Abstract
Sex identification of individuals is an important task in wildlife forensics as well as in conservation biology. It helps scientists understand population sex ratios with respect to maintaining genetic diversity, managing inbreeding depression and preventing the demographic consequences of sex-biased poaching. The literature on the use of mammalian molecular sex markers indicates that the success of accurate sex identification is variable across species. Very little is known about the effectiveness of such markers on the mammals of South and Southeast Asia. Therefore, we selected and tested three sets of universal primers for low-cost gel-based sex identification of mammals. We amplified different sets of markers—SRY (157 bp) and 12S rRNA (384 bp); Y-53-SRY (225 bp) and ZFX/ZFY (P1/P2; 445); SRY (157 bp) and 12S rRNA (151 bp)—to be used with different types (tissue, hair and skin) of samples from 20 mammalian species. All three sets of primers amplified the sex-specific fragment in a range of samples from hair to tissue. With an increasing number of field studies using non-invasively collected samples, this proposed low-cost gel-based method of molecular sexing may be applied in various aspects of the ecology and biology of South and Southeast Asian mammals, their conservation and forensics. We suggest that at least two sets of primers be used for any biological samples to avoid ambiguity.
Keywords: Sex identification, Duplex PCR, Internal marker, Wildlife forensic science, Mitochondrial DNA
BACKGROUND
Identification of the sex of mammals from a population facilitates behavioural, breeding system and evolutionary ecology studies of different species (Rosel 2003). Sex identification is also crucial for understanding the effects of sex-selective harvesting in sports and in dealing with the illegal trade of wildlife species of high conservation priority (Spong et al. 2000; Milner et al. 2007; Mondol et al. 2014), as a skewed population sex ratio may affect the mating system and biology of a species (Mysterud et al. 2002). Samples collected from the field provide information regarding the population dynamics and demographic structure of a species as well as sex-biased habitat use by the species (Brown et al. 1991; Gompper et al.1998; Hughes 1998; Eggert et al. 2003). Therefore, many markers and techniques have been proposed to identify the sex of an individual. These include cytogenetic analysis, detection of H-Y antigen and measurement of X-linked enzymes before Barr body formation (Bondioli 1992). PCR-based sex identification can be performed using random fragment length polymorphisms (RFLP) (Aasen and Medrano 1990; Palsboll et al. 1992), TaqMan Probe-based real-time PCR (Chou et al. 2010), fluorescent labelled sex-specific primers (Settin et al. 2008; Mukesh et al. 2013), and hybridization and ligation (Zoledziewska and Dobosz 2003). The random amplified polymorphic DNA (RAPD) technique has also been used for gender determination, but this technique has not been found to be reliable for sexing mammalian species because it is time consuming and there is a chance that the sex-specific band disappears during the reaction (Smith et al. 1994).
PCR has been used for sex determination by targeting sex-specific regions in the genome such as the sex-determining Y chromosome (SRY) gene (Pomp et al. 1995; McHale et al. 2008; Han et al. 2010), amelogenin (Sullivan et al. 1993) and the zinc finger protein encoding Zfx and Zfy genes (Aasen and Medrano 1990). Among these, SRY has been used for many mammalian species (Pomp et al. 1995; Garcia-Meunier et al. 2001; Mukesh et al. 2013). Successful amplification of the Y-specific region indicates male identity. However, in poor-quality samples (hair, degraded tissues, museum specimens and fecal samples) and even sometimes in samples of good quality, PCR may fail to amplify DNA due to the presence of an inhibitor or the amount of DNA and not due to the animal being female (Ortega et al. 2004). Some proposed solutions to this include co-amplification of mitochondrial DNA or a single copy of a nuclear gene in duplex PCR along with a sex-specific fragment in a single reaction (Kamimura et al. 1997; Ortega et al. 2004). A homologous gene, such as amelogenin, present in both X and Y chromosomes in mammalian species producing different-sized amplicons in males and females have also been used in sex identification. But the usability of this gene for sexing in some species of mustelid has not been confirmed (Hattori et al. 2003). However, Y-specific genes have also failed to amplify in some rodent species (Bryja and Konečný 2003). On the other hand, zinc finger proteins can be amplified using a single set of primers that are specific for identifying the sex of mammalian species (Palsboll et al. 1992; Aasen and Medrano 1990; Ortega et al. 2004; Xu et al. 2009). Most of the sex identification methods have been explored in the felids and other carnivores and only a few have been tested on other mammals (Wei et al. 2008; Mukesh et al. 2013; DeCandia et al. 2016).
