Abstract
Turmeric (Curcuma longa) is one of the most important ingredients in Indian and Chinese cuisine. Curcuminoids and volatile oils present in turmeric are known for their functional and nutraceutical properties. Health benefits attributed to curcuminoids have resulted in their wide utilization in food and pharmaceutical formulations. Therefore, characterization and estimation of the curcuminoids in fresh/dry turmeric, food and nutraceutical products are essential for their quality control during processing and storage. To meet the demand for analytical methods of curcuminoids, several methods have been developed for their quantification in turmeric powder and food formulations. In the present review, various analytical methods (spectrophotometric, chromatographic, capillary electrophoresis and biosensor techniques) which are used for monitoring curcuminoids have been thoroughly summarized and discussed. The spectrophotometric method is not useful when individual components of curcuminoids are required. Mobile phase optimization, the broadness of spots, plate-to-plate variations are significant limitations for TLC and HPTLC methods. Many analysts believe that HPLC method is the best choice for curcuminoids determination because of its rapid analysis. Spectrofluorimetry and Electrochemical methods are the more advanced methods with high sensitivity as well as rapid analysis. However, the selection of analytical method for curcuminoids analysis depends on the type of sample matrix, purpose of the analysis and limit of detection and limit of quantitation of the method.
Keywords: Turmeric, Curcuminoids analysis, Analytical methods, Quality control, Review
Introduction
Natural products from plant sources form the basis for many widely used nutraceuticals and functional foods (Nelson et al. 2017). Turmeric (Curcuma longa) is one of the most studied plant materials because of its promising health benefits. It belongs to the Zingiberaceae family. Tropical and subtropical parts of Asia and Africa are ideal places for its growth and cultivation (Priyadarsini 2014). Turmeric is being used in Ayurvedic medicines since ancient times for the treatment of diabetes, cough, anorexia and sinusitis (Jayaprakasha et al. 2005; Nelson et al. 2017). Furthermore, turmeric is also used as the main ingredient in various food preparations to impart characteristic color and flavor and it acts as preservative as well (Jayaprakasha et al. 2005).
The importance of turmeric mainly comes from its major phytochemical constituents, called curcuminoids. Curcuminoids content of turmeric varies between 2 and 9% based on its cultivar, soil and climatic conditions (Priyadarsini 2014). Curcuminoids mainly contain three chemically related components called as curcumin (C), demethoxycurcumin (DMC) and bis-demethoxycurcumin (BDMC) (Nelson et al. 2017; Meng et al. 2018). The chemical structure of these components is shown in Fig. 1. These three curcuminoids along with other components imparts the distinctive yellow color to the turmeric and turmeric extracts (Jayaprakasha et al. 2005). Among the curcuminoids, C is the most abundant, potent, and has been extensively explored for its functional and nutraceutical activities. However, new reports have revealed that DMC and BDMC also had similar or purportedly higher bioactive potential when compared to C (Peram et al. 2017). For example, in case of anticancer activities, BDMC is most potent compared to DMC and C (Ali et al. 2014). Furthermore, it is reported that a mixture of three curcuminoids (in a particular ratio) is more potent than individual curcuminoids, thereby, suggesting the possible synergistic effect (Peram et al. 2017). Although the curcumin was isolated from the turmeric two centuries ago, it is still attracting researchers from all over the world because of its functional properties (Priyadarsini 2014).
Fig. 1.
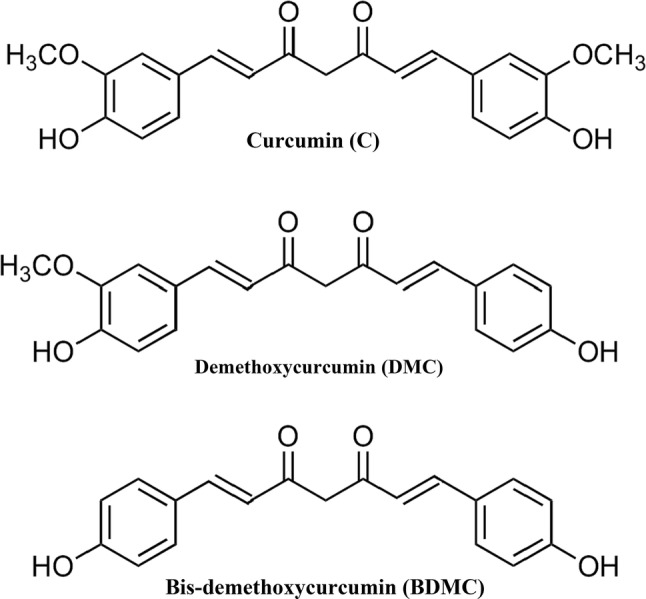
Structures of curcuminoids
Several studies showed that curcuminoids possess different functional and nutraceutical activities, including antioxidant, anticarcinogenic, antidiabetic and hypocholesteremic activities (Rao et al. 1970; Meng et al. 2018). As a result, turmeric has an ever-increasing demand for food, cosmetics, and pharmaceuticals. Many food companies are manufacturing various turmeric products in the form of extracts, powders, coloring agents, food supplements and drinks (Peram et al. 2017). Thus, nowadays, many researchers are trying to develop different functional and therapeutic foods with curcuminoids as an ingredient (Li et al. 2014a). Consequently, government institutions have established regulations for quality control of curcuminoids in finished products. For example, the maximum allowed curcumin in smoked fish is 0.1 g/kg, savory snack (0.1–0.2 g/kg), mustard, sauce, and seasoning (0.3–0.5 g/kg) in Europe (Lee and Choung 2011).
