Abstract
Ultrasound (US) is the main imaging modality for the evaluation of pediatric patients with musculoskeletal diseases; particularly, it is an appropriate and reliable tool for diagnosis, follow-up and treatment of several musculoskeletal pathologies affecting the pediatric age. High-frequency (10–15 MHz) and high-resolution probes provide very lofty quality images, allowing a detailed study of the pediatric musculoskeletal system. Among the well-known advantages of this technique—such as the absence of ionizing radiations, its low cost and wide availability—US can as well rely on some intrinsic characteristics of the pediatric musculoskeletal system that can improve its diagnostic capability. The unossified portions of the pediatric skeleton and the absence of a thickened adipose tissue allow US to be highly effective and reliable in the study of muscles, tendons and cartilage. Lower-frequency sectoral transducers can be required in the study of some joints such as the shoulder or the hip, as well as in the examination of deep soft-tissue lesions. Furthermore, both color and spectral Doppler play an important role in the examination of soft-tissue lesions and synovial phlogosis. In this pictorial essay the main pathological conditions of pediatric musculoskeletal system will be examined, such as painful hip, evolutionary hip dysplasia, osteochondrosis, trauma-related pathologies and juvenile idiopathic arthritis.
Keywords: Ultrasound examination, High-resolution probes, Musculoskeletal system, Pediatric age
Sommario
Nonostante i grandi sviluppi delle metodiche imaging degli ultimi anni, l’ecografia rappresenta al giorno d’oggi un valido ed affidabile strumento nella diagnosi, follow-up e nel trattamento di numerose patologie che interessano l’apparato muscolo scheletrico in età pediatrica. L’utilizzo di sonde lineari ad elevata frequenza (10-15 Mhz) e risoluzione risulta fondamentale nello studio dell’apparato muscolo-scheletrico pediatrico in quanto sono in grado di fornire immagini di altissimo dettaglio anatomico. Sonde settoriali a più bassa frequenza possono essere altresì richieste nello studio di alcune articolazioni come l’anca e la spalla o nello studio di lesioni dei tessuti molli profondi. Oltre ai già ben noti vantaggi di tale metodica di imaging, quali l’assenza di radiazioni ionizzanti, il basso costo e l’ampia disponibilità sul territorio, l’ecografia può sfruttare alcune caratteristiche intrinseche dell’apparato muscolo-scheletrico pediatrico, ampliando cosi notevolmente le sue capacità diagnostiche. In tal senso le porzioni non ancora ossificate dello scheletro pediatrico e la mancanza di uno spesso pannicolo adiposo forniscono una finestra acustica di studio ottimale, che rende così l’ecografia una metodica di primo livello di grande affidabilità nello studio delle articolazioni, tendini, muscoli e delle strutture cartilaginee. L’utilizzo sia del color Doppler che del Doppler spettrale svolge inoltre un ruolo fondamentale per la caratterizzazione di lesioni dei tessuti molli e per la valutazione della flogosi sinoviale. In questo pictorial essay verranno quindi esaminate le principali condizioni patologiche del sistema muscoloscheletrico pediatrico, come “l'anca dolorosa”, la displasia congenita evolutiva dell'anca, le più comuni osteocondrosi, alcune condizioni patologiche di natura traumatica e l’artrite giovanile idiopatica.
Introduction, technique and ultrasound aspects
Despite the huge advances of imaging techniques in the recent years, ultrasound (US) is an appropriate and reliable diagnostic tool for the follow-up and the treatment of several musculoskeletal pathologies in the pediatric age [1]. Such procedure is preferable to MR as it does not require sedation and allows for a dynamic study of the structures [1].
Among the well-known advantages of this procedure—such as the absence of radiation exposure, its low cost and wide availability—US can rely on some intrinsic characteristics of the pediatric musculoskeletal system as well, enabling the visualization of non-ossified cartilaginous and vascular structures and allowing dynamic imaging and quick contralateral comparison. Additionally, small children have not only thinner soft tissues, but they have long bones and midline spine structures whose ends are largely composed of cartilage, which provides an early opportunity to examine these regions by US [2–4].
All these features make US highly effective and reliable in the study of muscles, tendons and cartilage [2, 3].
US is also a useful and safe guidance during intra-articular procedures.