In view of the mixed findings regarding molecular sexing in the literature, we explored the use of PCR-based sex identification of mammals using the Y-specific gene. Most of these species are threatened by several factors such as illegal hunting, habitat destruction and climate change (Woodroffe 2000; Check 2006; Corlett 2007; Shepherd 2008). These threats affect their size and alter their population structures (Ginsberg and Milner-Gulland 1994). Our main focus in this study was to test and optimize the use of published universal primer sets for sex identification and suggest the applicability of these primer sets in identifying the sex of mammals distributed in South and Southeast Asian countries. Many biological samples obtained during field surveys, including surveys of elusive species, as well as wildlife forensics samples, are of poor quality and yield low-quality DNA (Taberlet et al. 1999; Teletchea et al. 2005; Pages et al. 2009), making the amplification of large fragments unreliable. Therefore, targeting smaller amplicon sizes in the sample types mostly encountered in the wildlife forensics—e.g. non-invasively collected or preserved in the formalin (Teletchea et al. 2005; Brinkman and Hundertmark 2009; Joshi et al. 2013)— increases the chance of amplification success from templates with low copy numbers. Therefore, we tested the applicability of a gradient of small to large (151–445 bp) amplicons using both degraded and good-quality samples from different mammals found in South and Southeast Asia.
MATERIALS AND METHODS
Selection of sex primers
We identified some robust primers based on their success in sexing and species identification as described in the literature. We selected universal primer sets of three markers 151–445 bp long (Table 1). Two mitochondrial primers (12S rRNAs) of different sizes and one ZFX-ZFY homologous sex marker (P1/P2) (Aasen and Medrano 1990) were used as internal markers to avoid the non-amplification of sex primers due to the presence of PCR inhibitors or low quality DNA, as recommended in the literature (Ortega et al. 2004).
Table 1.
List of primer sets used in this study
| Set | Primer | Size (bp) | Primer | Primer sequence | Reference |
| I | 12S rRNA | 384 | L1091 | 5ʹ-AAA AAG CTT CAA ACT GGG ATT AGA TAC CCC ACT AT-3 | Kocher et al.1989 |
| H1478 | 5ʹ-TGA CTG CAG AGG GTG ACG GGC GGT GTG T-3ʹ | ||||
| SRY | 157 | SRYA-5 | 5ʹ-TGAACGCAGTCATGGTGTGGT-3ʹ | Pomp et al. 1995 | |
| SRYA-3 | 5ʹ-AATCTCTGTGCCTCCTGGAA-3ʹ | ||||
| II | ZFX/ZFY | 445 | P15EZ | 5ʹ-ATAATCACATGGAGAGCCACAAGCT-3ʹ | Aasen and Medrano 1990 |
| P13EZ | 5ʹ-GCACTTCTTTGGTATCTGAGAAAGT-3ʹ | ||||
| SRY | 225 | Y53-3C | 5ʹ-CCCATGAACGCATTCATTGTGTGG-3ʹ | Fain and LeMay 1995 | |
| Y-53-3D | 5ʹ-ATTTTAGCCTTCCGACGAGGTCGATA-3ʹ | ||||
| III | 12S rRNA | 151 | 12Sa | 5ʹ-CTG GGG ATT AGA TAC CCC ACTA-3ʹ | Rohland et al.2004 |
| 12So | 5ʹ-GTC GAT TAT AGG ACA GGT TCC TCT A-3 | ||||
| SRY | 225 | Y53-3C | 5ʹ-CCCATGAACGCATTCATTGTGTGG-3ʹ | Fain and LeMay 1995 | |
| Y-53-3D | 5ʹ-ATTTTAGCCTTCCGACGAGGTCGATA-3ʹ |
The primers were selected based on the fact that most samples recovered from the wildlife trade (such as processed meat, bones, claws, tanned skins, carrion, hair, horn and ivory) are of poor quality (Jackson 1990; Mills 1993; Wasser et al. 2008; Milliken and Shaw 2012; TRAFFIC 2011). Such samples are difficult to amplify due to their prolonged exposure to ambient conditions, resulting in degradation of DNA through autolysis and bacterial action (Lindahl and Andersson 1972). Thus, only gene fragments of < 500 bp from these samples should be used to identify sex and species (Goyal et al. unpublished data). Therefore, we selected small primers with one internal marker (< 450 bp), which is within the range of most forensic and biological samples collected from field surveys or museum samples. Sex was identified based on the amplification of Y-linked sex-specific bands co-amplified with internal primers (12S rRNA and P1/P2), as suggested in the literature, to avoid ‘false positives’ for female samples (Ortega et al. 2004). This technique was used for both male and female animals, as two bands appeared in samples from male animals, one band being that of a Y-linked sex-specific primer and the second one being that of an internal primer. In samples from female animals, only the internal primer was amplified.
DNA extraction and quality assessment
We selected samples from 20 Indian mammal species that are also distributed in other parts of South and Southeast Asia and often reported to be affected by trade and habitat fragmentation. Species that we tested for sex identification are distributed in South and Southeast Asia and selected samples included tissues, hair and tanned skins (Table 2). The study was conducted from 2011–2013.
Table 2.