WHO has announced recommendations for quality control of herbal medicines, to ensure the identification of plant materials, purity and content (Baghel et al. 2017). Inevitably, characterization and quantification of three curcuminoids in turmeric powder or food products is essential for its quality control during processing and storage.
Several analytical methods have been developed for the detection of curcuminoids and each method has certain advantages and limitations as well (Cheng et al. 2010; Taha et al. 2015; Kadam et al. 2013; Dey et al. 2018). For example, the spectrophotometric method can only give total curcuminoids content whereas other advanced methods can simultaneously quantify each curcuminoid (Kadam et al. 2013). Therefore, selection of analytical method is crucial for obtaining better results. There are many critical reviews available on chemical, biological and pharmacological properties of curcuminoids. However, reviews on different analytical methods of curcuminoids are extremely limited. Therefore, the authors wish to give a flavor of some of the analytical methods for the determination of curcuminoids in turmeric and food formulations. It is hoped that this will encourage the general readers to select a suitable analytical method for specific requirements.
Spectrophotometric methods
UV–Vis spectrophotometry
The spectrophotometric method is simple and most commonly used method for the determination of curcumin in various sample matrices (Jayaprakasha et al. 2002). Methanol was found to be a suitable solvent for spectrophotometric measurements of curcumin in most of the sample matrices (Kadam et al. 2013). However, in this method, precision is low due to other color interfering pigments present in the sample (Pathania et al. 2006). Moreover, this method does not give the relative composition of individual curcuminoids, which is a major limitation for this method (Rohman 2012). Taylor and McDowell (1992) compared spectrophotometric and HPLC methods and reported that the spectrophotometric method had shown more curcumin content than HPLC method in turmeric rhizomes which is due to the fact that non-curcumin components were also absorbed at the same wavelength as curcumin.
To overcome this problem, a first-order differentiation of spectrum is performed, since it provides greater resolution. As a result, it is possible to analyze each component in the presence of other elements without any pretreatment or interference (Dave et al. 2007). Pundarikakshudu and Dave (2010) used first-order differential spectrophotometry for simultaneous determination of berberine and curcumin in combined methanolic extracts of Berberis aristata and Curcuma longa without any prior separation or purification step. Few other researchers used the extinction coefficient as a basis for analysis of curcuminoids using spectrophotometric method (Scotter 2009). The Joint Expert Committee on Food Additives (JECFA) prescribed that absorbance for curcumin in ethanol to be at 425 nm when determined by a spectrophotometric method of an assay with corresponding extinction coefficient as a reference value for the three curcuminoids together (Rohman 2012). From the above information, it is clear that spectrophotometric methods are useful when individual curcuminoids concentration is not a necessary quality parameter (Kadam et al. 2013).
Infrared (IR) Spectroscopy
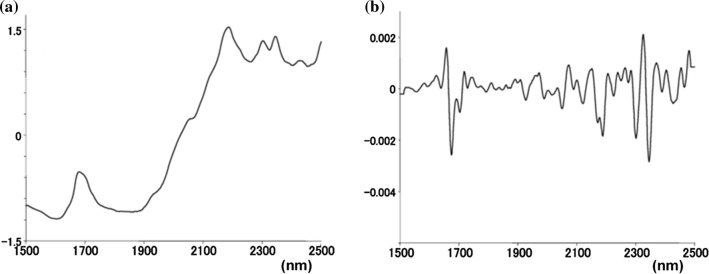
IR spectroscopy is the preferred method for the qualitative and quantitative analysis of physiologically active ingredients in food compositions (Kar et al. 2018). The primary mechanism involved in this method is arising of overtone and combination bands form fundamental chemical vibrations (Kasemsumran et al. 2014). This method can be combined with the chemometric tools, which could be used for analysis of food products both qualitatively and quantitatively. As a result, this technique allows fast, nondestructive analysis with minimal or no sample preparation and without the use of any chemical reagents (Roggo et al. 2007). Few researchers reported that IR spectrometry methods for determination of curcuminoids directly in turmeric powder and in some food formulations (Tanaka et al. 2008; Kim et al. 2014). The IR bands usually overlap and results into spectrum with broad peaks. However, IR spectra generally contain a significant amount of information about the molecular and physical structure of the sample. Thus, various multivariate statistical data analysis tool can be employed to extract the meaningful result. Tanaka et al. (2008) studied the profile of curcuminoids in 34 samples of turmeric using near infrared (NIR) spectroscopy and multidimensional statistics. Two characteristic absorptions of curcuminoids were detected in the second derivatives of the NIR spectra (Fig. 2) of turmeric samples and are around 1700 and 2300–2320 nm. Using Partial least-squares regression (PLS) method, they quantified curcuminoids content in turmeric sample. Although this technique is rapid and nondestructive, many samples from different sources are required to create a strong PLS model (Kasemsumran et al. 2014). Despite this, IR spectroscopy is suitable for quality control purpose in food industries due to its rapid and nondestructive nature of analysis.
