Abstract
Abstract
Saffron, obtained from dry stigmas of the flowers of Crocus sativus L. (fam. Iridaceae), is an ancient spice and a natural food colorant that has been used to treat various diseases in the long human history. Crocetin is of the main secondary metabolites of saffron and its curative properties for many ailments have been revealed in the previous scientific reports. The aim of this study was to evaluate the anticancer potentials of saffron extracts and its pure crocetin compounds against human cancer cells. The cytotoxic and antiproliferative activities along with lactate dehydrogenase activities of extracts and crocetin, a carotenoid derived from saffron, were assessed using A549, MCF-7 and HeLa human cancer cells, and compared to the non-malignant HUVECs. Additionally, apoptotic activity in the cells treated and untreated with the extracts and pure crocetin were determined in terms of DNA fragmentation. The results showed the extracts and crocetin from saffron induced cytotoxicity, enhanced cancer cell death as well as inhibited cancer cell growth in a concentration and time dependent manner. In addition, the results revealed that the tested compounds at different concentration had no cytotoxic effects on the non-malignant cells, whereas, it could significantly decrease the cell viability and proliferation in the malignant cells. As compared to anticancer potentials of the analyzed extracts and its pure crocetin compounds, crocetin was found as the more potent one. Overall, this research suggests that crocetin is a potential anticancer agent that can be used for cancer prevention and treatment.
Graphic abstract
Keywords: Antiproliferative, Cancer, Cell death, Crocetin, Crocus sativus L., Saffron
Introduction
Cancer, the second leading cause of death, is one of the major public health problem almost all around the world. It continues to have a significant incidence and mortality rates both in the developing and developed countries. Lack of the effective therapeutic strategies for control of the cancer is the main factor that accounting for its high mortality rates globally (Siegel et al. 2018; Gezici and Sekeroglu 2019a).
Treatment of cancer are generally classified into various categories: surgery, radiation therapy, hormone therapy, chemotherapy, targeted and herbal therapy. Among them, phytotherapy has become an extremely important strategy for cancer prevention in recent years, due to its superiority in the cancer treatment and prevention (Pan et al. 2010; Song et al. 2018). In fact, it has been clearly proved that medicinal plants are rich sources of bioactive compounds, which could provide significant benefits in managing the cancer. Therefore, current researches in cancer prevention have focused on developing new, safe, effective and selective anticancer agents obtained from these plants (Balunas and Kinghorn 2005; Tan and Norhaizan 2018).
From past to present, natural plant products have a wide range of uses for prevention and treatment of malignancies in traditional medicine, because of presence of many natural bioactive secondary metabolites. Additionally, the active ingredients of many cancer drugs currently in use have been isolated from medicinal plants and their natural compounds such as taxol, vinblastine, vincristine, podophyllotoxin, camptothecin, doxorubicin, resveratrol, paclitaxel, salvicine, curcumin, etc. Moreover, many compounds from natural products have been reported to inhibit the cell growth and progression of certain types of cancers, thus being potentially useful for tumor suppressing as chemoprevention agents (Reddy et al. 2003; Newman and Cragg 2016; Gezici and Sekeroglu 2017; Gezici et al. 2017; Roleira et al. 2018; Seca and Pinto 2018; Gezici and Sekeroglu 2019a; Gezici 2019).
The dried stigmas of Crocus sativus L. (Iridaceae), also known as Saffron, is native to Asia and cultivated in Greece, Spain, Iran, Italy and Turkey. Since ancient times, saffron has been widely used as a spice, tea, oil, food colorant and flavoring in diets (Giaccio 2004; Gutheil et al. 2012). Besides food purposes, it has been used in the traditional medicine for treatment of various diseases, including insomnia, cognitive deficits, cardiovascular diseases and cancer. Saffron possesses a wide range of bioactive constituents, particularly carotenoids that are crucial for human health. The major components of saffron include crocin, picrocrocein, safranal and crocetin. Among them, crocetin, a carboxylic carotenoid, have demonstrated as potential activities in chemoprevention, retinal function recovery and inhibition neurodegenerative diseases (Li et al. 2012; Bathaie et al. 2014; Festuccia et al. 2014).
Researches on exploring effect of anticancer and antitumor activities of saffron and its pure components to manage cancer development showed that they have several mechanisms including inhibition of polynucleotide biosynthesis and colony formation, inducing apoptosis through increasing caspases activation and interaction with cyclin-dependent growth factors, and also suppressing expressions of proto-oncogenes. Although, saffron and its pure components have multiple mechanisms involved in chemoprevention effects, but the previous studies indicated that saffron and its each components possess the difference in the mechanism of action. The effect of crocetin involved in cancer prevention metabolism have been elaborated against oxidative stress by reduction of malondialdehyde level, improving the levels of glutathione and anti-oxidant enzymes along with reducing lipid peroxidation. Moreover, carotenoids found highly in saffron are able to enhance cancer prevention mechanisms by acting as a strong antioxidant that have been revealed previously. On the other hand, chemoprevention activity of saffron and its pure components seem to occur through their anti-proliferative, anti-oxidant and anti-inflammatory effects (Gutheil et al. 2012; Christodoulou et al. 2015; Patel et al. 2017; Ashktorab et al. 2019).