High-frequency (10–15 MHz) high-resolution probes provide very high quality images, allowing a detailed study of the pediatric musculoskeletal system.
Lower-frequency sectorial transducers can be required in the study of some joints such as the shoulder or the hip, as well as in the examination of deep soft-tissue lesions.
Both color and spectral Doppler are crucial in the examination of soft-tissue lesions and synovial phlogosis. The examination has to be delivered in presence of a relative and in a warm, friendly and non-threatening environment avoiding long waits [3].
During the application, particular attention has to be paid to avoid anisotropic artifacts [5].
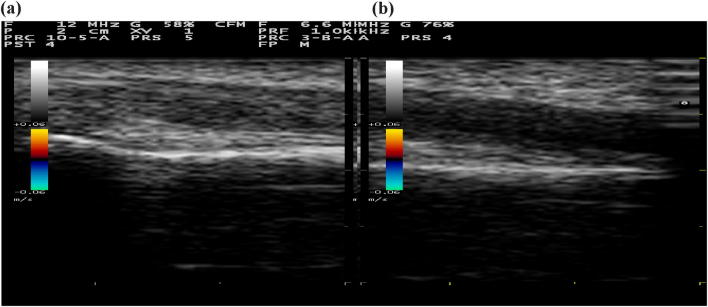
Such artifacts are the most common cause of misinterpretation of a musculoskeletal scan in all population. Indeed, the US band is often not perfectly perpendicular to the tendon fiber orientation. While a normal tendon is hyperechoic, anisotropy will cause the tendinous region to become anechoic, therefore, mimicking a lesion (Fig. 1) [5].
Fig. 1.
Anisotropic artifact: normal hyperechoic tendon (a), and ipoechoich appearance of the same tendon due to anisotropy artifact (b)
To recognize the main pathological processes affecting the pediatric musculoskeletal system, it is necessary to know the main ultrasonographic anatomical characteristics differentiating it from an adult's [3, 4]. The knowledge of age-related normal findings is essential when interpreting pathological findings such as those seen in juvenile idiopathic arthritis [6].
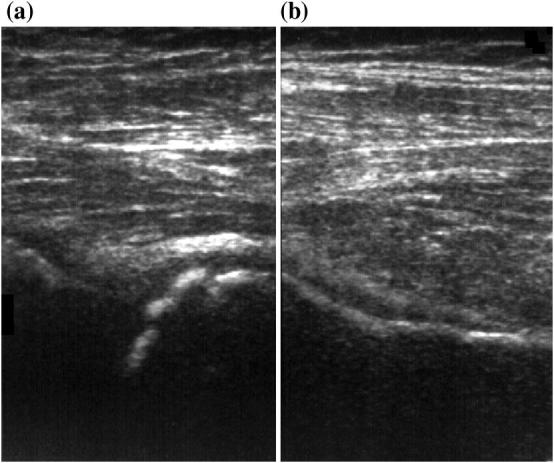
US is, therefore, very effective in the evaluation of the cartilaginous covering, which characterizes immature bones in pediatric patients (mainly at the level of the epiphysis). Ultrasonographic aspects of the epiphysis depend in fact on the stage of ossification. The epiphysis appears as a homogenously hypo/anechoic area, allowing the ultrasonographic band to see through the covering cartilage employed as an acoustic window for deeper structures. At birth, the primary diaphyseal ossification core of the long bones is ossified and the diaphysis appears on the US examination as a hyperechoic line with a rear shadow cone; the epiphyses and the apophyses are partially or totally cartilaginous, and appear as hypo-anechoic structures due to the high water content of the cartilage at US examination (Fig. 2). Accretion cartilage shows a dedicated vascularization visible under color Doppler. When the epiphysis starts its ossification process, articular cartilage, and accretion epiphyseal plate will be displayed as a gap in the cortical bone and should not be confused with erosion or fracture of the bone. The ultrasonographic aspects of the main musculoskeletal pediatric structures are reported in Table 1. Figure 3 shows a few examples of muscular, ligament and nerve scans.
Fig. 2.

2-Year-old child: the femoral diaphysis appears on the ultrasound examination as a hyperechoic line with a rear shadow cone; the epiphyses and the apophyses are partially or totally cartilaginous, and appear on the ultrasound examination as hypo-anechogenic structures due to the high water content of the cartilage
Table 1.
