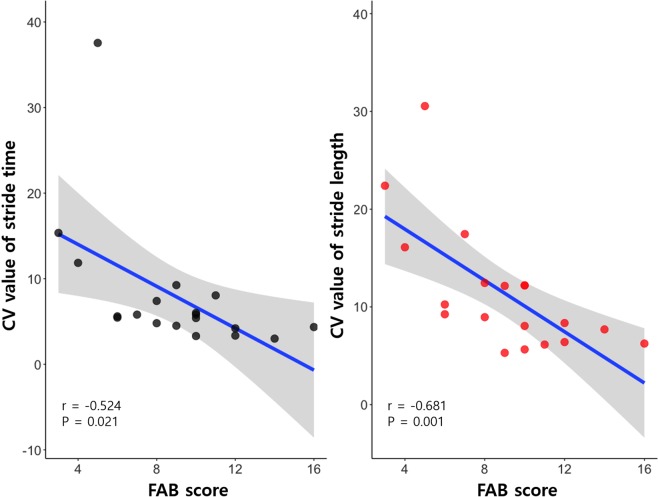
The FAB scores were negatively correlated with the CV value of stride time (r = −0.524; P = 0.021) and CV value of stride length (r = −0.681; P = 0.001) (Fig. 2).
Figure 2.
Scatterplots illustrating the relationships between stride time and stride length variability and FAB scores at baseline for INPH patients.
Discussion
Compared with age- and gender-matched healthy controls, the gait of patients with INPH was characterized by lower velocity, shorter stride length, and more broad-based gait. Further, patients with INPH had a longer stance phase with increased double-limb support in comparison to control subjects. Gait variability was also significantly higher in the INPH group relative to the control group.
A loss of consistency in the ability to produce a steady gait rhythm, resulting in higher stride-to-stride variability, has been related to balance impairments leading to falls21,22. It was reported that increased stride-to-stride variability in stride time and stride length was significantly associated with a high risk for falling in community dwelling older adults19,32. Falls are also important clinical problems in patients with INPH33. It was reported that more than half of the patients with INPH (56%) experienced falls33. We found that stride time and stride length variability were increased in the INPH group. We cautiously suggest that increased gait variability is one of the main risk factors for falls in INPH patients.
Previously unreported, our data showed that stride time and stride length variability were correlated with FAB score. Although the origin of the gait variability in INPH is not totally understood, associations between gait variability measurements and performance on the FAB suggest potentially overlapping processes involved in these functions. Several findings in the literature support these new results for INPH patients. In Alzheimer’s disease, reduction in mean regional cerebral blood flow in the prefrontal cortex was associated with increased stride-to-stride variability34,35. Further, some limited evidence in neurodegenerative diseases suggested that the prefrontal cortex was associated with gait variability35. Our results bear further connection to previous studies on INPH patients. For example, many previous reports on cerebral perfusion patterns in INPH patients point out diffuse or frontal-dominant reduction in cerebral blood flow15,16; in addition, frontal hypoperfusion and frontal subcortical white matter disintegration also have been associated with symptoms in INPH patients including urinary incontinence and gait disturbance15,36,37. Moreover, a previous study reported that the total FAB score was correlated with brain single photon emission CT (SPECT) perfusion in the prefrontal cortex independently of age, gender, and MMSE38. The study suggested further that the FAB might be useful for evaluating diseases correlated with frontal dysfunction38.
Generally, lower body parkinsonism is characteristic in INPH39. The aberrant ambulation observed in INPH is characterized by a slow, wide-based gait, and short shuffling steps40. Dysfunction of basal ganglia circuitry is known to be mainly responsible for the development of the cardinal features of Parkinson’s disease41,42. Ventricular enlargement may interrupt the cortical-subcortical basal ganglia loop, which connects the frontal cortex and basal ganglia, thus resulting in parkinsonism such as bradykinesia and short-stepped gait43,44. Considering the connection between cerebral perfusion (also referred to as cerebral blood flow) and brain function4,45, and the fact that significant reductions in mean cerebral blood flow of the basal ganglia and the thalamus were found in INPH patients compared with controls4, this may also explain the slowed, short-stepped gait observed in our patients.