Previous in vitro and in vivo various studies in regards of biological activities of C. sativus L. showed that it possesses comprehensive pharmacological properties such as antioxidant, antiatherosclerotic, cardioprotective, hepatoprotective, etc. (Abdullaev et al. 2003; Li et al. 2012; Bathaie et al. 2013; Kim et al. 2014). In view of the valuable biological activities of the saffron, the current study was aimed to investigate the activities of saffron on cytotoxicity, cell proliferation and necrosis in the human cancer cells by using in vitro test systems including MTT, trypan blue exclusion, and lactate dehydrogenase activity assays. To the best of our knowledge, this is the first comparative assessment of in vitro anticancer and antiproliferative activities against A549, MCF-7, and HeLa cancer cells treated with the extracts of saffron and its bioactive component, crocetin. From this point of view, this study includes the first comparative data for the literature.
Materials and methods
Chemicals and plant material
Crocetin, dimethyl sulfoxide (DMSO), phosphate buffer saline (PBS), fetal bovine serum (FBS) and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were purchased from Sigma (Sigma-Aldrich, USA). Roswell Park Memorial Institute Medium (RPMI 1640), Dulbecco’s modified Eagle medium (DMEM) and the other chemicals purchased from ThermoFisher Scientific (USA). The dried stigmas of C. sativus L. were obtained from a local herbal market, and a voucher specimen was deposited in the Biology Department of Kilis 7 Aralik University, Turkey.
Preparation of saffron extracts
The stigmas of the plant were dried at room temperature (25 °C) with no direct light, and powdered samples were extracted with distilled water and ethanol (70%) as previously described (Senol et al. 2018; Gezici and Sekeroglu 2019b). Extracts were filtered and concentrated under vacuum at 40 °C using a Rotary Evaporator (Heidolph, Laborota 4010), and then saffron extracts were kept at − 20 °C until use. The saffron extracts and crocetin were dissolved in DMSO at different concentrations for the analyses.
Cell lines and cell culture conditions
Human lung carcinoma (A549), breast adenocarcinoma (MCF-7), cervical cancer (HeLa), compared to the non-malignant human umbilical vein endothelial cell (HUVEC) lines, obtained from the American Type Culture Collection (ATCC, USA) were used for determination cytotoxic, antiproliferative, and lactate dehydrogenase enzyme activities of saffron extracts and crocetin. A549 and HeLa cells were cultured on RPMI 1640, supplemented with 10% (v/v) FBS, 1% antibiotics (100 U/ml penicillin/100 µg/ml streptomycin), and 1% l-glutamine, while HUVEC and MCF-7 cells were maintained in DMEM, containing with the same supplements. The cells were grown in the incubator at 37 °C equilibrated with 5% CO2.
Cytotoxicity assay
For determination cytotoxic potentials of extracts and crocetin from saffron, MTT assay was carried out as reported by Mosmann (1983), and incubation with the saffron extracts and crocetin were performed under the same conditions as described by the previous research (Gezici et al. 2017). All the assays were carried out in triplicate using the cell lines from passage 24 or less than, and the dose response curve was used to generate the IC50 (µg/mL) values for each cell line. The absorbance was measured at 570 nm with a Thermo Lab systems 408 Multiskan microplate spectrophotometer and the inhibition (%) was calculated as follow equilibration;
Trypan blue exclusion assay
Antiproliferative activities of the saffron extracts and crocetin were evaluated by using trypan blue exclusion method described previously by Strober (2015), with minor modification. Briefly, the cell suspensions (a density of 1 × 104 cells/well) were plated into a 96 well plates and incubated in 37 °C with 5% CO2 for 24 h. Afterwards, the cells were treated with the saffron extracts and crocetin with the concentration from 0, 100, 200, 500 to 1000 µg/mL at various time intervals (24, 48, and 72 h). In this assay, all the chemicals and conditions were same as the previous publication (Gezici 2018). The cell viability was determined microscopically (Nicon, Japan), and the viable cells were counted an automated cell counter (Cedex XS Analyzer).
Lactate dehydrogenase (LDH) assay
LDH release activity was conducted for determination of necrosis in the cultured cells described by Al-Qubaisi et al. (2011) previously. In brief, a density of 2 × 104 cells/well were seeded in 96-well plates. And then, the attached cells on the plates were treated with 0, 100, 200, 500 and 1000 µg/mL concentrations of the extracts and crocetin and incubated for 24, 48 and 72 h, respectively. All the solutions and process were performed in the same as previously described (Gezici 2018, 2019). For determination of the potential LDH activity of the tested samples, the plates were read at wavelength 340 nm using an ELISA microplate spectrophotometer system (Thermo Lab systems 408 Multiskan). The percentage of LDH release in medium was calculated as given below, and the IC50 (µg/mL) values for each cell line was also measured to generate the concentration of test sample needed for 50% inhibition of cell growth.