Fig. 2.
a Near IR spectra of demethoxycurcumin and b second order differential spectra of demethoxycurcumin (Tanaka et al. 2008)
Chromatographic methods
Thin layer chromatography (TLC) and high-performance thin layer chromatography (HPTLC)
Chromatography is a technique used for the separation of closely related components in a mixture by differential migration through immiscible stationery and mobile phases (Jupille and Perry 1977). Thus, successful separation of individual components of curcuminoids (C, DMC, and BDMC) by TLC method is possible using an optimized mobile phase. Janben and Gole (1984) were the first to report separation of curcuminoids in spices using chloroform:acetic acid (80:20 v/v) and silica gel 60 thin layer plates as mobile and stationary phases, respectively. They also determined curcuminoids content in spices by measuring the intensity of rubrocurcumin on the layer, which was obtained by treating boric-acetic acid reagent with curcumin. Few other authors also reported the TLC method (with many modifications) for separation and quantification of curcuminoids (Phattanawasin et al. 2009). Although the TLC method has some advantages (low cost, easy maintenance, and selectivity), its usage has gradually declined due to prolong separation time and poor resolution in the case of turmeric analysis. HPTLC method can overcome these limitations. It works on the same principle of TLC, but some changes have been made to increase resolution and to allow low detection levels like use of finer particles on the stationary phase of TLC plates and improved image scanning. Several HPTLC methods have been reported to determine curcuminoids as a single component or combination with others in turmeric and processed products. Those methods are detailed in Table 1.
Table 1.
TLC and HPTLC methods for determination of curcuminoids
| Sample | Stationary phase | Mobile phase | Solventa | Wavelength | LOD and LOQ | References |
|---|---|---|---|---|---|---|
| TLC methods | ||||||
| Turmeric powder | Silica gel | CHCl3:CH3COOH (80:20) | CH3OH | 436 nm | Not reported | Janben and Gole (1984) |
| Turmeric rhizomes | Silica gel plate 60F254 | C6H14:CHCl3:CH3OH (10:10:1 v/v/v) | CH3OH | 254 nm | 43–73 and 43–242 ng/spot | (Sotanaphun et al. (2009) |
| Turmeric rhizomes | Silica gel plate 60 F254 | CHCl3:C6H14:CH3OH (1:1:0.1 v/v/v) | CH2Cl2 | Image analysis | LOD: 158 and LOD: 525 ng/mL | Phattanawasin et al. (2009) |
| HPTLC methods | ||||||
| Turmeric rhizomes | LiChrosphere aluminium plates SI 60F254 | CHCl3:CH3OH (98:2 v/v | C3H6O | 366 nm | LOD: 40 and LOQ: 100 ng/mL | Pathania et al. (2006) |
| Turmeric rhizomes | Nano Silica gel 60F 254 | CHCl3:C4H8O2 (19:1 v/v) | C2H6O | 254 and 366 nm | Not reported | Green et al. (2008) |
| Turmeric rhizomes | Silica gel aluminum plate 60GF254 | CHCl3:CH3OH (48:2 v/v) | C6H6 | 425 nm | LOD: 0.1 µg/spot | Paramasivam et al. (2009) |
| Turmeric powder | Silica gel 60 F254 | CH2C12:CH3OH (99:1) combination | CH3OH | 427 nm | LOD: 49 and LOD: 148 ng/mL | Gantait et al. (2011) |
| Turmeric rhizomes | Silica gel plate 60 F254 | C7H8:C4H8O2:CH2O2 (9:6:0.4) | C2H6O | 200 and 700 nm | Not reported | Taha et al. (2015) |
| Polyherbal formulation | Silica gel aluminum plate 60 F254 | C7H8:C4H8O2:CH2O2 (4.5:4:0.1, v/v/v) | CH3OH | 250 nm | Not reported | Baghel et al. (2017) |
aUsed in sample preparation
HPTLC is one of the extensively used chromatographic techniques for quality analysis of food products because of its advantages. For instance, this is the only chromatographic method which gives results as an image (Attimarad et al. 2011). In addition to this, less solvent consumption, offline method and less warm up time for instrument to get ready for experimentation, makes the technique more beneficial when compared to HPLC (Ansari et al. 2005; Pathania et al. 2006). Methanol was found the most suitable solvent for maximum extraction of curcuminoids in HPTLC sample preparation (Paramasivam et al. 2009). Developing optimum mobile phase is the hardest part in HPTLC separation. Usually, it is a trial and error process. Ansari et al. (2005) first tried methanol:chloroform (0.5:9.5 v/v) which resulted in good results however typical peak nature was missing. Finally, a sharp, well-defined peak (Fig. 3) was provided by the mobile phase consisting of methanol:chloroform (0.75:9.25 v/v).