PCR amplification with different sets of primers (+) and reported distribution of mammalian species in South and Southeast Asian countries
| SN | Species | Common name | N | Set I | Set II | Set III | Southeast Asian countries |
South Asian countries |
||||||||||||||||||
| BR | CA | ET | IN | LA | MA | MY | PH | SI | TH | VI | AF | BA | BH | IND | MAL | NE | PA | SR | ||||||||
| Wild species1 | ||||||||||||||||||||||||||
| 1 | Panthera tigris | Tiger | 3# | + | + | +(2) | * | * | * | * | * | * | * | |||||||||||||
| 2 | Panthera pardus | Leopard | 7† | + | +(51) | +(2) | * | * | * | * | * | * | * | * | * | * | * | * | * | * | ||||||
| 3 | Prionailurus bengalensis | Leopard cat | 2‡ | + | + | + | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | |||||
| 4 | Lutra sp. | Otter | 4† | + | + (89) | +(3) | * | * | * | * | * | * | * | * | * | * | * | * | ||||||||
| 5 | Antilope cervicapra | Blackbuck | 2‡ | + | + | + | * | * | * | * | ||||||||||||||||
| 6 | Boselaphus tragocamelus | Nilgai | 2‡ | + | + | +(4) | * | * | * | * | ||||||||||||||||
| 7 | Bos gaurus | Gaur | 2‡ | + | + | + | * | * | * | * | * | * | * | * | * | |||||||||||
| 8 | Muntiacus muntjak | Barking deer | 2‡ | + | + | + | * | * | * | * | * | * | ||||||||||||||
| 9 | Capricornis thar | Serow | 2† | + | + | + | * | * | * | * | ||||||||||||||||
| 10 | Naemorhedus goral | Goral | 2‡ | + (14) | + (31) | + | * | * | * | * | ||||||||||||||||
| 11 | Hemitragus jemlahicus | Himalayan tahr | 2‡ | + | + | + | * | * | ||||||||||||||||||
| 12 | Gazella bennettii | Chinkara | 2‡ | + | + | + | * | * | ||||||||||||||||||
| 13 | Axis porcinus | Hog deer | 2‡ | + | + | + | * | * | * | * | * | * | ||||||||||||||
| 14 | Rusa unicolor | Sambar | 2‡ | + | + | +(20) | * | * | * | * | * | * | * | * | * | * | * | |||||||||
| 15 | Elephas maximus indicus | Elephant | 3# | + | + | +(2) | * | * | * | * | * | * | * | * | * | * | * | * | * | |||||||
| 16 | Sus scrofa | Wild pig | 2‡ | + | + | + | * | * | * | * | * | * | * | * | * | * | * | |||||||||
| Domestic species | ||||||||||||||||||||||||||
| 17 | Bubalus bubalis | Buffalo | 2‡ | + | + | + | ||||||||||||||||||||
| 18 | Equus ferus caballus | Horse | 2‡ | + | + | + | ||||||||||||||||||||
| 19 | Bos taurus | Cow | 2‡ | + | + | + | ||||||||||||||||||||
| 20 |
Capra aegagrus hircus |
Goat |
2‡ |
+ |
+ |
+ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total number of wild species found in countries | 44 | 14 | 171 | 33 | 3 | 7 | 9 | 7 | 7 | 1 | 3 | 9 | 7 | 5 | 12 | 10 | 16 | 13 | 9 | 6 | ||||||
1Based on native range description in IUCN Red List. N, Number of samples. ‡Tissues. †Tanned skins. # Hair and tissue. Values in parentheses indicate additional samples tested against respective species. BR, Brunei; CA, Cambodia; ET, East Timor; IN, Indonesia; LA, Laos; MA, Malaysia; MY, Myanmar; PH, Philippines; SI, Singapore; TH, Thailand; VI, Vietnam; AF, Afghanistan; BA, Bangladesh; BH, Bhutan; IND, India; MAL, Maldives; NE, Nepal; PA, Pakistan; SR, Sri Lanka.
DNA was extracted from the tissue samples using the Qiagen Tissue Kit according to the manufacturer’s protocol. We made some modifications to the protocol to extract DNA from the tanned skins. In the cases of tanned skins, Proteinase K (20 μl/ml) was added at 8–12 hour intervals and the samples were incubated for a long period (2 days) to increase the yield of DNA in the final extract. After the skins were digested completely, DNA was extracted according to the manufacturer’s protocol. DNA was extracted from the hair samples by chopping the hair into small pieces and placing the pieces into sterilized 1.5 mL Eppendorf tubes. Lysis buffer (Tris-Cl (10 mM), EDTA (10 mM), NaCl (100 mM)), 2% sodium dodecyl sulphate (SDS), 10 μl of Proteinase K and 10 μl of dithiothreitol (DTT, 10 mM) were added. The samples were incubated at 56°C in a water bath for 8 hours. Additional Proteinase K and DTT (10 μl each) were added during the incubation phase to digest the hair faster. After 8 hours, the samples were removed from the water bath and centrifuged at 8000g for 1 minute. The subsequent steps were carried out using the Qiagen Tissue Kit according to the manufacturer’s protocol. The quality of the DNA was tested on 0.8% gel and quantified using a 1 kb ladder (HiMedia). All the DNA samples were verified using gel electrophoresis and categorized as ‘good’, ‘moderate’, or ‘low’ quality or ‘no visible DNA’ (Fig. 1). Good-quality DNA was diluted 1:100 with elution buffer to reduce the concentration. Moderate-quality DNA was diluted 1:50, and low-quality DNA was diluted 1:20. Samples with no visible DNA were used directly (1 μl) to obtain a clear band. These were undertaken because failure to amplify the targeted genes may also be due to a high concentration of DNA or the presence of an inhibitor (Bryja and Koncny 2003).