Apoptotic DNA fragmentation assay
Apoptotic DNA-Ladder kit method was used to reveal apoptotic DNA cleavage according to the protocol described earlier (Chakraborty et al. 2010). In brief, a density of 10 × 104 cells/well were seeded onto a 24-well plate and incubated for 48 h at 37 °C. To isolate the DNA, the cells were harvested, lysed, washed and centrifuged according to the manufacturer’s protocol (QIAamp DNA mini kit, Qiagen). Then, concentrations of the DNA were measured at wavelength 260 nm using an ELISA microplate spectrophotometer system (Thermo Lab systems 408 Multiskan). Thereafter, oligonucleosomal fragmentation of DNA, a characteristic feature of apoptotic cell death, was analyzed electrophoretically at 60 V/cm in a 1.5% agarose gel, and the DNA fragmentations were imaged with UV transilluminator (Bio Rad, USA) when stained with ethidium bromide.
Statistics
The data were expressed as mean and standard error of mean (mean ± SEM) from at least triplicate analyses, for each test sample and each concentration. IC50 values were calculated using a linear regression analysis. The significance of differences between treated and control groups were evaluated by using the SPSS Statistics 20 software (IBM Corporation), and p < 0.05 was considered as statistically significant, p < 0.01 was also considered as statistically very significant.
Results
Cytotoxicity assay results
MTT assay was performed to test the cytotoxic effects of the extracts of saffron and its crocetin component on three human cancer cells including lung carcinoma (A549), breast adenocarcinoma (MCF-7), cervical cancer (HeLa), and non-tumor human umbilical vein endothelial cells (HUVEC). Cytotoxic activities of the samples based on their potential cell growth inhibition are given regarding of IC50 values as dose dependently after 48 h incubation period (Table 1).
Table 1.
Cytotoxic potentials of the extracts from saffron and its crocetin component
| Cell lines | Treatment | Concentrations (µg/mL)a | ||||
|---|---|---|---|---|---|---|
| 50 µg/mL | 100 µg/mL | 200 µg/mL | 500 µg/mL | 1000 µg/mL | ||
| A549 | Water extract | 69.80 ± 0.17** | 78.63 ± 0.43** | 67.08 ± 0.34* | 78.30 ± 0.51** | 70.42 ± 0.18* |
| Ethanol extract | 74.82 ± 0.12** | 72.25 ± 0.64* | 58.92 ± 0.35* | 60.57 ± 0.21** | 61.86 ± 0.06** | |
| Crocetin | 65.84 ± 0.08* | 56.09 ± 0.42** | 49.50 ± 0.17** | 46.21 ± 0.15** | 48.61 ± 0.22* | |
| MCF-7 | Water extract | 76.32 ± 0.52* | 70.03 ± 0.26** | 63.19 ± 0.56** | 70.14 ± 0.32** | 62.42 ± 0.31* |
| Ethanol extract | 71.02 ± 0.34* | 68.64 ± 0.51** | 50.72 ± 0.18** | 56.07 ± 0.15** | 48.28 ± 0.26* | |
| Crocetin | 66.10 ± 0.18** | 51.83 ± 0.21** | 48.14 ± 0.03** | 36.16 ± 0.12* | 38.19 ± 0.36** | |
| HeLa | Water extract | 84.19 ± 0.06** | 80.58 ± 0.21** | 76.03 ± 0.12** | 69.35 ± 0.11** | 40.75 ± 0.41* |
| Ethanol extract | 72.90 ± 0.29** | 64.41 ± 0.07** | 81.72 ± 0.39* | 50.24 ± 0.42** | 43.46 ± 0.21** | |
| Crocetin | 61.32 ± 0.18* | 49.81 ± 0.85** | 32.15 ± 0.16** | 38.60 ± 0.04* | 35.27 ± 0.56* | |
| HUVEC | Water extract | 18.32 ± 0.18** | 16.12 ± 0.06** | 12.80 ± 0.14* | 18.243 ± 0.61** | 14.92 ± 0.08* |
| Ethanol extract | 14.68 ± 0.28** | 14.02 ± 0.10** | 13.05 ± 0.02** | 12.14 ± 0.04* | 11.62 ± 0.06** | |
| Crocetin | 15.90 ± 0.09* | 12.52 ± 0.27** | 13.62 ± 0.10* | 11.60 ± 0.62** | 8.23 ± 0.14** | |
| Doxorubicinb | 4.28 ± 0.01 | 4.02 ± 0.15 | 3.48 ± 0.12 | 3.46 ± 0.04 | 3.08 ± 0.21 | |
**p value of < 0.01 and *p value of < 0.05
aValues are reported as IC50 ± SEM from after 48 h incubation period (n = 3)
bDoxorubicin. positive control
The results obtained from MTT assay demonstrated that both the extracts of saffron and its crocetin component had great cytotoxic potentials towards the tested human cancer cells, however the IC50 values were varied depending on the cancer cells. Additionally, treated concentration of the tested samples considerably affect the anticancer potentials of saffron on the human cancer cells. Among the water and ethanol extracts of saffron and its crocetin component, crocetin was found to have higher cytotoxic effects, than that of the extracts (Table 1).
