Abstract
We isolated an actinobacterium, Streptomyces sp. strain SP 85 from the marine sponge Dysidea avara. Polyphasic identification of the microorganism showed that the strain SP 85 had high 16S rRNA gene similarity (99%) with Streptomyces olivaceus strain NBRC 12805, while some physiological and biochemical differences were observed. A cytotoxic compound, SP 85 was isolated from the active culture extract of the strain SP 85 by bioassay-guided purification over silica gel column chromatography, preparative TLC, and HPLC. The structure elucidation based on the spectroscopic analysis, including UV, ESI–MS, and 13C NMR data revealed that SP 85 compound is an analog of anti-tumor drug, “olivomycin A”. The SP 85 compound showed high cytotoxic activity against three human cancer cell lines, including SW480, HepG2, and MCF7 with IC50 values of 16, 93, and 78 nM, respectively. SP 85 exhibited significantly (2–10 times) higher cytotoxicity against the tumor cell lines in comparison with HUVECs as the normal cell line, which also induced apoptosis in the tested cancerous cell line. This is the first report on the production of an “olivomycin A” derivative by a sponge-associated Streptomyces, showing the great potential of sponge-associated actinobacteria in producing cytotoxic natural products.
Keywords: Sponge-associated Streptomyces, Dysidea avara, Olivomycin, Anti-tumor antibiotics, Phylogenetic analysis
Introduction
Screening for new therapeutic agents, notably antitumor compounds, is in priority to alleviate the drug resistance crisis and to overcome the side effects of some approved synthetic drugs (David et al. 2015). More than half of the certified anti-cancer agents originate from natural products or their semisynthetic analogs (Kim 2015). Searching marine natural products from marine sponges provides a great opportunity to discover new drugs owning to their great structural diversity (Blunt et al. 2018; Nazemi et al. 2017).
Recent evidence revealed that microorganisms associated with marine sponges create a complex interaction with their hosts and comprise up to 60% of the sponge’s biomass (Hentschel et al. 2012). Therefore, it can be expected that their associated microorganisms primarily produce a considerable part of metabolites isolated from sponges. Sponge-associated bacteria inhabiting in the unexplored habitats such as the Persian Gulf, as one of the warmest body of water in the world, are promising sources of new bioactive compounds due to their metabolic adaptation to the unique environmental conditions (Patin et al. 2017; Sheppard et al. 1992; Strand et al. 2017). To the best of our knowledge, this is the first report investigating the anti-tumor activity of the sponge-associated actinobacteria isolated from the Persian Gulf.
Actinobacteria constitute major components of the sponge-associated microorganisms and continue to attract great interest for their potential in biosynthesis of bioactive metabolites (Horn et al. 2015). Approximately, 22% of the total bioactive natural products obtained from marine actinobacteria are synthesized by sponge-associated actinobacteria (Abdelmohsen et al. 2014). One of the most important members of actinobacteria is Streptomyces that produce 80% of the reported actinobacteria-derived natural products (Jacob and Weissman 2017).
A heterogeneous group of anti-neoplastic natural products are anti-tumor antibiotics (AA) that have attracted considerable attention of pharmacologists, because of their curative properties and their significant impact on oncologic practice (Carter et al. 2012). Some of the most important AA agents are widely prescribed as cancer chemotherapeutic medicines such as mitomycins, peptides, anthracyclines, carzinophilin, antimetabolites, enediynes, and aureolic acids (Olano et al. 2009). The aureolic acids are glycosylated aromatic polyketides, which are synthesized by various Streptomyces species. The aureolic acids are a family of AA, including mithramycin, chromomycin, olivomycin, and duramycin (Lombó et al. 2006). All of the aureolic acid glycosides, except chromocyclomycins, have a tricyclic aglycone with two aliphatic side chains attached to C-3 and C-7. Olivomycin and chromomycin have the same sugars, but different (homologous) aglycones, whereas the aglycones of mithramycin and chromomycin are identical.
In recent years, there are great interests in searching marine Streptomyces species as sources for new aureolic acid analogs. For instance, a new aureolic acid, “chromomycin B” was isolated from marine-derived Streptomyces sp. WBF16. This compound exhibited strong anti-tumor activity against COC1, A549, HepG2, HCT116, and SGC7901 cell lines (Lu et al. 2012). A series of chromomycin analogs, including chromomycin SA, chromomycin SA3, and aburamycin, which showed strong anti-tumor activities, were produced by marine-derived Streptomyces species (Hu et al. 2011; Lo Giudice et al. 2007). Some of these analogs showed higher anti-tumor activity compared to their parent compounds by exhibiting improved therapeutic efficacy such as less toxicity against normal cells (Baig et al. 2008; Núñez et al. 2012). Consequently, isolation and identification of the derivatives of anti-tumor antibiotics from sponge-associated actinobacteria collected from the Persian Gulf is a novel strategy to improve the therapeutic properties of these DNA-targeted drugs (Reinhold et al. 2017).
The aims of this study were: (1) to isolate and identify the most potent D. avara associated Streptomyces strain in terms of cytotoxic activity (2) to purify and characterize the cytotoxic compound from the culture extract of the most potent strain.
Materials and methods
Sample collection and isolation of actinobacteria
Marine sponge, Dysidea avara samples, (Porifera, Class Demospongiae, Order Choristida, Family Craniellidae) was collected from the Larak Island, Persian Gulf, by scuba diving at a depth of 15–20 m in February 2016 (Fig. 1). We deposited a voucher specimen (number DA 1021) in the sponge collection at the Persian Gulf and Oman Sea Ecological Research Institute. After rinsing with sterile seawater, the samples were cut into pieces of approximately 1 cm3 and homogenized in sterile-filtered seawater. The serially diluted samples were heated at 55 °C for 6 min (Gozari et al. 2019). Then, 100 µl of each dilution was inoculated on modified marine sponge agar (MSA) medium. The medium consisted of raffinose (10 g), dipotassium hydrogen phosphate (1 g), l-histidine (1 g), calcium carbonate (0.5 g), ferrous sulfate (0.01 g), aqueous sponge extract (100 ml), agar (15 g), natural seawater (900 ml). The medium pH was set at 7.8 and it was further supplemented with cycloheximide (100 mg/l) and nalidixic acid (20 mg/l). The inoculated media were incubated at 28 °C up to 4 weeks and the emerging colonies were purified by subculturing onto the MSA medium. Then, the colonies were preliminarily characterized using macroscopic and microscopic observations (Goodfellow et al. 2012).
Fig. 1.

