Abstract
The present study endeavoured to evaluate the nutritional, phytochemical and functional properties of outer and inner bracts of culinary banana flower which is a by-product of banana production. Both outer and inner bracts were found to be rich in dietary fibre (61.13 and 66.22%, respectively) along with other chemical compositions including proximate, minerals, and antioxidant-rich phenolics both free and bound. In addition, the functional properties including glucose dialysis retardation index (GDRI) of outer and inner bracts were also studied. The outer and inner bracts exhibited total polyphenols 7.56 and 9.44 mg phenols/g dry sample, respectively. The polyphenol profile by HPLC, revealed the presence of significant amount of free and bound phenolics in both outer and inner bracts. Functional properties of these dietary fibres-rich fractions of culinary banana flower exhibited lower bulk density, higher water-holding capacity, oil-holding capacity, and water-swelling capacity in outer and inner bracts than cellulose. The outer and inner bracts showed relatively higher GDRI compared to control and cellulose. The results revealed that both the outer and inner bracts of culinary banana flower are rich source of dietary fibre along with high antioxidant activity and could be one of the promising functional ingredients for low-calorie and high-fibre food product.
Keywords: Culinary banana flower, Dietary fibre, Chemical composition, Polyphenol profile, Functional properties
Introduction
Agricultural waste constitutes largely under-exploited residues and in recent years, waste valorization practices have attracted significant amount of attention by the researchers (Luque and Clark 2013). Banana flower is a by-product of post-harvest cultivation. The inner bract of banana flower is cooked in a variety of dishes in Asian countries such as curry, deep fried cutlet whereas the coloured outer bracts are generally disposed as residue after harvesting of banana flower (Leonard 2006). About 300 kg of coloured bracts per hectare are disposed as residues during harvesting of banana and is an excellent source of anthocyanin. The anthocyanin content of outer bract of culinary banana flower is 56.98 mg/100 g (Begum and Deka 2017).
By-product of fruits and vegetables has received high importance in recent years as a newer source of dietary fibre (DF) and varies according to the plant species, its genotype and even within the cultivar of the same species (Rodica et al. 2010). The composition of fibre, its organizational structure, physicochemical and surface properties, and associated bioactive (oligosaccharides and bound phenolics) compounds are mainly responsible for the physiological benefits of DF (Macagnana et al. 2016). Besides, polyphenols associated with DF is also responsible for beneficial effects, especially in the prevention and management of chronic and degenerative diseases (Aruna et al. 2017). Approximately 50% of total dietary antioxidant is comprised of these phenolics associated with plant polysaccharides (Macagnana et al. 2016).
In the present investigation, the comparative analysis of outer and inner bracts of culinary banana flower based on some chemical composition, its antioxidant activity, polyphenol profile, the free and bound (phenolics associated with polysaccharide) phenolics of different parts were done. In addition, the functional properties of DF-rich fractions of banana flower were evaluated which is related with the physiological effect. A detailed comparative analysis of nutrients, phytochemicals and DF composition of the outer bracts from its inner bracts is imperative for value addition of this agricultural waste.
Materials and methods
Chemicals
DPPH (2,2′-diphenyl-1-picrylhydrazyl), ABTS (2,2′-Azino-bis(3-ethylbenzthiazoline-6-sulfonic acid), TPTZ (2,4,6-tripyridyl-s-triazine), and Trolox were obtained from Merck Millipore. Enzymes (α-amylase, protease, amyloglucosidase), HPLC standards, HPLC-grade methanol and acetonitrile were purchased from Sigma Aldrich Corporation, USA and all the chemicals and reagents were of high purity analytical grade.
Collection and preparation of sample
Culinary banana (Musa ABB) flowers were obtained from Tezpur University Campus, Assam (India). It was washed immediately after collection and outer and inner bracts were separated and dried separately at 40 °C in a tray drier (Labotech, BDI-51, India). Samples were ground, packed in air tight container and stored at room temperature (25 ± 5 °C) until further analysis.
Chemical analysis
Moisture, ash, crude fat and crude fibre content were determined according to the method described in AOAC (1998). Protein content was determined following the method of Lowry et al. (1951). Starch content of the samples was determined using anthrone reagent (Sadasivam and Manickam 1992). Insoluble, soluble and total dietary fibres were determined following enzymatic–gravimetric method (Prosky et al. 1988). Cellulose and hemicellulose contents were determined according to Updegroff (1996) and pectin content was determined as calcium pectate (Ranganna 1986).
The mineral contents were estimated by atomic absorption spectrophotometer (Thermo Scientific, ICE 3000 Series, Newington, USA). Powder samples (0.5 g) were added to 15 mL concentrated nitric acid and perchloric acid in the ratio of 2:3. Samples were digested in a digester (KelPlus, Pelican Equipment, India), cooled and volume was made up to 100 mL with distilled water and concentrations were determined in the aqueous solution of acid digest.
Analysis of total polyphenol content and antioxidant activity
Preparation of extract
The outer and inner bracts of culinary banana flower were ground with 80% methanol and were placed in an ultrasonic bath (Bandelin sonorex, Germany) with frequency of 35 kHz for 15 min. The extracted samples were filtered through Whatmann No 1 filter paper and kept at 4 °C in the dark until further analysis.
Estimation of total phenols
Total phenol content was determined spectrophotometrically at 650 nm using Folin–Ciocalteau reagent with catechol as a standard (Bray and Thrope 1954). To estimate the total phenolic content, the extracts of bract samples (as mentioned in the “Preparation of extract” section) were taken, evaporated to dryness and the residues were dissolved in a known volume of distilled water. The aliquots (0.2 mL) were pipetted out into the test tubes and the volume was made up to 3 mL with water followed by addition of Folin–Ciocalteu reagent (0.5 mL). After 3 min, 2 mL of 20% Na2CO3 solution was also added to each tube and tubes were placed in boiling water for 1 min, cooled and the absorbance was measured at 650 nm against a reagent blank. A standard curve was prepared using different concentrations of catechol ().