Ultrasound appearance of the main musculoskeletal structures
| Structure | Ultrasound appearance |
|---|---|
| Cartilage | Anechoic |
| Fibrocartilage (labrum) | Hyperechoic |
| Tendon | Fibrillary hyperechoic |
| Ligament | Fibrillary hyperechoic |
| Bone surface | Hyperechoic line with a rear shadow cone |
| Sinovia | Iso-hypoechoic (ecogenicità intermediatra osso e cartilagine) |
| Joint capsule | The joint capsule is the anatomical structure included between the hypoechoic synovium and the synovial anecogenic fluid |
| Muscle | Iso-hypoechoic |
| Nerves | Iso-hyperechoic |
Fig. 3.
Few examples of muscular (a), tendon (b), ligament (c), and nerve scans (d)
Pathology
Several pathological conditions of either infectious, inflammatory or traumatic nature can involve the pediatric musculoskeletal system.
A list of the most common causes found in the clinical practice is reported below:
Painful hip,
evolutionary hip dysplasia,
osteochondrosis,
trauma-related and pathologies,
juvenile idiopathic arthritis.
Painful hip
Transient synovitis of the hip
Transient synovitis of the hip (TSH) is an unspecific inflammatory condition of the coxo-femoral articulation. It is probably one of the most common causes of hip pain in pediatric age.
Unproperly known as ‘hip fever’, it generally affects males aged 4–10 years with the boy-to-girl ratio of 2:1 [7]. Clinically, symptoms are: limited functionality of the affected limb, pain, and limping. Etiology is unknown, although some studies suggest the viral hypothesis [8]. The onset is unilateral, sudden and often associated with symptoms of gastroenteritis and common cold [8]. Passive motion causes intense pain that usually lasts several days and leaves no trace once it is over. US examination is the main diagnostic method of TSH, showing the presence of joint effusion at the anterior recess of the joint capsule (Fig. 4) [9]. In children, the distance between the outer margin of the hip capsule and the surface of the femoral neck should not be greater than 5 mm, or more than 2 mm thicker than the contralateral normal side [9].
Fig. 4.
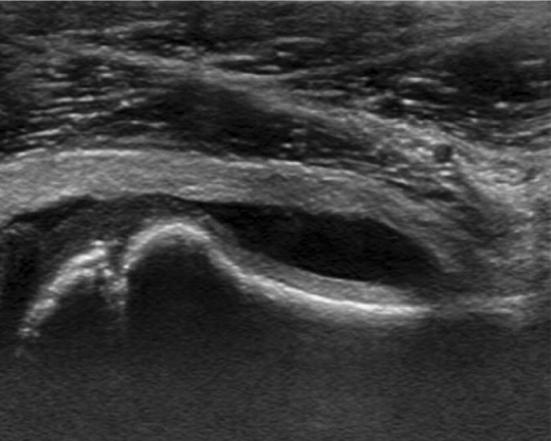
Six-year-old boy with fever, limping on the left side. Joint effusion in the anterior recess of the joint capsule in patient with transient synovitis of the hip
US is also used to monitor any evolutions as well as to report the resolution of a transitory hip synovitis effusion. In case of suspected hip joint aspiration, a US examination can be performed to rule out any septic hip arthritis.
Septic arthritis of the pediatric hip
Septic arthritis of the pediatric hip (SAPH) is an inflammatory alteration of bacterial etiology, mainly involving major joints such as the hip, knee and shoulder, with a prevalent involvement of the coxo-femoral joint. Staphylococcus aureus is the most common causative organism. In neonates, group B streptococci and coliform bacteria were previously common causative organisms [9]. In children aged from 3 months to 5 years, Haemophilus influenzae is an important cause, but the incidence has declined considerably due to the use of vaccinations [9]. SAPH is generally unilateral, except for the gonococcal type. Germal contamination can be favored by hematic samples, venous catetherism, intramuscular injections, infected umbilical wound and bowel infections.
Ultrasonographic examination is quite a reliable diagnostic tool for SAPH as it can verify the presence of corpusculated and inhomogeneous anterior joint recess effusion.
US shows hypoechoic joint fluid with or without echogenic debris in patients with clinical signs of joint infection (Fig. 5) [9].