Marine sponge Dysidea avara collected from Larak Island in the Persian Gulf
Identification of the SP 85 isolate
Morphological, physiological, and biochemical identification
The morphology and arrangement of spore chains and aerial hyphae of SP 85 isolate were investigated on ISP 2 agar medium after 14 days at 28 °C using the coverslip method (Kawato and Shinobu 1959). Spore surface ornamentation and spore chain morphology were examined with a field-emission scanning electron microscope (FE-SEM Merlin, Zeiss, Germany) (Bozzola and Russell 1999). Physiological and biochemical characterization of the SP 85 isolate was investigated using International Streptomyces Project (ISP) media (Shirling and Gottlieb 1966). The growth of the isolate on ISP 9 medium, supplemented with 1% various carbon sources, confirmed the assimilation of the carbohydrates and utilization of the nitrogen sources, H2S, and enzyme production were evaluated as described previously by Williams et al. (1989). Finally, the growth temperature ranges and the salinity tolerance were determined on ISP-2 medium.
Molecular identification and phylogenetic analysis
We extracted the genomic DNA of the SP 85 isolate according to CTAB procedure (Kieser 2000). Afterward, their16S rRNA gene was amplified by polymerase chain reaction (PCR) using universal primers 27F and 1492R (Heuer et al. 1997), and then the purified PCR product was sequenced by Macrogen Company (Seoul, Korea). Using the blastn program, the 16S rRNA sequence was compared to the same genes deposited at NCBI (National Centre for Biotechnology Information) GenBank (Zhang et al. 2000). Considering the similarity of the sequence of the purified SP 85 isolate gene with those presented in the GenBank, the phylogenetic tree was constructed using MEGA 7 program (Kumar et al. 2016) and according to the neighbor joining model (Saitou and Nei 1987). We submitted the 16S rRNA gene sequence to the NCBI GenBank database under the accession number: MF405180.1.
Extraction and purification of compound SP 85
The SP 85 strain was inoculated into the seeding medium, containing 2% glycerol, 1% malt extract, and 1% peptone, was incubated at 28 °C in a shaking incubator at 220 rpm for 48 h (Gozari et al. 2018). Consequently, the seeded culture was inoculated to the optimized production medium (10 g malt extract, 8 g starch, 1 g tryptone, 3 g yeast extract, 2 g glycerol, 100 mg methanolic extract of D. avara, 1 l filtered natural seawater, pH 7.3) and incubated for 5 days at 28 °C. After filtration, the aliquots of the fermentation broth were extracted with ethyl acetate twice (1:1 v/v) (Seidel 2005). The ethyl acetate fraction was evaporated in reduced pressure at 40 °C and the resulting crude extract was kept at − 20 °C until the purification process. To purify the bacterial extracts, we used different chromatographic methods, which were guided by the cytotoxic bioassay tests. The crude extract (250 mg) was chromatographed over silica gel column and eluted with CHCl3: MeOH with the ratios of 10:0; 9.5:0.5; 9:1; 8.5:1.5; 8:2; 7.5:2.5; and 7:3, respectively. The bioactive fractions were pooled, concentrated, and further separated by preparative TLC on silica gel using BuOH:AcOH:H2O (75:3:25). The purity of the compound was checked by reversed phase (RP-18-HPLC). The RP-18 HPLC analyses were performed on Knauer analytical HPLC consisted of a K-1001 pump and a four-channel K-2600 UV detector set at λ 210, 230, 254 and 320 nm. The HPLC column (Eurospher-100 C18, 250 × 4.6 mm, Knauer, Germany) was eluted with methanol (solvent B) and ultrapure water (solvent A) as the following solvent gradient program, 0–15 min, 0–40% of B; 15–20 min, 60% of B; 20–40 min, 100% of B; 40–45 min, 40% of B.
Determination of cytotoxic activity and apoptosis assays
The cytotoxic effects of the culture extracts or the pure compound were determined against human breast cancer cell line (MCF7) (NCBI Code: C135), colon cancer cell line (SW480) (NCBI Code: C506), and hepatoma G2 cells (HepG2) (NCBI Code: C158), as well as normal cell human umbilical vein endothelial cells (HUVECs) (NCBI Code: C554) by MTT assay (Peng and Zhao 2009). 100 µl of the cell suspensions containing 104 cells were transferred to each well of a 96-well microplate in DMEM or RPMI media supplemented with 10% FBS and 100 mg/l streptomycin and 100 IU/ml penicillin. The microplates were incubated in a humidified atmosphere with 5% CO2 at 37 °C for 24 h. Then the cultured cell lines were treated with 100 µl of SP 85, crude extract, or doxorubicin at final concentration of 100, 50, 25, 12.5, 6.25, 3.12, and 1.56 µg/ml and incubated for further 36 h. After the end of incubation time, 50 µl of the MTT solution (5 mg/ml) was added to each well and again the plate was incubated for another 4 h. After removing the MTT treated media from the insoluble formazan dye, 100 µl of a DMSO: ethanol (4:1) solution was added to each well and the microplates were shaken in a shaker at 200 rpm for an hour. The optical density (OD) of each well was measured at λ 550 nm using the microplate reader to measure the absorbance of the purple color of the dissolved formazan dye. The cell viability percentage was measured as follows:
| 1 |
The anti-cancer medicine, doxorubicin, was used as the positive control and the absorbance of the culture medium was taken as the blank control (ODBlank). The concentration of a test compound that causes 50% of the cells are killed or survived (cell viability %) is considered as a measure of cytotoxic potential of the test compound and is expressed as IC50.
Doxorubicin is a 14-hydroxylated derivative of daunorubicin, a tetracyclic polyketide natural product isolated from a Streptomyces species and used as a standard chemotherapy agent for the treatment of several cancers and also used as a positive control. Its cytotoxicity against the human tumor cell lines was compared to SP 85 compound, through IC50 calculation.
In the present study, the induction of apoptosis by SP 85 was determined using DNA fragmentation assay, since it is a hallmark of apoptosis. In apoptotic cells, DNA is cleaved by an endonuclease into nucleosomal units, which are multiples of 180 bp oligomers and appear as a DNA ladder, when run on an agarose gel. For DNA fragmentation assay one of the cancer cell lines, SW480 was treated with 250 μg/ml of SP 85 for 72 h. As the negative control, SW480 was treated with the medium without the addition of SP 85. The lysed cells were centrifuged and the supernatant was monitored for the presence of low molecular weight DNA on an agarose gel (Brady 2004).
