Abstract
To evaluate and compare the expression of HIF-1 Alpha (HIF-1α) in oral epithelial dysplasia (OED) and various grades of Oral squamous cell carcinoma (OSCC). 30 cases each of OEDand OSCC were stained with HIF-1α antibody. Quantification of HIF-1α positive cellswas carried out and the data was statistically analysed. The mean % HIF-1α labeling index (HIF-1α LI) increased significantly from mild OED (32.11%), moderate OED (55.07%), to severe OED (64.58%). There was a statistically significant increase in the expression of HIF-1α as grades of OED increased. The mean HIF-1α LI % in well differentiated OSCC was 46.3%, Moderately differentiated OSCC—76.31% and Poorly differentiated OSCC—89.9%. The mean HIF-1α LI was found to increase with increasing grades of OSCC which was statistically significant (P < 0.05). Further a comparison of mean HIF-1α LI in OED with different histologic grades of OSCC by Independent samples t test was performed. We found statistically significant difference between OED and moderately differentiated OSCC and OED and poorly differentiated OSCC (P = 0.000). Progressive increase in expression of HIF-1α was noted from OED to OSCC. It can be postulated that epithelial dysplastic lesions with increased HIF-1α expression are at greater risk of malignant transformation, suggesting that the expression of HIF-1α is an early event in oral carcinogenesis.
Keywords: HIF-1α, Oral epithelial dysplasia, OSCC, Immunohistochemistry
Introduction
Oral and pharyngeal cancers are considered an important part of the global burden of cancer [1]. Many Oral squamous cell carcinomas have been documented to be associated with or preceded by a precancerous lesion, especially leukoplakia [2]. The presence of dysplasia is more important in predicting malignant development than its clinical presentation such as leukoplakia, erythroplakia or leuko-erythroplakia [3]. It is observed that the malignant transformation potential increases with the severity of epithelial dysplasia with a malignant transformation rate ranging from 3 to 33% [4, 5]. Hypoxia is one of the hallmarks of cancer and has been demonstrated in different types of solid tumors including head and neck squamous cell carcinoma (HNSCC) [6, 7]. It reflects the imbalance between oxygen consumption by the rapidly proliferating cancer cells and the insufficient oxygen delivery due to poor vascularization and blood supply. Hypoxia causes cell death if it is severe or prolonged. However, cancer cells acclimatize to this hostile environment [8]. Protection against hypoxia in solid tumors is an important step in tumor development and progression. One system in hypoxia protection of tumor cells is represented by the hypoxia-inducible factor 1 (HIF-1) system [9]. The presence of hypoxic areas represents an independent prognostic factor in a diverse range of human cancers [10]. The relationship between HIF-1α expression, tumor progression and treatment response in Head and Neck cancer is still poorly understood [10].
Due to the scarcity of literature on role of HIF-1α in OSCC and OED in the population of Indian subcontinent and till date HIF-1α marker in OSCC and OED has not been studied in the population of Indian subcontinent, the present study was undertaken to assess the expression of HIF-1α in oral epithelial dysplasia and various histological grades of Oral squamous cell carcinoma.
Materials and Method
The present descriptive cross-sectional study was conducted in the Department of Oral Pathology and Microbiology among 30 cases each of oral epithelial dysplasia and OSCC from tertiary care centre, Igatpuri, Nashik. An Ethical clearance from the Institutional Ethical Committee and informed consent from patients was obtained for the present study. Biopsy was performed in each case, in order to obtain the histopathological diagnosis of the lesion. Histopathologic assessment of all clinically diagnosed premalignant lesions was done for degree of epithelial dysplasia according to WHO classification scheme (2005). The clinical staging of OSCC was done by Tumor-Node-Metastasis (TNM) system developed by American Joint Committee for Cancer staging and end result reporting (AJCCS) [11]. Histopathological grading of Oral Squamous Cell Carcinoma: Grading of OSCC was done according to Magne Bryne system (1989, 1992) for oral squamous cell carcinoma [12].