As given in the Table 1, crocetin exhibited the highest anticancer activity on HeLa cells with the IC50 values in range of 35.27 ± 0.56–61.32 ± 0.18 µg/mL, followed by MCF-7 and A549 cells with the IC50 values 51.83 ± 0.21–56.09 ± 0.42 µg/mL, respectively at 100 µg/mL concentration. As for the extracts of saffron, the water and ethanol extracts of saffron were also found to be highly cytotoxic potentials towards all the cancer cell lines with IC50 values between 64.41 ± 0.07 and 80.58 ± 0.21 µg/mL at 100 µg/mL concentration. The ethanol extract of saffron showed the highest cytotoxic effects against HeLa cells (IC50 = 64.41 ± 0.07 µg/mL), whilst the water extract of saffron demonstrated the highest one towards MCF-7 cells (IC50 = 70.03 ± 0.26 µg/mL, at 100 µg/mL concentration).
Based on the cytotoxicity assay, these findings demonstrated that saffron exhibited significant cytotoxicity against the tested human cancer cells by decreasing the cell growth as well as increasing cell death. In the light of the findings, it could be concluded that saffron and crocetin could have significant potential as anti-cancer agents.
Trypan blue assay results
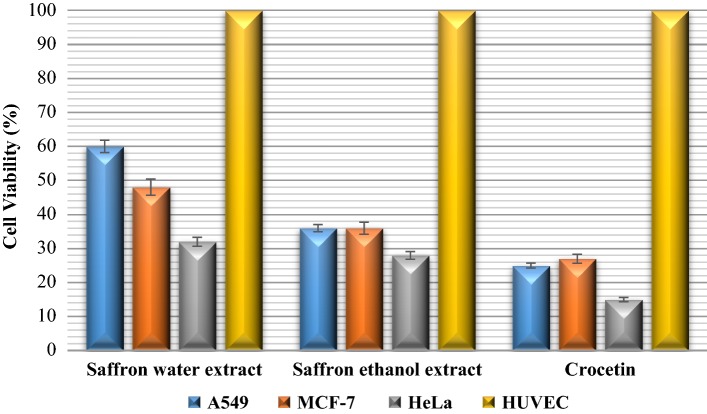
Antiproliferative effects of saffron extracts and crocetin at different concentrations were assessed by trypan blue assay for 24, 48, 72 h treatment. The assay was performed by cell counting that non-viable cells were visible as blue colored, while, viable cells were visible unstained under the microscope. The viability (%) percentage of A549, MCF-7, HeLa cancer cell lines after 48 h treatment with the extracts and crocetin were shown in the Fig. 1.
Fig. 1.
Antiproliferative assay results of the cells treated with 500 µg/mL concentration of the extracts and crocetin. Values are expressed as cell viability (%) from three independent experiment (n = 3). HUVEC cells were used as control and set as 100%
As consistent with cytotoxicity assay, results of antiproliferative assay revealed that both the extracts of saffron and its crocetin component inhibited cell growth and viability of all cancer cell lines in a time and dose dependent manner. Among the extracts of saffron and its crocetin component, crocetin caused much more reduction in the cell viability with the percentage of 15–27% in the cancer cells than that of the ethanol extract, following by the water extract (32–60% cell viability percentage). As can be seen from Fig. 1, all the tested samples showed greater inhibitory effects in cell growth of HeLa cells with survival percentage of 15–32%, as compared to that of A549 and MCF-cells.
The results obtained from trypan blue assay indicated that saffron exhibited great inhibitory effects on the cell growth in the treated human cancer cells, and the increasing exposure time and dose resulted in decreasing the cell viability in all the cell lines.