Structure elucidation of the compound SP 85
The structure of the isolated compound was elucidated using ESI–MS spectra, recorded on a Shimadzu LCMS-2010EV, with an ESI mass and a SPD-M20A diode array detector. The HPLC was coupled to the mass spectrometer equipped with an LC 20AD pump and a Phenomenex Luna 3 μm C18 (2) (100A, 150 × 3.00 mm) column. The LC solvent program was as follow; the flow rate set at 0.25 ml/min, solvent B was 0.01% formic acid in acetonitrile, and solvent A was 0.01% formic acid in water. The injection volume was 2 μl of a ten times diluted sample solutions that was used for the analytical HPLC. The column was eluted with 80% B for 15 min, then solvent composition changed to 100% B at 20 min, and kept at pure B for further 5 min at 25 min, then returned to the initial composition; 80% B at 30 min. Electro spray ionization (ESI) source was used in positive and negative modes. The MS detector, interface and CDL voltages were set at ± 1.5 kV, ± 4 kV, and ± 35 V, respectively, while the Q-array (Rf: ± 150 V) voltage uploaded from the tuning file. The scanning mass range was set between 100 and 1500 mass unit and N2 with flow rate of 1.5 l/min was used as the nebulizing gas. Finally, the heat block and CDL temperatures were set at 230 and 275 °C, respectively. The UV spectra of each peak on LC-PDA chromatogram were recorded on the DAD detector set at λ 190–600 nm. The 1H and 13C NMR spectra were acquired on a Bruker 500 NMR spectrometer (Bruker Biospin, Karlsruhe, Germany) operating at 500 MHz for 1H and 126 MHz for 13C using a 5 mm TCI cryoprobe.
Statistical analyses
All the experiments were performed in triplicate (three independent experiments). We performed one-way ANOVA, followed by least significant difference (LSD) test using SPSS program (Version 24) to analyze the obtained biological data and the significance level was set at p < 0.05. The cytotoxic activity was expressed as mean IC50 ± standard error (SE). The IC50 values of the samples were calculated using the linear regression between the final concentration of the samples and respective cell viability obtained from the Eq. (1) by the software GraphPad PRISM version 6 (GraphPad Software, San Diego, CA). The statistical significance of resulting phylogenetic tree topology was measured by bootstrap analysis based on 1000 replicates with MEGA 7 (Felsenstein 1985).
Results
Isolation and identification of strain SP 85
The strain SP 85 was isolated from D. avara samples on modified marine sponge agar medium by heat treatment. The phenetic characters of this strain are reported in Table 1. The SP 85 strain grew well on all media, except CDA and ISP IV media and the substrate mycelium color was yellowish, while the aerial mycelium was white. The melanoid pigments were not found on any experienced media, but it can produce soluble pigment. It produced spiral sporophores and the spores were rod-shaped (Fig. 2). The whole cell wall hydrolysates of strain SP 85 revealed that it contained l,l-diaminopimelic acid. In addition, the strain utilized all the examined carbon sources, except sucrose and rhamnose and assimilated arginine and asparagine as the sole nitrogen sources. Table 1 also shows that the optimum growth condition for the SP 85 strain was determined at 10–45 °C and NaCl concentration up to 10% (Table 1) with a wide pH range 5–9. The partial 16 s rRNA gene sequence (1397 bp) of strain SP 85 was compared with the gene sequences of the type strains in NCBI GenBank database. The results of blastn analysis showed that SP 85 has 99% similarity to Streptomyces olivaceus strain NBRC 12805. The phylogenetic tree revealed that strain SP 85 positioned into the same clade by their most close type strains, although strain SP 85 developed a different lineage along with the most similar strains (Fig. 3).
Table 1.
Morphological, biochemical, and physiological characterization of SP 85 isolate
| Characters | Results | Characters | Results |
|---|---|---|---|
| Color | Nitrogen sources utilization | ||
| Vegetative mycelia | Yellow | Valine | – |
| Aerial mycelia | White | Arginine | + |
| Growth and sporulation on | Ornithine | – | |
| ISP II | Good | Asparagine | + |
| ISP III | Good | Carbon sources utilization | |
| ISP IV | Good | ||
| ISP V | Low | Glucose | + |
| ISP VI | Good | Fructose | + |
| ISP VII | Good | Xylose | + |
| CDA | Low | Arabinose | + |
| BA | Good | Rhamnose | – |
| NA | Good | Sucrose | – |
| Spore morphology | Spirals | Raffinose | + |
| Melanoid pigment | – | Galactose | + |
| Diffusible pigment | + | Manitol | + |
| H2S production | – | Inositol | + |
| Catalase production | + | Growth temperature range | 10–45 °C |
| Oxidase production | – | Growth pH range | 5–9 |
| Nitrate reduction | + | NaCl tolerance | < 10% |
| DAP-isomer | LL | ||
Fig. 2.
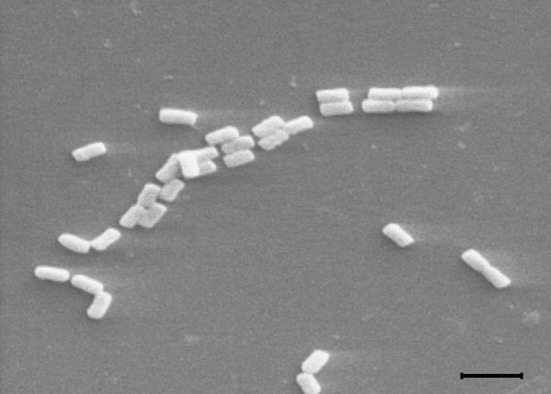
Scanning electron micrograph of strain SP 85 (×5000). The bar represents 2 µm
Fig. 3.