Fig. 5.

Five-year-old boy presenting with persistent fever and atraumatic right hip pain. Slightly corpusculated and inhomogeneous anterior joint recess effusion in septic arthritis of the pediatric hip
These US findings, if in agreement with clinical and laboratory parameters, can strongly support the diagnosis of SAPH. US can be used for the guided aspiration of joint fluid for the early diagnosis, to isolate the micro-organism of interest and treatment of SAPH [9].
Perthes
Legg–Calvé–Perthes disease is a degenerative pathology of the femur head, also known as femoral head osteochondrosis. It prevalently affects pediatric population, especially males aged from 3 to 12—mainly 7-years. The area of interest is the proximal epiphyseal nucleus of the femur head. It is usually unilateral though it can very slowly grow into being bilateral. The main symptoms are moderate to severe pain, difficult and increasingly limited movements, especially in regards to a femoral abduction and its intrarotation. Limping generally appears after physical activity, but can later become constant and even chronicize along with the pain caused by the local inflammation.
Pain can appear after running, jumping or other activities and can spread to the knee, causing antalgic posture as the patient moves all his body weight onto the healthy leg. A case of Perthes disease has to be suspected when a patient aged 4–7 years shows signs of limping and limited abduction. The diagnosis is clinical, though US can provide useful elements. US scans (Fig. 6a, b) highlight any signs of intra-joints effusion, thickening of the cartilage of the femur head as well as irregularity and fragmentation of the same and quadriceps hypotrophy on the affected side (Fig. 6a) [10, 11].
Fig. 6.
Irregular and fragmented aspect of the femoral head, and hypotrophy of the quadriceps of the affected side (a) compared to the healthy control (b) in a 5-year-old girl with persistent atraumatic left hip pain
Evolutionary hip dysplasia
Evolutionary hip dysplasia (EHD) is a wide spectrum of abnormalities concerning the development of the hip ranging from higher ligament laxity—and its consequent joints instability—to hip luxation (also known as congenital hip luxation) and it is also better known as developmental dysplasia of the hip. The condition affects 0.7–2 children out of 1000, with a clear predisposition of females.
It is bilateral 45% of the time. Early diagnosis is crucial as early treatment will promote easier and faster healing. Most patients will be completely cured and iatrogenic complications avoided. US has been widely validated as an appropriate diagnostic tool for the classification of EHD and recent clinical investigations have shown that US assessment of a hip clinically suspected to be dysplastic leads to a change in diagnosis for more than 50% of cases and a change in management for 32% of cases [12–14].
US examination within the first eight weeks of life is the gold standard for early screening of EHD. On the other hand, some recent studies claim that there is insufficient evidence to support universal US screening; instead, selective screening should be performed by 6–8 weeks of age on infants with risk factors of breech presentation, family history, or history of clinical hip instability [15].
US examination is carried out in four stages:
search of optimal study window;
morphological assessment;
quantitative assessment;
staging.
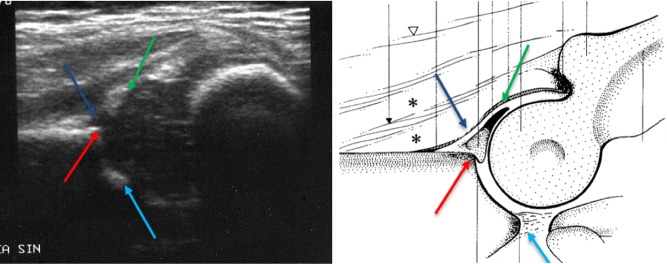
With the probe laying vertically, the strokes need to be applied backward and forward so as to find the joint’s ‘center’, allowing the visualization of the femur head, the ilium wing and the metaphysical ossification front as reported in Fig. 7.
Fig. 7.
Correct ultrasound scan for the study of hip dysplasia showing: labrum (green arrow), acetabular cartilage (blue arrow), bony rim of acetabulum (red arrow), and lower limb of the iliac bone (light blue arrow)
Morphological assessment takes three parameters into account:
Acetabulum conformation (good, scarce or insufficient).
Cotiloidal margin shape (angular, roundish or flat).
Acetabolic cartilage thickness (thin, thick or moved).