Fig. 3.
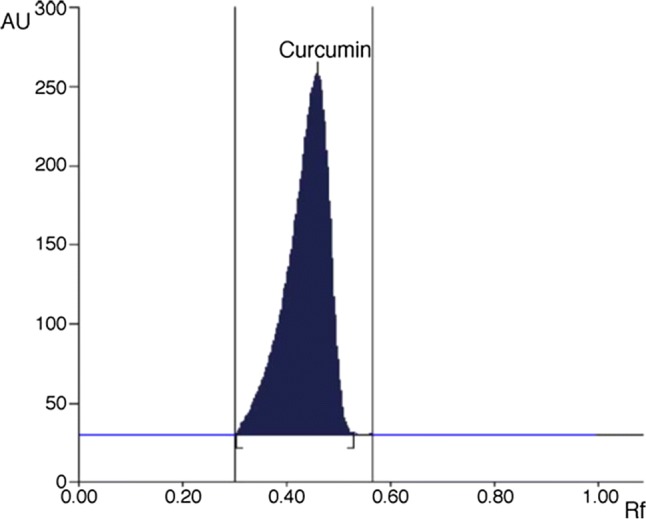
A typical HPTLC chromatogram of curcumin (Rf = 0.48) (Ansari et al. 2005)
The HPTLC chamber should be saturated with the mobile phase before experiment for clear spots on TLC plates (Ansari et al. 2005). The broadness of spots is the major problem in HPTLC method. Pathania et al. (2006) improved the HPTLC method by using LiChroshere plate that overcame the difficulties of the broadness of the spots. Limit of detection and variation from plate to plate are the main controlling aspects for the use of HPTLC method. However, these limitations can be overcome by pointing known amount of standard together with the unknown sample in each experiment (Pathania et al. 2006). Paramasivam et al. (2009) used the HPTLC method to find high-quality turmeric plant concerning curcuminoids. This method could be used for identifying high-quality turmeric cultivars for plant breeding program. Gantait et al. (2011) compared curcuminoids content in several market samples and in-house turmeric powder using HPTLC method and reported that turmeric powder available in market has less curcumin (LOD: 49 ng/ml; LOD: 148 ng/ml).
High-performance liquid chromatography (HPLC)
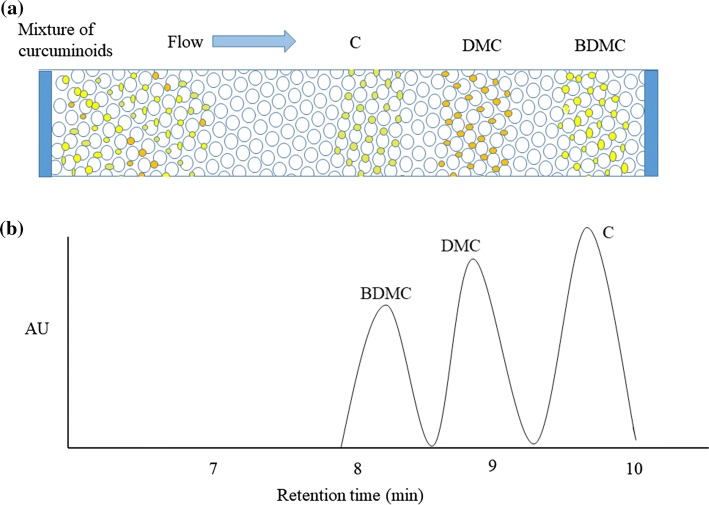
There are many HPLC methods to determine curcuminoids in turmeric and processed products, which are summarized in Table 2. Separation and quantitation of components in a mixture are the main properties of HPLC systems. These properties of HPLC overcome the drawbacks of the spectrophotometric method, which cannot quantify the individual components of curcuminoids. Since curcuminoids are thermally liable and less volatile in nature, separation and quantifying them with gas chromatography is also not popular (Rohman 2012). Although separation and quantification of curcuminoids are possible with TLC methods, these methods could not resolve curcuminoids completely. Figure 4 shows the schematic view of curcuminoids separation in C18 column and typical chromatogram of standard curcuminoids mixture. Inevitably, many analysts believe that HPLC method is the best choice for curcuminoids determination because of its rapid analysis and accuracy. In curcuminoids analysis, HPLC together with UV–VIS or photodiode array detector (PAD) are the routine methods (Jadhav et al. 2007). Tonnesen and Karlsen (1983) were the first to employ this method for estimation of curcumin and its related components. Jayaprakasha et al. (2002) modified the existing HPLC protocol with gradient elution of a solvent mixture of acetonitrile, methanol, and acetic acid for the determination of curcuminoids with a run time of 20 min.
Table 2.