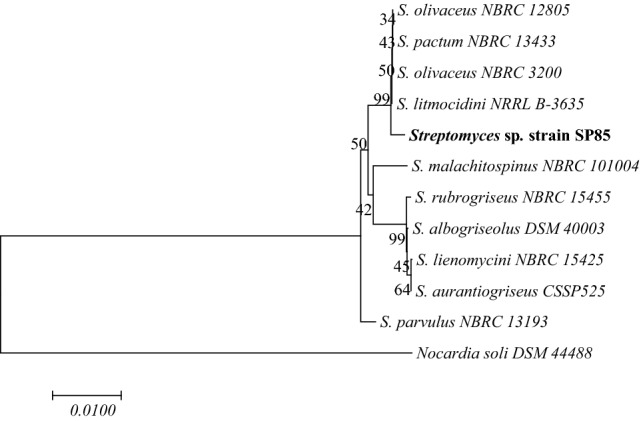
Phylogenetic tree of SP 85 strain showing the phylogenetic position of SP 85 strain and the most closely related strains. Nocardia soli was used as an out group. Bootstrap values are indicated at the relevant branching points. Number of branch node is bootstrap value based on 1000 resampling. Bar: 0.01 substitutions per nucleotide position
Purification and structure elucidation of the compound SP 85
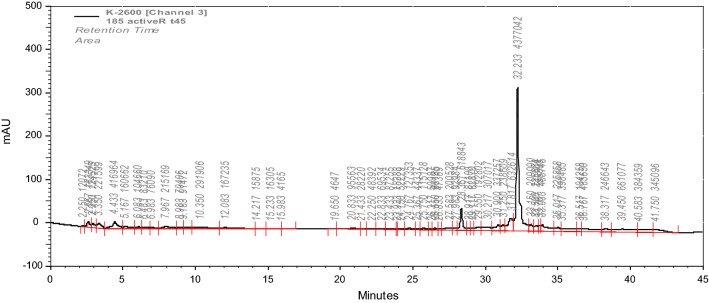
SP 85 was isolated from the culture extract of Streptomyces sp. strain SP 85 by bioassay-guided fractionation strategy, using cytotoxicity assay. Fractionations by solvent partitioning, silica gel column chromatography, preparative thin layer chromatography, and reversed-phase HPLC resulted in a yellow colored pure compound (20 mg). Compound SP 85 was easily soluble in chloroform, ethyl acetate, acetone, ethanol, methanol, and dimethyl sulfoxide, but insoluble in n-hexan. The HPLC chromatogram of compound SP 85 exhibited a main peak with retention time 32.23 min at 254 nm (Fig. 4). The LC-PDA analysis showed that UV λmax of the compound SP 85 recorded at 201 nm and 258 nm. The (−)-ESI–MS and (+)-ESI–MS indicate that the general molecular ions of compound SP 85 are at m/z 1187 [M−H]− and m/z 1210 [M+Na]+, respectively, which leads to a molecular weight 1188 g/mol (Fig. 5).
Fig. 4.
HPLC chromatogram of the SP 85 compound
Fig. 5.
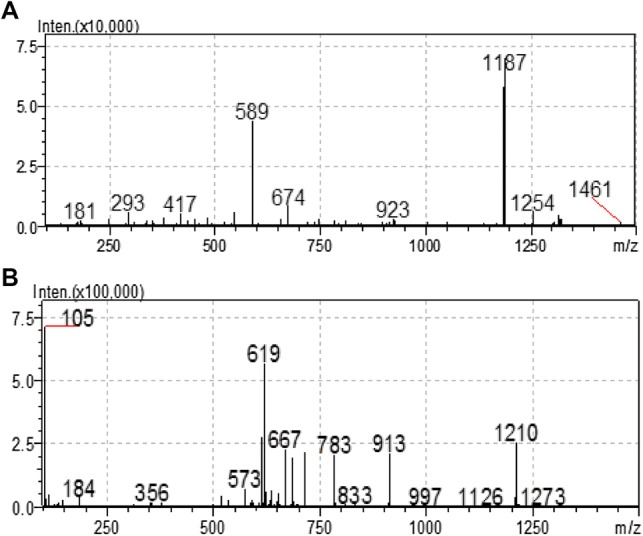
Electrospray ionization (ESI) mass spectrum of the SP 85 compound. a Negative ESI mode, b positive ESI mode
Biological assays for compound SP 85
SP 85 exhibited strong in vitro cytotoxicity against several human tumor cell lines. As can be seen in Fig. 6, SP 85 exhibited the highest cytotoxicity against SW480 with IC50 value of 16 nM. While the compound showed less cytotoxicity against HepG2 and MCF7 cell lines with IC50 values of 93 and 78 nM. Although compound SP 85 exhibited high cytotoxicity against all the tested human tumor cell lines, it was significantly 2–10 times (p < 0.05) less toxic against HUVECs as normal cell line in comparison to the cancerous cell lines (Fig. 6). In Fig. 6, the cytotoxicity of doxorubicin against different cells was also reported. SW480 cell line was the most susceptible cell to the cytotoxic activity (IC50 = 16 ± 0.3 nM) of SP 85, even several fold stronger than the standard chemotherapy drug, doxorubicin (IC50 = 69 ± 5 nM). SP 85 exhibited more than 14 times lower cytotoxicity (IC50 = 174 nM) against HUVECs than the positive control, doxorubicin (IC50 = 12 nM). The DNA fragmentation pattern, hallmark of the apoptosis, was observed in the conventional agarose gel electrophoresis (CAGE) and confirmed the apoptotic cell death in the treated cells with SP 85. The low molecular weight (LMW) DNA ladders were detected on the gel (Fig. 7), suggesting SP 85 to induce the apoptotic cell death in SW480 cell line.
Fig. 6.

Cytotoxic activity of SP 85 compound and doxorubicin against tumor and normal cell lines
Fig. 7.