Fig. 1.
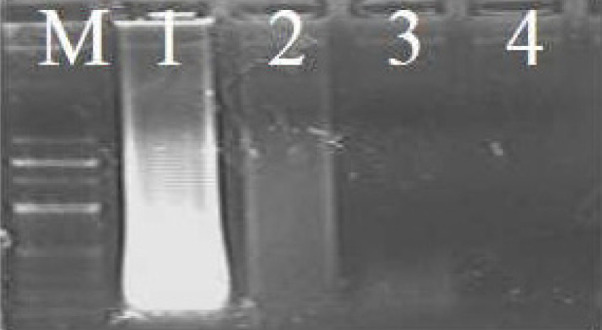
Quality of DNA extracted on 0.8% agarose gel. M, 100 bp leader; 1, good quality; 2, moderate quality; 3, low quality; 4, no visible DNA.
PCR amplification of sex primers
Sex identification of 20 mammalian species was performed using three sets of sex primers with one internal primer (Table 2). One known male positive sample and one female positive sample were used as controls during the PCR amplification of these sets of primer. The amplification of sex primers was performed with 2.5 mM MgCl2, 0.2 μM SRY primers, 0.3 μM12S rRNA and ZFX/ZFY (P1/P2) primers, 200 μM dNTPs, 0.5 U Taq polymerase (Fermantas) and 40–50 ng of each DNA sample in a 20 μl reaction volume. For the primers of Set I, the cycling included 40 cycles of denaturation at 95°C for 45 seconds, annealing at 55°C for 1 minute, extension at 72°C for 1 minute and a final extension cycle at 72°C for 20 minutes. For the primers of Set II and Set III, all the conditions were the same as those of Set I except that the primer concentration was 0.3 μM. The amplified PCR products were subjected to 2.5% agarose gel electrophoresis for physical identification. The gel was prepared using 1× TAE buffer (40 mMTris acetate, 1 mM EDTA, pH 8) containing 0.2 μg/mL EtBr, and photography was carried out under UV light in the gel documentation system. We identified the sex based on the presence or absence of Y-specific bands in the samples. In addition, after screening for positive amplification with all three sets of primers, a total of 218 samples (leopard, 53; otter, 93; goral, 31; chital, 13; sambar, 20; nilgai, 4; elephant, 2; tiger, 2) were also tested. The number of samples tested with each sex marker is provided against the respective primer set in table 2.
Validating the replicability of primers
To check the repeatability of co-amplified mitochondrial DNA as an internal primer for PCR amplification was also crosschecked using an X chromosome linked to a ZFX primer. After the primer set was successfully amplified for the 20 mammalian species, we also tested these primer sets with a larger number of samples (n = 218) of different mammalian species, the details of which are shown in table 2. Furthermore, the wide applicability of these markers have also been used to understand male-biased predation by tiger using single hair samples of four prey species obtained from tiger scat; these findings were published in a separate paper (De et al. 2018).
RESULTS
We tested the reliability of PCR-based sex identification assays using three sets of primers for 20 Indian mammalian species and suggested its applicability in mammals. The 44 samples from different mammalian species of known sexes were all amplified successfully with all three sets of primers, which had different amplicon sizes. The primers of Set I amplified one band of 12S rRNA (384 bp) and a second band, that of a sex-specific SRY primer (157 bp, Fig. 2; Set I). The upper band of P1/P2 (internal marker, 445 bp) and the lower band of SRY-Y53-3C and Y53-3D (225 bp) could be visualized (Fig. 2; Set II) using the primers of Set II. With the third set of primers (Set III), the lower band of 12s rRNA (151 bp) and the upper band of SRY-Y53-3C and Y53-3D (225 bp) were amplified (Fig. 2; Set III). In addition, after these primer sets were screened for amplification of all 20 mammalian species, they were used to identify the sex of different mammalian species using the different kind of samples (n = 218). The species tested are indicated against their respective primers (Table 2). We successfully extracted DNA from single hairs of tigers (n = 2), elephants (2), sambars (20), nilgais (4) and chitals (13) with the third set of primers. This set of primers has small amplicon sizes (151–225 bp) for both genes and can easily amplify DNA from degraded samples.
Fig. 2.