Lactate dehydrogenase (LDH) activity results
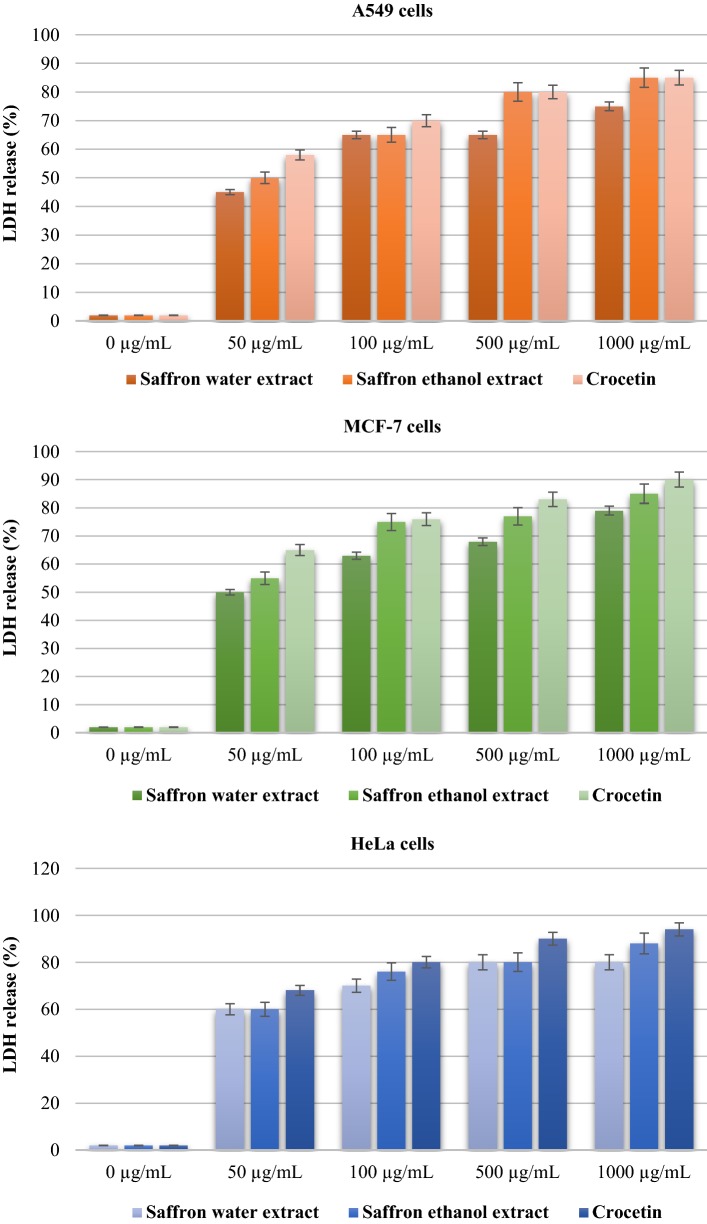
In this assay, the release of LDH from the tested human cancer cells was observed after treatment with varying concentrations of saffron extracts and crocetin, along with different time intervals, 24, 48 and 72 h, respectively. LDH release (% of total) activity results of the tested samples were shown in the Fig. 2 for each human cancer cells in a concentration dependent manner. Non-tumorous HUVEC cells were used as control, and doxorubicin as standard cytotoxic agent (Fig. 2).
Fig. 2.
LDH release (% of total) of A549, MCF-7 and HeLa cancer cells Values are expressed as mean ± SEM from three independent experiment (n = 3) after 48 h treatment
As presented in the Fig. 2, an increasing rate was observed in the percentage of LDH release in a dose dependent manner against the human cancer cells. As compared to LDH activities of saffron’s crocetin component, it showed the highest LDH leakage effects towards HeLa cells with the percentage from 60 to 94%, whereas, the lowest LDH leakage effects were observed on A549 cells with 45–85% LDH release percentage in a time and concentration dependently.
Moreover, the leakage of LDH was measured regarding of IC50 values for the cancer cells as comparing with non-tumor HUVECs depending the time. The water and ethanol extracts from the saffron were found to have lower LDH activity with IC50 values in range of 32.17 ± 0.09–45.06 ± 0.81 µg/mL and 24.90 ± 0.36–39.52 ± 0.21 µg/mL, respectively, while, the IC50 values of crocetin were estimated ranged between 6.21 ± 0.10 and 10.94 ± 0.08 µg/mL towards all the tested cancer cells, which is consistent with cytotoxicity and antiproliferative assay results. Additionally, the results were compered to HUVEC cells as untreated normal cell lines, and doxorubicin as positive control (IC50 = 2.01 ± 0.03 µg/mL). LDH assay results revealed that saffron could possess considerable effects on the cell death in the A549, MCF-7 and HeLa human cancer cells, as consistent with the results of the other assays.
Apoptotic DNA fragmentation assay results
Apoptotic activity induced in the saffron extracts and crocetin treated cancer cell lines were analyzed for DNA fragmentation profile in electrophoresis. The assay was carried out at least triplicate and a represented gel image is presented in the Fig. 3.
Fig. 3.