The study group comprised of 30 cases each of oral epithelial dysplasia and OSCC which were selected from Department of ENT, Tertiary Care Centre, Igatpuri, Nashik for quantification of HIF-1α positive cells after immunohistochemistry.
Formalin fixed paraffin embedded tissue sections were cut to 5 μm thickness. The immunohistochemical staining for HIF-1α using Universal Immuno Enzyme Polymer Technique was carried out. Sections were brought to distilled water and treated with hydrogen peroxide to eliminate endogenous peroxidase activity. Then antigen retrieval with trisodium citrate for HIF1α was carried out. The tissue was incubated sequentially with:
Primary antibodies, that is, HIF1α (Thermo fissure Scientific)
Novolink polymer detection system
3,3-Diaminobenzidine substrate solution
This resulted in formation of a colored precipitate at the tissue antigen sites. Visualization was aided by counterstaining with hematoxylin.
For the quantitative analysis of HIF-1alpha (HIF-1α) positive cells, immunostained slides were examined under high power (magnification: 400 ×) of light microscope (Olympus CH 20i). Cytoplasmic immunoreactivity was considered to be positive and HIF-1α expression was scored.
Cells were considered immunopositive if they presented with brown cytoplasmic staining, regardless of the intensity. HIF-1 α positive cells were counted per 1000 tumor cells on randomly selected microscopic fields. The number of positively stained cells were quantified as a percentage of total number of tumor cells counted, which is known as HIF-1α labeling index.
Statistical Analysis
Statistical analysis was done using Statistical Package of Social Science (SPSS Version 22; Chicago Inc., USA). Data comparison was done by applying specific statistical tests like Chi square test, Independent samples t test, ANOVA test, Tukey post hoc analysis test to find out the statistical significance of the comparisons. Quantitative variables were compared using mean values and qualitative variables using proportions. Significance level was fixed at P < 0.05.
Results
Table 1 shows comparison of HIF-1α Expression in Histopathological grades of OED. The mean HIF-1α LI % was found to be highest in severe dysplasia. There was statistically significant difference found in expression of HIF-1α between the grades of Oral epithelial dysplasia (P = 0.001).
Table 1.
Comparison of HIF-1α expression in histological grades of OED
| Grade | No. of cases | Mean ± SD (HIF-1α) | Mean HIF-1α LI in % | Median | Range |
|---|---|---|---|---|---|
| Mild | 13 | 32.11 ± 6.09 | 32.11 | 33.80 | 17.8–38.8 |
| Moderate | 11 | 55.07 ± 2.95 | 55.07 | 54.60 | 51.2–59.8 |
| Severe | 6 | 64.58 ± 2.39 | 64.58 | 64.15 | 62.2–68.4 |
| ANOVA F value | 25.006 | ||||
| Significance P value | 0.001 (HS) | ||||
Table 2 reveals Intragroup Comparison of HIF-1α according to grade of OED done by Tukey post hoc analysis test. It shows highly significant difference between mild and moderate, mild and severe and moderate and severe grades of Oral epithelial dysplasia (P = 0.001).
Table 2.
Intragroup comparison of HIF-1α according to grade of OED by Tukey post hoc analysis test
| Group | Mean difference (HIF-1α LI %) | P value |
|---|---|---|
| Mild versus Moderate | 22.95 | 0.001 (HS) |
| Mild versus Severe | 32.46 | 0.001 (HS) |
| Moderate versus Severe | 9.51 | 0.001 (HS) |
Table 3 reveals Comparison of HIF-1α Expression in Histological grades of OSCC. The mean HIF-1α LI was found to be highest in poorly differentiated squamous cell carcinoma. There was statistically significant difference found in expression of HIF-1α and the Histological grades of OSCC (P = 0.001).
Table 3.