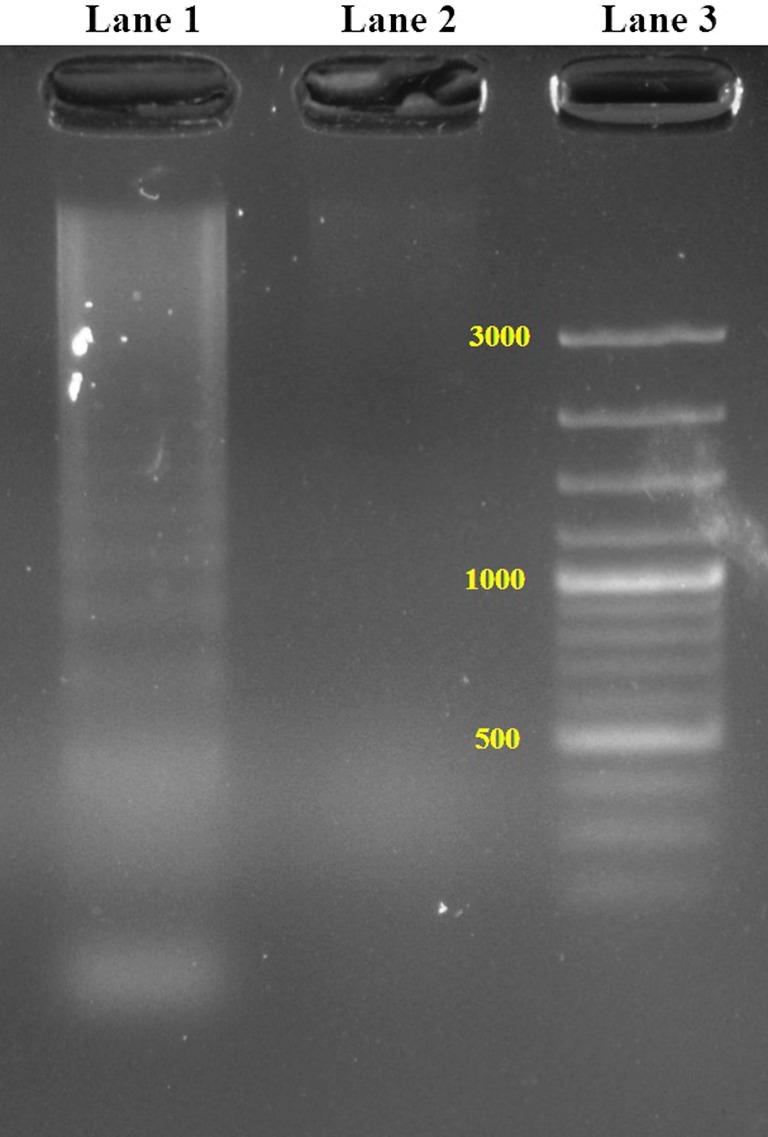
Apoptosis detection by DNA fragmentation in SW480 cells. Lane 1 represent DNA laddering pattern in SW480 cells treated with SP 85. Lane 2 shows no laddering pattern in untreated control SW480 cells. Lane 3 is a DNA marker of 100 bp
Discussion
Isolation and taxonomy of strain SP 85
In addition to our selective isolation of Streptomyces sp. strain SP 85 from the marine sponge, D. avara, other bacterial isolation was reported such as those from Iotrochota sp. (Jiang et al. 2008), Dendrilla nigra (Selvin et al. 2009), Haliclona simulans (Kennedy et al. 2009), and Hymeniacidon perleve (Xi et al. 2012), confirming that Streptomyces species are major and specific cultivable actinobacteria associated with marine sponges. According to the polyphasic taxonomical studies, it became clear that the 16S rRNA gene sequence of Streptomyces sp. strain SP 85 had a 99% similarity to S. olivaceus. However, strain SP 85 and S. olivaceus are slightly different in their physiological and biochemical properties (Table 1). Unlike S. olivaceus, the Streptomyces sp. strain SP 85 cannot utilize rhamnose as the sole carbon source and can tolerate more than 10% NaCl concentrations. This high NaCl tolerance might be acquired due to the adaptation of SP 85 strain with marine habitat during its evolution. Consequently, the SP 85 strain might be diverged from S. olivaceus. Phylogenetic analysis revealed that strain SP 85 developed a disparate lineage along with the closest type strains. Therefore, it can confirm the previous finding that the strain SP 85 experienced mutations during adaptation with microenvironments in its sponge host (Orr 2005). These mutations could also occur in biosynthesis gene clusters of this strain and could change its biosynthetic pathways to synthesize new bioactive derivatives. Recent studies on actinobacteria provided new evidence about the relationship between taxonomy and secondary metabolite production, especially for the Streptomyces genus (Salcedo et al. 2016; Ziemert et al. 2014).
Purification and structure elucidation of the compound SP 85
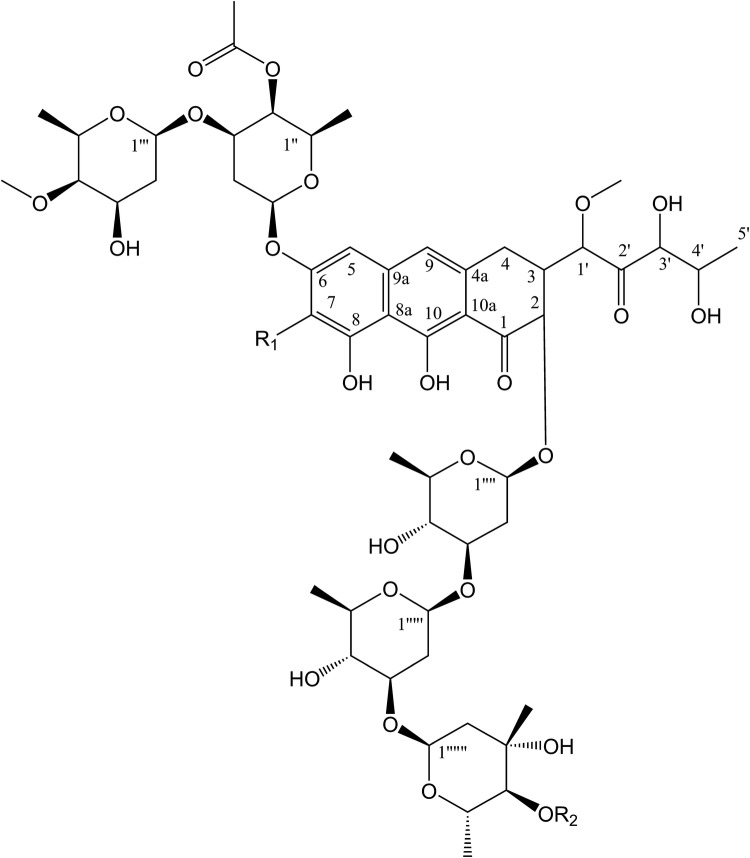
We purified compound SP 85 as a cytotoxic agent and elucidated its structure, mainly based on its 13C NMR spectral data. The spectral data are similar to olivomycin A (1) analog, and members of aureolic acid family, especially chromomycin A3 (2) (Fig. 8 ) (Koenuma et al. 1988; Yoshimura et al. 1988). Fourteen signals of the tricyclic aromatic moiety together with the side chain signals at C-3 were in good agreement with the signals in 13C NMR recorded for the compounds, except for the recorded signal of C-2′ at 206 ppm. Five anomeric signals at δ 97–102 ppm together with the signals for the deoxy sugars at C-2″, C-6″, were in good agreement for those recorded for both compounds. The main reason why we suggest the structure of the compound as a derivative of olivomycin A, was due to the absence of a signal at δ 111 and 8 ppm for C-7 and its substituted methyl group, respectively, in its 13C NMR spectrum. However, the main difference was the shift of C-2′ at 206, ppm for the side chain in our compound in comparison to the original data of the olivomycin A with the carbonyl shift at 212 ppm. Additionally, the molecular formula of C58H84O26 for compound 1 was compatible with the 13C NMR spectra of compound SP 85 (Table 2, Fig. 8).
Fig. 8.
Olivomycin A (1): R1=H, R2=COCH3 and Olivomycin A3 (2): R1=Me; R2=COC(CH3)2
Table 2.