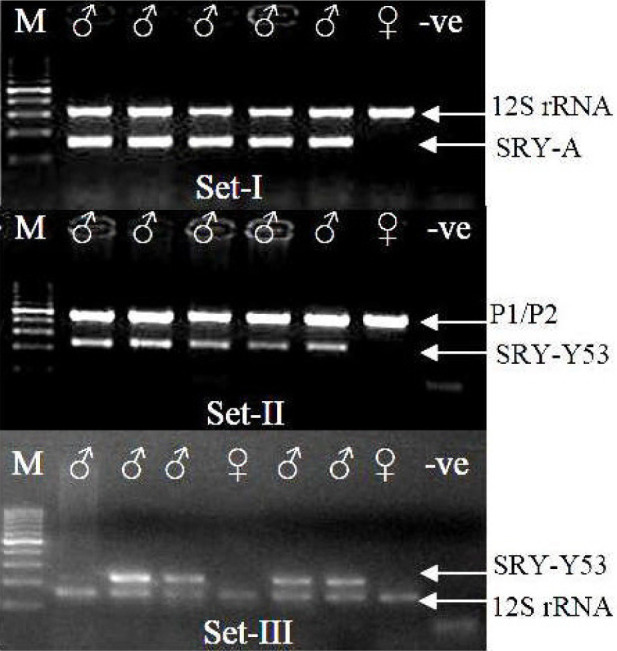
PCR amplification of sex primer with three different sets of primers. ♂, male; ♀, female; +♂ and +♀, positive male and positive female; -ve, negative control; M, 100 bp leader. The samples amplified with Set-I and Set-II in figure 2 are identical; Set-III is depicted amplifying a different set of samples.
DISCUSSION
The method of PCR-based sex identification using three sets of primers described in the foregoing can be used for different mammalian species. It has potential use in identifying sex from small quantity samples, which is useful because samples in wildlife forensics and non-invasive genetics often have lower quality DNA. Such poor-quality DNA, especially with the presence of inhibitors, make obtaining large amplicons difficult.
Most wildlife forensics and endangered species samples collected during status surveys (including non-invasive genetic samples) yield low-quality DNA in small quantities. Set I and Set II are useful for identifying sex from a wide range of samples that are encountered in wildlife forensics, such as blood strains, tissues and tanned skins (Hsieh et al. 2006; Banks and Wright 2007; Caniglia et al. 2010; Jun et al. 2011). Set III has amplicons of small sizes, and hence it can be used with degraded samples, such as those from articles made with hair (paint and shaving brushes, also tested in this study) (Domingo-Roura et al. 2006) and tanned skin (clothes, purses, ties, belts, etc.). Set III can also be used with samples such as antlers, horns, ivory and bones, which are frequently encountered in the illegal trade market (Banks and Wright 2007; TRAFFIC 2011). This primer set also has a wide applicability in amplifying low-quality carnivore and herbivore DNA obtained from different samples (Farrell et al. 2000; Brinkman and Hundertmark 2009), especially in hair obtained from snares or prey species in carnivore scat (De et al. 2018); it has been proven that only a single hair is needed for DNA-based examination.
With molecular tools being used more and more in wildlife science, our study provides a method for low-cost molecular sexing of mammals in South and Southeast Asian countries using various biological samples. All three sets of primers may be used in wildlife forensic work and the conservation biology of South and Southeast Asian mammals, as most of the sample types yielded DNA < 500 bp long (Baker et al. 2001; Butler et al. 2003; Brinkman and Hundertmark 2009; Goyal et al. unpublished data). We suggest that at least two sets of primers be used for any biological samples to avoid any ambiguity.
CONCLUSIONS
Low-cost molecular sexing was performed on 20 mammalian species distributed in South and Southeastern countries. Three sets of universal primers were checked and successfully amplified in all the species, and they exhibited the potential to identify the sex of these species using the Y chromosome. Most samples utilized in conservation genetics are of poor quality and it is difficult to perform a single run amplification of larger fragments of DNA. Therefore, each set of primers was targeted based on the type of sample; the primers generated fragments of 151–445 bp long. The use of a combination of sex-linked genes and internal (sex-linked and mitochondrial) DNA primers provide unambiguous results for sex identification (Ortega et al. 2004). The method describes here has a wide applicability for conservation genetics and wildlife forensics in understanding sex-biased poaching and sex ratios in populations as a low-cost method. Among the different sets of primers used, Set III is the most effective for identifying sex using poor quality samples, such as single hairs from carnivore scats (De et al. 2018), and understanding biased sex ratios in carnivore diets.
Acknowledgments
We extend our sincere thanks to the Director and Dean of the Wildlife Institute of India for their consistent support and for encouraging this work. We also thank the Nodal Officer, Wildlife Forensics Cell for their support. We would like to express our gratitude to our lab mates, who shared their personal experiences with us. We thank the two anonymous reviewers who reviewed this manuscript and provided valuable input. We also thank the technical staff of the Wildlife Forensic and Conservation Genetics Cell for their support during the study.