Antioxidant activity determination
DPPH free radical scavenging activity was determined following the method of Brand-Williams et al. (1995) with slight modifications. A stock solution was prepared by dissolving 24 mg DPPH in 100 mL methanol. The working solution was prepared by diluting the stock solution until the absorbance of 1.1 ± 0.01 units at 515 nm was obtained. Extracts from outer and inner bracts (150 µL) were allowed to react with DPPH solution (2850 µL) and after 30 min; the absorbance was read at 515 nm. The standard curve was prepared with Trolox, concentrations ranging from 25 to 800 µM and results were expressed in µM Trolox equivalents (TE)/g fresh mass. The IC50 for various concentrations of outer and inner bracts was determined and compared with standard.
The ABTS assay was performed according to the method of Arnao et al. (2001) with slight modifications. After preparation of ABTS+ (7.4 mM) and K2S2O8 (2.6 mM) solutions, the working solution was prepared by mixing equal amount of them and allowed to react for 12 h in dark. The solution was diluted with methanol to obtain an absorbance of 1.1 ± 0.01 units at 734 nm. Extract of 150 µL each from outer and inner bracts was allowed to react with 2850 µL of working solution for 2 h and the absorbance was taken at 734 nm. The standard curve was prepared with Trolox (concentration ranging from 25 to 600 µM) and results were expressed in µM Trolox equivalents (TE)/g fresh mass. The IC50 for various concentrations of outer and inner bracts was determined and compared with standard.
FRAP assay was performed following the method of Benzie and Strain (1996) with some modifications. The working solution include 25 mL acetate buffer (300 mM, pH 3.6), 2.5 mL TPTZ (2,4,6-tripyridyl-s-triazine) solution (10 mM) and 2.5 mL FeCl3·6H2O solution (20 mM) and heated at 37 °C. Extract of 150 µL each from outer and inner bracts was allowed to react with 2850 µL of FRAP solution for 30 min and the absorbance was read at 593 nm. The standard curve was prepared with Trolox, concentrations ranging from 25 to 800 µM and results were expressed in µM Trolox equivalents (TE)/g fresh mass.
HPLC analysis of phenolic extract
Extraction of free and bound phenolics was done according to the method described by Okarter et al. (2010) with some modifications.
For free phenolic extraction, the outer and inner bracts powder were defatted with n-hexane (bp 70 °C) and extracted with 80% methanol at room temperature (25 ± 5 °C) and sonicated using ultrasonic bath (Bandelin sonorex, Germany) at 35 kHz for 15 min and the extraction continued until the solvent became colourless. The extracts were filtered through Whatman No. 1 filter paper and the solvent was removed using rotary vacuum evaporator (EYELA, NCB-1200, Tokyo) at 40 °C and stored under refrigeration until further analysis.
Bound phenolics were extracted from the residue of free phenolics. The residues of outer and inner bracts were hydrolyzed with 2 M NaOH under nitrogen atmosphere for 1 h. The pH was adjusted to 2.0 and then phenolics were extracted with ethyl acetate. It was then evaporated to dryness using rotary vacuum evaporator at 40 °C and dissolved in 10 mL methanol and stored under refrigeration for analysis.
The HPLC analysis was conducted following the method of Rodriguez-Delgado et al. (2001) with some modifications. Detection was done using HPLC system (Dionex, Ultimate 3000, Germany). The column used was Acclaim 120-C18 column (5 µm, 120 Å) with size of 4.6 × 250 mm. A mixture of methanol:acetic acid:water (10:2:88, v/v) and methanol:acetic acid:water (90:2:8, v/v) were used as solvent A and solvent B, respectively. The gradient program was 0–15 min 15% B, 16–20 min 50% B, 21–35 min 70% B, 36–50 min 100% B and 51–60 min 15% B. The injection volume was 20 µL, and the flow rate was 1 mL/min. Detection was done at 280 nm and sample peaks were identified by comparing with retention times of standard peaks.
Functional properties
Bulk density of the sample was determined by the method of Wang and Kinsella (1976). Powdered sample (3 g) was placed in a 50 mL graduated cylinder and packed by tapping the cylinder on a rubber sheet until a constant volume was obtained and the bulk density was expressed as g of sample/mL.
To determine water holding capacity (WHC) or oil holding capacity (OHC) of the banana bracts and cellulose, 25 mL of distilled water or olive oil were added to 250 mg of dry sample followed by stirring and incubation at room temperature (25 ± 5 °C) for 1 h. Tubes were centrifuged at 3000g for 20 min and the supernatant was decanted and tubes were allowed to drain for 10 min to ensure the proper removal of water or oil but not the residue. The residue was weighed and WHC or OHC was calculated as g water or oil per g dry sample, respectively as per the method described by Rodríguez et al. (2006) with slight modification. To determine water swelling capacity (WSC), samples (200 mg) were mixed with 10 mL distilled water in calibrated cylinders and incubated at room temperature (25 ± 5 °C) for 18 h. The bed volume was recorded. The WSC was determined as mL per g of dry sample following the method described by Robertson et al. (2000).
Glucose dialysis retardation index (GDRI) of outer and inner bracts, and cellulose was determined according to the method described by Lecumberri et al. (2007). Outer bracts, inner bracts and cellulose (400 mg each) were extracted with 80% ethanol to ensure the total removal of soluble sugar and mixed with 15 mL of distilled water followed by constant stirring for 1 h at room temperature (25 ± 5 °C). An equal amount of glucose solution (2 mg/mL) was added to both samples before transferring to 15 cm portions of previously hydrated dialysis bags. An equal volume of glucose solution without sample was placed in a dialysis bag and considered as control. Samples were dialyzed against 400 mL of distilled water in separate beakers and incubated in a shaking water bath at 37 °C for 60 min. After 20, 40, and 60 min, 0.5 mL of dialysate were collected from each sample and glucose concentration was determined using anthrone method (Sadasivam and Manickam 1992). The retardation of glucose diffusion from the dialysis bag into the dialysate was calculated as below.