Comparison of HIF-1α Expression in Histological grades of OSCC
| Grade | No of cases | Mean ± SD (HIF-1α) | Mean HIF-1α LI in % | Median | Range |
|---|---|---|---|---|---|
| WDSCC | 9 | 46.30 ± 32.41 | 46.3 | 47.60 | 41.2–50.8 |
| MDSCC | 16 | 76.31 ± 60.07 | 76.31 | 75.80 | 62.3–84.2 |
| PDSCC | 5 | 89.90 ± 40.47 | 89.9 | 88.90 | 86.8–96.8 |
| ANOVA F value | 147.073 | ||||
| Significance P value | 0.001 (HS) | ||||
Table 4 shows Intragroup Comparison of HIF-1α according to grade of OSCC done by Tukey post hoc analysis test.It shows highly significant difference between WDSCC versus MDSCC, WDSCC versus PDSCC and MDSCC versus PDSCC grades of OSCC (P = 0.001).
Table 4.
Intragroup Comparison of HIF-1α according to grade of OSCC by Tukey post hoc analysis test
| Group | Mean difference (HIF-1α LI %) | p value |
|---|---|---|
| WDSCC versus MDSCC | 30.013 | 0.001 (HS) |
| WDSCC versus PDSCC | 43.6 | 0.001 (HS) |
| MDSCC versus PDSCC | 13.587 | 0.001 (HS) |
Tables 5 and 6 reveals comparison of mean HIF-1α LI in Oral epithelial dysplasia with different histologic grades of OSCC by Independent sample t test. There was no statistically significant difference between OED and well differentiated OSCC (P = 0.998). However, there was statistically significant difference found between OED and moderately differentiated OSCC (P = 0.001) and OED and poorly differentiated OSCC (P = 0.000).
Table 5.
Comparison of HIF-1α expression in Oral Epithelial Dysplasia (OED) and histologic grades of OSCC
| Group | No of cases | Mean ± SD | Mean HIF-1α LI (%) | Range |
|---|---|---|---|---|
| OED | 30 | 47.02 ± 14.39 | 47.02 | 17.8–68.4 |
| Well differentiated OSCC | 9 | 46.30 ± 3.24 | 46.30 | 41.2–50.8 |
| Moderately differentiated OSCC | 16 | 76.31 ± 6.07 | 76.31 | 62.3–84.2 |
| Poorly differentiated OSCC | 5 | 89.90 ± 40.4 | 89.90 | 86.8–96.8 |
| ANOVA F value | 42.499 | |||
| P value | 0.001 (HS) |
Table 6.
Intragroup comparison of HIF-1α expression in Oral Epithelial Dysplasia (OED) with different histologic grades of OSCC by Tukey post hoc analysis test
| Group | Mean difference (HIF-1α LI %) | P value |
|---|---|---|
| OED versus well differentiated OSCC | 0.726 | 0.998 (NS) |
| OED versus moderately differentiated OSCC | 24.285 | 0.001 (HS) |
| OED versus poorly differentiated OSCC | 42.873 | 0.001 (HS) |
Table 7 reveals comparison of HIF-1α expression between OED group and OSCC group done by Independent sample t test.
Table 7.
Comparison of mean HIF-1α expression in oral epithelial dysplasia (OED) with oral squamous cell carcinoma (OSCC) group by independent sample t test
| Sr. No. | Variable | Mean ± SD | Mean HIF-1α-LI in % | t value | P value |
|---|---|---|---|---|---|
| 1 | OED | 47.027 ± 14.3996 | 47.027 | 5.482 | 0.001 (HS) |
| 2 | OSCC | 69.573 ± 16.9900 | 69.573 |
The mean HIF-1α LI in Oral epithelial dysplasia was 47.02%, while in OSCC it was 69.57%. The HIF-1α LI was, thus, significantly higher (P = 0.000, 95% confidence level) in OSCC as compared to OED.
Table 8 shows comparison of expression of HIF-1α scores with stages of OSCC by ONE WAY ANOVA. There was a highly significant difference between the mean HIF-1α LI values between the 4 stages (P = 0.002).The HIF-1α expression was highest in stage IV followed by stage III, II and I.
Table 8.
