Abstract
Medial knee pain is common in clinical practice and can be caused by various conditions. In rare cases, it can even be by calcific bursitis of the medial collateral ligament (MCL). Treatment of calcific bursitis and/or calcification of the MCL classically includes observation, local injections, shockwave therapy and surgical resection. We report a case of nontraumatic medial knee pain poorly responsive to conservative treatments. Ultrasound (US) imaging revealed a massive lobed hyperechoic formation with partial acoustic shadow in the MCL context compatible with calcific bursitis, and magnetic resonance imaging (MRI) confirmed the presence of the bursa’s calcific deposit surrounded by hyperintense signal compatible with pericalcific edema. We performed a double-needle ultrasound-guided percutaneous lavage (UGPL), which is today a fairly common treatment for many musculoskeletal disorders, such as rotator cuff calcific tendinopathy and elbow extensor tendons pathology, but regarding the knee, it is not part of ordinary care. This report shows the clinical and imaging presentation of calcific bursitis of the MCL and describes in detail the technique to perform the UGPL with a system of two needles, two syringes and a double connection to ensure a correct lavage of the calcium deposit without significant intrabursal pressure increase and consequently without pain during the procedure.
Keywords: Medial collateral ligament, Pain, Voshell’s bursa, Calcific bursitis, Ultrasound, Treatment
Sommario
Il dolore localizzato alla regione mediale del ginocchio è di frequente riscontro nella pratica clinica e può essere causato da diverse condizioni patologiche, in rari casi anche da una borsite calcifica del legamento collaterale mediale (LCM). Il trattamento di una borsite calcifica e/o di una calcificazione del LCM prevede classicamente il monitoraggio clinico, le infiltrazioni locali, la terapia con onde d’urto ed infine la rimozione chirurgica. Riportiamo un caso di dolore localizzato alla regione mediale del ginocchio, non traumatico, scarsamente responsivo ai trattamenti conservativi. La valutazione ecografica ha rivelato la presenza di una voluminosa formazione iperecogena polilobata con parziale cono d’ombra posteriore nel contesto del LCM, compatibile con una borsite calcifica intra-ligamentosa; le scansioni di risonanza magnetica nucleare (RMN) hanno confermato la presenza del deposito endo-bursale circondato da segnale iperintenso compatibile con edema peri-calcifico. Abbiamo quindi eseguito una procedura di lavaggio percutaneo eco-guidato con due aghi, che ad oggi rappresenta un trattamento ampiamente utilizzato per diverse patologie del sistema muscolo scheletrico come la tendinopatia calcifica della cuffia dei rotatori della spalla e la patologia calcifica degli estensori di gomito, mentre per il ginocchio non rientra nei trattamenti eseguiti di routine. Questo articolo illustra le caratteristiche cliniche ed ecografiche della borsite calcifica del LCM ed ha l’obiettivo di descrivere in dettaglio la tecnica per eseguire un lavaggio percutaneo eco-guidato con un sistema di due aghi, due siringhe e un duplice tubo di raccordo per assicurare un completo lavaggio del deposito calcifico senza significativo aumento della pressione intra-bursale con l’obiettivo di minimizzare il dolore in corso di procedura.
Introduction
Medial knee pain is common in clinical practice and can be caused by various conditions, such as diseases of the medial meniscus, medial plica syndrome, saphenous nerve entrapment, pes anserine syndrome [1, 2] and, in rare cases, MCL calcification and/or calcific bursitis [3–5].
The bursa of the MCL was first described in 1943 by Brantigan and Voshell [6]. It can be identified in 90% of subjects between the meniscotibial ligament (distal deep portion of the MCL) and the superficial fibre contingent of the MCL [7]. This bursa, if distended by liquid or calcific material, can increase in size, expanding mainly in the proximal direction towards the medial femoral condyle (Fig. 1).
Fig. 1.