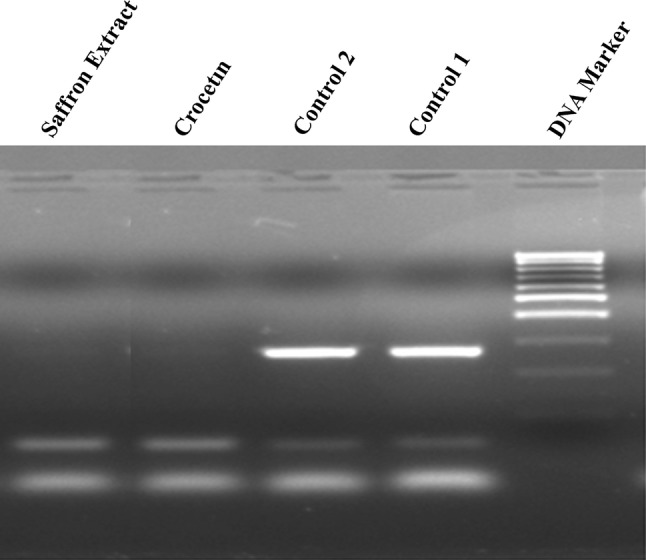
Apoptotic DNA fragmentation
Based on the presented data it could be clearly concluded that saffron extracts and crocetin caused the visible changes in DNA fragmentation, which determined the last point of the apoptosis.
Discussion
According to global statistics, cancer has become a critical health problem in both developed and developing countries with high rate of deaths. It is also estimated that it will continue to be one of the top killer diseases almost all around the world in the next years (Siegel et al. 2018; Song et al. 2018; Gezici and Sekeroglu 2019a).
However, there has been a variety of conventional therapeutic approaches to prevent cancer, recently the researchers have focused on developing plant-derived natural products for fighting cancer effectively. Furthermore, plant-derived natural products and bioactive compounds offer to prevent and treat various cancers with little or no side effects than the conventional cancer therapies (Dhar et al. 2009; Bolhassani et al. 2014; Gezici 2019; Gezici and Sekeroglu 2019b). It is noteworthy that combination of cytotoxic, antiproliferative and LDH activities of saffron extracts and its crocetin ingredients have been closely related to find an effective anticancer agent. On this basis, this research has already been carried out to identify anticancer effects of the extracts and crocetin against the A549, MCF-7 and HeLa human cancer cell lines.
As far as the results obtained from this work, saffron extracts and crocetin possessed cytotoxic and antiproliferative activities on the cancer cells, even though there had no cytotoxicity on the non-malignant (healthy) cells. On the other hand, it was observed that saffron caused much more inhibitory effects on cell growth and viability in cancer cells versus non-malignant HUVEC cells. In this regard, that would be a positive factor to suggest saffron may offer additional benefit in order to interfere with decreasing the amount of cancer cells. Meanwhile, the antiproliferative assay findings were revealed that saffron inhibited the growth in the cancer cells as a dose-time dependent manner.
It is clear that the results of the current research were consistent with the previously anticancer studies with saffron and its constituents. According to Tavakkol-Afshari et al. (2008), ethanol extracts of saffron were analyzed for their cytotoxic and apoptogenetic properties on epithelial-like human hepatocellular carcinoma cell125s (HepG-2) and human cervical carcinoma cells (HeLa), and the extracts were found to have selectively cytotoxic effects on the cancer cells. Another work was conducted to analyze anticarcinogenic properties of the major carotenoids of saffron including crocin and safranal towards HepG-2 cells (Amin et al. 2011). Another study also revealed that crocetin showed significant reduction of cell proliferation in breast cancer cells (MCF-7 and MDA-MB-231), which is more similar to the presented research (Dhar et al. 2009).
In addition to anticancer and antiproliferative assays, the extracts and crocetin were also analyzed their potential lactate dehydrogenase enzyme activity, which enzyme releases from the necrotic cell membranes when the cell membrane is damaged. While, the data produced from lactate dehydrogenase activity points to the percentage of dead and necrotic cells, as observed in this study. The presented results were also in agreement with researches revealing the anticancer and growth inhibitory potentials of saffron may be due to the fact that its strong free radical scavenger capacities, which can cause to cell death by enhancing necrosis and apoptosis in the cells (Li et al. 2012; Bathaie et al. 2014; Bolhassani et al. 2014; Festuccia et al. 2014; Kim et al. 2014). Taken together, it can be clearly suggested that saffron with both of the extracts and isolated compounds could offer significant potential in the management of cancer cases.
Conclusion
The presented research was conducted to compare potential anticancer and antiproliferative activities of the saffron extracts and its crocetin component each other against A549, MCF-7 and HeLa cells. The findings have revealed that the extracts of saffron, as well as its crocetin component may have been used to inhibit the cell viability and cell growth in cancer cells in a time and concentration dependent manner, even at lower concentration and minimum exposure time. As compared to anticancer potentials of the extracts, crocetin was found to have more powerful activity than the extracts. Additionally, the findings highlight the potential usage of saffron extracts in the prevention of cancer as an effective chemoprevention agent. Considering saffron extracts could have many useful natural compounds, it should also be investigated in the future studies that the pure crocetin compound may cause probable adverse side effects on human health. However, further exhaustive in vitro and in vivo studies should be performed to identify molecular mechanisms of potential anticancer effects of saffron extracts and its other bioactive components.