Footnotes
Authors’ contributions: BDJ designed the study, performed DNA extraction and further laboratory procedures such as sequencing and data analysis, and wrote the MS; RD helped in laboratory procedures and co-wrote the MS. SPG conceptualized the idea, provided the laboratory support, supervised the work, edited and commented of the MS.
Competing interests: BDJ, RD and SPG declare that they have no conflict of interest.
Availability of data and materials: All required information are given in the Materials and Methods section.
Consent for publication: Not applicable.
Ethics approval consent to participate: No ethical permission required as all the samples used from the reference repository of Wildlife Institute of India.
References
- Aasen E, Medrano JF. 1990. Amplification of the ZFY and ZFX genes for sex identification in humans, cattle, sheep and goats. Biotechno 8:1279–1281. [DOI] [PubMed]
- Baker LE, McCormick WF, Matteson KJ. 2001. A silica-based mitochondrial DNA extraction method applied to forensic hair shafts and teeth. J Forens Sci 46:126–130. [PubMed]
- Banks D, Wright B. 2007. Use and Availability of Asian Big Cats Skins at the Litang Horse Festival, Sichuan, August 2007. Environmental Investigation Agency and Wildlife Protection Society of India, London, UK and New Delhi, India.
- Bondioli KR. 1992. Embryo sexing: A review of current techniques and their potential for commercial application in livestock production. J Ani Sci 70(2):19–29. doi:10.2527/1992.70suppl_219x.
- Brinkman TJ, Hundertmark KJ. 2009. Sex identification of northern ungulates using low quality and quantity DNA. Conserv Genet 10:189–1193. doi:10.1007/s10592-008-9747-2.
- Brown MW, Helbig R, Boag PT, Gaskin DE, White BN. 1991. Sexing beluga whales (Delphinapterus leucas) by means of DNA markers. Canad J of Zool 69:1971–1976. doi:10.1139/z91-273.
- Bryja J, Konečný A. 2003. Fast sex identification in wild mammals using PCR amplification of the Sry gene. Folia Zool 52(3):269– 274.
- Butler JM, Shen Y, McCord BR. 2003. The development of reduced size STR amplicons as tools for analysis of degraded DNA. J Forens Sci 48(5):1054–1064. [PubMed]
- Caniglia R, Fabbri E, Greco C, Galaverni M, Randi E. 2010. Forensic DNA against wildlife poaching: identification of a serial wolf killing in Italy. Forensic Sci Int Genet 4(5):334–338. doi:10.1016/j.fsigen.2009.10.012. [DOI] [PubMed]
- Check E. 2006. Conservation biology: The tiger’s retreat. Nature 441:927–930. doi:10.1038/441927a. [DOI] [PubMed]
- Chou TC, Yao CT, Su SH, Hung YC, Chen WS, Cheng CC, Tseng CN, Wang HM, Chou YC, Li SS, Gu DL, Chang HW. 2010. Validation of Spilornis cheela hoya TaqMan probes for potential gender identification of many Accipitridae species. Theriogenol 73(3):404–411. doi:10.1016/j.theriogenology.2009.09.024. [DOI] [PubMed]
- Corlett RT. 2007. The impact of hunting on mammalian fauna of tropical tropical Asian forests. Biotropica 39:292–303. doi:10.1111/j.1744-7429.2007.00271.x.
- De R, Joshi BD, Shukla M, Pandey M, Singh R, Goyal SP. 2018. Understanding predation behaviour of tiger (Panthera tigris tigris) in Ranthambore Tiger Reserve, Rajasthan, India: Use of low cost gel based molecular sexing of prey hairs from scats. Conser Genet Resour 11(1):97–104. doi:10.1007/s12686-017-0963-2.
- DeCandia A, Gaughran S, Caragiulo A. 2016. A novel molecular method for noninvasive sex identification of order Carnivora. Conser Genet Resour 8:119–121. doi:10.1007/s12686-016-0525-z.
- Domingo-Roura X, Marmi J, Ferrando A, López-Giráldez F, Macdonald DW, Jansman HAH. 2006. Badger hair in shaving brushes comes from protected Eurasian badgers. Biol Conserv 128:425–430. doi:10.1016/j.biocon.2005.08.013.
- Eggert L, Eggert JA, Woodruff D. 2003. Estimating population sizes for elusive animals: The forest elephants of Kakum National Park, Ghana. Mol Ecol 12:1389–1402. doi:10.1046/j.1365-294X.2003.01822.x. [DOI] [PubMed]
- Fain SR, LeMay JP. 1995. Gender identification of humans and mammalian wildlife 162 species from PCR amplified sex linked genes. Proc Am Acad Forensic Sci 1:34.
- Farrell LE, Roman J, Sunquist ME. 2000. Dietary separation of sympatric carnivores identified by molecular analysis of scats. Mol Ecol 9:1583–1590. doi:10.1046/j.1365-294x.2000.01037.x. [DOI] [PubMed]
- Garcia-Meunier P, Pastout L, Chevalier G, Guinet C. 2001. Détermination rapide du sexe chezdes embryons de ragondin, Myocastor coypus, dãs les premiers stades de gestation. CR Acad Sci Paris, Sci de la vie/Life Sci 324:321–325. [DOI] [PubMed]
- Ginsberg JR, Milner-Gulland EJ. 1994. Sex-biased harvesting and population-dynamics in ungulates: Implications for conservation and sustainable use. Conserv Biol 8:157–166.