Statistical analysis
The data were analyzed using Paired-Samples T Test and expressed as mean values from three replicates with standard deviations and the confidence interval was 95% (P < 0.05).
Results and discussion
The chemical compositions of the outer and inner bracts of culinary banana flower are presented in Table 1. DF was the major component in both outer and inner bracts among all the components (Table 1). The insoluble DF was found to be higher compared to soluble DF for both outer and inner bracts. The outer and inner bracts contained higher total DF of 61.13 and 66.22%, respectively. The results are in line with the findings of Bhaskar et al. (2012) who reported the DF content of banana (Musa sp. var. elakki bale) flower as 65.6%. Ma and Mu (2016) reported total DF content of rice bran (27.04%), peach DF concentrate (30.7%), oranges DF (35.4–36.9%) and sesame coat DF concentrate (31.64%). Hence, the total DF content of outer and inner bracts of culinary banana flower was higher than several fibre-rich foods. Different components of DF viz., cellulose, hemicellulose and pectin contents were also analysed and are shown in Table 1.
Table 1.
Chemical composition of outer and inner bract of culinary banana flower
| Component | Outer bract | Inner bract |
|---|---|---|
| Moisture content (g/100 g) | 8.47 ± 0.02a | 8.44 ± 0.02a |
| Crude fat (g/100 g) | 4.78 ± 0.30a | 7.02 ± 0.03b |
| Protein (g/100 g) | 2.06 ± 0.15a | 2.55 ± 0.05b |
| Crude fibre (g/100 g) | 9.52 ± 0.20a | 10.73 ± 0.20b |
| Ash (g/100 g) | 14.00 ± 0.10b | 10.1 ± 0.64a |
| Starch (mg/100 g) | 7.2 ± 0.17a | 16.8 ± 0.10b |
| Insoluble dietary fibre (g/100 g) | 53.9 ± 0.10a | 61.86 ± 0.14b |
| Soluble dietary fibre (g/100 g) | 7.23 ± 0.25b | 4.36 ± 0.55a |
| Total dietary fibre (g/100 g) | 61.13 ± 0.15a | 66.22 ± 0.67b |
| Cellulose (g/100 g) | 5.47 ± 0.25a | 13.19 ± 0.26b |
| Hemicellulose (g/100 g) | 18.83 ± 0.76b | 14.36 ± 0.32a |
| Pectin as calcium pectate (g/100 g) | 5.31 ± 0.20b | 3.97 ± 0.11a |
| Total polyphenol content (mg phenols/g dry sample) | 7.56 ± 0.11a | 9.44 ± 0.05b |
| K (mg/100 g) | 1800.01 ± 0.017b | 1087.92 ± 0.088a |
| Ca (mg/100 g) | 651.86 ± 0.007b | 317.83 ± 0.076a |
| Mg (mg/100 g) | 567.56 ± 0.005b | 305.09 ± 0.026a |
| Na (mg/100 g) | 61.26 ± 0.031b | 17.94 ± 0.014a |
| Fe (mg/100 g) | 26.99 ± 0.026b | 14.51 ± 0.027a |
| Zn (mg/100 g) | 28.62 ± 0.054b | 9.13 ± 0.036a |
| Cu (mg/100 g) | 79.59 ± 0.003b | 5.53 ± 0.012a |
Mean with different superscript letters in the same row represent a significant difference at P < 0.05 based on paired t test, values represent mean ± SD; n = 3
The mineral content viz., K, Ca, Mg, and Na of outer bracts was significantly higher compared to inner bracts, among which K content was the highest (Table 1). The micronutrients viz., Fe, Cu and Zn were also found higher in outer bracts compared to inner one. Sheng et al. (2010) also reported banana flower (cvs. Baxijiao (AAA) and Paradisica (AAB)) as a good source of minerals viz., Mg, Fe, Cu and K.
The chemical compositional analysis showed that culinary banana bracts are rich source of nutrients and contains significant amount of minerals (Table 1). Incorporation of fibre-rich bracts into a wide range of products has the potential to contribute to the development of functional foods that are currently in high demand. Furthermore, the bracts of culinary banana flower could be commercially utilized for extraction of DF and utilization of it in food product will unleash a new source of fibre coupled with nutritional and dietary fibre requirements in a ready-to-eat form. Apart from this, bracts of culinary banana yields considerable amount of cellulose and water-soluble polysaccharide (WSP) which could also be a good source of bio-based packaging material and therefore utilization of it will give high value to the agricultural waste of culinary banana bracts.