Quantitative assessment is based on the geometrical evaluation as proposed by Graaf: a few lines are traced upon the above scan which are essential for the Alpha and Beta angle calculus.
Basal line from the hyaline cartilage apex a line is traced tangentially to the lateral portion of the ileum (generally parallel to the scanned line).
Bone roof line from the inferior ileum margin a tangent is traced toward the exterior portion of the bone roof.
Cartilage roof line between the cotyle margin and the middle of the acetabular labrum. If the cotyle margin is round, it corresponds to the junction point of the acetabular cavity with the cotyle margin convexity. These procedures provide two different angles.
α angles generated within the intersection of the basal line and the bone roof line: it constitutes a parameter for bone component development.
β angle generated in the intersection of the lineal base with the cartilage roof line: it constitutes a parameter for the evaluation of the disposition of cartilage components and the acetabular roof in relation to the bone structures.
On the basis of these parameters and age of the infant it is possible to obtain different angles that are indicative of the more or less advanced state of maturation of the development of the hip, from mature and well-centered joints up to dysplasia and dislocation as indicated in Graaf classification (Table 2).
Table 2.
Graaf’s classification
| Type | Maturity | Bony roof | Bony angle | Bony rim | Cartilage roof | β-angle | Age |
|---|---|---|---|---|---|---|---|
| Type I | Mature | Good | α ≥ 60° | Sharp | Good coverage femoral head | Ia = β < 55° | All |
| Type II a+ | Immature but appropriate for age | Adequate | 50°–59° | Blunt | Coverage femoral head | Ib = β < 55° | < 3 months |
| Type II a- | Immature but inappropriate for age | Deficient | 50°–59° | Rounded | Coverage femoral head | < 3 months | |
| Type II b | Delay in development | Deficient | 50°–59° | Rounded | Coverage femoral head | > 3 months | |
| Type II c | Stable or unstable | Severely deficient | 50°–59° | Rounded/flat | Stile coverage femoral head | β > 77° | All |
| Type D | Decentring hip | Severely deficient | 43°–49° | Rounded/flat | Displaced | β > 77° | All |
| Type III | Eccentric hip | Poor | < 43° | Flat | Labrum pressed upwards | All | |
| Type IV | Eccentric hip | Poor | < 43° | Flat | Labrum pressed downwards | All |
The alpha-angle, which is a measurement of the bony roof of the acetabulum, mainly determines the hip type. Actually, the beta angle is only used to differentiate between type Ia and Ib (both normal hips) and between type IIc and type D.
Figures 8a, b, 9a, b, and 10a, b, show exemplary images of the main types of hips according to Graaf ‘s classification.
Fig. 8.
Hip type Ia (a) and type Ib (b) according to Graaf classification
Fig. 9.
Hip type IIa+ (a) and type IIb (b) according to Graaf classification
Fig. 10.
Hip type IIc (a) and type III (b) according to Graaf classification
Hip dysplasia screening is the main indication for US scanning in prenatal age.
The rationale of such screening is based on the observation that there is a pre-clinical period in which a diagnosis is possible and the natural evolution of the condition can be reversed through early intervention. After hip dysplasia is diagnosed, US can provide important information about the resolution or persistence of hip abnormalities after harnessing. US may identify a persistently unstable hip not detected on physical examination and may direct clinicians to consider further harnessing [16].
Osteochondrosis
Osgood–Schlatter
Osgood–Schlatter disease (OSD) is a type of osteochondrosis of the anterior tibial tubercle affecting children aged 10–14 years, especially boys who practice sports such as soccer, race, volleyball and gymnastics. The condition is linked to traumatic action caused by constant traction of the patellar tendon within the insertion of the tibial apophysis—still partially made of cartilage—during contraction of the femoral quadriceps muscle [17]. This leads to cartilage microfractures and inflammation, with consequent pain (especially after physical efforts) and tumefaction. Clinical diagnosis is based on relevance of spontaneous pain and tumefaction at the level of the anterior tibial tubercle. OSD is usually self-limited as symptoms disappear with complete ossification of the tibial apophysis [18]. US examination allows the staging of the disease: effusion of the deep serum infrapatellar bursa (Fig. 11a), cartilage tumefaction, accretion nucleus fragmentation, and thickening of the tendon (Fig. 11b) [17, 18].
