Abstract
Three forms of modified tapioca starch, i.e. phosphorylated cross-linked tapioca starch (CLTS), octenyl succinic anhydride substituted tapioca starch (OSTS) and hydroxypropylated tapioca starch (HPTS) were studied and used as stabilizers (0.5, 1 and 1.5%) in industrial liquid kashk, and their effects on the physicochemical and sensory properties of products were examined during 60 days of refrigerated storage. When combined with the highest concentration of each stabilizer, the kashk reached its highest acidity, hardness, adhesiveness, viscosity and overall acceptability, while the lowest value of syneresis was obtained. Moreover, the highest values of viscosity was observed after incorporating CLTS into the samples, and other samples with HPTS showed the highest syneresis, hardness, adhesiveness and overall acceptability. During storage, there were significant trends of increase in the values of acidity, syneresis and hardness, whereas the viscosity, pH and adhesiveness decreased significantly. Several sensory attributes such as texture, odour value and overall acceptability were influenced by the type of stabilizer. In general, among the three kinds of modified tapioca starch, the HPTS was the most suitable form at the concentration of 1.5%, and this was most appropriate for the production of industrial liquid kashk with respect to high-quality physicochemical and sensory properties.
Keywords: Modified tapioca starch, Kashk, Stabilizer, Physicochemical, Sensory evaluation
Introduction
Kashk is an acid-fermented dairy product that is widely consumed in many parts of the Middle East, especially in Iran. This product is basically a dried low-fat yogurt, without any cereal additives. Since ancient times, it has been used extensively in Iran as a traditional product (Shiroodi et al. 2012). Its ingredients and preparation methods vary from place to place, and therefore different names are used for this fermented dry yogurt, names such as Tarhana (in Turkey and Greece), Kishk (in Lebanon and Egypt), Kushuk (in Iraq), Madeer-Oggt (in Saudi Arabia), Kichk (in India), Talkuna (in Finland), Tahanya (in Hungary) and Atole (in Scotland) (Damir et al. 1992). This nutritious food product is recommended for use in the diets of children, pregnant and lactating women. It contains high quality proteins and high amounts of calcium, along with its subordinate role as a protein supplement in livestock and poultry feed (Soltani and Güzeler 2013).
This product is available in a semi-liquid or dried form. The latter needs to be soaked and softened before it can be used in cooking. In recent years, two types of industrial liquid kashk have been introduced into the Iranian market (Shiroodi et al. 2012). One is called the industrial liquid kashk which, according to the national standard No. 6127, is produced after fermenting and concentrating milk or its by-products via industrial methods, and is followed by adding the rest of the ingredients (whey powder and salt), heat treatment and packaging (Iranian National Standards 2009). The second type of traditional liquid kashk is prepared according to the national standard No. 2452, by soaking dry kashk for a sufficient amount of time, followed by adding a certain amount of water and, if necessary, salt. Then, the process involves rubbing the kashk with industrial or semi-industrial equipment and then exposing it to a heat treatment before packaging (Iranian National Standards 1990). Obviously, the industrial liquid kashk (type I) has more health benefits and better microbiological quality.
The production and maintenance of a liquid kashk with optimum consistency and stability is a major issue in the industry. Stabilizers are often added to dairy products and other foods to increase viscosity, stability and sensory attributes among which starch is one of the most prevalent ingredients used in food products because of its low price and sufficient availability (Diamantino et al. 2014). The tapioca starch is a suitable type of starch that can be used on food, especially on dairy products. Since dairy products are mildly flavoured, tapioca starch is the best choice because it adds a creamy texture and a unique flavour to the final food product (Schmidt et al. 2001). This starch contains lower levels of amylase (16.3%) compared to high-amylose starches. It also has less protein (0.5%), fat (0.5%) and moisture content (7.5%) compared to the starch found in corn and potato. The tapioca starch is characterized by specific functional properties. Compared to cereal starch, it is capable of forming pastes at lower temperatures while being able to gelatinize at higher temperatures because of its higher degree of crystallinity. These qualities contribute to the formation of softer, less springy, gummy and chewy gels, compared to those created by cereal starch and tuber starch (Mishra and Rai 2006). However, native forms of starch are often modified to achieve desirable functional properties. Cross-linking and substitution are two types of modifications mostly used in cultured dairy products, regardless of the botanical sources of starch (Klemaszewski 2011). There have been various cases of research on the application of modified starch in cultured dairy products. Examples include the octenyl succinylated waxy maize starch in minas fresh cheese (Diamantino et al. 2014), acetylated cross-linked, hydroxypropylated, or hydroxypropylated cross-linked starch in set yogurt (Schmidt et al. 2001) and cross-linked acetylated starch in set yogurt (Cui et al. 2014). However, no amount of research has so far considered the use of modified starch in liquid kashk. Accordingly, the main objective of this study is to investigate the effects of three modified tapioca starch at three different concentrations on the physicochemical, sensory and microbial properties of this acid-fermented dairy product, the kashk.
Materials and methods
Materials
Skimmed milk powder (Mani Mas Dairy Co., Iran), sodium caseinate (DMV Co., Holland), whey protein powder (Sabah Dairy Co., Iran) and freeze-dried lactic cultures (Mediterranea Biotecnologie srl, Italia) were used in order to prepare the kashk variations.
Three kinds of chemically modified food starch were refined from tapioca (Vedan, Vietnam). Specifically, they included phosphorylated cross-linked tapioca starch (Geli CT69, sample code: 15128406), octenyl succinic anhydride substituted tapioca starch (Geli KS43C, sample code: 14102012) and hydroxypropylated stabilized tapioca starch with DS 0.046% (Geli CB58, sample code: 15113006). All chemicals in the experiment were purchased from representatives of the Merck Company (Germany).