Acknowledgements
The author would like to thank Gaziantep University, Medical Biology Department, Genetics Research Laboratory for their technical support.
Compliance with ethical standards
Conflict of interest
The author declares that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abdullaev FI, Riveron-Negrete L, Caballero-Ortega H, Hernández JM, Perez-Lopez I, et al. Use of in vitro assays to assess the potential antigenotoxic and cytotoxic effects of saffron (Crocus sativus L.) Toxicol In Vitro. 2003;17(5–6):731–736. doi: 10.1016/S0887-2333(03)00098-5. [DOI] [PubMed] [Google Scholar]
- Al-Qubaisi M, Rozita R, Yeap SK, Omar AR, Ali AM, et al. Selective cytotoxicity of goniothalamin against hepatoblastoma HepG2 cells. Molecules. 2011;16(4):2944–2959. doi: 10.3390/molecules16042944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin A, Hamza AA, Bajbouj K, Ashraf SS, Daoud S. Saffron: a potential candidate for a novel anticancer drug against hepatocellular carcinoma. Hepatology. 2011;54(3):857–867. doi: 10.1002/hep.24433. [DOI] [PubMed] [Google Scholar]
- Ashktorab H, Soleimani A, Singh G, Amr A, Tabtabaei S, Latella G, et al. Saffron: the golden spice with therapeutic properties on digestive diseases. Nutrients. 2019;11(5):943. doi: 10.3390/nu11050943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balunas MJ, Kinghorn D. Drug discovery from medicinal plants. Life Sci. 2005;78(5):431–441. doi: 10.1016/j.lfs.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Bathaie SZ, Hoshyar R, Miri H, Sadeghizadeh M. Anticancer effects of crocetin in both human adenocarcinoma gastric cancer cells and rat model of gastric cancer. Biochem Cell Biol. 2013;91(6):397–403. doi: 10.1139/bcb-2013-0014. [DOI] [PubMed] [Google Scholar]
- Bathaie SZ, Bolhassani A, Tamanoi F. Anticancer effect and molecular targets of saffron carotenoids. Enzymes. 2014;36:57–86. doi: 10.1016/B978-0-12-802215-3.00004-5. [DOI] [PubMed] [Google Scholar]
- Bolhassani A, Khavari A, Bathaie SZ. Saffron and natural carotenoids: biochemical activities and anti-tumor effects. Biochim Biophys Acta (BBA) Rev Cancer. 2014;1845(1):20–30. doi: 10.1016/j.bbcan.2013.11.001. [DOI] [PubMed] [Google Scholar]
- Chakraborty A, Gupta N, Ghosh K, Roy P. In vitro evaluation of the cytotoxic, anti-proliferative and anti-oxidant properties of pterostilbene isolated from Pterocarpus marsupium. Toxicol In Vitro. 2010;24(4):1215–1228. doi: 10.1016/j.tiv.2010.02.007. [DOI] [PubMed] [Google Scholar]
- Christodoulou E, Kadoglou NP, Kostomitsopoulos N, Valsami G. Saffron: a natural product with potential pharmaceutical applications. J Pharm Pharmacol. 2015;67(12):1634–1649. doi: 10.1111/jphp.12456. [DOI] [PubMed] [Google Scholar]
- Dhar A, Mehta S, Dhar G, Dhar K, Banerjee S, et al. Crocetin inhibits pancreatic cancer cell proliferation and tumor progression in a xenograft mouse model. Mol Cancer Ther. 2009;8(2):315–323. doi: 10.1158/1535-7163.MCT-08-0762. [DOI] [PubMed] [Google Scholar]
- Festuccia C, Mancini A, Gravina GL, Scarsella L, Llorens S, et al. Antitumor effects of saffron-derived carotenoids in prostate cancer cell models. Biomed Res Int. 2014 doi: 10.1155/2014/135048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gezici S. Promising anticancer activity of lavender (Lavandula angustifolia Mill) essential oil through induction of both apoptosis and necrosis. Ann Phytomed. 2018;7(2):38–45. doi: 10.21276/ap.2018.7.2.5. [DOI] [Google Scholar]
- Gezici S. Anticancer, antiproliferative, lysosomal and lactate dehydrogenase inhibitory effects of fruit extracts from sumac (Rhus coriaria L.) on human lung cancer cells. Acta Oncol Turc. 2019;52(1):160–168. doi: 10.5505/aot.2019.09326. [DOI] [Google Scholar]
- Gezici S, Sekeroglu N. Regulation of MicroRNAs by natural products and bioactive compounds obtained from common medicinal plants: novel strategy in cancer therapy. Indian J Pharm Educ Res. 2017;51(3):483–488. doi: 10.5530/ijper.51.3s.71. [DOI] [Google Scholar]
- Gezici S, Sekeroglu N. Current perspectives in the application of medicinal plants against cancer: novel therapeutic agents. Anticancer Agents Med Chem. 2019;19(1):101–111. doi: 10.2174/1871520619666181224121004. [DOI] [PubMed] [Google Scholar]