Fig. 11.
Ten-year-old boy with Osgood Schlatter disease presenting with pain in the front lower part of the left knee. US examination shows: effusion of the infrapatellar bursa (a); cartilage tumefaction, accretion nucleus fragmentation, and thickening of the tendon (b)
Sinding Larsen Johanson
Sinding–Larsen–Johansson's disease is a rare syndrome characterized by osteochondrosis affecting the inferior patellar pole. It is represented by an insertional tendinitis of the distal pole of the patella, whose etiology is due to both repeated microtraumas and falls implying an impact on the patellar apex. It is often bilateral and usually affects male teenagers aged 10–14 who practice intense sport activity. Patients complain of pain during the contraction of the quadriceps femoris. Other clinical features are swelling of the infrapatellar soft tissues and functional limitation [17]. US findings are similar to those in OSD: cartilage swelling, patellar tendon swelling at its proximal insertion and patellar fragmentation at its distal pole (Fig. 12) [19].
Fig. 12.
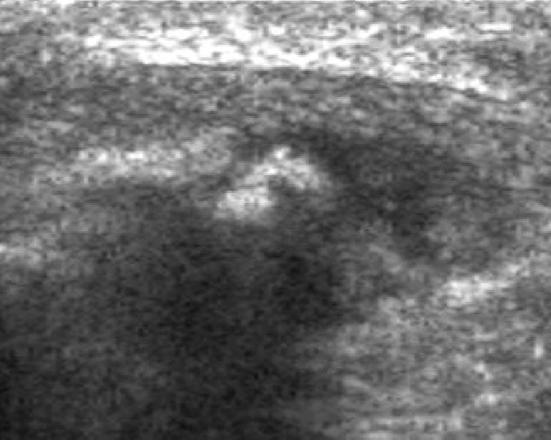
Sinding–Larsen–Johansson disease in a 11-year-old girl with anterior, spontaneous knee pain and swelling at the inferior pole of the patella. US examination demonstrates fragmentation of the lower pole of the patella and the signs of tendinopathy at insertion of the patella tendon
Trauma
Epiphyseal detachments
Epiphyseal detachments are fractures affecting the metaphyseal accretion cartilage with relative detachment of the proximal or distal (epiphysis) bone segment. Pathogenic noxa mimics classic fractures: a force that, acting (directly or indirectly) on the structure, overcomes its resistance.
In most cases traumatic pathology affects children practicing sports but also several epiphyses birth traumas have been reported. Injuries of the proximal humerus in infants are often missed or misinterpreted because of the non ossification of the epiphysis. US allow direct visualization of the proximal humeral epiphysis, metaphysis, joint space, and relationship with the glenoid cavity. US diagnosis should be considered as the first imaging modality if traumatic epiphyseal dislocation is suspected, also in a newborn [20].
Bare in mind damaged accretion cartilage can lead to malformation of the developing bone.
Figure 13a, b show a case of antero-inferior iliac spine detachment [21, 22] in a patient referred to diagnostic imaging department with history of recent trauma, unilateral hip pain, absence of joint effusion with lameness, pain and suspected coxite.
Fig. 13.
Longitudinal scan images of detachment of the SIAI (antero-inferior iliac spine) with (a) and without (b) color Doppler in a patient with history of recent trauma, unilateral hip pain, absence of joint effusion with lameness, pain and suspected coxite
Juvenile idiopathic arthritis
Juvenile idiopathic arthritis (JIA) is a chronic disease characterized by persistent joints inflammation. It is an idiopathic arthritis encompassing a heterogeneous group of inflammatory arthritis, which is currently divided into seven specific subtypes [23]. Typical symptoms are pain, swelling and difficulties in the movement. JIA defines any arthritis of unknown cause affecting patients aged < 16 that lasts more than 6 weeks and does not show other causes [23]. The current International League Against Rheumatism (ILAR) classification system comprises seven different types of JIA, based on the number of affected joints and the presence of extra-articular manifestations [23]:
Systemic (15% incidence),
oligoarticular (50%)
persistent,
extended,
poliartrhitis (17%)
psoriatic (5%)
arthritis/enthesitis (10%)
The most common is the oligoarticular form, which is typical of pediatric age. Both the persistent and the extended phase are characterized by precocious onset (< 5 year olds) and prevalence in females. Asymmetry is associated with high risk of uveitis (30%) and positive ANA antibodies (> 80%).