Methods
Sample preparation
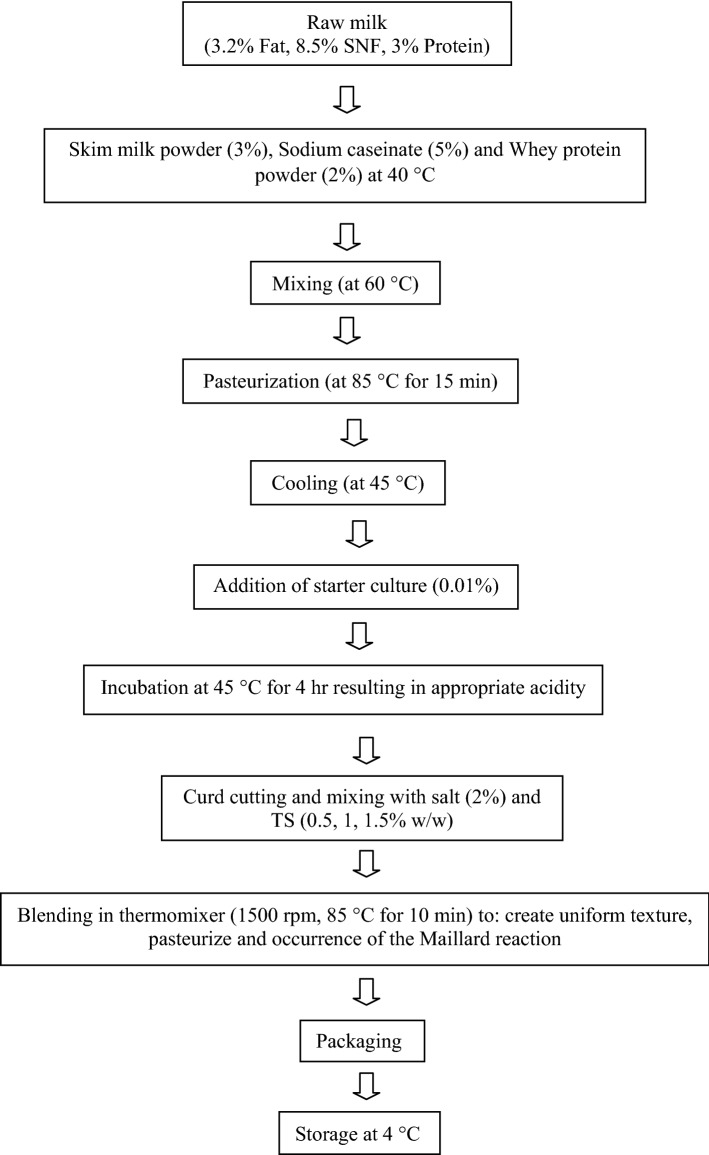
The industrial liquid kashk was prepared in the Behin Azma Food Production Co., according to the process illustrated in Fig. 1. Three types of modified tapioca starch were used at three different concentrations (0.5, 1 and 1.5%). Their role was considered as stabilizers. Samples with no stabilizer were considered as the control group. All kashk variations were stored at 4 °C for 60 days until the completion of physiochemical, microbial and sensory experiments.
Fig. 1.
Schematic production of the industrial liquid kashk
Chemical composition
On the first day of preparation, the kashk variations were analyzed for protein, fat, moisture and ash content (AOAC 2000). The salt content was determined according to the Iranian National Standards (1990). All analyses were carried out in triplicate.
Determination of pH and titratable acidity
The change in pH of the kashk samples during storage was measured using a pH meter (Model 3520, Jenway, England) at room temperature. The titratable acidity (TA) was quantified in terms of the percentage of lactic acid, and was measured according to its procedure (AOAC 2000). The experiments that follow hereafter in this article were performed on days 1, 15, 30, 45 and 60 of the storage period.
Determination of syneresis
The syneresis in the kashk samples was measured according to the method proposed by Keogh and O’Kennedy (1998). Kashk samples (14 g) were centrifuged (Model Funke Gerber, Funk, Germany) at 222 rpm and at 4 °C for 10 min. The clear supernatant was poured off, weighed and recorded as the percentage of weight observed in relation to the original weight of the kashk. Each sample was measured to obtain an average for the triplicate.
Viscosity measurement
The viscosity values (cP) of the kashk variants (100 mL) were measured after mixing the sample for 5 min at room temperature, whereby a Brookfield viscometer was used (Model DVII, Brookfield, USA) along with a CP51 spindle (LV mode) at 20 rpm. All samples were measured in triplicate.
Texture analysis
The textural properties of the kashk variant samples were measured with a Texture Analyzer (Model CT3, Brookfield, USA), equipped with a TA4/1000 cylindrical probe (38.1 mm diameter, 20 mm length). The speed of the compression was 0.5 mm/s, and the distance target was 15 mm. The maximum force in the first peak (g) was used as a measure to determine firmness, whereas the negative force area after the first compression (N g) was used in order to evaluate the adhesiveness. The test was performed at 20 °C.
Color
Using a colorimeter (Model ZE6000, Nippon Denshoku Co., Japan), the color of kashk variants were measured under controlled conditions on days 1, 30 and 60 of storage. The measured values of L*, a* and b* were used as indicators of lightness, redness and yellowness, respectively. The total color difference (ΔE*) was also calculated in each period of time with respect to the control sample. The calculations employed the characteristic parameters of CIELAB, including L*, a*, and b* as follows:
| 1 |
Sensory evaluation
Sensory evaluation of the samples was performed by 20 untrained panelists using a five-point hedonic scale. The panelists received anonymous samples, identified only with random coded digits, and were asked to rate their sensory properties (taste, odor, mouthfeel, adhesiveness, texture and overall acceptability) as (1) extreme dislike; (2) dislike; (3) neutral; (4) like; and (5) extreme like.
Statistical analysis
Data were analyzed using statistical software of SPPS v.18 and the charts were plotted in Microsoft Excel 2016. Experiments were carried out according to a completely randomized design (3-Factor Factorial). One-way ANOVA identified significant differences among mean values (p ≤ 0.05). The mean values of the samples were compared with the mean values obtained by Duncan’s Multiple Range Test. All experiments were performed in triplicate.
Result and discussion
Chemical composition
Table 1 shows the average composition of the kashk variations on a dry basis, compared with commercial samples, according to national standards. Kashk is recognized as a source of high quality protein with high biological value, in addition to its high content of calcium and vitamin D (Jafari et al. 2015). In the present study, the protein and fat contents in all variations ranged between 8.05–8.1%, and 3.10–3.13%, respectively. Both ranges can be considered as an acceptable standard. There were, however, no significant differences in the fat and protein content of the samples stabilized with different TS and their concentrations (p ≤ 0.05). According to the results, the moisture content of kashk samples decreased when adding modified tapioca starch and all were in the acceptable limit. The control sample had the highest moisture content (79.9% w.b.), i.e. the lowest amount of dry matter, and was therefore significantly different from all other treatments, including the one with the lowest amount of moisture, i.e. the highest dry matter, which was observed in the kashk sample containing 1.5% modified tapioca starch (78.6–78.9% w.b) (p < 0.05). The moisture content in the samples decreased parallel to the increase in the concentration of the stabilizer, and this can be attributed to the water holding capacity of stabilizers and their ability to increase the viscosity of kashk. Moreover, there was no significant difference between different types of modified starch at the same concentration. A similar result pertaining to moisture content was reported by Mbaeyi-Nwaoha et al. (2017) in yogurt, where local forms of stabilizer were used. Here, however, the dry matter of the kashk samples corresponded negatively with moisture content. This result is in agreement with previous findings reported by Olorunnisomo et al. (2015) who also reported the increase in solid contents of yoghurt when stabilizers were added. They attributed this to the high solid contents of the stabilizers used for producing yogurt, compared to those in milk. The ash content of kashk samples varied from 2.15 to 2.17%, and the percentage increased significantly when stabilizers were added, especially when added at higher concentrations. The highest ash content was recorded in OSTS, and the least was observed in the control. In this regard, Mbaeyi-Nwaoha et al. (2017) reported that yoghurts which had been formulated from ofo and achi stabilizers exhibited higher ash content compared to the control, presumably because of the high content of calcium and phosphorous in achi. The salt content of kashk samples varied from 2 to 2.13%. According to Shiroodi et al. (2012), the addition of 3% salt to the formulation of kashk, supplemented with 0.1–0.5% tragacanth gum, causes the neutralization of the gum. It also inhibits complex formations between gum and protein molecules. However, the presence of lower levels of salts (0.5–0.6%) in the formulation of doogh—an Iranian yogurt drink—was reported not to be enough for the neutralization of the tragacanth gum (Ghorbani Gorji et al. 2010).