- Gompper ME, Gittleman JL, Wayne RK. 1998. Dispersal, philopatry, and genetic relatedness in a social carnivore: Comparing males and females. Mol Ecol 7:157–163. doi:10.1046/j.1365-294x.1998.00325.x. [DOI] [PubMed]
- Han S-H, Yang BC, Ko MS, Oh HS, Lee SS. 2010. Length difference between equine ZFX and ZFY genes and its application for molecular sex determination. J Assist Reprod Genet 27:725–728. doi:10.1007/s10815-010-9467-7. [DOI] [PMC free article] [PubMed]
- Hattori K, Burdin AM, Onuma M, Suzuki M, Ohtaishi N. 2003. Sex determination in the sea otter (Enhydra lutris) from tissue and dental pulp using PCR amplification. Can J Zool 81:52–56. doi:10.1139/z02-219.
- Hsieh HM, Huang LH, Tsai LC, Liu CL, Kuo YC, Hsiao CT, Linacre A, Lee JC. 2006. Species identification of Kachuga tecta using the cytochrome b gene. J Forensic Sci 51(1):52–56. doi:10.1111/j.1556-4029.2005.00004.x. [DOI] [PubMed]
- Hughes C. 1998. Integrating molecular techniques with field methods in studies of social behavior: A revolution results. Ecology 79:383–399. doi:10.1890/0012-9658(1998)079[0383:IMTWFM ]2.0.CO;2.
- Jackson P. 1990. Chinese medicine threatens Asia’s last tigers. Cat News 13:7–9.
- Joshi BD, Mishra S, Singh SK, Goyal SP. 2013. An effective method for extraction and polymerase chain reaction (PCR) amplification of DNA from formalin preserved tissue samples of snow leopard. African J Biotech 12(22):3399–3404. doi:10.5897/AJB12.2759.
- Jun J, Han SH, Jeong T-J, Park HC, Lee B, Kwak M. 2011. Wildlife forensics using mitochondrial DNA sequences: species identification based on hairs collected in the field and confiscated tanned Felidae leathers. Genes Genom 33(6):721–726. doi:10.1007/s13258-011-0080-7.
- Kamimura S, Nishiyama N, Ookutsu S, Goto K, Hamana K. 1997. Determination of bovine fetal sex by PCR using fetal fluid aspirated by transvaginal ultrasound-guided amniocentesis. Theriogen 47:1563–1569. doi:10.1016/S0093-691X(97)00161-1. [DOI] [PubMed]
- Kocher TD, Thomas WK, Meyer A, Edwards SV, Paabo S, Villablanca FX, Wilson AC. 1989. Dynamics of mitochondrial DNA evolution in animals: Amplification and sequencing with conserved primers. Proc Natl Acad Sci USA 86:196–200. doi:10.1073/pnas.86.16.6196. [DOI] [PMC free article] [PubMed]
- Lindahl T, Andersson A. 1972. Rate of depurination of native deoxyribonucleic acid. Biochem 11:3618–3623. doi:10.1021/bi00769a019. [DOI] [PubMed]
- McHale M, Broderick D, Ovenden JR, Lanyon JM, 2008. A multiplexed PCR assay for sex assignment in dugong (Dugong dugon) and West Indian manatee (Trichechus manatus). Mol Ecol Notes 8:669–670. doi:10.1111/j.1471-8286.2007.02041.x. [DOI] [PubMed]
- Milliken T, Shaw J. 2012. The South Africa–Viet Nam rhino Horn Trade Nexus: A Deadly Combination of Institutional Lapses, Corrupt Wildlife Industry Professionals and Asian Crime Syndicates. TRAFFIC, Johannesburg, South Africa.
- Mills JA. 1993. Tiger bone trade in South Korea. Cat News 19:13–16.
- Milner JM, Nilsen EB, Andreassen HP. 2007. Demographic side effects of selective hunting in ungulates and carnivores. Conserv Biol 21:36–47. doi:10.1111/j.1523-1739.2006.00591.x. [DOI] [PubMed]
- Mondol S, Sridhar V, Yadav P, Gubbi S, Ramakrishnan U. 2014. Tracing the geographic origin of traded leopard body parts in the Indian subcontinent with DNA-based assignment tests. Conserv Biol 29(2):556–64. doi:10.1111/cobi.12393. [DOI] [PubMed]
- Mukesh, Sharma LK, Charoo SA, Sathyakumar S. 2013 An improved and reliable molecular sexing technique for Asiatic black bears, Ursus thibetanus. Conserv Genet Resour 5(6):1079–1082. doi:10.1007/s12686-013-9988-3.