Both the step width and the foot angle have been generally considered as balance-related gait parameters13,14. It is believed that phenomena such as enlarged step width and outward rotated feet can be interpreted as a protective strategy to stabilize gait13,14. Cerebellar circuits are well known to be involved in controlling balance46. It was also suggested that hydrocephalus may directly compress and therefore impede frontopontocerebellar fibers as they descend close to the lateral ventricle43. Considering the fact that a significant reduction in mean cerebral blood flow of the cerebellum was also found for INPH patients compared with controls4, our finding showing a broad-based gait pattern with outward rotated feet in INPH patients is not surprising. In our study, patients with INPH may also increase the stance phase and the double-support period to stabilize their inefficient gait control. It has been suggested that both the stance phase and double-limb support are stabilizing factors during normal gait in the elderly47.
The CSFTT is usually thought of as an acute treatment for INPH13. It has been suggested that CSF movement is not a CSF circulation from the brain ventricles along the entire CSF system to its absorption site in the cortical subarachnoid space, but a permanent rhythmic systolic-diastolic CSF pulsation in all directions along all CSF spaces48. CSF production and absorption (CSF exchange) might be constant and present everywhere in the CSF system49. Removing 30–50 ml CSF from the lumbar CSF space, as in the CSFTT, may create for a certain period a situation identical to the definitive ventricular shunt operation5. Further, the clinical parameters that improve during the CSFTT can be very specific to INPH13. Interestingly, our INPH patients showed significant improvements in various gait parameters (especially in the gait velocity, stride length, and step width). At the same time, there were significant decreases in the stride time and stride length variability in our study after CSF removal. Although certain gait parameters, such as the gait velocity and stride length, also improved after the CSFTT in INPH13, no previous study has analyzed changes in both stride time and stride length variability after CSF removal. Our results bear further connection to previous studies on INPH patients. For example, the cerebral blood flow in INPH shunt-responders increased postoperatively in the periventricular white matter and the caudate head, a part of the basal ganglia50. Additionally, after shunt surgery in INPH patients, local cerebral blood flow increased toward normal, particularly in frontal white matter and basal ganglia51. It was suggested that motor function recovery in INPH patients after CSF removal was related to a reversible suppression of frontal periventricular cortico-basal ganglia-thalamo-cortical circuits52.
INPH patients were selected consecutively from our prospectively enrolled INPH registry. We tried to minimize any bias related to evaluation before and after the CSFTT by using various objective grading scales. The first limitation of this study is that INPH patients with a negative response to the CSFTT were not included. However, the motivation for this was to enhance diagnostic certainty of INPH by restricting our study to CSFTT responders. In addition, INPH patients that were CSFTT non-responders were generally more likely to have other cerebral comorbidities, which could affect the analysis53. Our findings encourage future studies with larger study populations, including both CSFTT responders and non-responders, and quantitative gait parameters to investigate the possibility to utilize a quantitative gait analysis as a neurophysiological biomarker to predict CSFTT response. A second limitation was that we did not investigate quantitative neuroimaging results in our INPH patients. Combining quantitative gait and neuroimaging investigations of INPH patients may help us understand those associations and potentially any underlying pathophysiological interrelationships. Third, this study included a relatively small number of participants. Because of the limited sample size, the results of this study need to be replicated in future studies. To our knowledge, there have been only 2 studies investigating changes in quantitative gait parameters before and after the CSFTT in INPH patients, but these previous studies included only 10 or 11 INPH participants13,14.