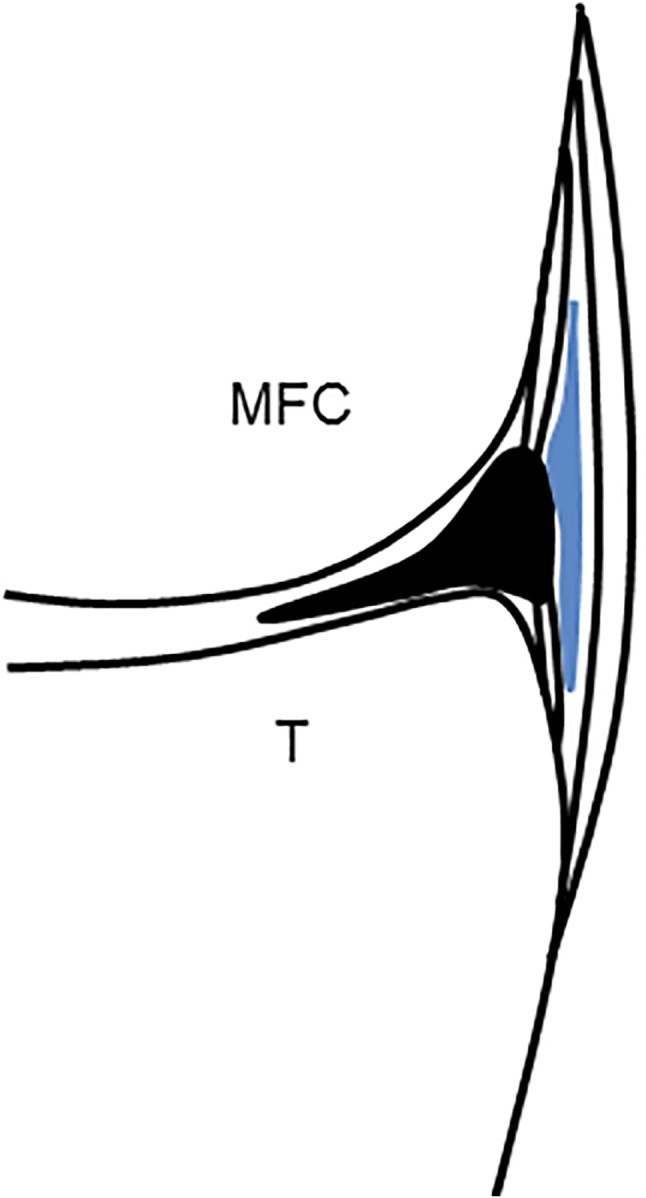
Schematic drawing shows a coronal section of the medial knee with a distended Voshell’s bursa (blue) between the superficial and the deep contingent of fibres of the MCL. MFC medial femoral condyle, T: tibia
The treatment of calcification and/or calcific bursitis of the MCL classically includes observation, local injections, shockwave therapy and surgical resection [4]. Ultrasound-guided percutaneous lavage is today a fairly common treatment for many musculoskeletal disorders, such as rotator cuff calcific tendinopathy [8–10] and elbow extensor tendons pathology [11], with a good profile of effectiveness and safety. Regarding the knee, we have found only a few cases of UGPL of calcific bursitis of the medial collateral ligament in the literature [5].
Calcific tendinopathy is characterized by deposition of calcium, predominantly hydroxyapatite crystals, in the tendons [12–14]. It has been extensively described in the literature over the years not only in the rotator cuff of the shoulder [12] but also in other tendons, such as the Achilles and patellar tendons. In the rotator cuff, this condition is common (2.5–7.5% of healthy shoulders in adults) and occurs in women in about 70% of cases, especially during the fourth and fifth decades of life [15, 16]. It seems not to be connected to physical activity [17, 18].
The pathogenesis is still not fully understood, but it seems to be related to areas of hypoxia in tendons that lead to fibrocartilaginous metaplasia and cellular necrosis, followed by formation of a calcium deposit, typically in an intact tendon. Although considered a self-healing condition, it can cause acute/chronic pain and functional disability depending on the stage of the disease [12, 13]. Uhthoff et al. hypothesized that a favourable environment permits an active process of cell-mediated calcification, usually followed by spontaneous phagocytic resorption [19, 20].