13C NMR data of SP 85, olivomycin A (1) and chromomycin A3 (2) (Koenuma et al. 1988; Yoshimura et al. 1988)
| Position | δc (ppm) | Position | δc (ppm) | ||||
|---|---|---|---|---|---|---|---|
| SP 85 | 1 | 2 | SP 85 | 1 | 2 | ||
| Aglycon | 1″′ | 98.0 | 95.4 | 95.2 | |||
| 1 | 201.6 | 202.3 | 202.1 | 2″′ | 33.8 | 33.5 | 33.5 |
| 2 | 75.2 | 75.9 | 75.9 | 3″′ | 65.4 | 65.9 | 65.9 |
| 3 | 43.1 | 43.5 | 43.7 | 4″′ | 82.2 | 81.5 | 81.5 |
| 4 | 25.7 | 27.0 | 26.9 | 5″′ | 66.4 | 67.0 | 67.0 |
| 5 | 101.7 | 102.9 | 100.7 | 6″′ | 16.9 | 17.3 | 17.3 |
| 6 | 157.3 | 160.8 | 159.6 | 4″′-OCH3 | 62.1 | 62.4 | 62.3 |
| 7 | 101.2 | 102.0 | 111.6 | Sugar C | |||
| 8 | 157.3 | 165.6 | 156.1 | 1″″ | 101.2 | 100.3 | 100.3 |
| 9 | 167.3 | 159.6 | 165.2 | 2″″ | 37.4 | 37.5 | 37.5 |
| 10 | 119.2 | 117.1 | 117.0 | 3″″ | 83.2 | 82.3 | 82.3 |
| 4a | 135.7 | 135.9 | 134.5 | 4″″ | 75.1 | 75.1 | 75.1 |
| 8a | 108.0 | 108.1 | 108 | 5″″ | 71.9 | 72.3 | 72.3 |
| 9a | 108.9 | 108.4 | 108 | 6″″ | 18.2 | 18.0 | 18.0 |
| 10a | 141.3 | 140.8 | 138.3 | Sugar D | |||
| 7-CH3 | – | – | 8.2 | 1″″′ | 101.2 | 99.7 | 99.7 |
| 1′ | 82.1 | 81.6 | 82.0 | 2″″′ | 36.7 | 37.1 | 37.1 |
| 2′ | 206.2 | 211.0 | 211.2 | 3″″′ | 78.6 | 80.7 | 80.5 |
| 3′ | 78.4 | 78.2 | 78.4 | 4″″′ | 75.2 | 75.3 | 75.3 |
| 4′ | 68.3 | 68.0 | 67.9 | 5″″′ | 71.4 | 72.2 | 72.2 |
| 5′ | 19.6 | 20.5 | 20.5 | 6″″′ | 17.8 | 17.8 | 17.8 |
| 1′-OCH3 | 57.3 | 59.6 | 59.7 | Sugar E | |||
| Sugar A | 1″″″ | 97.0 | 97.2 | 97.0 | |||
| 1′’ | 98.3 | 97.1 | 97.4 | 2″″″ | 43.1 | 43.9 | 43.8 |
| 2″ | 33.9 | 32.9 | 33.0 | 3″″″ | 70.9 | 70.6 | 70.6 |
| 3″ | 68.5 | 70.1 | 69.9 | 4″″″ | 78.6 | 79.4 | 79.6 |
| 4″ | 66.3 | 67.3 | 67.3 | 5″″″ | 66.7 | 66.7 | 66.7 |
| 5″ | 69.0 | 69.8 | 69.7 | 6″″″ | 17.8 | 17.9 | 17.8 |
| 6″ | 16.9 | 16.8 | 16.8 | 3″″″–CH3 | 23.2 | 23.0 | 23.0 |
| CH3CO | 20.2 | 20.8 | 20.8 | CH3 C | 18.9 | 18.9 | 20.9 |
| CH3CO | 171.3 | 170.9 | 170.9 | CH3 C | 19.3 | 19.0 | – |
| Sugar B | CH3 CH–CO | 34.4 | 34.4 | – | |||
| CH3 CO | 176.6 | 177.5 | 171.6 | ||||
Measured at 125 MHz in CDCl3
Biological assays for compound SP 85
The common feature in the antibacterial–anticancer activity mechanisms for the members of aureolic acids seems to be their interaction with DNA. However, DNA-targeted therapeutics such as AA preferentially destroy cancerous cells due to their genomic instability and high proliferation rate. In the treatment process, the normal cells can also be affected (Tacar et al. 2013). Due to the abovementioned limitations, drug development program of new DNA-targeted compounds with less toxicity on normal cells has been considered by different research groups (Glorieux et al. 2017; Reinhold et al. 2017). Interestingly, compound SP 85 exhibited the highest and lowest cytotoxicities against SW480 and the normal cell lines, respectively (Fig. 6). The above-mentioned different activity spectrum can be considered as a therapeutic advantage. In addition to cytotoxic activity, compound SP 85 exhibited strong and selective antibacterial activity against gram-positive bacteria, Micrococcus lutes and Bacillus cereus (data not shown). Recent studies showed that some FDA-approved antibiotics classes can be used to selectively target cancer stem cells (CSCs) across multiple tumor types (Lamb et al. 2015). Therefore, AAs by molecular disruption of mitochondrial biogenesis can represent a novel therapeutic strategy for the eradication of cancerous cells. The appearance of 180–200 bp oligosomal fragments (hallmark of apoptosis) after treatment with SP 85 compound revealed that SP 85 compound could activate endonucleases via induction of caspase enzymes (Pelicano et al. 2003). In previous work, the cytotoxic effect of sesquiterpene isolated from marine actinobacterium Streptomyces sp. VITJS8 against HepG2 cell line was studied (Naine et al. 2016). The cytotoxic effect of VITJS8 was comparable to SP 85 and both compounds could induce DNA fragmentation pattern at 250 μg/ml concentration.
Conclusion
SP 85 is a strong cytotoxic agent against several human tumor cell lines with low activity against normal cells; hence, can be considered as a suitable anticancer agent. This compound is the analog of olivomycin A, an antitumor drug from aureolic acid family. Therefore, the D. avara associated actinobacterium; Streptomyces sp. strain SP 85 that produced this valuable AA might become a potential tool for genetically based combinatorial methods for the biosynthesis of bioactive compounds. Although further work needs to be done to determine the exact structure of the active substances, the detection of this cytotoxic agent from the strain SP 85 provided another evidence for importance of sponge-associated bacteria in bioactive compounds discovery.
Acknowledgements
The authors are thankful to Prof. Bernd Schneider at Max Planck Institute for chemical ecology, Jena, Germany for the NMR measurement, Mrs. Najmeh Khalighian for her technical help and research council of Shiraz University of medical sciences for using the analytical measurements. The authors wish to thank Mr. H. Argasi at the Research Consultation Center (RCC) of Shiraz University of Medical Sciences for his invaluable assistance in editing this manuscript.