Comparison of HIF-1α expression in stages of OSCC
| Stages of OSCC | Mean ± SD | Mean HIF-1α LI in % |
|---|---|---|
| Stage I | 516.43 ± 105.555 | 51.643 |
| Stage II | 704.78 ± 164.410 | 70.478 |
| Stage III | 765.08 ± 126.356 | 76.508 |
| Stage IV | 968.18 ± 45.54 | 96.8 |
| ANOVA F value | 6.619 | |
| Significance P value | 0.002 (HS) |
Discussion
Oral cancers arise from sustained, stepwise accumulations of mutations resulting in transition of normal mucosa to dysplasia to invasive carcinoma over time [13]. Identification of high-risk oral premalignant disorders and intervention at premalignant stages could constitute one of the keys to reducing the mortality, morbidity and cost of treatment associated with SCC. Those most commonly encountered are erythroplakia and leukoplakia [14].
Solid malignant tumors, such as OSCC, have the potential for rapid and unlimited growth. Hypoxia is a common feature and contributes to local and systemic cancer progression, resistance to therapy and poor outcome [15]. Oxygen concentrations are markedly reduced in many human cancers compared with normal tissue and a major mechanism mediating adaptive responses to reduced oxygen availability (hypoxia) is the regulation of transcription by hypoxia-inducible factor 1 (HIF-1) [16]. Neovascularization and increased glycolysis represent adaptations to a hypoxic microenvironment that are correlated with tumor invasion and metastasis [17].
Development of hypoxia microenvironment is caused by the imbalance between oxygen consumption and oxygen delivery. The rapidly proliferating head and neck squamous cell carcinoma has insufficient vascularization with poor blood supply. The hypoxic stress stimulates solid tumors to upregulate expression of a variety of oncogenes such as HIF and vascular endothelial growth factor which enhances irregular vascular endothelial cell proliferation and differentiation. The adaptation of cells to hypoxia appears to be mediated via hypoxia inducible factor1α (HIF-1α). HIF-1α is said to be associated with malignant transformation of epithelium in other sites. It appears that HIF1α plays a significant role in both prostate and cervical carcinogenesis at early stages [18].
Little is known about the role of HIF-1α in early events of carcinogenesis of the oral cavity. Therefore, the aim of our study was to evaluate the expression of HIF-1α in Oral epithelial dysplasia and different histologic grades of Oral squamous cell carcinoma by immunohistochemistry.
In both the study groups, the age of patients varied between 20 and 70 years with the mean age of 43.3 years in OED and 49.3 years in OSCC. We did not find a statistically significant correlation between patient’s age, sex, site and HIF-1α LI in both the categories.
Further, we assessed the expression of HIF-1α in histologic grades of Oral Epithelial Dysplasia. The mean HIF-1α labeling index significantly increased from mild to moderate dysplasia and from moderate to severe dysplasia (Table 1). Staining was seen in the basal cell layer to the middle spinous layer for samples in mild and moderate dysplasia (Figs. 1, 2) and almost the entire thickness of the dysplastic epithelium in severe dysplasia (Fig. 3). The expression in OED differed from normal epithelium where it is reported to stain only in the lower two-third of suprabasal cells (Lin et al. [19]) or is rarely expressed (Chaudhary et al. [18]).
Fig. 1.
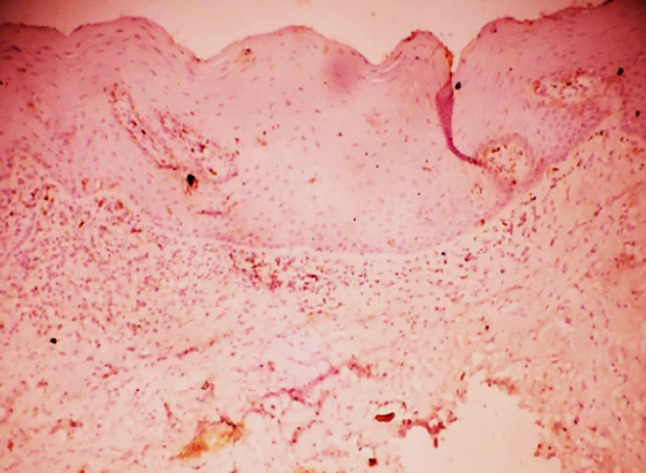
HIF-1α expression in mild dysplasia
Fig. 2.