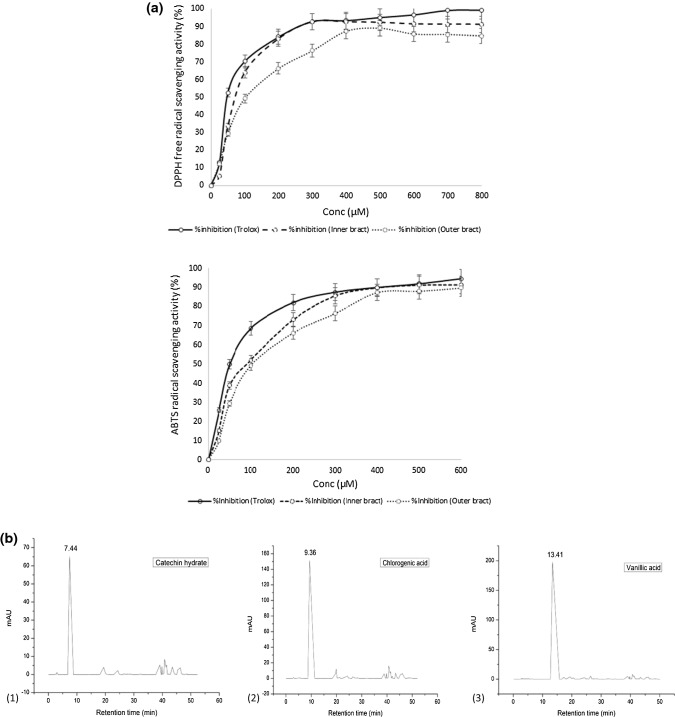
The antioxidant activity was determined by DPPH, ABTS and FRAP assay (Table 2). The percent inhibition of DPPH free radical and ABTS radical of inner and outer bracts are illustrated in Fig. 1a. Both inner and outer bracts potentially scavenged DPPH and ABTS radical and it increased in a dose dependent manner (Fig. 1a). The per cent inhibition of DPPH free radical and ABTS radical of inner bract increased much faster by increasing the concentration followed by outer bract and are very close to the positive control (Trolox). The concentration at 50% inhibition (IC50) of DPPH free radical and ABTS radical was determined from the standard curve (Fig. 1a). The IC50 values of DPPH free radical scavenging activity and ABTS radical scavenging activity (Table 2) revealed that inner bracts showed greater antioxidant activity (27.96 and 24.53 µmol TE/g fresh mass of inner and outer bract, respectively) than outer bracts with lesser IC50 value (163.50 and 219.06 µM of inner and outer bract, respectively) for DPPH assay. The IC50 value of Trolox was 105.66 µM and similar results were observed for ABTS assay with lower IC50 value (241.83 and 296.46 µM of inner and outer bracts, respectively) in inner bracts when compared to the outer bracts. The lower IC50 value indicates higher scavenging free radical activity of an antioxidant (Zhang et al. 2015) and higher antioxidant activity for inner bracts (20.6, 8.85 µmol TE/g fresh mass of inner and outer bract, respectively) was also evidenced by FRAP assay.
Table 2.
Antioxidant activity of outer and inner bracts of culinary banana flower
| DPPH | ABTS | FRAP | |||
|---|---|---|---|---|---|
| Antioxidant activity (µmol TE/g fresh mass) | IC50 (µM) | Antioxidant activity (µmol TE/g fresh mass) | IC50 (µM) | Antioxidant activity (µmol TE/g fresh mass) | |
| Outer bract | 24.53 ± 0.04a | 219.06 ± 0.05c | 29.62 ± 0.10a | 296.46 ± 0.47c | 8.85 ± 0.04a |
| Inner bract | 27.96 ± 0.11b | 163.50 ± 0.41b | 30.66 ± 0.15b | 241.83 ± 0.76b | 20.6 ± 0.45b |
| Positive control (Trolox) | 105.66 ± 0.57a | 148.60 ± 0.52a | |||
Mean with different superscript letters in the same column represent a significant difference at P < 0.05 based on paired t test, values represent mean ± SD; n = 3
IC50: Concentration at 50% radical scavenging activity
Fig. 1.
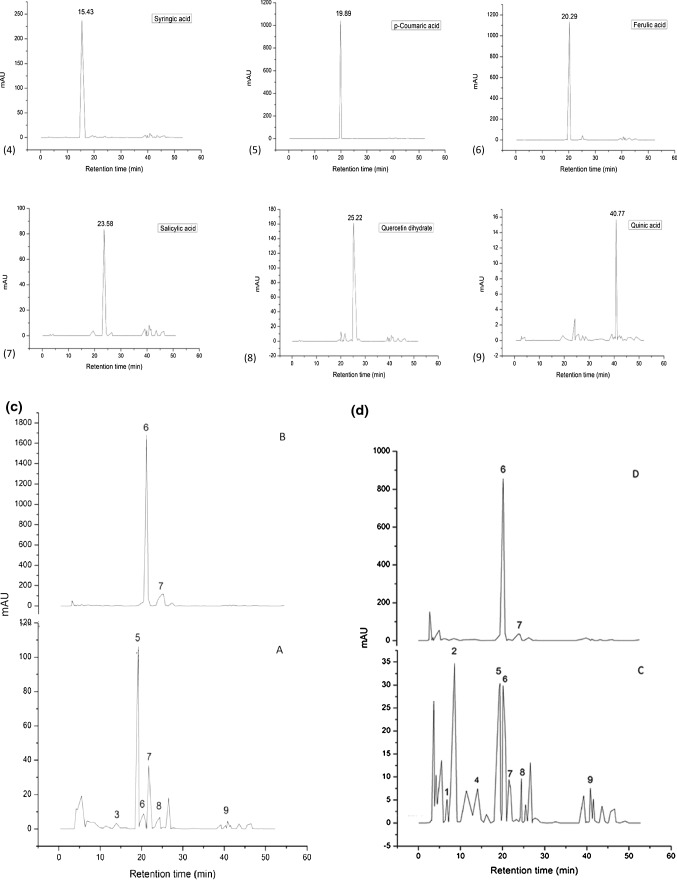
a DPPH free radical and ABTS radical scavenging activity of Trolox, inner and outer bracts of culinary banana flower at varying concentration. b HPLC chromatogram of standard ((1) catechin hydrate, (2) chlorogenic acid, (3) vanillic acid, (4) syringic acid, (5) p-coumaric acid, (6) ferulic acid, (7) salicylic acid, (8) quercetin dihydrate, and (9) quinic acid). c HPLC chromatogram of outer bract (A) Free phenolics; (B) Bound phenolics. d HPLC chromatogram of inner bract (C) Free phenolics; (D) Bound phenolics
The DPPH assay of antioxidant is based on the activity of quenching free radicals or H-donor capability of the antioxidant. Increased concentration of the extracts of inner and outer bracts resulted in enhancement of the scavenging activity of DPPH free radicals. This indicated the increased ability of the compound present in the extracts to donate hydrogen ions. Zhang et al. (2015) reported that the IC50 value for DPPH free radical scavenging activity of different natural and synthetic standards such as propyl gallate (PG), gallic acid (GA), quercetin (QCT) and Trolox were 35.5 ± 2.8, 36.3 ± 2.0, 55.0 ± 2.7 and 128.3 ± 6.3 µmol/L, respectively. The IC50 of butylated hydroxytoluene (BHT) was over 1000 µmol/L. Furthermore, the inner and outer bracts evinced good ability to scavenge the ABTS radical. FRAP assay is a singlet electron transformer method which is based on the reduction capability of ferrous ion (Fe3+) to ferric ion (Fe2+) of a test sample. Du et al. (2014) reported that the reduction capability of miracle berry (Synsepalum dulcificum) flesh extract was 22.9 mmol/100 g. The higher reduction capability attributed to the higher levels of phenolics and ascorbic acid, which is considered to be stronger reductants in donating electrons. The average antioxidant activity of methanolic extracts of different varieties of guava (white-fleshed “Allahabad Safeda”, pink-fleshed “Fan Retief”, “Ruby Supreme”, “Advanced Selection”) as determined by ABTS, DPPH and FRAP assays were 31.1, 25.2, and 26.1 µM TE/g, respectively. The antioxidant activity of 12 fresh fruits (melon, pear, tomato, apple, banana, white and pink grape, pink grapefruit, orange, kiwi, plum, strawberry) ranged from less than 1 µM TE/g for melon up to 15 µM TE/g for strawberry (Thaipong et al. 2006). The antioxidant activity of inner and outer bracts was found to be very close to that of different guava varieties. These exceptionally high antioxidant activity of bracts extract as obtained by three different antioxidant (DPPH, ABTS and FRAP) assays might be due to the pronounced total phenolic content and phenolic compounds present in the bracts extract, which were evidenced by HPLC analysis. Sheng et al. (2010) reported banana flowers (cvs. Baxijiao (AAA) and Paradisiaca (AAB)) are good sources of antioxidants including phenolics and flavonoids. The ethyl acetate and methanol extract of Musa paradisiaca (Nendran variety) inflorescence also showed significant ABTS radical scavenging activity (Aruna et al. 2017).