Four stages of the disease have been described in the Uhthoff cycle: precalcific phase, in which fibrocartilaginous transformation occurs within the tendon fibres, usually asymptomatic (stage 1); formative phase, in which calcifications are formed, usually poorly symptomatic, including sub-acute low-grade pain increasing at night (stage 2); resorptive phase, in which the tendon develops increased vascularization and calcium deposits are usually removed by multinucleated cells, but calcifications may migrate into the adjacent tissues with intense pain (stage 3); and postcalcific phase, in which self-healing and repair of the tendon fibres occur, lasting for several months, and may be associated with pain and limited function (stage 4) [12, 20].
Nakase et al. [21] clarified the nature of the multinucleated cells located near the calcium deposits, which were positive for cathepsin K, showing a typical osteoclast phenotype.
Case report
A 65-year-old male patient was seen because of pain and stiffness of the left knee which appeared a week before. The pain, localized in the medial region of the knee, was worse at night and during loading activity. He denied any trauma and declared that steroidal anti-inflammatory drugs, also injectives, had been partially effective. Physical examination revealed medial knee pain proximally and posteriorly to the tibiofemoral joint and stiffness, especially in extension movements. Valgus stress test and Apley’s compression test were also positive.
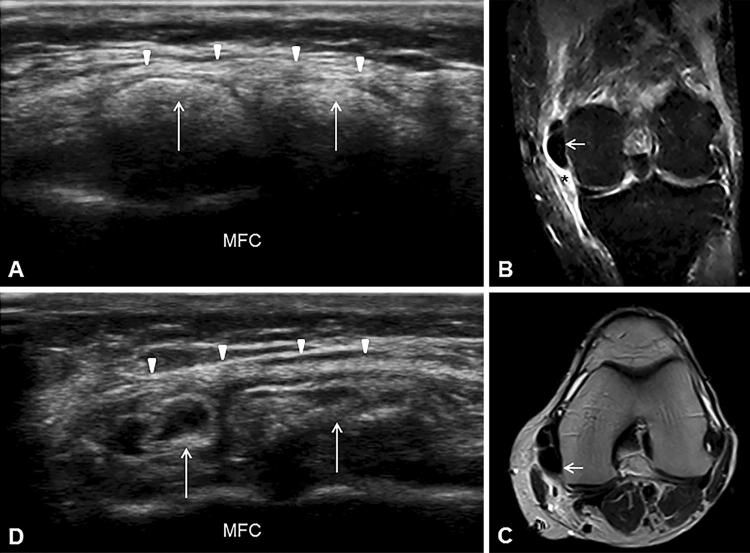
US examination was performed in accordance with the traditional scans for medial knee [7]. While a normal echostructure of the medial meniscus was seen in coronal plane, a massive (maximum diameter 25 mm) hyperechoic formation with partial acoustic shadow was observed proximally and posteriorly to the tibiofemoral joint in the MCL context (Fig. 2a). The polylobed structure was compatible with calcific bursitis of the MCL, and MRI confirmed the presence of a massive lobed formation adjacent to the medial femoral condyle, extended posteriorly and surrounded by hyperintense signal caused by pericalcific edema (Fig. 2b, c).
Fig. 2.
Ultrasound imaging of the medial knee in coronal view shows the lobed calcific deposit (white arrows) in the MCL context (white arrowheads) with partial acoustic shadow (a). STIR coronal MRI of the left knee shows the calcification (white arrow) adjacent to the medial femoral condyle and the hyperintense pericalcific edema (asterisk) around it (b). T2-weighted axial MRI shows the extension of the calcific deposition in the posterior region of the knee (white arrow) (c). Ultrasound examination during the procedure of lavage shows the reduction of the calcification size and changes of the echostructure (white arrows) with disappearance of the partial acoustic shadow (d). MFC medial femoral condyle
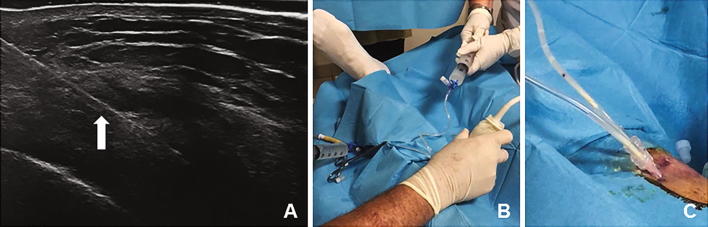
A double-needle ultrasound-guided percutaneous lavage was performed with a first step of local anaesthesia (Chirocaine injected in the soft tissues above the calcification with a 13 mm and 26-gauge needle), a second step of needles placement (two needles of 40 mm and 18 gauge were inserted under ultrasound guidance within the calcification area) and a last step of lavage (saline solution pushed under pressure through a 60-cc syringe from one needle and aspirated from the second needle with a syringe of the same size). The procedure was carried out using sterile sheet, sterile gloves, probe cover and polyvinyl chloride (PVC) sterile tubes connected to the needles. The connecting tubes allowed to create a closed circuit without communication with the external environment to guarantee complete sterility within the procedure (Fig. 3b, c). The use of two needles, one to insufflate and one to recover the saline solution, allowed us to keep intrabursal pressure low without pain complained by the patient (Fig. 4). The procedure ended when the saline solution came out clean and without calcific fragments, with a total duration of approximately 30 min.