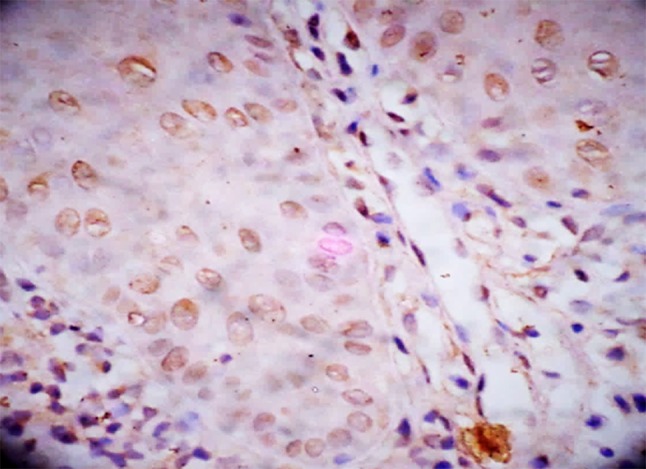
HIF-1α expression in moderate dysplasia
Fig. 3.
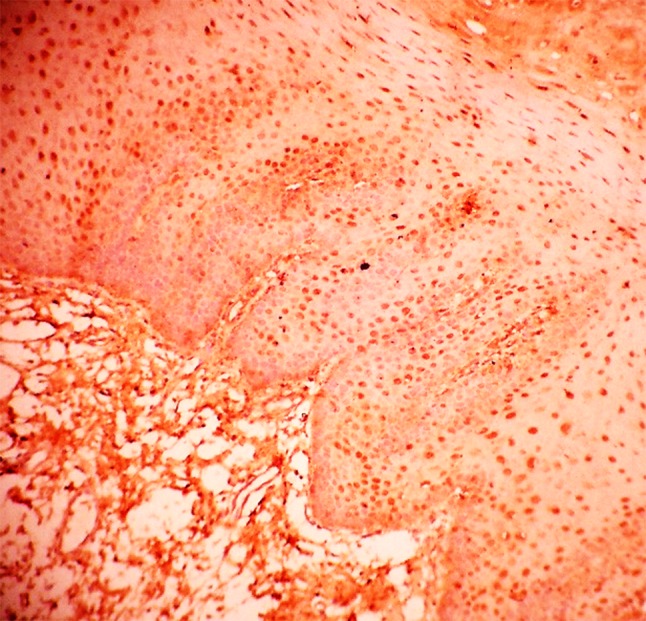
HIF-1α expression in severe dysplasia
On intragroup comparison of HIF-1α LI and histologic grades of OED, we found highly significant difference between mild dysplasia and moderate dysplasia, moderate dysplasia and severe dysplasia and severe dysplasia and mild dysplasia (P = 0.001) (Table 2). Our study revealed overall significantly increased HIF-1α expression as the grades of dysplasia increased.
These results are similar to those of Lin et al. [19] who found that there was a significant elevation of HIF-1α LI from mild OED to moderate to severe OED. They had suggested that elevated HIF-1α is an early event in oral carcinogenesis [19].
Further in our study, all the cases of OSCC were clinically staged according to the AJCCS known as the TNM system. We observed that stage IV had maximum HIF-1α LI (96.8%), followed by stage III (76.50%), stage II (70.47%) and stage I (51.64%). As our study has shown higher expression of HIF-1α in stage IV cases of OSCC, it can be inferred that higher HIF-1α expression correlates with increased lymph node metastasis and is significantly associated with advanced clinical stage.