Table 1.
Chemical composition of the kashk variations (mean ± SD)
| Sample code | Protein (%) | Fat (%) | Moisture (%) | Dry matter (%) | Ash (%) | Salt (%) |
|---|---|---|---|---|---|---|
| C | 8.10 ± 0.0A | 3.10 ± 0.00A | 79.90 ± 0.01A | 20.10 ± 0.01E | 2.15 ± 0.00G | 2.02 ± 0.00CD |
| CLTS 0.5 | 8.10 ± 0.0A | 3.13 ± 0.06A | 79.47 ± 0.01B | 20.53 ± 0.01D | 2.16 ± 0.00D | 2.06 ± 0.05BCD |
| HPTS 0.5 | 8.10 ± 0.0A | 3.10 ± 0.00A | 79.47 ± 0.01B | 20.53 ± 0.01D | 2.15 ± 0.00E | 2.00 ± 0.01D |
| OSTS 0.5 | 8.10 ± 0.0A | 3.13 ± 0.06A | 79.48 ± 0.01B | 20.52 ± 0.01D | 2.15 ± 0.00F | 2.05 ± 0.05CD |
| CLTS 1.0 | 8.10 ± 0.0A | 3.13 ± 0.06A | 79.02 ± 0.02C | 20.98 ± 0.02C | 2.16 ± 0.00B | 2.08 ± 0.04ABC |
| HPTS 1.0 | 8.10 ± 0.0A | 3.13 ± 0.06A | 79.02 ± 0.02C | 20.98 ± 0.02C | 2.16 ± 0.00D | 2.08 ± 0.05ABC |
| OSTS 1.0 | 8.10 ± 0.0A | 3.10 ± 0.00A | 79.01 ± 0.01C | 20.99 ± 0.01C | 2.16 ± 0.00D | 2.13 ± 0.02A |
| CLTS 1.5 | 8.05 ± 0.0B | 3.13 ± 0.06A | 78.91 ± 0.01D | 21.39 ± 0.01B | 2.17 ± 0.00A | 2.06 ± 0.04BCD |
| HPTS 1.5 | 8.05 ± 0.0B | 3.10 ± 0.00A | 78.60 ± 0.01E | 21.40 ± 0.01A | 2.16 ± 0.00C | 2.08 ± 0.03ABC |
| OSTS 1.5 | 8.05 ± 0.0B | 3.13 ± 0.06A | 78.59 ± 0.01E | 21.11 ± 0.01A | 2.16 ± 0.00C | 2.12 ± 0.02A |
| Acceptable limit* | Min 8.0% | Min 1.0% | Max 82.0% | – | Max 2.5% | Max 2.0% |
Different uppercase letters in columns means significant differences at probability level of 5%
*According to ISIRI national standard No. 2452
Acidity and pH
Table 2 shows the changes in acidity and pH of the kashk samples supplemented with different concentrations of starch variations compared to the control (without stabilizer) during 60 days of storage at 4 °C. On the first day of storage, adding starch stabilizer at various levels (0.5, 1 and 1.5%, wt/vol) increased the values of acidity significantly, and this occurred in all kashk variations, except the ones which had been supplemented with HPTS. However, the acidity of all kashk variations increased significantly (p < 0.05) as the storage time went from day 1 to day 60, but the variations supplemented with HPTS showed significantly lower acidity values than others. This was associated with a decrease in pH value which reached a range of 3.58–3.87 in the samples. Nonetheless, the range is considered to be an acceptable range for commercial kashk varieties. The acidity (%lactic acid) of yogurt and the application of heat treatment during the production of the industrial liquid kashk may influence its titratable acidity (Soltani and Güzeler 2013). Lactic acidification by yogurt starters at the end of incubation, as well as post-acidification during storage, can effectively increase the acidity of kashk variations. According to Soukoulis et al. (2007), enriching yogurts with polysaccharides can increase their acidity. This could be attributed to an enhanced growth and survival of probiotic bacteria that might induce a more rapid transformation of lactose into lactic acid. However, the minimum increase of acidity and the decrease in the pH of kashk variations was observed in the ones supplemented with HPTS. This may be attributed to the preservation of functional groups containing a negative charge via acetyl or hydroxylpropyl groups in this type of starch. This result is in agreement with other reports on kashk (Damir et al. 1992), yogurt (Basiri et al. 2018; Kim et al. 2011; Lobato-Calleros et al. 2014; Mbaeyi-Nwaoha et al. 2017; Sameen et al. 2016) and cheese products (Diamantino et al. 2014).
Table 2.
Titratable acidity (%) and pH (Mean ± SD) of kashk variations during 60 days of storage at 4 °C
| Sample code | Titratable acidity (%) | pH | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Storage time (days) | Storage time (days) | |||||||||
| 0 | 15 | 30 | 45 | 60 | 0 | 15 | 30 | 45 | 60 | |
| C | 1.72 ± 0.00Ee | 1.73 ± 0.01Fd | 1.75 ± 0.00Ec | 1.79 ± 0.01Eb | 1.80 ± 0.00Da | 3.87 ± 0.01Aa | 3.80 ± 0.00ABb | 3.80 ± 0.01Ab | 3.72 ± 0.01Ac | 3.70 ± 0.00Ad |
| CLTS 0.5 | 1.78 ± 0.01De | 1.79 ± 0.00Dd | 1.80 ± 0.01Dc | 1.85 ± 0.00Bb | 1.87 ± 0.01Aa | 3.79 ± 0.01DEa | 3.72 ± 0.00DEb | 3.71 ± 0.01DEb | 3.62 ± 0.01Gc | 3.62 ± 0.01Fc |
| HPTS 0.5 | 1.71 ± 0.00Fe | 1.72 ± 0.00Gd | 1.73 ± 0.01Fc | 1.79 ± 0.01Eb | 1.80 ± 0.00Da | 3.87 ± 0.01Aa | 3.80 ± 0.01Ab | 3.80 ± 0.00Ab | 3.70 ± 0.01Bc | 3.70 ± 0.00Ac |
| OSTS 0.5 | 1.78 ± 0.01De | 1.79 ± 0.00Dd | 1.80 ± 0.01Dc | 1.83 ± 0.01Cb | 1.85 ± 0.01Da | 3.80 ± 0.01Ca | 3.72 ± 0.01Eb | 3.71 ± 0.00Eb | 3.67 ± 0.00Dc | 3.66 ± 0.01Dd |
| CLTS 1.0 | 1.79 ± 0.00Ce | 1.82 ± 0.00Bd | 1.84 ± 0.00ABc | 1.86 ± 0.00Ab | 1.88 ± 0.01Aa | 3.79 ± 0.01CDa | 3.73 ± 0.01Db | 3.72 ± 0.00Dc | 3.62 ± 0.01Gd | 3.61 ± 0.00Ge |
| HPTS 1.0 | 1.72 ± 0.00Ee | 1.73 ± 0.00Fd | 1.75 ± 0.00Ec | 1.78 ± 0.00Fb | 1.80 ± 0.01Da | 3.87 ± 0.01Aa | 3.79 ± 0.01Bb | 3.78 ± 0.01Bc | 3.69 ± 0.01Cd | 3.69 ± 0.00Bd |
| OSTS 1.0 | 1.80 ± 0.01Bd | 1.74 ± 0.00Ec | 1.83 ± 0.01BCb | 1.83 ± 0.00Cb | 1.85 ± 0.00Ba | 3.80 ± 0.01Ca | 3.72 ± 0.00DEb | 3.71 ± 0.01DEc | 3.64 ± 0.00Ed | 3.63 ± 0.00Ee |
| CLTS 1.5 | 1.80 ± 0.00Be | 1.81 ± 0.01Cd | 1.83 ± 0.00Cc | 1.85 ± 0.00Bb | 1.88 ± 0.01Aa | 3.78 ± 0.00Ea | 3.71 ± 0.01Eb | 3.71 ± 0.01Ec | 3.63 ± 0.00Fd | 3.60 ± 0.00He |
| HPTS 1.5 | 1.72 ± 0.00Ee | 1.73 ± 0.00Fd | 1.75 ± 0.01Ec | 1.80 ± 0.00Db | 1.82 ± 0.00Ca | 3.86 ± 0.00Ba | 3.78 ± 0.01Cb | 3.77 ± 0.01Cc | 3.68 ± 0.01Dd | 3.68 ± 0.00Cd |
| OSTS 1.5 | 1.72 ± 0.00Ae | 1.83 ± 0.00Ac | 1.85 ± 0.01Ab | 1.80 ± 0.01Bb | 1.88 ± 0.00Aa | 3.79 ± 0.01CDa | 3.70 ± 0.00Fb | 3.70 ± 0.01Fb | 3.87 ± 0.00Hc | 3.58 ± 0.00Id |
Different lower case letters in rows and different uppercase letters in columns means significant differences at probability level of 5%
Syneresis