In conclusion, this study demonstrated that the CSFTT for INPH might improve the so-called balance-related gait parameter (ie, step width). Stride time and stride length variability also responded to the CSFTT. Our findings suggest future studies are needed to investigate whether CSF removal in INPH patients decreases a risk of falling. Further, association between FAB scores and both stride time and stride length variability suggests frontal lobe functions and gait variability in INPH patients may involve similar circuits.
Acknowledgements
The authors would like to thank Wade Martin of Emareye for his critical English revision. This research was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (Ministry of Science and ICT) (No. 2017R1C1B5018354), the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2018R1A6A1A03025109), and the Ministry of Education of the Republic of Korea and the National Research Foundation of Korea (NRF-2016S1A5B5A01025253).
Author contributions
Design and conceptualization of the study: Y.-H.L., P.-W.K., H.-W.L. and K.K. Acquisition of data: K.-S.P., S.-K.H., S.H.K. and K.K. Analysis and interpretation of the data: Y.-H.L., J.H., U.Y. and K.K. Drafting and revising the manuscript for content: Y.-H.L., P.-W.K. and H.-W.L. Final approval of manuscript: K.K.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author upon request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Yong-Hyun Lim, Pan-Woo Ko, Ho-Won Lee and Kyunghun Kang.
Contributor Information
Ho-Won Lee, Email: neuromd@knu.ac.kr.
Kyunghun Kang, Email: kangkh@knu.ac.kr.
References
- 1.Cabral D, et al. Frequency of Alzheimer’s disease pathology at autopsy in patients with clinical normal pressure hydrocephalus. Alzheimers Dement. 2011;7:509–513. doi: 10.1016/j.jalz.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim MJ, et al. Differential diagnosis of idiopathic normal pressure hydrocephalus from other dementias using diffusion tensor imaging. AJNR. American journal of neuroradiology. 2011;32:1496–1503. doi: 10.3174/ajnr.A2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adams RD, Fisher CM, Hakim S, Ojemann RG, Sweet WH. Symptomatic occult hydrocephalus with “normal” cerebrospinal-fluid pressure: a treatable syndrome. N Engl J Med. 1965;273:117–126. doi: 10.1056/NEJM196507152730301. [DOI] [PubMed] [Google Scholar]
- 4.Owler BK, et al. Normal pressure hydrocephalus and cerebral blood flow: a PET study of baseline values. J Cereb Blood Flow Metab. 2004;24:17–23. doi: 10.1097/01.WCB.0000093326.88757.49. [DOI] [PubMed] [Google Scholar]
- 5.Wikkelsø C, Andersson H, Blomstrand C, Lindqvist G. The clinical effect of lumbar puncture in normal pressure hydrocephalus. J Neurol Neurosurg Psychiatry. 1982;45:64–69. doi: 10.1136/jnnp.45.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ishikawa M, et al. Guidelines for management of idiopathic normal pressure hydrocephalus. Neurol Med Chir (Tokyo) 2008;48(suppl):S1–S23. doi: 10.2176/nmc.48.s1. [DOI] [PubMed] [Google Scholar]
- 7.Menz HB, Latt MD, Tiedemann A, Mun San Kwan M, Lord SR. Reliability of the GAITRite walkway system for the quantification of temporo-spatial parameters of gait in young and older people. Gait Posture. 2004;20:20–25. doi: 10.1016/s0966-6362(03)00068-7. [DOI] [PubMed] [Google Scholar]