- Mysterud A, Coulson T, Stenseth NC. 2002. The role of males in the dynamics of ungulate populations. J Anim Ecol 71:907–915. doi:10.1046/j.1365-2656.2002.00655.x.
- Ortega J, Franco M, Adams BA, Ralls K, Maldonado JE. 2004. A reliable, non-invasive method for sex determination in the endangered San Joaquin kit fox (Vulpes macrotis mutica) and other canids. Conserv Genet 5:715–718. doi:10.1007/s10592-004-1862-0.
- Pages M, Maudet C, Bellemain E, Taberlet P, Hughes S, Hanni C. 2009. A system for sex determination from degraded DNA: a useful tool for palaeogenetics and conservation genetics of ursids. Conserv Genet 10:897–907. doi:10.1007/s10592-008-9650-x.
- Palsboll PJ, Vader A, Bakke I, Raafatel-Gewely M. 1992. Determination of gender in cetaceans by the polymerase chain reaction. Canad J of Zool 70:2166–2170. doi:10.1139/z92-292.
- Pomp D, Good BA, Geisert RD, Corbin CJ, Conley AJ. 1995. Sex identification in mammals with polymerase chain reaction and its use to examine sex effects on diameter of day-10 or -11 pig embryos. J of Anim Sci 73:1408–1415. [DOI] [PubMed]
- Rohland N, Siedel H, Hofreiter M. 2004. Nondestructive DNA extraction method for mitochondrial DNA analyses of museum specimens. BioTechni 36:814–821. doi:10.2144/04365ST05. [DOI] [PubMed]
- Rosel PE. 2003. PCR based sex determination in odontocete cetaceans. Conserv Genet 4:647–649. doi:10.1023/A:1025666212967.
- Settin A, Elsobky E, Hammad A, Al-Erany A. 2008. Rapid Sex Determination Using PCR Technique Compared to Classic Cytogenetics. International J of Health Scien 2(1):49–52. [PMC free article] [PubMed]
- Shepherd CR. 2008. Civets in trade in Medan, North Sumatra, Indonesia (1997–2001) with notes on legal protection. Small Carniv Conserv 38:34–36.
- Smith JL, Scott-Craig JS, Leadbetter JR, Bush GL, Roberts DL, Fulbright DW. 1994. Characterization of random amplified polymorphic DNA (RAPD) products from Xanthomonas campestris and some comments on the use of RAPD products in phylogenetic analysis. Mol Phyl Evol 3:135–145. doi:10.1006/mpev.1994.1016. [DOI] [PubMed]
- Spong G, Hellborg L, Creel S. 2000. Sex ratio of leopards taken in trophy hunting: Genetic data from Tanzania. Conserv Genet 1:169–171. doi:10.1023/A:1026543308136.
- Sullivan K, Mannucci A, Kimpton C, Gill P. 1993. A rapid and quantitative DNA test: Fluorescence-based PCR analysis of X–Y homologous gene amelogenin. Biotechni 15:636–642. [PubMed]
- Taberlet P, Waits LP, Luikart G. 1999. Noninvasive genetic sampling: look before you leap. Trends Ecol Evol 14(8):323–327. doi:10.1016/S0169-5347(99)01637-7. [DOI] [PubMed]
- Teletchea F, Maudet C, Hänni C. 2005. Food and forensic molecular identification: update and challenges. Trends Biotechnol 23(7):359–366. doi:10.1016/j.tibtech.2005.05.006. [DOI] [PubMed]
- TRAFFIC. 2011. Seizures and prosecutions: March 1997–October 2013. TRAFFIC Bulletin http://www.traffic.org/mediareports. Accessed 19 October 2013.
- Wasser S, Clark WJ, Drori O, Kisamo ES, Mailand C, Mutayoba B, Stephens M. 2008. Combating the illegal trade in African elephant ivory with DNA forensics. Conserv Biol 22:1065–1071. doi:10.1111/j.1523-1739.2008.01012.x. [DOI] [PubMed]
- Wei K, Zhang Z, Zhang W. 2008. PCR-CTPP: a rapid and reliable genotyping technique based on ZFX/ZFY alleles for sex identification of tiger (Panthera tigris) and four other endangered felids. Conserv Genet 9:225–228. doi:10.1007/s10592-006-9279-6.
- Woodroffe R. 2000. Predators and people: Using human densities to interpret declines of large carnivores. Anim Conserv 3:165–173. doi:10.1111/j.1523-1739.2008.01012.x.
- Xu X, Li Y, Wang X, Wei K, Zhang W, Zhang Z, Shen F, Yue B. 2009. Zinc-finger Intron 7: A new locus for sex identification of giant panda (Ailuropoda melanoleuca). Zoo Bio 28:1–6. doi:10.1002/zoo.20274. [DOI] [PubMed]
- Zoledziewska M, Dobosz T. 2003. Gender determination in highly degraded DNA samples. Intern Cong Ser 1239:593–595. doi:10.1016/S0531-5131(02)00565-4.