Author contributions
MG designed and preformed the isolation, screening, and identification of bacterial strain under the supervision of NB. Bioassays were carried out under the supervision of EE. MG performed extraction and purification of the bioactive compound under the guidance of ARJ. ARJ designed and analyzed the spectroscopic analysis and elucidation of the bioactive compound. The manuscript was written by MG and revised by ARJ, NB, MSM, and EE.
Funding
This research article is a part of the PhD thesis entitled “Evaluation, identification and structure elucidation of cytotoxic and antioxidant secondary metabolites from Persian Gulf and Oman Sea derived Actinobacteria” that was supported by Islamic Azad University, Shiraz branch.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Abdelmohsen UR, Bayer K, Hentschel U. Diversity, abundance and natural products of marine sponge-associated actinomycetes. Nat Prod Rep. 2014;31(3):381–399. doi: 10.1039/c3np70111e. [DOI] [PubMed] [Google Scholar]
- Baig I, Perez M, Braña AF, Gomathinayagam R, Damodaran C, Salas JA, Méndez C, Rohr J. Mithramycin analogues generated by combinatorial biosynthesis show improved bioactivity. J Nat Prod. 2008;71(2):199–207. doi: 10.1021/np0705763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blunt JW, Carroll AR, Copp BR, Davis RA, Keyzers RA, Prinsep MR. Marine natural products. Nat Prod Rep. 2018;35:8–53. doi: 10.1039/c7np00052a. [DOI] [PubMed] [Google Scholar]
- Bozzola JJ, Russell LD. Electron microscopy: principles and techniques for biologists. 2. Sudbury: Jones and Bartlett Publishers; 1999. [Google Scholar]
- Brady HJ. Apoptosis methods and protocols. Berlin: Springer; 2004. [Google Scholar]
- Carter SK, Umezawa H, Douros J, Sakurai Y. Antitumor antibiotics. Berlin: Springer Science & Business Media; 2012. [Google Scholar]
- David B, Wolfender J-L, Dias DA. The pharmaceutical industry and natural products: historical status and new trends. Phytochem Rev. 2015;14:299–315. [Google Scholar]
- Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- Glorieux M, Dok R, Nuyts S. Novel DNA targeted therapies for head and neck cancers: clinical potential and biomarkers. Oncotarget. 2017;8:81662. doi: 10.18632/oncotarget.20953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodfellow M, Kämpfer P, Busse H-J, Trujillo ME, Suzuki K-I, Ludwig W, Whitman WB. Bergey’s manual® of systematic bacteriology. New York: Springer; 2012. [Google Scholar]
- Gozari M, Bahador N, Jassbi A, Mortazavi M, Eftekhar E. Antioxidant and cytotoxic activities of metabolites produced by a new marine Streptomyces sp. isolated from the sea cucumber Holothuria leucospilota. Iran J Fish Sci. 2018;17:413–426. [Google Scholar]
- Gozari M, Zaheri A, Jahromi ST, Gozari M, Karimzadeh R. Screening and characterization of marine actinomycetes from the northern Oman Sea sediments for cytotoxic and antimicrobial activity. Int Microbiol. 2019;22:521–530. doi: 10.1007/s10123-019-00083-3. [DOI] [PubMed] [Google Scholar]
- Hentschel U, Piel J, Degnan SM, Taylor MW. Genomic insights into the marine sponge microbiome. Nat Rev Microbiol. 2012;10:641–654. doi: 10.1038/nrmicro2839. [DOI] [PubMed] [Google Scholar]
- Heuer H, Krsek M, Baker P, Smalla K, Wellington E. Analysis of actinomycete communities by specific amplification of genes encoding 16S rRNA and gel-electrophoretic separation in denaturing gradients. Appl Environ Microbiol. 1997;63:3233–3241. doi: 10.1128/aem.63.8.3233-3241.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn H, Hentschel U, Abdelmohsen UR. Mining genomes of three marine sponge-associated actinobacterial isolates for secondary metabolism. Genome Announc. 2015;3:e01106–e01115. doi: 10.1128/genomeA.01106-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Espindola APD, Stewart NA, Wei S, Posner BA, MacMillan JB. Chromomycin SA analogs from a marine-derived Streptomyces sp. Bioorg Med Chem. 2011;19:5183–5189. doi: 10.1016/j.bmc.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob C, Weissman KJ. Unpackaging the roles of Streptomyces natural products. Cell Chem Biol. 2017;24:1194–1195. doi: 10.1016/j.chembiol.2017.09.013. [DOI] [PubMed] [Google Scholar]
- Jiang S, Li X, Zhang L, Sun W, Dai S, Xie L, Liu Y, Lee KJ. Culturable actinobacteria isolated from marine sponge Iotrochota sp. Mar Biol. 2008;153(5):945–952. [Google Scholar]
- Kawato N, Shinobu R. On Streptomyces herbaricolor sp. nov supplement: a simple technique for the microscopic observation. Mem Osaka Univ Lib Arts Educ B Nat Sci. 1959;8:114–119. [Google Scholar]
- Kennedy J, Baker P, Piper C, Cotter PD, Walsh M, Mooij MJ, et al. Isolation and analysis of bacteria with antimicrobial activities from the marine sponge Haliclona simulans collected from Irish waters. Mar Biotechnol. 2009;11(3):384–396. doi: 10.1007/s10126-008-9154-1. [DOI] [PubMed] [Google Scholar]
- Kieser T. Practical streptomyces genetics. Norwich: John Innes Foundation; 2000. [Google Scholar]
- Kim S-K (2015) Handbook of anticancer drugs from marine origin, vols 22–98. Springer, Berlin
- Koenuma M, Uchida N, Yamaguchi K, Kawamura Y, Matsumoto K. New aureolic acid antibiotics. J Antibiot. 1988;41:45–52. doi: 10.7164/antibiotics.41.45. [DOI] [PubMed] [Google Scholar]
- Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33(7):1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb R, Ozsvari B, Lisanti CL, Tanowitz HB, Howell A, Martinez-Outschoorn UE, et al. Antibiotics that target mitochondria effectively eradicate cancer stem cells, across multiple tumor types: treating cancer like an infectious disease. Oncotarget. 2015;6(7):4569–4584. doi: 10.18632/oncotarget.3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Giudice A, Bruni V, Michaud L. Characterization of Antarctic psychrotrophic bacteria with antibacterial activities against terrestrial microorganisms. J Basic Microbiol. 2007;47:496–505. doi: 10.1002/jobm.200700227. [DOI] [PubMed] [Google Scholar]