- 8.van Uden CJ, Besser MP. Test-retest reliability of temporal and spatial gait characteristics measured with an instrumented walkway system (GAITRite) BMC Musculoskelet Disord. 2004;5:13. doi: 10.1186/1471-2474-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McDonough AL, Batavia M, Chen FC, Kwon S, Ziai J. The validity and reliability of the GAITRite system’s measurements: A preliminary evaluation. Arch Phys Med Rehabil. 2001;82:419–425. doi: 10.1053/apmr.2001.19778. [DOI] [PubMed] [Google Scholar]
- 10.Nelson AJ, et al. The validity of the GaitRite and the Functional Ambulation Performance scoring system in the analysis of Parkinson gait. NeuroRehabilitation. 2002;17:255–262. [PubMed] [Google Scholar]
- 11.Bugalho P, Guimaraes J. Gait disturbance in normal pressure hydrocephalus: a clinical study. Parkinsonism Relat Disord. 2007;13:434–437. doi: 10.1016/j.parkreldis.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 12.Bugalho P, Alves L, Miguel R. Gait dysfunction in Parkinson’s disease and normal pressure hydrocephalus: a comparative study. J Neural Transm (Vienna) 2013;120:1201–1207. doi: 10.1007/s00702-013-0975-3. [DOI] [PubMed] [Google Scholar]
- 13.Stolze H, et al. Gait analysis in idiopathic normal pressure hydrocephalus–which parameters respond to the CSF tap test? Clin Neurophysiol. 2000;111:1678–1686. doi: 10.1016/s1388-2457(00)00362-x. [DOI] [PubMed] [Google Scholar]
- 14.Stolze H, et al. Comparative analysis of the gait disorder of normal pressure hydrocephalus and Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2001;70:289–297. doi: 10.1136/jnnp.70.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sasaki H, et al. Cerebral perfusion pattern of idiopathic normal pressure hydrocephalus studied by SPECT and statistical brain mapping. Ann Nucl Med. 2007;21:39–45. doi: 10.1007/bf03033998. [DOI] [PubMed] [Google Scholar]
- 16.Ishii K, et al. A multicenter brain perfusion SPECT study evaluating idiopathic normal-pressure hydrocephalus on neurological improvement. Dement Geriatr Cogn Disord. 2011;32:1–10. doi: 10.1159/000328972. [DOI] [PubMed] [Google Scholar]
- 17.Mori E. Gait disturbance in idiopathic normal pressure hydrocephalus. Brain and nerve = Shinkei kenkyu no shinpo. 2008;60:219–224. [PubMed] [Google Scholar]
- 18.Stephenson J, et al. Gait and balance in adults with Friedreich’s ataxia. Gait Posture. 2015;41:603–607. doi: 10.1016/j.gaitpost.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 19.Maki BE. Gait changes in older adults: predictors of falls or indicators of fear. J Am Geriatr Soc. 1997;45:313–320. doi: 10.1111/j.1532-5415.1997.tb00946.x. [DOI] [PubMed] [Google Scholar]
- 20.Hausdorff JM, Rios DA, Edelberg HK. Gait variability and fall risk in community-living older adults: a 1-year prospective study. Arch Phys Med Rehabil. 2001;82:1050–1056. doi: 10.1053/apmr.2001.24893. [DOI] [PubMed] [Google Scholar]
- 21.Balasubramanian CK, Neptune RR, Kautz SA. Variability in spatiotemporal step characteristics and its relationship to walking performance post-stroke. Gait Posture. 2009;29:408–414. doi: 10.1016/j.gaitpost.2008.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hausdorff JM. Gait dynamics in Parkinson’s disease: common and distinct behavior among stride length, gait variability, and fractal-like scaling. Chaos (Woodbury, N.Y.) 2009;19:026113. doi: 10.1063/1.3147408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dubois B, Slachevsky A, Litvan I, Pillon B. The FAB: a Frontal Assessment Battery at bedside. Neurology. 2000;55:1621–1626. doi: 10.1212/WNL.55.11.1621. [DOI] [PubMed] [Google Scholar]