One of the parameters that indicate the instability of gel polymer networks is syneresis. On grounds of product quality, it is suggested that this parameter be observed and controlled during storage. The syneresis of gel-like dairy products may be influenced by the type and concentration of the hydrocolloid, and by the storage time (Temesgen and Yetneberk 2015). Uncharged hydrocolloids are known to reduce the syneresis by increasing the viscosity of the continuous phase and the anionic hydrocolloids. This is achieved by interacting with the positive charges of the casein micelles, by the formation of protein–polysaccharide complexes and by strengthening the casein network. However, the use of uncharged polysaccharides at low concentrations may lead to higher syneresis due to the depletion of flocculation (Shiroodi et al. 2012). Table 3 summarizes the values of syneresis in all kashk variations during 2 months of storage at 4 °C. At the beginning of the storage (i.e. on the first day), the addition of modified starch at different concentrations acted to reduce the syneresis significantly, whereas the addition of CLTS 0.5, HPTS 0.5 and HPTS 1.0 increased the syneresis (compared to the control). However, throughout the storage period, the percent of wheying-off (or syneresis) in the following samples increased less, compared to the control. Accordingly, the syneresis of the control group was the highest among all other variations when the first month had elapsed. This difference between the following types of modified starch during storage may be attributed to the fewer number of hydrogen bonds between starch and water on the first day of storage, and therefore the syneresis had a higher value. However, throughout the storage period, the number of hydrogen bonds increased, more water molecules were entrapped, and so the syneresis became weaker. Numerous studies have shown that the addition of salt in concentrated yogurt products can increase protein–protein interactions, thereby enhancing syneresis during storage (Abu-Jdayil and Mohameed 2002; Lucey et al. 1997). The significant decrease in the syneresis of kashk variations with the addition of modified starch may be attributed to the stability of the system via the increase in the viscosity of the continuous phase when high concentrations of modified starch exist (Shiroodi et al. 2012). Another explanation could be the emulsion stability and the prevention of fat globule coalescence by centrifugal force through modified starch. This is known to cause no phase separation, and so the syneresis decreases. Akin to the current report, previous studies have considered incorporating various compounds into dairy products, i.e. the addition of 0.5% tragacanth gum to kashk (Shiroodi et al. 2012) and xanthan gum to yogurt (El-Sayed et al. 2002). Furthermore, the decrease in whey syneresis can occur by using gelatin, pectin and sodium alginate (Kumar and Mishra 2004) or by using quince seed mucilage (Nikoofar et al. 2013), cress seed mucilage and guar gum (Hassan et al. 2015). Comparing kashk variations with different types of modified starch, the one incorporated with OSTS and HPTS demonstrated the lowest and highest degree of syneresis, respectively. This phenomenon may be attributed to the type of modification in starch. Adding eight-carbon chains with hydrophobic (octenyl) and hydrophilic (acetate) properties in amylose and amylopectin chains can cause emulsifying properties. It can also enable more absorption of water by starch granules which may result in increasing the viscosity and the reduction of syneresis in kashk variations containing OSTS.
Table 3.
Syneresis values (%) and viscosity (cP) (mean ± SD) of kashk variations during 60 days of storage at 4 °C
| Sample code | Syneresis (%) | Viscosity (cP) | ||||||
|---|---|---|---|---|---|---|---|---|
| Storage time (days) | Storage time (days) | |||||||
| 0 | 15 | 30 | 45 | 60 | 0 | 30 | 60 | |
| C | 6.84 ± 0.02De | 8.24 ± 0.04Bd | 13.84 ± 0.04Ac | 19.40 ± 0.02Ab | 22.80 ± 0.07Aa | 38.25 ± 0.14Ha | 34.04 ± 0.82Eb | 30.35 ± 0.15Gc |
| CLTS 0.5 | 7.49 ± 0.02Be | 8.30 ± 0.02Bd | 9.08 ± 0.03Cc | 10.62 ± 0.05Cb | 10.93 ± 0.04Ca | 40.83 ± 0.35Fa | 36.86 ± 0.66CDEb | 32.67 ± 0.18Ec |
| HPTS 0.5 | 8.50 ± 0.02Ae | 9.39 ± 0.08Ad | 10.19 ± 0.02Bc | 11.08 ± 0.02Bb | 11.57 ± 0.10Ba | 39.98 ± 0.20Ga | 36.21 ± 0.64CDEb | 32.07 ± 0.15Ec |
| OSTS 0.5 | 1.96 ± 0.06He | 2.08 ± 0.06Gd | 2.22 ± 0.02Hc | 2.38 ± 0.03Hb | 2.53 ± 0.06Ha | 38.28 ± 0.31Ha | 34.60 ± 0.20DEb | 30.55 ± 0.04Gc |
| CLTS 1.0 | 5.53 ± 0.03Ed | 5.63 ± 0.04Dd | 5.85 ± 0.05Ec | 6.50 ± 0.08Eb | 6.80 ± 0.10Ea | 48.98 ± 0.68Da | 43.41 ± 2.82Bb | 37.92 ± 0.46Bc |
| HPTS 1.0 | 7.10 ± 0.05Ce | 7.66 ± 0.05Cd | 8.21 ± 0.03Dc | 8.70 ± 0.02Db | 8.86 ± 0.06Da | 44.10 ± 0.13Ea | 38.21 ± 0.64Cb | 32.25 ± 0.13Ec |
| OSTS 1.0 | 1.51 ± 0.02Ie | 1.57 ± 0.02Hd | 1.61 ± 0.02Ac | 1.72 ± 0.03Ib | 1.81 ± 0.03Ia | 43.77 ± 0.08Ea | 37.86 ± 0.43CDb | 31.74 ± 0.15Fc |
| CLTS 1.5 | 2.07 ± 0.06Gd | 2.23 ± 0.10Fc | 2.51 ± 0.07Gb | 2.87 ± 0.06Ga | 2.94 ± 0.04Ga | 56.84 ± 0.07Aa | 48.91 ± 4.99Ab | 40.84 ± 0.14Ac |
| HPTS 1.5 | 3.92 ± 0.05Fc | 3.95 ± 0.07Ec | 4.04 ± 0.04Fbc | 4.15 ± 0.04Fab | 4.22 ± 0.11Fa | 50.41 ± 0.04Ba | 42.47 ± 0.93Bb | 34.41 ± 0.04Cc |
| OSTS 1.5 | 0.54 ± 0.03Jc | 0.60 ± 0.03Ibc | 0.67 ± 0.01Jab | 0.72 ± 0.10Jab | 0.77 ± 0.11Ja | 49.57 ± 0.08Ca | 41.79 ± 0.27Bb | 33.63 ± 0.07Dc |
Different lower case letters in rows and different uppercase letters in columns means significant differences at probability level of 5%
Viscosity