Fig. 3.
In-plane visualization of the needle (white full arrow) during the procedure of lavage on the medial aspect of the knee (a). Real-time use of the two syringes, one to infuse under pressure the saline solution in the bursa and the other one to aspirate the washing liquid. This system allows controlling the intrabursal pressure and carrying out a minimally painful procedure for the patient (b). Clearance of the calcified fragments during the procedure of lavage (Cc)
Fig. 4.
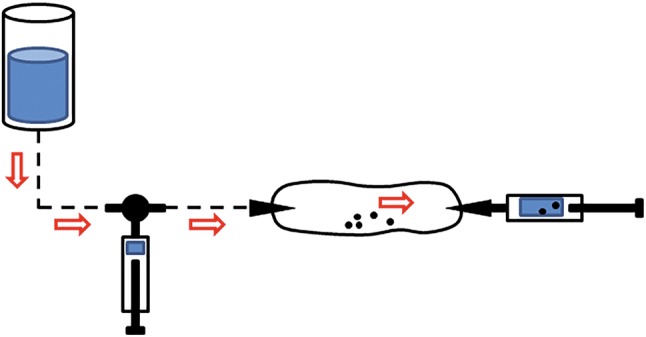
Schematic drawing shows a closed circuit with two needles and connecting tubes. The technique involves the use of two syringes, one to infuse under pressure the saline solution in the bursa and the other one to aspirate the washing liquid. This system allows controlling the intrabursal pressure and carrying out a minimally painful procedure for the patient
After the procedure, the patient reported immediate reduction of knee stiffness and pain (NRS pain preprocedure 8/10, postprocedure 2/10), further improved at 4-week follow-up (NRS pain 0/10). The US examination performed during the procedure revealed remarkable reduction of the calcification size and changes of the echostructure with disappearance of the partial acoustic shadow previously described (Fig. 2d).
Discussion
Calcific tendinopathy is characterized by deposition of calcium, primarily hydroxyapatite crystals, in the tendons, and it is common for the deposits to migrate to adjacent structures with pain and functional restrictions. This condition is usually self-limiting, and for this reason, the initial management of pain is typically conservative and involves rest, physical therapy and oral administration of nonsteroidal anti-inflammatory drugs. For cases in which conservative treatment has failed, nonsurgical therapeutic options may be used, such as extracorporeal shock wave therapy (ESWT), steroid injection (ultrasound-guided or not guided) and ultrasound-guided percutaneous lavage (UGPL).
Extracorporeal shock wave therapy (ESWT) is based on the application of repetitive impulses over the affected site. The results are variable, and the exact underlying mechanism of the therapeutic effect on calcific tendinopathy is still being discussed. It seems to be related to the phagocytosis of calcium deposition induced by neovascularization response and leukocyte chemotaxis [22]. Moreover, extracorporeal shock wave therapy is painful, expensive and not widely available.
The use of conservative treatments or ESWT in patients with acute pain given by calcific tendinopathy in resorption phase seems to be suboptimal and often fails because the symptoms significantly impact the quality of life [23], and many patients may not be able to tolerate the duration of time to resolution [24]. Thus, minimally invasive interventional techniques, such as steroid injections (ultrasound-guided or not guided) and UGPL [25], may be carried out.