Polyarticular (< 5 joints) form onset generally happens in kids aged 3–6 or 10–14 years. Affecting prevalently females, it is characterized by fast ankylosis. Psoriatic form is characterized by the combination of psoriasis and arthritis or arthritis in combination with two of the following conditions: dactylitis, ungual anomalies and history of psoriasis in at least one first grade relative. US play a very important role in diagnosis and follow-up of these patients. Data obtained from the eco B-mode and eco-color Doppler (Fig. 13) are fundamental both for diagnosis and follow-up of patients affected by JIA. The main US findings, as illustrated in Fig. 14a–c, are represented by synovial effusion and thickening (a), synovial phlogosis (b) and cartilage and marginal bones erosion (c) [1,6].
Fig. 14.
US findings in an 8-year-old boy with Juvenile idiopathic arthritis of the left knee presenting with functional limitation, pain and low grade fever: synovial effusion and thickening (a), synovial flogosis (b) and cartilage and marginal bones erosion (c)
Conclusions
US is the main imaging modality for the evaluation of pediatric patients affected by musculoskeletal diseases. Due to its high-resolution in the evaluation of muscles, tendons and cartilage as well as in the detection of synovial fluid effusion, US is often preferred to MRI for its low costs, wide availability and since it does not require patient sedation. All these issues make US suitable and effective for diagnosis, follow-up and assessment of the response to treatment in the majority of pediatric musculoskeletal pathologies as well as to guide intra-articular therapeutic procedures.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, and its late amendments.
Human and animal rights
This article does not contain any studies with human or animal subjects performed by any of the authors.
Informed consent
Additional informed consent was obtained from all patients for which identifying information is not included in this article.
References
- 1.Thapa M, Vo J-N, Shiels WE., II Ultrasound guided musculoskeletal procedures in children. Pediatr Radiol. 2013;43(Suppl):S55–S60. doi: 10.1007/s00247-012-2599-4. [DOI] [PubMed] [Google Scholar]
- 2.Di Pietro MA, Leschied JR. Pediatric musculoskeletal ultrasound. Pediatr Radiol. 2017;47:1144–1154. doi: 10.1007/s00247-017-3919-5. [DOI] [PubMed] [Google Scholar]
- 3.Marc S. Keller musculoskeletal sonography in the neonate and infant. Pediatr Radiol. 2005;35:1167–1173. doi: 10.1007/s00247-005-1550-3. [DOI] [PubMed] [Google Scholar]
- 4.Karmazyn B. Ultrasound of pediatric musculoskeletal disease: from head to toe. Semin Ultrasound CT MR. 2011;32:142–150. doi: 10.1053/j.sult.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 5.Hryhorczuk AL, Restrepo R, Lee EY. Pediatric musculoskeletal ultrasound: practical imaging approach. AJR Am J Roentgenol. 2016;206:W62–W72. doi: 10.2214/AJR.15.15858. [DOI] [PubMed] [Google Scholar]
- 6. Windschall D, Trauzeddel R, Haller M, et al. Pediatric musculoskeletal ultrasound: age and sex related normal B-mode findings of the knee. Rheumatol Int. 2016;36:1569–1577. doi: 10.1007/s00296-016-3528-x. [DOI] [PubMed] [Google Scholar]
- 7.Dubois-Ferrière V, Belaieff W, Lascombes P, de Coulon G, Ceroni D. Transient synovitis of the hip: which investigations are truly useful? Swiss Med Wkly. 2015;21(145):w14176. doi: 10.4414/smw.2015.14176. [DOI] [PubMed] [Google Scholar]
- 8.Kastrissianakis K, Beattie TF. Transient synovitis of the hip: more evidence for a viral aetiology. Eur J Emerg Med. 2010;17:270–273. doi: 10.1097/MEJ.0b013e32832b1664. [DOI] [PubMed] [Google Scholar]