Table 3 also shows the changes in viscosity of all kashk variants during 60 days of storage at 4 °C. The value of viscosity ranged from 38.25 cP (control) to 56.84 cP (sample CLTS 1.5). The viscosity of kashk increased significantly with the addition of all three modified forms of starch. Moreover, at a specific period of storage, the viscosity value of kashk samples increased parallel to the increase in the concentration of each modified starch. The higher value of viscosity in the samples can be explained by the binding of water with the starch granules and by the interactions between the casein micelles and the modified starch through electrostatic coupling. Indeed, the addition of modified starch reduced interactions between the casein micelles due to the presence of hydrophilic substituents (Cui et al. 2014). According to Takeuchi (1969), starch-protein interactions can be found in the form of electrostatic interactions. At an acidic pH (< 4.6)—which resembles the normal pH of all kashk variations—the casein molecules have positive charges that can interact with negatively-charged starch molecules. Moreover, as the pH value decreases, the calcium ions in the casein micelles also become more soluble and thus become more capable of interacting with negatively-charged starch molecules, thereby leading to the dominance of their gelling structure (Schmidt et al. 2001). However, some researchers have suggested that the interaction between casein and some forms of starch may lead to an increase in viscosity, but this is also accompanied by a gradual phase separation. In this regard, Kim et al. (2011) reported that the addition of a lower concentration (0.2% wt/vol) of YPT (Yam powder Thunb) in yogurt may reduce the phase separation which has a higher value of viscosity. This, however, was in contradiction with our results, as the kashk variant incorporated with OSTS had the lowest viscosity and syneresis. The increase in viscosity has been previously reported when adding FSM and FSM + CMC to yogurt (Basiri et al. 2018), adding various forms of plant starch to yogurt (Alim et al. 2016) and adding GT to kashk (Shiroodi et al. 2012). Furthermore, Sameen et al. (2016) reported that yogurts enriched with chemically extracted plant sources of starch at 0.3–0.4% (Sweet potato) and 0.2–0.3% (Taro) showed better results in terms of lowering the syneresis and increasing the water holding capacity, as well as the viscosity, compared to the yoghurt containing stabilizers such as gelatin. According to Cui et al. (2014), as the concentration of the cross-linked acetylated starch increased, so did the apparent viscosity of the set yoghurt. This was explained by the solubility of the cross-linked acetylated starch granules and the increased swelling stability thereof. By adding these forms of starch, reductions were observed in the interactions between the polymer chains (including casein micelles) because hydrophilic substituents were present. Furthermore, it can be hypothesized that electrostatic coupling helped with the assumption that casein micelles can interact with cross-linked acetylated starch.
In this study, the samples enriched with CLTS and OSTS had the highest and the lowest viscosity, respectively, at all three temporal points of measurement during storage, compared to other samples. This observation was more obvious (or significant) at higher concentrations, which is presumably due to the more stable viscosity of starch granules at high temperature, low pH values, and mechanical shear in CLTS. Indeed, the swelling of granules in this type of modified starch is more controllable, and the granules maintain their structure during heat treatment, mixing and under acidic conditions. Accordingly, compared to other modified forms of starch, the kashk that was enriched with CLTS was observed to better maintain its viscosity after the second pasteurization. The decrease in viscosity during storage may be due to the increased acidity and syneresis. Sameen et al. (2016) and Basiri et al. (2018) also reported the same patterns of change in viscosity vis-à-vis storage time.
Color
The changes in color parameters of different kashk variations stored at 4 °C during 60 days indicated that adding modified starch caused a significant increase in the lightness of the kashk variations compared to the control (p < 0.05). However, adding different kinds of modified tapioca starch with different concentrations did not have significant effects on the b* and a* values of kashk samples (XXX). The type of starch modification did affect the light scattering properties of the kashk, which subsequently caused differences in the lightness of the kashk samples. Incorporating OSTS into the kashk, compared with the other two modifications, caused the highest L* value which was more obvious, especially at higher concentrations (i.e. 1.0 and 1.5%). Moreover, with increasing the concentration of OSTS in kashk samples, the L* increased significantly, unlike the CLTS and HPTS which, parallel to the increase in their concentrations, caused the L* to decrease significantly. Substituting the hydroxyl group with octenyl succinate in OSTS may lead to steric hindrance in starch chains, and this keeps the chains away from each other, hence the higher transparency of their gel. Therefore, the samples that were enriched with this kind of modified starch had a smaller value of lightness. Moreover, the L*, a* and b* values for all of the samples did not change considerably during storage for 60 days (data not shown). The magnitude of color difference between the two food (kashk) samples is best represented by the total color difference value (ΔE). According to Table 4, a significant difference was observed between the different kashk variations regarding ΔE (p < 0.05). The sample which was enriched with OSTS at the highest test concentration (1.5%) shows the highest ΔE which confirms our previous data. Kim et al. (2011) reported that by adding YPT to yogurt, the L* and a* color values were not remarkably influenced, whereas the b* values significantly increased at all concentrations on day 0 of the storage, probably due to the original yellow color of yam powder. Schmidt et al. (2001) examined the effect of different stabilizers on the physicochemical properties of yogurt, and the result was that gelatin-stabilized yogurt had the highest L* value (similar to values of yogurts stabilized with the cross-linked starch), whereas the HP-stabilized yogurt had the lowest L* value. The a* value of gelatin-, NWS- and HPCL stabilized yogurts and the b* value of the HP-stabilized yogurt were significantly more than the other yogurt samples.
Table 4.