The literature reports that UGLP is a superior technique compared with steroid injections in the calcific tendinitis of the rotator cuff [26], and it is always indicated in the acute phase of the pathology with soft or fluid calcifications. The percutaneous treatment is not indicated when patients are asymptomatic or little symptomatic or the calcification is smaller than 5 mm and of hard consistency [12, 27].
Different techniques have been reported in the recent literature, and all include the use of a fluid (local anaesthetic or saline solution) to dissolve the calcium deposits, and one or two needles are used to inject and take back the fluid to dissolve the calcific deposits.
Recent evidence showed that a double-needle approach might be more appropriate to treat harder calcific deposits because it allows fragmenting them, while one needle may be more useful in treating fluid calcifications [28]. Primary advantages of UGLP are that the procedure does not require any hospitalization and is performed under local anaesthesia, the patient can return home about 30 min after the procedure and can return to work early, and there is no need of postprocedural immobilization [29, 30].
Arthroscopic treatment of calcific tendinitis accounts selected cases. Indeed, it is the last option in chronic conditions in which conservative or less invasive approaches have failed. Calcification removal techniques differ regarding the type of tendon incision and the instruments used to remove the calcium deposit [31]. The surgery allows to remove calcification, and at the same time, to treat any associated joint diseases but requires hospitalization, general anaesthesia or sedation, and quite a long rehabilitation period after invasive treatment [32].
In our case, the patient needed significant doses of analgesic and anti-inflammatory drugs, moreover, with poor results in controlling pain and consequent impossibility to work activity. At the ultrasound evaluation, we have identified a hyperechoic formation with partial acoustic shadow in the MCL context, in which the polylobed structure was compatible with calcific bursitis, also confirmed by the MRI. Hence, we have decided to perform an UGPL due to the failure of the conservative treatment to control the intense pain of the patient. We used a double-needle technique for two main reasons: first, the morphological features of the calcification (i.e., the large size and the polylobed structure) that imply a high risk of obstructing the needle during the procedure and/or a partial removal of the deposition located in multiple cavities, and second, concerning the technical details because the simultaneous infusion–aspiration carried out with two-needle guarantees control of the endo-bursal pressure, which is not obtainable with a single-needle modality, even if well executed, because the two operations (insufflation/aspiration) cannot be performed at the same time.
Conclusion
The calcific tendinopathy is a common pathology, especially in the rotator cuff of the shoulder, but it can also be a cause of pain in the medial knee, although rarely. Therefore, it is important that the physician, in the examination of a patient with intense medial knee pain, also atraumatic, poorly responsive to common anti-inflammatory and analgesic treatments, considers the possibility of calcific bursitis and/or calcification of the MCL. The conservative, mini-invasive or surgical treatment depends on the Uhthoff stages, on the anatomical localizations of the calcium deposits and on the clinical conditions of the patient. With regard to the mini-invasive treatment of MCL calcification and/or calcific bursitis, particular attention must be paid to the course of the medial genicular arteries and the saphenous nerve.
In conclusion, ultrasound-guided percutaneous lavage can be an effective therapy of calcific bursitis of the medial collateral ligament of the knee, avoiding the known side effects of the common anti-inflammatory drugs and accelerating the physiological resolution of the calcium deposit. This procedure is little invasive, minimally painful, low-cost, fast and burdened by fewer complications compared with the surgical treatment. Moreover, it is an innovative technique that involves the use of two syringes, one to infuse under pressure the saline solution in the bursa and the other to aspirate the washing liquid, which limits the increase in intrabursal pressure and carries out a minimally painful procedure for the patient.