- Gezici S, Sekeroglu N. Neuroprotective potential and phytochemical composition of acorn fruits. Ind Crops Prod. 2019;128:13–17. doi: 10.1016/j.indcrop.2018.10.082. [DOI] [Google Scholar]
- Gezici S, Sekeroglu N, Kijjoa A. In vitro anticancer activity and antioxidant properties of essential oils from Populus alba L. and Rosmarinus officinalis L. from South Eastern Anatolia of Turkey. Indian J Pharm Educ Res. 2017;51(3):498–503. doi: 10.5530/ijper.51.3s.74. [DOI] [Google Scholar]
- Giaccio M. Crocetin from saffron: an active component of an ancient spice. Crit Rev Food Sci Nutr. 2004;44(3):155–172. doi: 10.1080/10408690490441433. [DOI] [PubMed] [Google Scholar]
- Gutheil GW, Reed G, Ray A, Anant S, Dhar A. Crocetin: an agent derived from saffron for prevention and therapy for cancer. Curr Pharm Biotechnol. 2012;13(1):173–179. doi: 10.2174/138920112798868566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Lee JM, Kim SC, Park CB, Lee PC. Proposed cytotoxic mechanisms of the saffron carotenoids crocin and crocetin on cancer cell lines. Biochem Cell Biol. 2014;92(2):105–111. doi: 10.1139/bcb-2013-0091. [DOI] [PubMed] [Google Scholar]
- Li CY, Huang WF, Wang QL, Wang F, Cai E, et al. Crocetin induces cytotoxicity in colon cancer cells via p53-independent mechanisms. Asian Pac J Cancer Prev. 2012;13(8):3757–3761. doi: 10.7314/APJCP.2012.13.8.3757. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65(1–2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Newman DJ, Cragg GM. Natural products as sources of new drugs from 1981 to 2014. J Nat Prod. 2016;79(3):629–661. doi: 10.1021/acs.jnatprod.5b01055. [DOI] [PubMed] [Google Scholar]
- Pan L, Chai H, Kinghorn AD. The continuing search for antitumor agents from higher plants. Phytochem Lett. 2010;3(1):1–8. doi: 10.1016/j.phytol.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Sarwat M, Khan TH. Mechanism behind the anti-tumour potential of saffron (Crocus sativus L.): the molecular perspective. Crit Rev Oncol Hematol. 2017;115:27–35. doi: 10.1016/j.critrevonc.2017.04.010. [DOI] [PubMed] [Google Scholar]
- Reddy L, Odhav B, Bhoola KD. Natural products for cancer prevention: a global perspective. Pharmacol Ther. 2003;99(1):1–13. doi: 10.1016/S0163-7258(03)00042-1. [DOI] [PubMed] [Google Scholar]
- Roleira FM, Varela CL, Costa SC, Tavares-da-Silva EJ. Phenolic derivatives from medicinal herbs and plant extracts: anticancer effects and synthetic approaches to modulate biological activity. Stud Nat Prod Chem. 2018;57:115–156. doi: 10.1016/B978-0-444-64057-4.00004-1. [DOI] [Google Scholar]
- Seca A, Pinto D. Plant secondary metabolites as anticancer agents: successes in clinical trials and therapeutic application. Int J Mol Sci. 2018;19(1):263. doi: 10.3390/ijms19010263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senol FS, Sekeroglu N, Gezici S, Kilic E, Orhan IE. Neuroprotective potential of the fruit (acorn) from Quercus coccifera L. Turk J Agric For. 2018;42(2):82–87. doi: 10.3906/tar-1711-18. [DOI] [Google Scholar]
- Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin. 2018;68(1):7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- Song M, Vogelstein B, Giovannucci EL, Willett WC, Tomasetti C. Cancer prevention: molecular and epidemiologic consensus. Science. 2018;361(6409):1317–1378. doi: 10.1126/science.aau3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strober W. Trypan blue exclusion test of cell viability. Curr Protoc Immunol. 2015;111(1):A3.B.1–A3.B.3. doi: 10.1002/0471142735.ima03bs111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan BL, Norhaizan ME. Plant-derived compounds in cancer therapy: traditions of past and drugs of future. In: Akhtar M, Swamy M, editors. Anticancer plants: properties and application. Singapore: Springer; 2018. pp. 91–127. [Google Scholar]
- Tavakkol-Afshari J, Brook A, Mousavi SH. Study of cytotoxic and apoptogenic properties of saffron extract in human cancer cell lines. Food Chem Toxicol. 2008;46(11):3443–3447. doi: 10.1016/j.fct.2008.08.018. [DOI] [PubMed] [Google Scholar]