Our results were in accordance with the study done by Zhou et al. [20] who concluded that increased expression of HIF-1α protein was significantly associated with larger tumor size (T3/T4), more advanced TNM stage (III/IV), and lymph node metastasis, but not with poor differentiation. This suggested an association of HIF-1α overexpression with the biological behavior of OSCC [20].
Zhou et al. [20] stated that a significant association between HIF-1α overexpression and poor prognosis was found only in Asian population (HR = 2.33, 95% CI = 1.72–3.15), without significant heterogeneity in this respect.
HIF-1α expression was evaluated in histologic grades of OSCC. HIF-1α LI increased as the grade of OSCC progressed from well differentiated to moderately to poorly differentiated OSCC and was found to be statistically highly significant (P = 0.001) (Table 4; Figs. 4, 5, 6).
Fig. 4.
HIF-1α expression in well differentiated squamous cell carcinoma
Fig. 5.
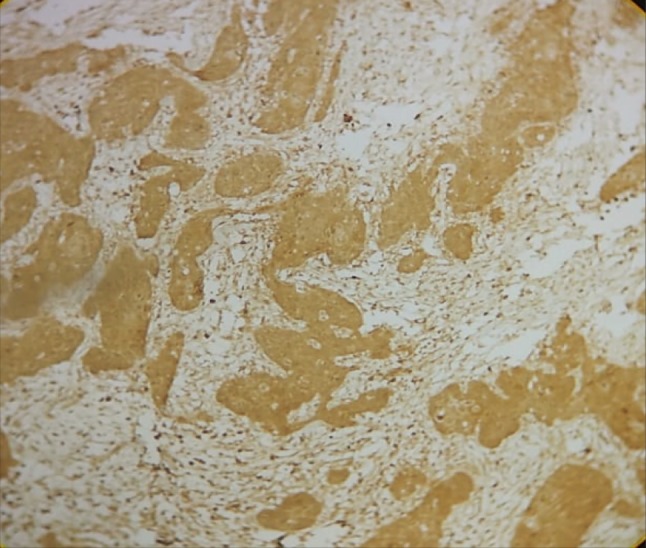
HIF-1α expression in moderately differentiated squamous cell carcinoma
Fig. 6.
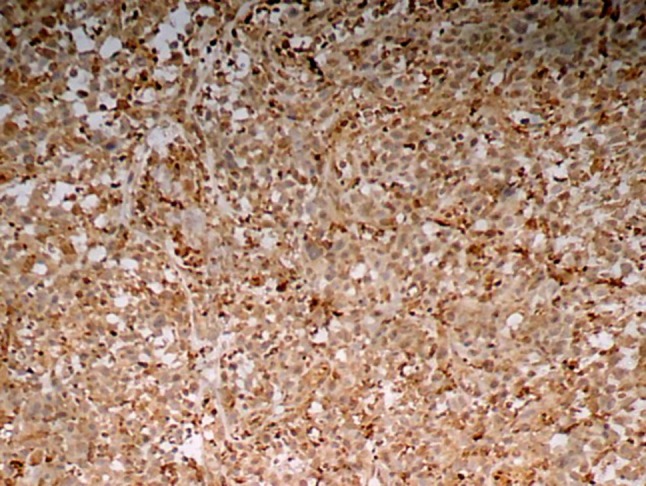
HIF-1α expression in poorly differentiated squamous cell carcinoma
We went ahead to compare the HIF-1α LI between individual grades. Our study found a highly significant difference in the HIF-1α LI between WDSCC and MDSCC, MDSCC and PDSCC, WDSCC and PDSCC (P = 0.001) (Tables 5, 6).
Our results were in agreement with the study carried out by Lin et al. [19] who stated that the augmented expression of HIF-1α in oral cancers may be in part due to the stimulation from certain growth factors like EGF, cytokines (like PGE 2) and Ras protein leading to an increase of HIF-1α expression in human cancer cells. Loss of wild—type p53 protein also enhances HIF-1α level in OSCC cells.
Our results were also similar with the study done by Zhu et al. [21] who observed that HIF-1α expression was significantly associated with histologic differentiation.