Total color difference and TPA parameters (mean ± SD) of kashk variations during 60 days of storage at 4 °C
| Sample code | Delta E | Hardness (g) | Work of adhesion (g s) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Storage time (days) | Storage time (days) | Storage time (days) | |||||||
| 0 | 30 | 60 | 0 | 30 | 60 | 0 | 30 | 60 | |
| C | 0.00 ± 0.00Ga | 0.00 ± 0.00Ca | 0.00 ± 0.00Ea | 448.33 ± 2.89Ia | 448.33 ± 2.89Ia | − 222.00 ± 12.17Ec | − 222.00 ± 12.17Ec | − 180.67 ± 1.15Bb | − 155.67 ± 6.03Aa |
| CLTS 0.5 | 2.35 ± 0.43Da | 2.20 ± 1.10BCa | 2.85 ± 0.53Ca | 695.00 ± 5.00Hb | 702.67 ± 4.62Hab | − 192.33 ± 2.52BCc | − 192.33 ± 2.52BCc | − 179.67 ± 0.58Bb | − 167.00 ± 1.73Ba |
| H PTS 0.5 | 4.28 ± 0.26Ba | 3.08 ± 1.80Ba | 4.16 ± 0.07Ba | 785.00 ± 5.00Eb | 792.67 ± 2.31Eb | − 197.33 ± 2.08BCDc | − 197.33 ± 2.08BCDc | − 180.67 ± 0.58Bb | − 152.33 ± 2.52Aa |
| OSTS 0.5 | 1.99 ± 0.25EDb | 2.92 ± 0.44Ba | 1.52 ± 0.18Db | 735.00 ± 5.00Gb | 740.83 ± 1.44Gb | − 172.00 ± 2.65Ab | − 172.00 ± 2.65Ab | − 170.67 ± 1.15Ab | − 167.33 ± 2.08Ba |
| CLTS 1.0 | 1.14 ± 0.24Fa | 1.69 ± 0.47BCa | 1.61 ± 0.34Dba | 865.00 ± 5.00Cb | 871.67 ± 1.53Cb | − 198.33 ± 2.08CDc | − 198.33 ± 2.08CDc | − 185.33 ± 0.58Cb | − 177.33 ± 0.58Ca |
| HPTS 1.0 | 2.14 ± 0.80Db | 2.80 ± 0.49Bab | 3.31 ± 0.26BCa | 800.33 ± 5.51Db | 807.00 ± 7.00Db | − 280.00 ± 1.00Fc | − 280.00 ± 1.00Fc | − 260.33 ± 0.58Gb | − 245.33 ± 1.53Fa |
| OSTS 1.0 | 3.05 ± 0.09Ca | 2.76 ± 1.60Ba | 3.43 ± 0.61BCa | 769.00 ± 3.61Fb | 773.83 ± 2.89Fb | − 190.33 ± 0.58Bb | − 190.33 ± 0.58Bb | − 189.33 ± 0.58Db | − 185.67 ± 2.08Da |
| CLTS 1.5 | 1.280 ± 0.24Fb | 2.69 ± 0.83Ba | 1.38 ± 0.37Db | 915.00 ± 5.00Ba | 917.83 ± 2.47Bab | − 204.33 ± 2.08Dc | − 204.33 ± 2.08Dc | − 195.67 ± 0.58Eb | − 187.00 ± 1.00Da |
| HPTS 1.5 | 1.41 ± 0.00EFa | 2.29 ± 1.90BCa | 1.55 ± 0.63Da | 9993.33 ± 4.16Aa | 994.50 ± 3.46Ab | − 301.67 ± 1.15Gc | − 301.67 ± 1.15Gc | − 284.33 ± 0.58Hb | − 260.67 ± 1.53Ga |
| OSTS 1.5 | 5.90 ± 0.45Aa | 5.49 ± 2.18Aa | 6.32 ± 1.22Aa | 799.67 ± 1.53Db | 805.00 ± 4.33Db | − 227.00 ± 2.00Eb | − 227.00 ± 2.00Eb | − 224.67 ± 0.58Fb | − 220.00 ± 1.00Ea |
Different lower case letters in rows and different uppercase letters in columns means significant differences at probability level of 5%
TPA analysis
Hardness
Hardness and adhesiveness values of the kashk variations enriched with different forms of tapioca starch at various levels over the storage period are shown in Table 4. Hardness is defined as the force necessary to crush the sample in the mouth. It can be seen that the hardness value increases by adding different forms of modified tapioca starch, and by increasing their concentrations (p < 0.05). An increased value of hardness among the different varieties of kashk may be attributed to the increase in the dry matter content, which happens by adding starch stabilizers. Moreover, the involvement of reactive groups with the amylase/amylopectin part of the starch chain may contribute to its emulsifying property which leads to a higher level of water absorption, greater viscosity in the continuous phase and a firmer texture in the treated kashk. Previous results reported by Alim et al. (2016), Schmidt et al. (2001) and Cui et al. (2014) confirm the use of adding modified starch (with or without homogenization), gelatin, native or modified wheat starch and cross-linked acetylated starch to yogurt. On the contrary, Michael et al. (2010), Nikoofar et al. (2013) and Basiri et al. (2018) reported a decrease in the hardness value of yogurt when enriching it with plant extracts, quince seed mucilage and flaxseed mucilage, either independently or in combination with carboxymethyl cellulose. According to our results, the type of starch modification appeared to affect the hardness of kashk variations. Among samples of the same concentrations, the ones enriched with HPTS and OSTS were respectively the most and the least firm samples. Indeed, OSTS has both hydrophilic and hydrophobic natures derived from the octenyl succinate functional groups located on it. Therefore, the OSTS has emulsifying properties and thus can absorb more water, which may allow the starch to remain more hydrated. This would account for the softer gel texture observed in the kashk samples containing OSTS. Another explanation is the lower occurrence of retro-gradation in this type of modified starch, which is actually the re-association of amylose chains and the formation of the gel structure. These factors cause the starch-enriched kashk to be more stable and less firm at lower temperatures. However, this was contrary to previous results reported by Schmidt et al. (2001), where the type of starch modification did not affect the gel firmness of yogurt. They suggested that the negatively-attached hydroxypropylated or acetylated functional groups may interfere with the amylose–amylose associations which causes the starch to remain hydrated, stable and account for the weaker gel texture (Yook et al. 1993). Our results suggest that, over the period of storage, the hardness of each treatment group increased, but the differences were not significant (p < 0.05) in most treatment groups, especially until the first month of storage. The changes in arrangement and the binding of proteins with each other may affect the increasing trend of firmness during storage. By the end of storage (on day 60), the control sample had the lowest value of firmness, and the one enriched with HPTS (1.5%) had the highest value of firmness. Similarly, Salvador and Fiszman (2004) reported that the textural properties (hardness) of whole and skimmed flavored set-type yogurt increased during long periods of storage.
Work of adhesion
Work of adhesion (g.s) refers to the work needed to overcome the attractive forces between the surface of the food and other substances with which the food is in contact. As shown in Table 4, on the first day of storage, the different forms of modified tapioca starch significantly reduced the work of adhesion in kashk samples, while the ones enriched with HPTS (1%), HPTS (1.5%) had higher values of adhesion work compared to the control. However, by the end of the storage period (on day 60), all samples had significantly higher values of adhesion work, compared to the control sample, especially at higher concentrations of starch.