- Lombó F, Menéndez N, Salas JA, Méndez C. The aureolic acid family of antitumor compounds: structure, mode of action, biosynthesis, and novel derivatives. Appl Microbiol Biotechnol. 2006;73:1–14. doi: 10.1007/s00253-006-0511-6. [DOI] [PubMed] [Google Scholar]
- Lu J, Ma Y, Liang J, Xing Y, Xi T, Lu Y. Aureolic acids from a marine-derived Streptomyces sp. WBF16. Microbiol Res. 2012;167:590–595. doi: 10.1016/j.micres.2012.06.001. [DOI] [PubMed] [Google Scholar]
- Naine SJ, Devi CS, Mohanasrinivasan V, Doss CGP, Kumar DT. Binding and molecular dynamic studies of sesquiterpenes (2R-acetoxymethyl-1, 3, 3-trimethyl-4t-(3-methyl-2-buten-1-yl)-1t-cyclohexanol) derived from marine Streptomyces sp. VITJS8 as potential anticancer agent. Appl Microbiol Biotechnol. 2016;100:2869–2882. doi: 10.1007/s00253-015-7156-2. [DOI] [PubMed] [Google Scholar]
- Nazemi M, Moradi Y, Rezvani Gilkolai F, Ahmaditaba M, Gozari M, Salari Z. Antimicrobial activities of semi polar-nonpolar and polar secondary metabolites of sponge Dysidea pallescens from Hengam Island. Persian Gulf Iran J Fish Sci. 2017;16:200–209. [Google Scholar]
- Núñez LE, Nybo SE, González-Sabín J, Pérez M, Menéndez N, Braña AF, et al. A novel mithramycin analogue with high antitumor activity and less toxicity generated by combinatorial biosynthesis. J Med Chem. 2012;55:5813–5825. doi: 10.1021/jm300234t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olano C, Méndez C, Salas JA. Antitumor compounds from actinomycetes: from gene clusters to new derivatives by combinatorial biosynthesis. Nat Prod Rep. 2009;26:628–660. doi: 10.1039/b822528a. [DOI] [PubMed] [Google Scholar]
- Orr HA. The genetic theory of adaptation: a brief history. Nat Rev Genet. 2005;6:119–127. doi: 10.1038/nrg1523. [DOI] [PubMed] [Google Scholar]
- Patin NV, Schorn M, Aguinaldo K, Lincecum T, Moore BS, Jensen PR. Effects of actinomycete secondary metabolites on sediment microbial communities. Appl Environ Microbiol. 2017;83:e02676-16. doi: 10.1128/AEM.02676-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelicano H, Feng L, Zhou Y, Carew JS, Hileman EO, Plunkett W, et al. Inhibition of mitochondrial respiration a novel strategy to enhance drug-induced apoptosis in human leukemia cells by a reactive oxygen species-mediated mechanism. J Biol Chem. 2003;278:37832–37839. doi: 10.1074/jbc.M301546200. [DOI] [PubMed] [Google Scholar]
- Peng S, Zhao M. Pharmaceutical bioassays: methods and applications. New York: Wiley; 2009. [Google Scholar]
- Reinhold WC, Thomas A, Pommier Y. DNA-targeted precision medicine; have we been caught sleeping? Trends Cancer. 2017;3:2–6. doi: 10.1016/j.trecan.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Salcedo RG, Olano C, Gómez C, Fernández R, Braña AF, Méndez C, et al. Characterization and engineering of the biosynthesis gene cluster for antitumor macrolides PM100117 and PM100118 from a marine actinobacteria: generation of a novel improved derivative. Microb Cell Fact. 2016;15(1):44–62. doi: 10.1186/s12934-016-0443-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidel Véronique. Methods in Molecular Biology. Totowa, NJ: Humana Press; 2012. Initial and Bulk Extraction of Natural Products Isolation; pp. 27–41. [DOI] [PubMed] [Google Scholar]
- Selvin J, Gandhimathi R, Kiran GS, Priya SS, Ravji TR, Hema T. Culturable heterotrophic bacteria from the marine sponge Dendrilla nigra: isolation and phylogenetic diversity of actinobacteria. Helgol Mar Res. 2009;63:239–247. [Google Scholar]
- Sheppard C, Price A, Roberts C. Marine ecology of the Arabian region: patterns and processes in extreme tropical environments. London: Academic Press; 1992. [Google Scholar]
- Shirling Et, Gottlieb D. Methods for characterization of Streptomyces species. Int J Syst Evol Microbiol. 1966;16:313–340. [Google Scholar]
- Strand R, Whalan S, Webster NS, Kutti T, Fang JK-H, Luter HM, Bannister R. The response of a boreal deep-sea sponge holobiont to acute thermal stress. Sci Rep. 2017;7:1660. doi: 10.1038/s41598-017-01091-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tacar O, Sriamornsak P, Dass CR. Doxorubicin: an update on anticancer molecular action, toxicity and novel drug delivery systems. J Pharm Pharmacol. 2013;65:157–170. doi: 10.1111/j.2042-7158.2012.01567.x. [DOI] [PubMed] [Google Scholar]
- Williams ST, Goodfellow M, Alderson G. Genus Streptomyces Waksman and Henrici 1943, 339AL. In: Williams ST, Sharpe ME, Holt JG, editors. Bergey’s manual of systematic bacteriology. Baltimore: Williams and Willkins; 1989. pp. 2453–2492. [Google Scholar]
- Xi L, Ruan J, Huang Y. Diversity and biosynthetic potential of culturable actinomycetes associated with marine sponges in the China seas. Int J Mol Sci. 2012;13:5917–5932. doi: 10.3390/ijms13055917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura Y, Koenuma M, Matsumoto K, Tori K, Terui Y. NMR studies of chromomycins, olivomycins, and their derivatives. J Antibiot. 1988;41:53–67. doi: 10.7164/antibiotics.41.53. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Schwartz S, Wagner L, Miller W. A greedy algorithm for aligning DNA sequences. J Comput Biol. 2000;7:203–214. doi: 10.1089/10665270050081478. [DOI] [PubMed] [Google Scholar]
- Ziemert N, Lechner A, Wietz M, Millán-Aguiñaga N, Chavarria KL, Jensen PR. Diversity and evolution of secondary metabolism in the marine actinomycete genus Salinispora. Proc Natl Acad Sci USA. 2014;111:E1130–E1139. doi: 10.1073/pnas.1324161111. [DOI] [PMC free article] [PubMed] [Google Scholar]