The total polyphenol content of outer and inner bracts was 7.56 and 9.44 mg phenols/g dry sample, respectively (Table 1). The polyphenol profiles of free and bound phenolics of outer and inner bracts were evaluated by HPLC (Fig. 1c, d) and revealed the presence of 6 free phenolics (vanillic, p-coumaric, ferulic, salicylic, quercetin dihydrate, and quinic acid) in outer bracts and 8 free phenolics (catechin hydrate, chlorogenic, syringic, p-coumaric, ferulic, salicylic, quercetin dihydrate and quinic acid) in inner bracts. Moreover, both outer and inner bracts contained significant amount of bound phenolics (ferulic and salicylic acid) as illustrated in Fig. 1c, d. The standard peaks are shown separately in Fig. 1b. Bound phenolics were also identified and quantified in the present study and retention time (RT) for each phenolic compound is shown in Table 3. Higher amount of bound phenolics in outer bracts was observed as compared to inner bracts which may be referred as phenolics associated with DF. Phenolic acids (PA) occur as soluble free acids, as soluble conjugates that are esterified to sugars and other low molecular mass compounds, and as insoluble bound PAs that are mostly ester linked to cell wall polymers such as polysaccharides and lignin. The extracted PAs after alkali hydrolysis were obtained in natural forms which might be present as ester linked to cell wall polymers of banana bracts. However, based on RT of standards, the PAs in the extracts of bound phenols were identified as ferulic and salicylic acid. Several authors (Bhaskar et al. 2012; Aruna et al. 2017) have also reported that ferulic acid was obtained upon alkali hydrolysis of hemicelluloses (HA and HB) of banana (Musa sp var. elakki bale) flower and ferulic acid as free phenolics in Musa paradisiaca inflorescence. The phenolic profile by HPLC exhibited that inner and outer bracts contained substantial amount of free and bound phenolics. These phenolics provide beneficial effects on oxidative stress related disorders. The bound phenolics are relevant constituents of DF and act as carrier of dietary polyphenols to the colon and contribute to intestinal health by promoting an antioxidant environment in the colon (Macagnana et al. 2016). Moreover, these bound phenolics are mainly related with the beneficial effects of DF in the prevention and management of chronic and degenerative diseases (Aruna et al. 2017).
Table 3.
Estimation and quantification of free and bound phenolics in outer and inner bract of culinary banana flower
| Free phenolics | Retention time (min) | Outer bract (ppm) | Inner bract (ppm) |
|---|---|---|---|
| (1) Catechin hydrate | 7.44 | ND | 2.51 ± 0.01 |
| (2) Chlorogenic acid | 9.36 | ND | 6.55 ± 0.32 |
| (3) Vanillic acid | 13.41 | 1.19 ± 0.06 | ND |
| (4) Syringic acid | 15.43 | ND | 1.40 ± 0.02 |
| (5) p-coumaric acid | 19.89 | 34.80 ± 0.17b | 6.05 ± 0.1a |
| (6) Ferulic acid | 20.29 | 1.86 ± 0.05a | 2.79 ± 0.09b |
| (7) Salicylic acid | 23.58 | 9.86 ± 0.47b | 6.23 ± 0.21a |
| (8) Quercetin dihydrate | 25.22 | 5.18 ± 0.6b | 1.81 ± 0.09a |
| (9) Quinic acid | 40.77 | 3.63 ± 0.05a | 9.17 ± 0.11b |
| Bound phenolics | Retention time (min) | Outer bract (ppm) | Inner bract (ppm) |
|---|---|---|---|
| (6) Ferulic acid | 20.29 | 265.26 ± 0.73b | 141.41 ± 0.55a |
| (7) Salicylic acid | 23.58 | 95.75 ± 0.86b | 32.92 ± 0.14a |
Mean with different superscript letters in the same row represent a significant difference at P < 0.05 based on paired t test, values represent mean ± SD; n = 3
ND not detected
The functional properties of DF-rich outer, inner bracts and cellulose (Table 4) revealed significantly lower bulk density and higher WHC, OHC, and WSC of outer and inner bracts as compared to cellulose. Bulk density of outer and inner bracts was observed 0.45 and 0.51 g/mL, respectively which is significantly lower than cellulose (0.55 g/mL). Bulk density is associated to the structural characteristics and particle size of a material which is related to physicochemical properties (Benítez et al. 2017). WHC of outer and inner bracts was 12.06 and 7.53 g water/g dry sample, respectively and was significantly higher compared to cellulose (5.8 g water/g dry sample). WHC of both outer and inner bracts was higher than that of wheat bran (5.2 g/g), corn (2.05 g/g), potato peel fibre (5.32 g/g), citrus fruits (1.65 g/g), apple (1.87 g/g), grapes (2.09 g/g) and banana (1.71 g/g) (Fernando et al. 2005). DF with high WHC avoids syneresis and improves the viscosity and texture of foods (Grigelmo-Miguel and Martina-Belloso 1999).