Moreover, during storage, the work of adhesion of all samples decreased significantly. It is believed that keeping liquid kashk samples over time in the refrigerator makes amylose become more likely to bind with casein micelles, and therefore a stronger gel structure and a three-dimensional network is formed with more stiffness which cause the work of adhesion to decrease. This result is similar to the study reported by Tavakolipour et al. (2014) regarding higher concentrations of gelatin (0.025, 0.05 and 0.1 w/w) and modified starch (1 and 1.5 w/w) which were incorporated into low fat concentrated flavored yogurt. The result was a significant decrease in adhesiveness and an increase in hardness (p < 0.05). Their results revealed a negative relationship between adhesiveness and hardness. According to the report, the decrease in adhesiveness is attributed to the formation of a weak three-dimensional network caused by an increase in the hydrocolloid concentration. The high content of gelatin caused low values of adhesiveness in those samples, and therefore the yogurt acquired a soft and very low rubbing texture. Regarding the type of starch modification and the effect of each starch, it can be stated that the HPTS showed a significantly higher value of work of adhesion in kashk samples. The more the stabilizer effect, the greater the work of adhesion. This can be attributed to the acetyl and hydroxypropyle groups on HPTS and its ability to create a more uniform texture in kashk.
Sensory evaluation
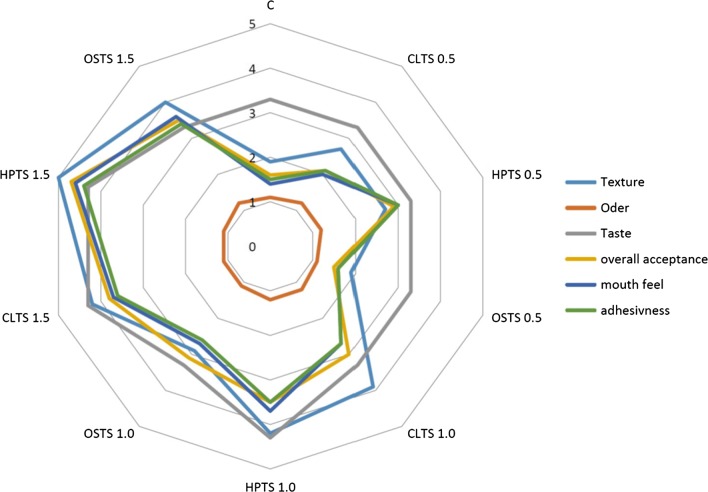
The average scores for sensory evaluation of kashk variations with or without different modified tapioca starch at 4 °C are shown in Fig. 2. The statistical analysis revealed that while there was no significant difference between the texture and odor of all kashk variations during the storage period, the scores for adhesiveness, mouthfeel, flavor and overall acceptability decreased significantly during the storage period (data not shown). Similar to our results, the sensory indices of yogurts containing CMC, FSM or both (Basiri et al. 2018), and different concentrations of starch (Sameen et al. 2016) decreased with storage. On day 1, the kashk enriched with HPTS (1.5%) and the control, respectively, had the highest and lowest sensory attributes regarding flavor, mouth feel, texture, adhesiveness and overall acceptability, while odor was an exception. Generally, stabilizers settle in the aqueous phase of the product, and this causes less water syneresis during storage. Moreover, they play an important role in the uniform distribution of flavoring materials, preventing air bubble collapse and creating a good creamy mouth-feel. It has been stated that the texture can influence flavor perception, and an increase in the concentration of hydrocolloids generally leads to a decrease in aroma and taste (Tournier et al. 2007). Similar to the following sentence, according to our results, we realize that increasing the concentration of different forms of tapioca modified starch leads to an increase in the values of texture, adhesiveness, mouthfeel, flavor and overall acceptability of kashk variations. In agreement with our results, Basiri et al. (2018) reported that, after 21 days of storage, the taste, mouth feel and overall acceptance of yogurt, enriched with CMC, decreased significantly. However, the appearance and texture of yogurt did not change significantly. They attributed this decrease to the decrease in the amount of acetaldehyde which is the main aromatic compound in yogurt. In general, based on the scores of our panelists, the kashk samples enriched with HPTS showed the highest score of texture, adhesiveness and overall acceptance, and the ones enriched with OSTS showed the highest score of odor. But regarding flavor and mouth feel, no clear trend was observed.
Fig. 2.
A graphic representation of the sensory evaluation for kashk variations
Conclusion
The present study showed that all three forms of modified tapioca starch (CLTS, OSTS and HPTS) can be used with the aim of improving the physicochemical, sensory properties and overall acceptability of the kashk samples. The acidity, hardness and viscosity increased, whereas the syneresis decreased as a result of adding the different forms of modified tapioca starch. The mentioned trends intensified when higher concentrations of starch were used. The kashk enriched with HPTS had the highest value of hardness, adhesiveness and syneresis, while the sample with CLTS had the highest value of viscosity. According to the sensory scores, it was observed that the use of HPTS (1.5%) can be an optimal option which can yield a broader range of acceptance among the consumers of liquid kashk, while the samples without stabilizers (the control) and the ones with OSTS (0.5%) are the least preferred. This research can provide opportunities for future applications of the modified tapioca starch at an industrial level, and it can be used for the production of industrial liquid kashk. Future research can examine the possible application of these modified tapioca starch on other dairy products. Specifically, the potential for a broader marketability of kashk variants enriched with these types of starch can be investigated.
Acknowledgements
The authors are grateful to Mohsen Hamedpour-Darabi for editing the English research language.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abu-Jdayil B, Mohameed H. Experimental and modelling studies of the flow properties of concentrated yogurt as affected by the storage time. J Food Eng. 2002;52:359–365. doi: 10.1016/S0260-8774(01)00127-3. [DOI] [Google Scholar]
- Alim MA, Wadehra A, Singh AK. Effect of various plant starches on the quality characteristics of starch-based sweetened cow milk yoghurt. J Bangladesh Agric Univ. 2016;14(1):119–126. doi: 10.3329/jbau.v14i1.30606. [DOI] [Google Scholar]
- AOAC . Official methods of analysis. Washington, DC: Association of Official Analytical Chemists; 2000. [Google Scholar]
- Basiri S, Haidary N, Shekarforoush SS, Niakousari M. Flaxseed mucilage: a natural stabilizer in stirred yogurt. Carbohydr Polym. 2018;187:59–65. doi: 10.1016/j.carbpol.2018.01.049. [DOI] [PubMed] [Google Scholar]
- Cui B, Lu Y-m, Tan C-p, Wang G-q, Li G-H. Effect of cross-linked acetylated starch content on the structure and stability of set yoghurt. Food Hydrocoll. 2014;35:576–582. doi: 10.1016/j.foodhyd.2013.07.018. [DOI] [Google Scholar]