HPLC and LC–MS/MS methods for determination of curcuminoids
| Sample matrix | Mobile phase | Column and detector | Separation time (min) | LOD and flow rate | Reference |
|---|---|---|---|---|---|
| HPLC methods | |||||
| Turmeric powder | C2H5OH:H2O (96:04 v/v) | RP-5-NH2, UV 280 nm | 20.73 | −; 1 mL/min | Khurana and Ho (1988) |
| Fresh turmeric extract | (A) H2O (0.25% CH3COOH) and (B) CH3CN, 0–17 min, 40– 60% B; 17–32 min, 60–100% B; 32–38 min, 100% B; 38–40 min, 100–40% B | C18, UV 200–500 nm | 14.0 | –; 0.2 mL/min | He et al. (1998) |
| Turmeric and spent oleoresin | CH3OH–2% CH3COOH– CH3CN | C18, UV 425 nm, UV 425 nm | 6.75 | 0.05 µg/mL, 1 mL/min | Jayaprakasha et al. (2002) |
| Turmeric powder | CH3CN–0.1% CF3COOH– (50:50 v/v), (pH adjusted to 3.0 with NH3) | C18, UV–VIS 420 nm | 9.0 | 27.99, 31.91 and 21.81 ng/mL for C, DMC and BDMC; 1.5 mL/min | Jadhav et al. (2007) |
| Turmeric extracts |
Isocratic elution of CH3CN and 2% v/v CH3COOH (40:60v/v) |
RP-Alltima C18 Column |
13.6 | 0.90, 0.84, 0.08 µg/mL for C, DMC and BDMC; 2.0 mL/min | Wichitnithad et al. (2009) |
| Turmeric powder and tablet | 0.1 M of acetate buffer (pH 4.0)–CH3CN (57:43 v/v) |
C18, fluorescence detector RF-10AXL |
~28 | 1.5, 0.9 and 0.09 ng/mL for C, DMC and BDMC; 1 mL/min | Zhang et al. (2009) |
| Turmeric oleoresin | C3H8O:H2O (95:05 v/v) | Exil-Amino column, UV–VIS 425 nm | 11 | 0.3 µg/mL; 1 mL/min | Naidu et al. (2009) |
| Curcuminoids-loaded liposome | 1% H3PO4–CH3CN | C18, UV–VIS 425 nm | 6.36 | 2.5 mg/mL; 1.0 mL/min | Jangle and Thorat (2013) |
| Turmeric powder | ACN–MeOH–H2O (40:20:40 v/v | RP-phenyl column, UV–VIS | 10.5 | 0.30–0.50 ng/mL; 1.0 mL/min | Ali et al. (2014) |
| Turmeric powder | 10 mM Na2HPO4:H3PO4 (pH 5.0) (50:50 v/v) | C18, Electrochemical detection cell | 15.0 | 0.208, 0.197 and 0.227 µM for C, DMC and BDMC; 1.0 mL/min | Long et al. (2014) |
| Turmeric colour pigments, curry powder | CH3OH (A):C4H8O (B): 0.1 g/100 ml H3PO4 in H2O (C). Gradient system: 0–15 min, 10–15% A, 30–40% B, and 60–45% C; 15–20 min, 15–55% A, 40–10% B, and 45–35% C | C18, UV 425 nm | 17.13 | 0.27, 0.18, 0.23 µg/mL for C, DMC and BDMC; 1.0 mL/min | Li et al. (2014b) |
| Turmeric extract and emulsion | CH3CN:CH3OH:H2O (40:20:40 v/v/v) | C18, UV/VIS detector | 6.67 | 0.305 µg/mL; 1.5 mL/min | Syed et al. (2015) |
| Turmeric rhizome | Acetonitrile:water (70:30 v/v). | C18, DAD | 15.08 | 1.0 µg/mL; 0.8 mL/min | Hwang et al. (2016) |
| In different commercial products | 0.1% CH3COOH in H2O(A): CH3CN with 0.1% CH3COOH (B) Gradient Program:0 min,45% of solvent B; Step 1: 1.5 min, 65% solvent B; Step 2: 2.5 min, 90% solvent B; Step 3: 4.0 min, 90% | C18, Photodiode array detector | 1.3 | 0.40, 0.20, 0.19 µg/mL for C, DMC and BDMC 2.5 mL/min | Osorio-Tobón et al. (2016) |
| In different commercial products | CH3CN: 0.1% CH2O2 in H2O | C18, Photodiode array detector | 9.18 | 7.40, 9.24 and 6.48 ng/mL for C, DMC and BDMC; 0.8 mL/min | Peram et al. (2017) |
| Java turmeric | Gradient elution, acetonitrile- 0.001% formic acid | Phenomenex C18, UV–VIS 425 nm | 10.72 | 0.0250, 0.0166, 0.0119 µg/mL for C, DMC and BDMC;1 mL/min | Erpina et al. (2017) |
| LC–MS/MS methods | |||||
| Equine plasma | 0.1% (v/v) CH2O2 in C2H3N (A) and 0.1% (v/v) CH2O2 in H2O (B); isocratic program (43% A: 57% B) |
XBridge BEH C18, MS, electrospray ionization |
9 | 1 ng/mL; 0.2 mL/min | Liu et al. (2018b) |
| Bacterial culture medium | 10 mM C2H7NO2 and 0.1% (v/v) CH2O2 in H2O (A) or in acetonitrile (B). Gradient elution: 0–5 min, 20% B; 5–8.1 min, 80% B; 8.1–10.0 min, 20% B. |
Poroshell 120 EC-C8 column , MS, electrospray ionization |
10 | 0.1 µM; 0.3 mL/min | Tan et al. (2015) |
| Turmeric rhizome | 5 mM NH4HCO2, 0.1% CH2O2, in H2O (A); C2H3N (B); Gradient (in buffer A): 0–2 min, 5% B; 2–57 min, 5–100% B; 57–60 min, 100% B; 60–65 min, 100–5% B; 65–75 min, 5% B | Discovery HS C18; MS Sustained off-resonance irradiation fragmentation (SORI) | 75 | –; 0.25 mL/min | Jiang et al. (2006a) |