Table 4.
Functional properties of outer and inner bracts of culinary banana flower
| Functional properties | Outer bract | Inner bract | Cellulose |
|---|---|---|---|
| Bulk density (g/mL) | 0.45 ± 0.02a | 0.51 ± 0.01b | 0.55 ± 0.01c |
| WHC (g water/g dry sample) | 12.06 ± 0.40c | 7.53 ± 0.55b | 5.8 ± 0.17a |
| OHC (g oil/g dry sample) | 5.46 ± 0.45c | 3.50 ± 0.57a | 3.7 ± 0.26b |
| WSC (mL/g) | 15.51 ± 0.50c | 8.90 ± 0.36b | 4.5 ± 0.08a |
Mean with different superscript letters in the same row represent significant difference at P < 0.05 based on Duncan’s multiple range test, values represent mean ± SD; n = 3
The OHC of outer and inner bracts were 5.46 and 3.5 g oil/g dry sample, respectively and was found higher than wheat bran (2.18 g/g), corn (0.87 g/g), citrus fruits (1.81 g/g), grape fruits (1.20–1.52 g/g) and apples (0.60–1.45 g/g) (Grigelmo-Miguel and Martina-Belloso 1999). DF with improved OHC can prevent fat loss during food processing and also reduce cholesterol level in serum (Navarro-Gonzalez et al. 2011).
The WSC of outer and inner bracts was 15.51 and 8.9 mL/g, respectively and was found significantly higher when compared to cellulose (4.5 mL/g). WSC is again directly related to the soluble DF content (especially of pectin content). The WSC of outer and inner bracts revealed that it is more than several prominent vegetables viz., peas (5.26 mL/g), chickpeas (4.28 mL/g) and edible seaweed (5.7–10.5 mL/g) (Gomez-Ordonez et al. 2010). Functional properties of DF viz., WHC, OHC, WSC are important from both physiological and technological perspectives (Al-Sheraji et al. 2011). Culinary banana bracts with high WHC can be used in food system to improve density of the product, minimizing shrinkage and to improve the texture and appeal of the food products. Moreover, culinary banana bracts exhibited relatively higher OHC than several fibre rich foods, which could be useful for emulsification of some products. Additionally, due to the high hydration properties of banana bracts fibre, it can be used as anticaking and antisticking agent, reduce syneresis and in stabilizing food system.
The retardation of glucose diffusion at different points of time (20, 40 and 60 min) for inner bract, outer bract, cellulose (reference) and control was taken and are illustrated in Fig. 2. The dialysate glucose content of control increased gradually (0.16 to 0.38 mg) with time (20 to 60 min). However, the dialysate glucose content of outer and inner bracts increased in a lower rate as compared to control and increased from 0.1 mg to 0.14 mg and 0.09 mg to 0.15 mg, respectively with time 20 to 40 min (Fig. 2). Hence, the glucose diffusion from dialysis tubing was potentially reduced by outer and inner bracts and was significantly (P < 0.05) lower than cellulose. GDRI is a useful in vitro index which simulates the action of fibre on delaying glucose absorption in the gastrointestinal tract. It is related to several other properties of fibres, such as structural and surface properties, soluble DF content and uronic acid content (Hasnaoui et al. 2014). The soluble DF of bracts also affected the retardation of glucose diffusion. Thus, GDRI of inner and outer bracts is quite higher than cellulose which is a pure insoluble DF.
Fig. 2.
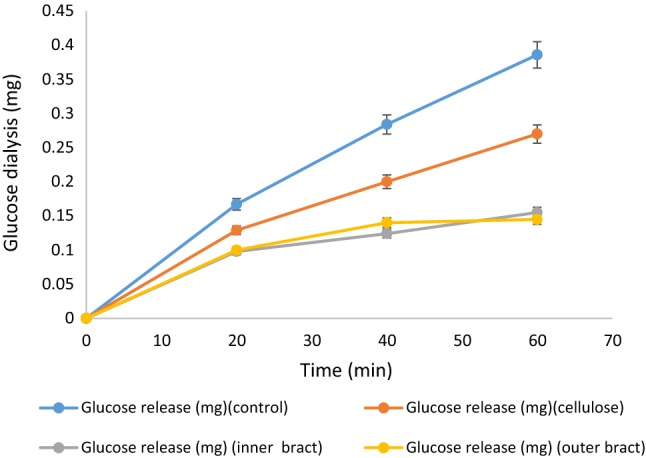
Glucose dialysis in presence of cellulose, inner and outer bracts of culinary banana flower
The GDRI of inner and outer bracts along with cellulose are presented in Table 5. The GDRI of outer and inner bracts at 60 min was 62.51 and 59.82%, respectively and these values are strikingly higher compared to several fibre-rich byproducts, such as artichoke fibre (27%), mango peel (21%) and asparagus fibre (18–47%) as reported by Hasnaoui et al. (2014). The plausible reasons might be attributed to the antihyperglycemic effects of banana flower. Banana flower (Musa sp. var. elakki bale) was found to have antidiabetic properties and inhibit the formation of AGEs (Advanced Glycation End Products) in streptozotocin induced diabetic rats (Bhaskar et al. 2011). Moreover, the results suggested that different parts of culinary banana bracts have potential in controlling postprandial blood glucose level and could be used in antidiabetic remedies formulations. This could also be used as low-calorie and fibre-rich ingredients for dietetic snacks.