Funding
No funding was received.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bellary SS, Lynch G, et al. Medial plica syndrome: a review of the literature. Clin Anat. 2012;25(4):423–428. doi: 10.1002/ca.21278. [DOI] [PubMed] [Google Scholar]
- 2.Morganti CM, McFarland EG, Cosgarea AJ. Saphenous neuritis: a poorly understood cause of medial knee pain. J Am Acad Orthop Surg. 2002;10(2):130. doi: 10.5435/00124635-200203000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Siddiq MAB, Jahan I. Medial collateral ligament calcification: a rare knee pain entity with literature review. Acta Radiol Open. 2017 doi: 10.1177/2058460117738549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Themistoklis V, Filon A, et al. Massive non-traumatic calcification of the medial collateral ligament of the knee. BMJ Case Rep. 2016 doi: 10.1136/bcr-2016-217743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Del Castillo-González F, Ramos-Álvarez JJ, et al. Ultrasound-guided percutaneous lavage of calcific bursitis of the medial collateral ligament of the knee: a case report and review of the literature. Skeletal Radiol. 2016;45:1419–1423. doi: 10.1007/s00256-016-2442-3. [DOI] [PubMed] [Google Scholar]
- 6.Brantigan OC, Voshell AF. The tibial collateral ligament: its function, its bursae, and its relation to the medial meniscus. J Bone Joint Surg Am. 1943;25:121–131. [Google Scholar]
- 7.Stella SM, Ciampi B. Ginocchio, richiami di anatomia e semeiotica ecografica. In: Galletti S, editor. Atlante di anatomia ecografica e biomeccanica muscoloscheletrica. Padova: Piccin; 2017. pp. 402–459. [Google Scholar]
- 8.Lanza E, Banfi G, Serafini G, et al. Ultrasound-guided percutaneous irrigation in rotator cuff calcific tendinopathy: what is the evidence? A systematic review with proposals for future reporting. Eur Radiol. 2015;25:2176–2183. doi: 10.1007/s00330-014-3567-1. [DOI] [PubMed] [Google Scholar]
- 9.Galletti S, Magnani M, Rotini R, et al. The echo-guided treatment of calcific tendinitis of the shoulder. Chir Organi Mov. 2004;89(4):319–323. [PubMed] [Google Scholar]
- 10.Galletti S, Serafini G, Minetti V, Galletti R, Mignani G. Tendini, appendice: trattamento percutaneo ecoguidato della tendinopatia calcifica di spalla. In: Galletti S, editor. Ecografia patologica muscolo scheletrica, testo e atlante. Padova: Piccin; 2018. pp. 376–386. [Google Scholar]
- 11.Abate M, Salini V, Schiavone C. Ultrasound-guided percutaneous lavage in the treatment of calcific tendinopathy of elbow extensor tendons: a case report. Malays Orthop J. 2016;10(2):53–55. doi: 10.5704/MOJ.1607.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Serafini G, Sconfienza M, Lacelli F, et al. Rotator cuff calcific tendonitis: short-term and 10-year outcomes after two needle US-guided percutaneous treatment—non randomized controlled trial. Radiology. 2009;252(1):157–164. doi: 10.1148/radiol.2521081816. [DOI] [PubMed] [Google Scholar]
- 13.Bianchi S, Martinoli C. Ultrasound of the musculoskeletal system. Berlin: Springer; 2007. pp. 198–332. [Google Scholar]
- 14.Tagliafico A, Russo G, Boccalini S, et al. Ultrasound-guided interventional procedures around the shoulder. Radiol Med. 2014;119(5):318–326. doi: 10.1007/s11547-013-0351-2. [DOI] [PubMed] [Google Scholar]
- 15.Clavert P, Sirveaux F, Sociètè française d’arthroscopie Shoulder calcifying tendinitis. Rev Chir Orthop Reparatrice Appar Mot. 2008;94:336–355. doi: 10.1016/j.rco.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 16.Barile A, Bruno F, Mariani S, et al. Follow-up of surgical and minimally invasive treatment of Achilles tendon pathology: a brief diagnostic imaging review. Musculoskeletal Surg. 2017;101:51–61. doi: 10.1007/s12306-017-0456-1. [DOI] [PubMed] [Google Scholar]
- 17.Uhthoff HK, Sarkar K. Calcifying tendinitis. Baillieres Clin Rheumatol. 1989;3:567–581. doi: 10.1016/S0950-3579(89)80009-3. [DOI] [PubMed] [Google Scholar]