- Damir AA, Salama AA, Safwat Mohamed M. Acidity, microbial, organic and free amino acids development during fermentation of skimmed milk, Kishk. Food Chem. 1992;43:265–269. doi: 10.1016/0308-8146(92)90210-S. [DOI] [Google Scholar]
- Diamantino VR, Beraldo FA, Sunakozawa TN, Penna ALB. Effect of octenyl succinylated waxy starch as a fat mimetic on texture microstructure and physicochemical properties of Minas fresh cheese. LWT Food Sci Technol. 2014;56:356–362. doi: 10.1016/j.lwt.2013.12.001. [DOI] [Google Scholar]
- El-Sayed E, Abd El-Gawad I, Murad H, Salah S. Utilization of laboratory-produced xanthan gum in the manufacture of yogurt and soy yogurt. Eur Food Res Technol. 2002;215:298–304. doi: 10.1007/s00217-002-0551-9. [DOI] [Google Scholar]
- Ghorbani Gorji E, Mohammadifar MA, Ezzatpanah H. Influence of gum tragacanth, Astragalus gossypinus, addition on stability of nonfat Doogh, an Iranian fermented milk drink. Int J Dairy Technol. 2010;64:262–268. doi: 10.1111/j.1471-0307.2010.00658.x. [DOI] [Google Scholar]
- Hassan LK, Haggag HF, ElKalyoubi MH, AbdEL-Aziz M, El-Sayed MM, Sayed AF. Physico- chemical properties of yogurt containing cress seed mucilage or guar gum. Ann Agric Sci. 2015;60(1):21–28. doi: 10.1016/j.aoas.2014.11.021. [DOI] [Google Scholar]
- Iranian National Standards (1990) Specification for liquid kashk. Number 2452 (in Persian)
- Iranian National Standards (2009) Industrial liquid kashk specifications. Number 6127 (in Persian)
- Jafari M, Ghaisari HR, Jahed Khaniki G, Shariatifar N. Comparison of chemical and microbiological characteristics between traditional and industrial kashks in the Fars Province. Ann Food Sci Technol. 2015;16(2):462–470. [Google Scholar]
- Keogh M, O’Kennedy B. Rheology of stirred yogurt as affected by added milk fat, protein and hydrocolloids. J Food Sci. 1998;63:108–112. doi: 10.1111/j.1365-2621.1998.tb15687.x. [DOI] [Google Scholar]
- Kim SH, Lee SY, Palanivel G, Kwak HS. Effect of Dioscorea opposita Thunb. (yam) supplementation on physicochemical and sensory characteristics of yogurt. J Dairy Sci. 2011;94(4):1705–1712. doi: 10.3168/jds.2010-3918. [DOI] [PubMed] [Google Scholar]
- Klemaszewski J. Hydrocolloids in cultured dairy products (chapter 7) In: Laaman TR, editor. Hydrocolloids in food processing. Hoboken: Blackwell; 2011. pp. 141–164. [Google Scholar]
- Kumar P, Mishra HN. Mango soy fortified set yogurt: effect of stabilizer addition on physicochemical, sensory and textural properties. Food Chem. 2004;87:501–507. doi: 10.1016/j.foodchem.2003.12.022. [DOI] [Google Scholar]
- Lobato-Calleros C, Ramírez-Santiago C, Vernon-Carter EJ, Alvarez-Ramirez J. Impact of native and chemically modified starches addition as fat replacers in the viscoelasticity of reduced-fat stirred yogurt. J Food Eng. 2014;131:110–115. doi: 10.1016/j.jfoodeng.2014.01.019. [DOI] [Google Scholar]
- Lucey JA, Van Vliet T, Grolle K, Geurts T, Walstra P. Properties of acid casein gels made by acidification with glucono-δ-lactone. 1. Rheological properties. Int Diary J. 1997;7:381–388. doi: 10.1016/S0958-6946(97)00027-7. [DOI] [Google Scholar]
- Mbaeyi-Nwaoha IE, Nnagbo CL, Obodoechi CM, Nweze BC, Okonkwo TM. Production and evaluation of yoghurt contained local stabilizers—Brachystegia eurycoma (‘Achi’) and Detarium microcarpuim (‘Ofo’) Int J Biotechnol Food Sci. 2017;5(2):23–31. [Google Scholar]
- Michael M, Phebus RK, Schmidt KA. Impact of a plant extract on the viability of Lactobacillus delbrueckii ssp. bulgaricus and Streptococcus thermophilus in nonfat yogurt. Int Diary J. 2010;20:665–672. doi: 10.1016/j.idairyj.2010.03.005. [DOI] [Google Scholar]
- Mishra S, Rai T. Morphology and functional properties of corn, potato and tapioca starches. Food Hydrocoll. 2006;20(5):557–566. doi: 10.1016/j.foodhyd.2005.01.001. [DOI] [Google Scholar]
- Nikoofar E, Hojjatoleslami M, Shariaty MA. Surveying the effect of quince seed mucilage as a fat replacer on texture and physicochemical properties of semi fat set yogurt. Int J Farm Alli Sci. 2013;2(20):861–865. [Google Scholar]
- Olorunnisomo OA, Ososanya TO, Adedeji AY. Influence of stabilizers on composition, sensory properties and microbial load of yoghurt made from zebu milk. Int J Dairy Sci. 2015;10(5):243–248. doi: 10.3923/ijds.2015.243.248. [DOI] [Google Scholar]
- Salvador A, Fiszman S. Textural and sensory characteristics of whole and skimmed flavored set-type yogurt during long storage. J Dairy Sci. 2004;87:4033–4041. doi: 10.3168/jds.S0022-0302(04)73544-4. [DOI] [PubMed] [Google Scholar]
- Sameen A, Umair Sattar M, Javid A, Ayub A, Issa Khan M. Quality evaluation of yoghurt stabilized with sweet potato (Ipomoea batatas) and taro (Colocassia esculenta) starch. Int J Food Allied Sci. 2016;2(1):23–29. doi: 10.21620/ijfaas.2016123-29. [DOI] [Google Scholar]
- Schmidt KA, Herald TJ, Khatib KA. Modified wheat starches as stabilizers in set-style yogurt. J Food Qual. 2001;24:421–434. doi: 10.1111/j.1745-4557.2001.tb00620.x. [DOI] [Google Scholar]
- Shiroodi SG, Mohammadifar MA, Gorji EG, Ezzatpanah H, Zohouri N. Influence of gum tragacanth on the physicochemical and rheological properties of kashk. J Dairy Res. 2012;79(1):93–101. doi: 10.1017/S0022029911000872. [DOI] [PubMed] [Google Scholar]
- Soltani M, Güzeler N. The production and quality properties of liquid kashks. GIDA. 2013;38(1):1–7. [Google Scholar]
- Soukoulis C, Panagiotidis P, Koureli R, Tzia C. Industrial yoghurt manufacture: monitoring of fermentation process and improvement of final product quality. J Dairy Sci. 2007;90:2641–2654. doi: 10.3168/jds.2006-802. [DOI] [PubMed] [Google Scholar]
- Takeuchi I. Interaction between protein and starch. Cereal Chem. 1969;46:570–576. [Google Scholar]
- Tavakolipour H, Vahid-moghadam F, Jamdar F. Textural and sensory properties of lowfat concentrated flavored yogurt by using modified waxy corn starch and gelatin as a fat replacer. Int J Biosci. 2014;5(6):61–67. doi: 10.12692/ijb/5.6.61-67. [DOI] [Google Scholar]
- Temesgen M, Yetneberk S. Effect of application of stabilizers on gelation and synersis in yoghurt. Food Sci Qual Mang. 2015;37:90–102. [Google Scholar]
- Tournier C, Sulmont-Rosse C, Guichard E. Flavour perception: aroma, taste and texture interactions. Food. 2007;1(2):246–257. [Google Scholar]
- Yook C, Pek UH, Park KH. Gelatinization and retrogradation characteristics of hydroxypropylated and cross-linked rice. J Food Sci. 1993;58(2):405–407. doi: 10.1111/j.1365-2621.1993.tb04285.x. [DOI] [Google Scholar]