- 9.Kang YR, Koo J. Ultrasonography of the pediatric hip and spine. Ultrasonography. 2017;36:239–251. doi: 10.14366/usg.16051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Terjesen T. Ultrasonography in the primary evaluation of patients with Perthes disease. J Pediatr Orthop. 1993;13:437–443. doi: 10.1097/01241398-199307000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Wirth T, LeQuesne GW, Paterson DC. Ultrasonography in Legg–Calvé–Perthes disease. Pediatr Radiol. 1992;22(7):498–504. doi: 10.1007/BF02012992. [DOI] [PubMed] [Google Scholar]
- 12.Ashby E, Roposch A. Diagnostic yield of sonography in infants with suspected hip dysplasia: diagnostic thinking efficiency and therapeutic effciency. AJR Am J Roentgenol. 2015;204:177–181. doi: 10.2214/AJR.14.12477. [DOI] [PubMed] [Google Scholar]
- 13.Pillai A, Joseph J, McAuley A, Bramley D. Diagnostic accuracy of static Graf technique of ultrasound evaluation of infant hips for developmental dysplasia. Arch Orthop Trauma Surg. 2011;131:53–58. doi: 10.1007/s00402-010-1100-9. [DOI] [PubMed] [Google Scholar]
- 14.Graf R. Classification of hip joint dysplasia by means of sonography. Arch Orthop Trauma Surg. 1984;102:248–255. doi: 10.1007/BF00436138. [DOI] [PubMed] [Google Scholar]
- 15.Schaeffer EK, Study Group I. Mulpuri K. Developmental dysplasia of the hip: addressing evidence gaps with a multicentre prospective international study. Med J Aust. 2018;208:359–364. doi: 10.5694/mja18.00154. [DOI] [PubMed] [Google Scholar]
- 16.Carmichael KD, Longo A, Yngve D, et al. The use of ultrasound to determine timing of Pavlik harness discontinuation in treatment of developmental dysplasia of the hip. Orthopedics. 2008;31:988. [PubMed] [Google Scholar]
- 17.Draghi F, Danesino GM, Coscia D, Precerutti M, Pagani C. Overload syndromes of the knee in adolescents: sonographic findings. J Ultrasound. 2008;11:151–157. doi: 10.1016/j.jus.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Demirag B, Ozturk C, Yazici Z, Sarisozen B. The pathophysiology of Osgood–Schlatter disease: a magnetic resonance investigation. J Pediatr Orthop B. 2004;13:379–382. doi: 10.1097/01202412-200411000-00006. [DOI] [PubMed] [Google Scholar]
- 19.De Flaviis L, Nessi R, Scaglione P, Balconi G, Albisetti W, Derchi LE. Ultrasonic diagnosis of Osgood-Schlatter and Sinding–Larsen–Johansson diseases of the knee. Skelet Radiol. 1989;18:193–197. doi: 10.1007/BF00360969. [DOI] [PubMed] [Google Scholar]
- 20,Schmit P, Hautefort P, Raison-Boulley AM. Ultrasonographic diagnosis of an epiphyseal detachment of the upper end of the humerus due to birth injury. J Radiol. 1999;80:466–468. [PubMed] [Google Scholar]
- 21.Blankenbaker DG, De Smet AA. The role of ultrasound in the evaluation of sports injuries of the lower extremities. Clin Sports Med. 2006;25:867–897. doi: 10.1016/j.csm.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 22.Suzue N, Matsuura T, Iwame T, et al. State of the art ultrasonographic findings in lower extremity sports injuries. J Med Investig. 2015;62:109–113. doi: 10.2152/jmi.62.109. [DOI] [PubMed] [Google Scholar]
- 23.Stoll ML, Cron RQ. Treatment of juvenile idiopathic arthritis in the biologic age. Rheum Dis Clin N Am. 2013;39:751–766. doi: 10.1016/j.rdc.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 24.Collado P, Jousse-Joulin S, Alcalde M, Naredo E, D’Agostino MA. Is ultrasound a validated imaging tool for the diagnosis and management of synovitis in juvenile idiopathic arthritis? A systematic literature review. Arthritis Care Res (Hoboken) 2012;64:1011–1019. doi: 10.1002/acr.21644. [DOI] [PubMed] [Google Scholar]
- 25.Spârchez M, Fodor D. What's new in musculoskeletal ultrasound in pediatric rheumatology? Med Ultrason. 2018;20:371–378. doi: 10.11152/mu-1604. [DOI] [PubMed] [Google Scholar]