Further, comparison of expression of HIF-1α LI in Oral epithelial dysplasia and different histologic grades of Oral squamous cell carcinoma was done using Tukey post hoc analysis test. The mean HIF-1α LI in oral epithelial dysplasia was 47.02%, while in WDSCC it was 46.3%. The HIF-1α LI was not found to be statistically significant between these two categories and this may be probably due to variation in sample size. The mean HIF-1α LI in oral epithelial dysplasia was further compared with other histologic grades of OSCC that is MDSCC and PDSCC and the difference was found to be statistically significant (P < 0.05) (Tables 5, 6).
Further, we compared the expression of HIF-1α in Oral epithelial dysplasia and Oral squamous cell carcinoma by employing Independent sample t test.
The mean HIF-1α LI in oral epithelial dysplasia was 47.02%, while in OSCC it was 69.57%. The HIF-1α LI was, thus, significantly higher (P < 0.05) in OSCC as compared to OED (Table 7). Patients with OSCC had high proportion of cells expressing HIF-1α as compared to dysplastic epithelia. Therefore, increase in HIF-1α expression is a predictor for greater risk of malignant transformation in many oral premalignant disorders.
Our results were in accordance with the study done by Lin et al. [19] who reported that HIF-1α expression increased from OED to OSCC. This may indicate that HIF-1α upregulation occurs in early carcinogenesis and is an essential step before acquiring invasive phenotype.
Conclusion
Hypoxia is a common feature in HNSCC, which contributes to the development of tumorous aggression and metastasis. The hypoxic condition should be an important target in treatment of HNSCC. Removing the hypoxic microenvironment by enhancing the oxygen delivery and improving the hypoxic cell death rate by using hypoxic cell radiosensitizer and hypoxic cell cytotoxin have proved to overcome the poor prognosis of HNSCC.Therefore, expression of HIF-1α in OSCC is likely to be of great value to predict the prognosis of OSCC and could provide important information for a targeted treatment program. It can be also concluded that HIF-1α could be an independent prognostic marker in patients with OSCC.
Funding
The present study was not funded by any agency, it was self funded and there was no conflict of interest between authors.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Standards
An Ethical clearance was obtained from the Institutional Ethical Committee. All procedure performed on human participant were in accordance with the ethical standards of the Institutional Ethical Committee.
Informed Consent
Informed consent from patients was obtained prior to examination.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Saman DM. A review of the epidemiology of oral and pharyngeal carcinoma: update. Head and Neck Oncology. 2012;4:1. doi: 10.1186/1758-3284-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feller L, Lemmer J. Oral squamous cell carcinoma. Journal of Cancer Therapy. 2012;3:263–268. doi: 10.4236/jct.2012.34037. [DOI] [Google Scholar]
- 3.Sadiq H, Gupta P, Singh N, Thakar SS, Prabhakar I, Thakral J. Various grading systems of the oral epithelial dysplasia: a review. International Journal of Advanced Health Sciences. 2015;1(11):20–26. [Google Scholar]
- 4.Ishida K, Ito S, Wada N, Deguchi H, Hata T, Hosoda M, Nohno T. Nuclear localization of beta – catenin involved in precancerous change in oral leukoplakia. Molecular Cancer. 2007;6:62–68. doi: 10.1186/1476-4598-6-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scully C, Bagan JV, Hopper C, Epstein JB. Oral cancer: current and future diagnostic techniques. Am J Dent. 2008;21:199–209. [PubMed] [Google Scholar]