| Plasma samples | 10.0 mM NH4HCO2 (pH 3.0) (A) and CH3OH (B) Gradient elution: 0–3.0 min (25–90% B), 3.0–7.50 min (90–90% B), 7.50–7.51 min (90–25% B), 7.51–11.0 min (25–25% B) | Xterra MS C18, MS, electrospray ionization | 11.0 | 2.50–179 ng/mL; 0.25 mL/min | Kunati et al. (2018) |
| Cell medium and mouse plasma | 0.1% CH2O2 in C2H3N (50%) (A) and H2O (B); isocratic mode | Beta basic C8 column, MS ion reaction monitoring | 12 | 1 ng/mL; 0.2 mL/min | Vijaya Saradhi et al. (2010) |
| Human plasma | 0.1% CH2O2 in C2H3N (50%) (A) and H2O (B); isocratic mode | BetaBasic C8 column, MS, electro-spray ionization | 5 | 2.0 ng/ml; 0.2 mL/min | Chen et al. (2012) |
Fig. 4.
a Separation of mixture of curcuminoids and b HPLC chromatograms of the standard mixture of curcuminoids
In the HPLC method, the food matrix is an essential factor that affects the extraction efficiency of curcuminoids in food samples. Lee and Choung (2011) reported recovery rates of 16 food types. Among these, solid foods showed the highest recovery rates (above 90%) whereas beverages and some other liquid foods showed less than 3.3%. In any analytical method, extraction of target components from the sample matrix is the most critical step. For extraction of phenolic, the solvent type has a unique role even though other factors (e.g., particle size, pH, solute to solvent ratio and temperature etc.) also effect the efficiency of extraction (Lee and Choung 2011). In the case of curcuminoids extraction, methanol was found to be an appropriate solvent (Paramasivam et al. 2009). Lee and Choung (2011) compared three different solvents (ethyl acetate, acetonitrile, and methanol) for extraction of curcuminoids and reported methanol followed by acetonitrile and ethyl acetate showed better efficiency in isolation of curcuminoids from the sample matrix. C, DMC, and BDMC are powerful complexing agents; they can form inter and intera molecular bonds. Therefore, separation in chromatographic methods entirely depends upon the bonding between a reactive di-ketone group of curcuminoids and stationary phase. Usually, some interaction is necessary between curcuminoids and stationary phase to get successful separation (Jadhav et al. 2007; Mudge et al. 2016; Peram et al. 2017). Due to the liable characteristics of curcuminoids, mostly, C-18 columns are preferred for determination of curcumin using HPLC systems. However, for better separation with this column, pH must be very low which damages the column in the long run. Moreover, the temperature of the column has to be maintained for the peak shape and low retention time of the curcuminoids (Mudge et al. 2016; Peram et al. 2017). Usage of other types of columns were also reported, for example, RP-5NH2 (Khurana and Ho 1988) TSK-GEL ODS 80 TS (Inoue et al. 2008) and Chromolith column (Malasoni et al. 2013), but these columns do not show any advantage compared to those of C18 columns. For the first time, (Ali et al. 2014) used a phenyl column for resolving curcuminoids, and their results showed that this column is beneficial, as it can successfully separate curcuminoids at normal temperature, works under the acid-free condition and has better performance. Type of stationary phase and specific surface area are major factors that will affect separation of curcuminoids(Jadhav et al. 2007). Major indicators for poor separation of curcuminoids are elevation of the baseline along with broad tailing of peaks (Peram et al. 2017). The mobile phase is an important factor that affects the separation process in the HPLC system. Gradient elution mobile phase has shown better separation when compared to that of isocratic elution mobile phase. Due to the hydrophilic nature of curcuminoids, HPLC separation is mostly done on reverse phase (RP) silica phases using a mixture of water, acetonitrile, ethanol, and methanol (Jayaprakasha et al. 2002; Jadhav et al. 2007; Naidu et al. 2009). Several authors reported that addition of formic acid (0.1%) to water resulted in sharp and well-defined peaks (Peram et al. 2017). However, gradient elution mobile phase is a very complex mix of different solvents and need more experimentation to find the optimized combination (Naidu et al. 2009).