- 24.Relkin N, Marmarou A, Klinge P, Bergsneider M, Black PM. Diagnosing idiopathic normal-pressure hydrocephalus. Neurosurgery. 2005;57(Suppl. 3):S4–16. doi: 10.1227/01.NEU.0000168185.29659.C5. [DOI] [PubMed] [Google Scholar]
- 25.Kang Y, Na DL, Hahn S. A validity study on the Korean Mini-Mental State Examination (K-MMSE) in dementia patients. J Korean Neurol Assoc. 1997;15:300–308. [Google Scholar]
- 26.Choi SH, et al. Estimating the validity of the Korean version of expanded Clinical Dementia Rating (CDR) scale. J Korean Neurol Assoc. 2001;19:585–591. [Google Scholar]
- 27.Kubo Y, et al. Validation of grading scale for evaluating symptoms of idiopathic normal-pressure hydrocephalus. Dement Geriatr Cogn Disord. 2008;25:37–45. doi: 10.1159/000111149. [DOI] [PubMed] [Google Scholar]
- 28.Rossier P, Wade DT. Validity and reliability comparison of 4 mobility measures in patients presenting with neurologic impairment. Arch Phys Med Rehabil. 2001;82:9–13. doi: 10.1053/apmr.2001.9396. [DOI] [PubMed] [Google Scholar]
- 29.Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142–148. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 30.Bohannon RW, Andrews AW, Thomas MW. Walking speed: reference values and correlates for older adults. J Orthop Sports Phys Ther. 1996;24:86–90. doi: 10.2519/jospt.1996.24.2.86. [DOI] [PubMed] [Google Scholar]
- 31.Ishikawa M, Hashimoto M, Mori E, Kuwana N, Kazui H. The value of the cerebrospinal fluid tap test for predicting shunt effectiveness in idiopathic normal pressure hydrocephalus. Fluids Barriers CNS. 2012;9:1. doi: 10.1186/2045-8118-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hausdorff JM, Edelberg HK, Mitchell SL, Goldberger AL, Wei JY. Increased gait unsteadiness in community-dwelling elderly fallers. Arch Phys Med Rehabil. 1997;78:278–283. doi: 10.1016/s0003-9993(97)90034-4. [DOI] [PubMed] [Google Scholar]
- 33.Selge C, et al. Gait analysis in PSP and NPH: Dual-task conditions make the difference. Neurology. 2018;90:e1021–e1028. doi: 10.1212/WNL.0000000000005168. [DOI] [PubMed] [Google Scholar]
- 34.Nakamura T, et al. Postural and gait disturbance correlated with decreased frontal cerebral blood flow in Alzheimer disease. Alzheimer Dis Assoc Disord. 1997;11:132–139. doi: 10.1097/00002093-199709000-00005. [DOI] [PubMed] [Google Scholar]
- 35.Tian Q, et al. The brain map of gait variability in aging, cognitive impairment and dementia-A systematic review. Neuroscience and biobehavioral reviews. 2017;74:149–162. doi: 10.1016/j.neubiorev.2017.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Denays R, Tondeur M, Noel P, Ham HR. Bilateral cerebral mediofrontal hypoactivity in Tc-99m HMPAO SPECT imaging. Clin Nucl Med. 1994;19:873–876. doi: 10.1097/00003072-199410000-00006. [DOI] [PubMed] [Google Scholar]
- 37.Kanno S, et al. White matter involvement in idiopathic normal pressure hydrocephalus: a voxel-based diffusion tensor imaging study. J Neurol. 2011;258:1949–1957. doi: 10.1007/s00415-011-6038-5. [DOI] [PubMed] [Google Scholar]
- 38.Guedj E, et al. Frontal Assessment Battery is a marker of dorsolateral and medial frontal functions: A SPECT study in frontotemporal dementia. J Neurol Sci. 2008;273:84–87. doi: 10.1016/j.jns.2008.06.035. [DOI] [PubMed] [Google Scholar]