- 6.Rohwer N, Cramer T. Hypoxia-mediated drug resistance: novel insights on the functional interaction of HIFs and cell death pathways. Drug Resist Updates. 2011;14:191–201. doi: 10.1016/j.drup.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Li JZ, Gao W, Wai Chan JY, Kuen Ho W, Sze Wong T. Hypoxia in head and neck squamous cell carcinoma. ISRN Otolaryngology. 2012;2012:708974. doi: 10.5402/2012/708974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uehara M, Sano K, Ikeda H, Nonaka M, Asahina I. Hypoxia-inducible factor 1 alpha in oral squamous cell carcinoma and its relation to prognosis. Oral Oncol. 2009;45:241–246. doi: 10.1016/j.oraloncology.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 9.Fillies T, Werkmeister R, Van Diest PJ, Brandt B, Joos U, Buerger H. HIF-1 alpha overexpression indicates a good prognosis in early stage squamous cell carcinoma of the oral floor. BMC Cancer. 2005;5:84. doi: 10.1186/1471-2407-5-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Santos MD, Cunha Mercante AM, Louro ID, Goncalves AJ, Carvalho MB, Tajara da Silva EH, et al. HIF-1 Alpha expression predicts survival of patients with squamous cell carcinoma of the oral cavity. PLoS ONE. 2012;7:9. doi: 10.1371/journal.pone.0045228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greene FL, Page DL, Fleming ID, Fritz AG, Balch CM, Haller DG, et al. AJCC cancer staging manual. 6. Chicago: Springer; 2002. [Google Scholar]
- 12.Bryne M, Koppang HS, Lilleng R, Stene T, Bang G, Dabelsteen E. New malignancy grading is a better prognostic indicator than Broder’s grading in oral squamous cell carcinoma. J Oral Pathol Med. 1989;18:432–437. doi: 10.1111/j.1600-0714.1989.tb01339.x. [DOI] [PubMed] [Google Scholar]
- 13.Gillenwater A, Papadimitrakopoulou V, Kortum RR. Oral premalignancy: new methods of detection and treatment. Curr Oncol Rep. 2006;8(2):146–154. doi: 10.1007/s11912-006-0050-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dionne KR, Warnakulasuriya S, Zain RB, Cheong S. Potentially malignant disorders of the oral cavity: current practice and future directions in the clinic and laboratory. Int J Cancer. 2015;136:503–515. doi: 10.1002/ijc.28754. [DOI] [PubMed] [Google Scholar]
- 15.Oliveira De Lima P, Jorge CC, Oliveira DT, Pereira MC. Hypoxic condition and prognosis in oral squamous cell carcinoma. Anticancer Res. 2014;34:605–612. [PubMed] [Google Scholar]
- 16.Semenza GL. Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene. 2010;29:625–634. doi: 10.1038/onc.2009.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hockel M, Vaupel P. Tumor hypoxia: definitions and current clinical, biologic, and molecular aspects. J Natl Cancer Inst. 2001;93:266–276. doi: 10.1093/jnci/93.4.266. [DOI] [PubMed] [Google Scholar]
- 18.Chaudhary M, Bajaj S, Bohra S, Swastika N, Hande A. The domino effect: role of hypoxia in malignant transformation of oral submucous fibrosis. Journal of Oral and Maxillofacial Pathology. 2015;19(2):122–127. doi: 10.4103/0973-029X.164519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin PY, Yu CH, Wang JT, Chen HH, Cheng SJ, Kuo MYP, et al. Expression of hypoxia-inducible factor-1a is significantly associated with the progression and prognosis of oral squamous cell carcinomas in Taiwan. J Oral Pathol Med. 2008;37:18–25. doi: 10.1111/j.1600-0714.2007.00571.x. [DOI] [PubMed] [Google Scholar]
- 20.Zhou J, Huang S, Wang L, Yuan X, Dong Q, Zhang D, et al. Clinical and prognostic significance of HIF-1α overexpression in oral squamous cell carcinoma: a meta-analysis. World J Surg Oncol. 2017;15(1):104. doi: 10.1186/s12957-017-1163-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu G, Tang Y, Li L, Zheng M, Jiang J, Li XY, et al. Hypoxia inducible factor 1α and hypoxia inducible factor 2α play distinct and functionally overlapping roles in oral squamous cell carcinoma. Clin Cancer Res. 2010;16(19):4732–4741. doi: 10.1158/1078-0432.CCR-10-1408. [DOI] [PubMed] [Google Scholar]