Recently many improved HPLC methods, which are validated as per the guidelines of International Conference on Harmonization (ICH), are reported (Wichitnithad et al. 2009; Ali et al. 2014). Most of the HPLC methods reported in the literature for quantification of curcuminoids had certain limitations such as high flow rates (Osorio-Tobón et al. 2016), long run times (Li et al. 2011), complicated gradient elution (Li et al. 2014a), buffer solutions in mobile phase and high limits of detection (Jangle and Thorat 2013). Besides, these methods are restricted for quantification of curcuminoids in one particular type of turmeric products (Peram et al. 2017). Thus, nowadays researchers are improving HPLC methods for rapid identification, differentiation, and quantification of curcuminoids (Lechtenberg et al. 2004). However, overcoming all the limitations mentioned above is not achieved yet.
Ultra high-performance liquid chromatography (UHPLC)
Although HPLC method is most convenient among the chromatographic methods for analysis of curcuminoids, there are several limitations to the HPLC approach (discussed in the previous section). For the better performance of chromatographic methods, several improvements have been made to existing technology. As a result, the UHPLC method has become one of the most promising technique in the field of fast chromatography (Taibon et al. 2015). The columns of UHPLC are modified with sub micrometer (sub-2 µm) particles as a stationary phase. These small particles are responsible for the radical increase in resolution per time because the rate of flow of mobile phase and performance of chromatography are inversely related to the size of UHPLC column particles (Jerkovich et al. 2005). Figure 5 shows the comparison of chromatograms of curcuminoids obtained from HPLC and UHPLC methods. Cheng et al. (2010) developed a new RP-UHPLC method and validated according to ICH guidelines. In this report, authors also compared UHPLC with HPLC for different parameters like sensitivity, time of analysis and efficiency. Overall, this method is suitable for quick and accurate quality control of curcuminoids. Avula et al. (2012) used UHPLC-UV–MS method for determination of curcuminoids in turmeric rhizome tougher with C18 column and acetonitrile:water as mobile phase. They achieved clear separation of curcuminoids in the column within 3.5 min only. This proves the method is rapid and accurate for quality control of curcuminoids. Jia et al. (2017) used UHPLC- QTOF –MS/MS (UHPLC quadrupole time of flight tandem mass spectrometry) to analyze curcuminoids in turmeric rhizome. According to them, separation of isomers that belong to the same type of curcuminoids could be done which is not possible by any other chromatographic methods. Although UHPLC method is simple, economical, rapid and most reliable method for analysis of curcuminoids for quality control, there are only a few reports available in the literature about UHPLC application.
Fig. 5.
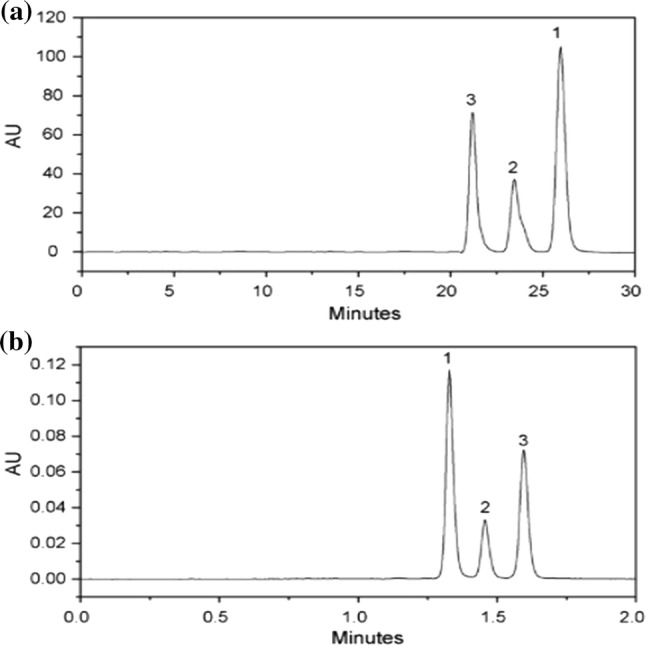
Comparison of chromatograms of (1) curcumin; (2) desmethoxycurcumin; (3) bis desmethoxycurcumin obtained from a HPLC and b UPLC (Cheng et al. 2010)
Liquid chromatography-mass spectrometry (LC–MS)
Although the above-mentioned methods can determine curcuminoids in turmeric powder or in its processed products, these methods cannot determine very low levels of curcuminoids. In this regard, the LC–MS method is a promising method that can detect very low levels of curcuminoids in any given sample matrix. In this method, LC follows the same principle as in the HPLC, but the coupling of the MS detector to LC makes these methods more efficient as compared to HPLC and HPTLC methods (Pitt 2009). Time-consuming chemical modifications can be eliminated by this method, which permits MS analysis of non-volatile, thermally labile, or charged molecules. A typical mass spectrum of standard curcuminoids is shown in Fig. 6. He et al. (1998) reported first LC–MS method for analysis of curcuminoids in turmeric rhizome. In this study, they have used electro-spray mass spectrometry and UV-diode-array to analyze curcuminoids however complete structural details were not reported. This is because of using a single dimensional LC–MS method with only one mode of ionization. Thus, using tandem mass spectrometry can overcome this problem. The fragmentation behavior of the three curcuminoids in ion trap LC–MS/MS was investigated by Jiang et al. (2006a) in both positive and negative mode electrospray ionization. They also used to sustain off-resonance irradiation (SORI) in a Fourier Transform Ion Cyclotron resonance (FTICR) mass spectrometer. In this study, the analysis of all three curcuminoids in turmeric rhizome was identified along with other minor curcuminoids, and their origins were given. Jiang et al. (2006b) used LC–ESI–MS/MS coupled to Diode Array Detection (DAD) to identify known and unknown diarylheptanoids in fresh turmeric rhizome extracts, and they identified 12 new diarylheptanoids. Many studies proved that metabolites of curcuminoids also have pharmacological activity similar to curcuminoids (Prasad et al. 2014). Thus, several methods were reported for determining curcuminoids metabolites content along with curcuminoids using LC–MS/MS in biological matrices (Table 2).