- 39.Espay AJ, Narayan RK, Duker AP, Barrett ET, Jr., de Courten-Myers G. Lower-body parkinsonism: reconsidering the threshold for external lumbar drainage. Nat Clin Pract Neurol. 2008;4:50–55. doi: 10.1038/ncpneuro0688. [DOI] [PubMed] [Google Scholar]
- 40.Gallia GL, Rigamonti D, Williams MA. The diagnosis and treatment of idiopathic normal pressure hydrocephalus. Nat Clin Pract Neurol. 2006;2:375–381. doi: 10.1038/ncpneuro0237. [DOI] [PubMed] [Google Scholar]
- 41.Obeso JA, et al. The basal ganglia in Parkinson’s disease: current concepts and unexplained observations. Ann Neurol. 2008;64(Suppl 2):S30–46. doi: 10.1002/ana.21481. [DOI] [PubMed] [Google Scholar]
- 42.Wu T, et al. Basal ganglia circuits changes in Parkinson’s disease patients. Neurosci Lett. 2012;524:55–59. doi: 10.1016/j.neulet.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee PH, Yong SW, Ahn YH, Huh K. Correlation of midbrain diameter and gait disturbance in patients with idiopathic normal pressure hydrocephalus. J Neurol. 2005;252:958–963. doi: 10.1007/s00415-005-0791-2. [DOI] [PubMed] [Google Scholar]
- 44.Curran T, Lang AE. Parkinsonian syndromes associated with hydrocephalus: case reports, a review of the literature, and pathophysiological hypotheses. Mov Disord. 1994;9:508–520. doi: 10.1002/mds.870090503. [DOI] [PubMed] [Google Scholar]
- 45.Tohgi H, et al. Cerebral blood flow and oxygen metabolism in senile dementia of Alzheimer’s type and vascular dementia with deep white matter changes. Neuroradiology. 1998;40:131–137. doi: 10.1007/s002340050553. [DOI] [PubMed] [Google Scholar]
- 46.Mosconi MW, Wang Z, Schmitt LM, Tsai P, Sweeney JA. The role of cerebellar circuitry alterations in the pathophysiology of autism spectrum disorders. Front Neurosci. 2015;9:296. doi: 10.3389/fnins.2015.00296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kwon MS, Kwon YR, Park YS, Kim JW. Comparison of gait patterns in elderly fallers and non-fallers. Technol Health Care. 2018;26:427–436. doi: 10.3233/THC-174736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Klarica M, Rados M, Oreskovic D. The Movement of Cerebrospinal Fluid and Its Relationship with Substances Behavior in Cerebrospinal and Interstitial Fluid. Neuroscience. 2019;414:28–48. doi: 10.1016/j.neuroscience.2019.06.032. [DOI] [PubMed] [Google Scholar]
- 49.Oreskovic D, Rados M, Klarica M. Role of choroid plexus in cerebrospinal fluid hydrodynamics. Neuroscience. 2017;354:69–87. doi: 10.1016/j.neuroscience.2017.04.025. [DOI] [PubMed] [Google Scholar]
- 50.Ziegelitz D, et al. Pre-and postoperative cerebral blood flow changes in patients with idiopathic normal pressure hydrocephalus measured by computed tomography (CT)-perfusion. J Cereb Blood Flow Metab. 2016;36:1755–1766. doi: 10.1177/0271678X15608521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Meyer JS, et al. Pathogenesis of normal-pressure hydrocephalus–preliminary observations. Surg Neurol. 1985;23:121–133. doi: 10.1016/0090-3019(85)90329-5. [DOI] [PubMed] [Google Scholar]
- 52.Lenfeldt N, et al. Idiopathic normal pressure hydrocephalus: increased supplementary motor activity accounts for improvement after CSF drainage. Brain. 2008;131:2904–2912. doi: 10.1093/brain/awn232. [DOI] [PubMed] [Google Scholar]
- 53.Kang K, Ko PW, Jin M, Suk K, Lee HW. Idiopathic normal-pressure hydrocephalus, cerebrospinal fluid biomarkers, and the cerebrospinal fluid tap test. J Clin Neurosci. 2014;21:1398–1403. doi: 10.1016/j.jocn.2013.11.039. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author upon request.