Table 5.
Glucose dialysis retardation index (GDRI) of outer and inner bracts of culinary banana flower
| Time (min) | Outer bract (%) | Inner bract (%) | Cellulose (%) |
|---|---|---|---|
| 20 min | 40.12 ± 0.10b | 41.33 ± 0.12c | 22.75 ± 0.05a |
| 40 min | 50.79 ± 0.17b | 56.67 ± 0.17c | 29.50 ± 0.03a |
| 60 min | 62.51 ± 0.07c | 59.82 ± 0.06b | 30.30 ± 0.50a |
Mean with different superscript letters in the same row represent significant difference at P < 0.05 based on Duncan’s multiple range test, values represent mean ± SD; n = 3
Conclusion
The present study revealed that both outer and inner bracts are potential sources of DF along with other nutrients and phytochemicals. Both outer and inner bracts contained significant amount of bound polyphenols and could be considered as antioxidant rich DF. Moreover, better functional properties (e.g. WHC, OHC, WSC and GDRI) evidenced that outer and inner bracts are superior to cellulose and many other DF rich sources. All-inclusive results evinced that the outer bracts along with the inner bracts constitute a good source of DF associated with polyphenols and its excellent functional properties render it more suitable source of DF. The results of the present investigation has the credentials to support that both the outer and inner bracts of culinary banana flower could be commercially exploited for extraction of DF coupled with high antioxidant activity and has the potential as functional food to promote and maintain human health.
Acknowledgements
Financial assistance received as MAN Fellowship from UGC, New Delhi, Govt. of India during the study is duly acknowledged.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Al-Sheraji SH, Ismail A, Manap MY, Mustafa S, Yusof RM, Hassan FA. Functional properties and characterization of dietary fiber from Mangifera pajang Kort. fruit pulp. J Agric Food Chem. 2011;59:3980–3985. doi: 10.1021/jf103956g. [DOI] [PubMed] [Google Scholar]
- Arnao MB, Cano A, Acosta M. The hydrophilic and lipophilic contribution to total antioxidant activity. Food Chem. 2001;73:239–244. doi: 10.1016/S0308-8146(00)00324-1. [DOI] [Google Scholar]
- Aruna KB, Thomas S, Reshmitha TR, Akhila GC, Nishaa P. Dietary fibre and phenolic-rich extracts from Musa paradisiaca inflorescence ameliorates type 2 diabetes and associated cardiovascular risks. J Funct Foods. 2017;31:198–207. doi: 10.1016/j.jff.2017.02.001. [DOI] [Google Scholar]
- Association of Official Analytical Chemists (AOAC) Official methods of analysis of the association of analytical chemists. 16. Washington, DC: Association of Official Analytical Chemists; 1998. [Google Scholar]
- Begum YA, Deka SC. Stability of spray-dried microencapsulated anthocyanins extracted from culinary banana bract. Int J Food Prop. 2017;20(12):3135–3148. doi: 10.1080/10942912.2016.1277739. [DOI] [Google Scholar]
- Benítez V, Mollá E, Martín-Cabrejas MA, Aguilera Y, Esteban RM. Physicochemical properties and in vitro antidiabetic potential of fibre concentrates from onion by-products. J Funct Foods. 2017;36:34–42. doi: 10.1016/j.jff.2017.06.045. [DOI] [Google Scholar]
- Benzie IFF, Strain JJ. The ferric reducing activity of plasma (FRAP) as measured of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Bhaskar JJ, Shobha MS, Sambaiah K, Salimath PV. Beneficial effects of banana (Musa sp. var. elakki bale) flower and pseudostem on hyperglycemia and advanced glycation end-products (AGEs) in streptozotocin-induced diabetic rats. J Physiol Biochem. 2011;67:415–425. doi: 10.1007/s13105-011-0091-5. [DOI] [PubMed] [Google Scholar]
- Bhaskar JJ, Paramahans SV, Nandin CD. Banana (Musa sp. var. elakki bale) flower and pseudostem: dietary fiber and associated antioxidant capacity. J Agric Food Chem. 2012;60(1):427–432. doi: 10.1021/jf204539v. [DOI] [PubMed] [Google Scholar]
- Brand-Williams W, Cuvelier ME, Berset C. Use of a free-radical method to evaluate antioxidant activity. LWT Food Sci Technol. 1995;28(1):25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- Bray HG, Thrope WV. Analysis of phenolic compounds of interest in metabolism. Methods Biochem Anal. 1954;1:27–52. doi: 10.1002/9780470110171.ch2. [DOI] [PubMed] [Google Scholar]
- Du L, Shen Y, Zhang X, Prinyawiwatkul W, Xu Z. Antioxidant-rich phytochemicals in miracle berry (Synsepalum dulcificum) and antioxidant activity of its extracts. Food Chem. 2014;153:279–284. doi: 10.1016/j.foodchem.2013.12.072. [DOI] [PubMed] [Google Scholar]
- Fernando F, Maria LH, Ana-Maria E, Chifelle I, Asenjo F. Fibre concentrates from apple pomace and citrus peel as potential source for food enrichment. Food Chem. 2005;91(3):395–401. doi: 10.1016/j.foodchem.2004.04.036. [DOI] [Google Scholar]
- Gomez-Ordonez E, Jimenez-Escrig A, Ruperez P. Dietary fibre and physicochemical properties of several edible seaweeds from northwestern Spanish coast. Food Res Int. 2010;43(9):2289–2294. doi: 10.1016/j.foodres.2010.08.005. [DOI] [Google Scholar]