- 18.Barile A, La Marra A, Arrigoni F, et al. Anaesthetics, steroids and platelet-rich plasma (PRP) in ultrasound-guided musculoskeletal procedures. Br J Radiol. 2016;89(1065):20150355. doi: 10.1259/bjr.20150355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oliva F, Giai Via A, Maffulli N. Physiopathology of intratendinous calcific deposit. BMC Med. 2012;10:95. doi: 10.1186/1741-7015-10-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uhthoff HK, Loehr JW. Calcific tendinopathy of the rotator cuff: pathogenesis, diagnosis and management. J Am Acad Orthop Surg. 1997;5:183–191. doi: 10.5435/00124635-199707000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Nakase T, Takeuchi E, Sugamoto K, et al. Involvement of multinucleated giant cells synthesizing cathepsin K in calcified tendinitis of the rotator cuff tendons. Rheumatology. 2000;39:1074–1077. doi: 10.1093/rheumatology/39.10.1074. [DOI] [PubMed] [Google Scholar]
- 22.Kim YS, Lee HJ, Kim YV, Kong CG. Which method is more effective in treatment of calcific tendinitis in the shoulder? Prospective randomized comparison between ultrasound-guided needling and extracorporeal shock wave therapy. J Shoulder Elbow Surg. 2014;23:1640–1646. doi: 10.1016/j.jse.2014.06.036. [DOI] [PubMed] [Google Scholar]
- 23.Robotti G, Canepa MG, Bortolotto C, Draghi F. Interventional musculoskeletal US: an update on materials and methods. J Ultrasound. 2013;16(2):45–55. doi: 10.1007/s40477-013-0018-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cura JL, et al. Sonographically guided percutaneous needle lavage in calcific tendinitis of the shoulder: short- and long-term results. AJR Am J Roentgenol. 2007;189(3):W128–W134. doi: 10.2214/AJR.07.2254. [DOI] [PubMed] [Google Scholar]
- 25.De Zordo T, Ahmad N, Ødegaard F, et al. US-guided therapy of calcific tendinopathy: clinical and radiological outcome assessment in shoulder and non-shoulder tendons. Ultraschall Med. 2011;32(Suppl 1):S117–S123. doi: 10.1055/s-0029-1245333. [DOI] [PubMed] [Google Scholar]
- 26.Witte PB, Selten JW, Navas A, et al. Calcific tendinitis of the rotator cuff: a randomized controlled trial of ultrasound-guided needling and lavage versus subacromial corticosteroids. Am J Sports Med. 2013;41(7):1665–1673. doi: 10.1177/0363546513487066. [DOI] [PubMed] [Google Scholar]
- 27.Sconfienza LM, Albano D, Messina C, et al. How, When, Why in Magnetic Resonance Arthrography: an International Survey by the European Society of Musculoskeletal Radiology (ESSR) Eur Radiol. 2018;28(6):2356–2368. doi: 10.1007/s00330-017-5208-y. [DOI] [PubMed] [Google Scholar]
- 28.Orlandi D, Mauri G, Lacelli F, et al. Rotator cuff calcific tendinopathy: randomized comparison of us-guided percutaneous treatments by using one or two needles. Radiology. 2017;285:518–527. doi: 10.1148/radiol.2017162888. [DOI] [PubMed] [Google Scholar]
- 29.Draghi F, Robotti G, Jacob D, et al. Interventional musculoskeletal ultrasonography: precautions and contraindications. J Ultrasound. 2010;13(3):126–133. doi: 10.1016/j.jus.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cocco G, Draghi F, Schiavone C. Ultrasonographic diagnosis and percutaneous treatment of insertional calcific tendinopathy of iliotibial band case report. Euro Rad. 2018 doi: 10.1594/eurorad/case.15881. [DOI] [Google Scholar]
- 31.Rebuzzi E, Coletti N, Schiavetti S, Giusto F. Arthroscopy surgery versus shock wave therapy for chronic calcifying tendinitis of the shoulder. J Orthop Traumatol. 2008;9:179–185. doi: 10.1007/s10195-008-0024-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fields LK, Muxlow CJ, Caldwell PE. Arthroscopic treatment of subscapularis cacific tendonitis. Arthrosc Tech. 2014;3:e571-3. doi: 10.1016/j.eats.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]