- Grigelmo-Miguel N, Martina-Belloso O. Characterization of dietary fibre from orange juice extraction. Food Res Int. 1999;131:355–361. [Google Scholar]
- Hasnaoui N, Wathelet B, Jiménez-Araujo A. Valorization of pomegranate peel from 12 cultivars: dietary fibre composition, antioxidant capacity and functional properties. Food Chem. 2014;160:196–203. doi: 10.1016/j.foodchem.2014.03.089. [DOI] [PubMed] [Google Scholar]
- Lecumberri E, Mateos R, Izquierdo-Pulido M, Ruperez P, Goya L, Bravo L. Dietary fibre composition, antioxidant capacity and physicochemical properties of a fibre-rich product from cocoa (Theobroma cacao L.) Food Chem. 2007;104:948–954. doi: 10.1016/j.foodchem.2006.12.054. [DOI] [Google Scholar]
- Leonard DB. Medicine at your feet: healing plants of the hawaiian kingdom bidens spp. (Kïnehi) Kapaa-Princeville: Roast Duck Producktion; 2006. pp. 1–15. [Google Scholar]
- Lowry OH, Rosebrouugh NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Luque R, Clark JH. Valorization of food residues: waste to wealth using green chemical technologies. Sustain Chem Process. 2013;1:10. doi: 10.1186/2043-7129-1-10. [DOI] [Google Scholar]
- Ma M, Mu T. Effects of extraction method and particle size distribution on the structural, physicochemical, and functional properties of dietary fibre from deoiled cumin. Food Chem. 2016;194:237–246. doi: 10.1016/j.foodchem.2015.07.095. [DOI] [PubMed] [Google Scholar]
- Macagnana FT, da Silvab LP, Hecktheuer L. Dietary fibre: the scientific search for an ideal definition and methodology of analysis, and its physiological importance as a carrier of bioactive compounds. Food Res Int. 2016;85:144–154. doi: 10.1016/j.foodres.2016.04.032. [DOI] [PubMed] [Google Scholar]
- Navarro-Gonzalez I, Garcia-Valverde V, Garcia-Alonso J, Periago J. Chemical profile, functional and antioxidant properties of tomato peel fibre. Food Res Int. 2011;44(5):1528–1535. doi: 10.1016/j.foodres.2011.04.005. [DOI] [Google Scholar]
- Okarter N, Liu CS, Sorrells ME, Liu RH. Phytochemical content and antioxidant activity of six diverse varieties of whole wheat. Food Chem. 2010;119(1):249–257. doi: 10.1016/j.foodchem.2009.06.021. [DOI] [Google Scholar]
- Prosky L, Asp NG, Schweizer TF, De Vries JW, Furda I. Determination of insoluble, soluble and total dietary fiber in foods and food products: collaboration study. J Assoc Off Anal Chem. 1988;71(5):1017–1023. [PubMed] [Google Scholar]
- Ranganna S. Handbook of analysis and quality control for fruit and vegetable products. 2. New Delhi: Tata McGraw-Hill Publishing Ltd.; 1986. [Google Scholar]
- Robertson JA, de Monredon FD, Dysseler P, Guillon F, Amado R, Thibault J. Hydration properties of dietary fibre and resistant starch: a European collaborative study. LWT-Food Sci Technol. 2000;33:72–79. doi: 10.1006/fstl.1999.0595. [DOI] [Google Scholar]
- Rodica C, Adrian C, Calin J. Biochemical aspects of non-starch polysaccharides. J Anim Sci Biotechnol. 2010;43:368–375. [Google Scholar]
- Rodríguez R, Jimenez A, Fernandez-Bolanos J, Guillen R, Heredia A. Dietary fibre from vegetable products as source of functional ingredients. Trends Food Sci Technol. 2006;17:3–15. doi: 10.1016/j.tifs.2005.10.002. [DOI] [Google Scholar]
- Rodriguez-Delgado MA, Malovana S, Perez JP, Borges T, Montelongo FJG. Separation of phenolic compounds by high-performance liquid chromatography with absorbance and fluorimetric detection. J Chromatogr A. 2001;912:249–257. doi: 10.1016/S0021-9673(01)00598-2. [DOI] [PubMed] [Google Scholar]
- Sadasivam S, Manickam A. Biochemical methods for agricultural sciences. New Delhi: Wiley Eastern Limited; 1992. pp. 11–12. [Google Scholar]
- Sheng Z, Ma W, Jin Z, Bi Y, Sun Z, Dou H, Gao J, Li J, Han L. Investigation of dietary fibre, protein, vitamin E and other nutritional compounds of banana flowers of two cultivars grown in China. Afr J Biotechnol. 2010;9(25):3888–3895. [Google Scholar]
- Thaipong K, Boonprakob U, Crosby K, Cisneros-Zevallos L, Byrne DH. Comparison of DPPH, ABTS, FRAP and ORAC assays for estimating antioxidant activity from guava fruit extracts. J Food Compost Anal. 2006;19:669–675. doi: 10.1016/j.jfca.2006.01.003. [DOI] [Google Scholar]
- Updegroff DM. Semimicro determination of cellulose in biological materials. Anal Biochem. 1996;32(3):420–424. doi: 10.1016/S0003-2697(69)80009-6. [DOI] [PubMed] [Google Scholar]
- Wang JC, Kinsella JE. Functional properties of novel proteins: alfalfa leaf protein. J Food Sci. 1976;41(2):286–292. doi: 10.1111/j.1365-2621.1976.tb00602.x. [DOI] [Google Scholar]
- Zhang Y, Shen Y, Zhu Y, Xu Z. Assessment of the correlations between reducing power, scavenging DPPH activity and anti-lipid-oxidation capability of phenolic antioxidants. LWT-Food Sci Technol. 2015;63:569–574. doi: 10.1016/j.lwt.2015.03.047. [DOI] [Google Scholar]