Fig. 6.
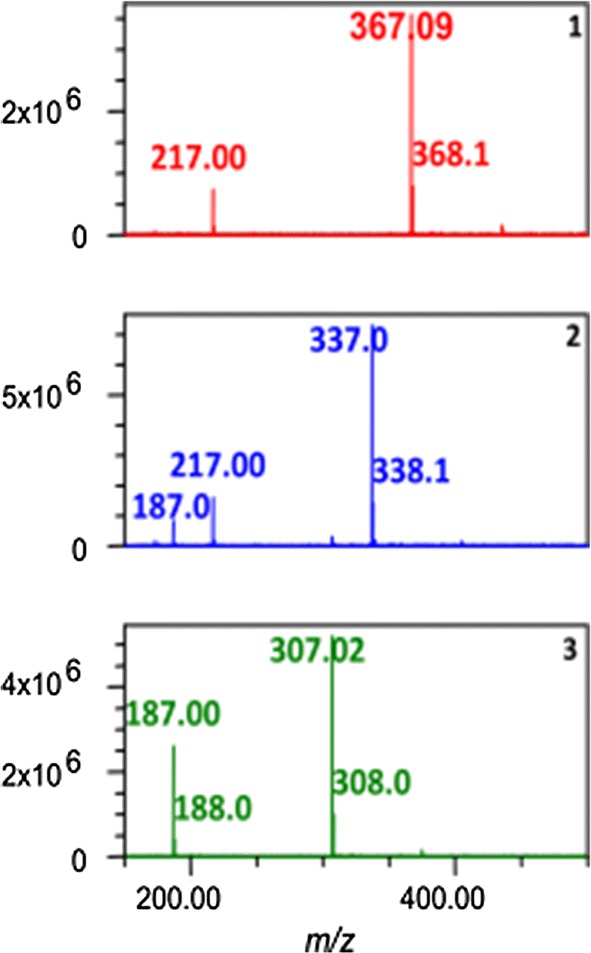
A typical mass spectrum of an of standard curcuminoids: (1) curcumin, (2) demethoxycurcumin and (3) bis-demethoxycurcumin (Avula et al. 2012)
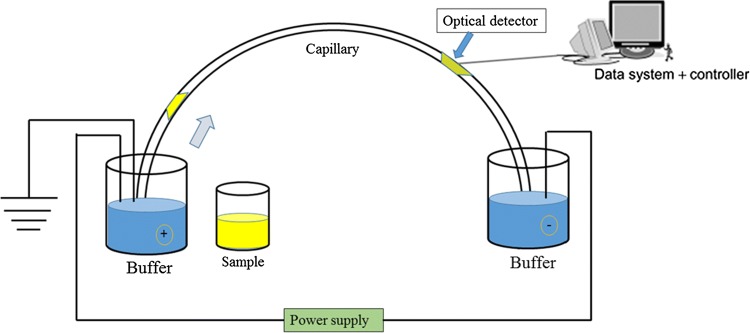
Capillary electrophoresis (CE)
Many chromatographic methods are available for the separation and analysis of curcuminoids (discussed in the previous section). Their primary disadvantages are either elaborated sample preparation and highly dependent on the sample matrix, or requirement of sophisticated detectors for analysis, especially in the case of LC–MS/MS. Thus, there is a requirement for more advanced methods, which could allow efficient separation and quantification of curcuminoids in the variety of sample matrices. CE method possesses many advantages such as rapid separation, inexpensive, low solvent and sample requirement for analysis (Sun et al. 2002).The main mechanism of CE is separation of components present in the sample based on different migration properties of electrically charged components through the capillary. The most general set up CE is shown in Fig. 7. It consists of a capillary tube fused with silica and the two ends of capillary tube placed in buffer reservoirs of inlet and outlet. However, there are some disadvantages with CE, such as small injection volume and low detection sensitivity due to the small inner diameter (Wu et al. 2018). For the determination of curcuminoids other CE method such as micellar electrokinetic chromatography (MEKC) and microemulsion electrokinetic chromatography have also been used coupled with different detectors (UV–VIS, PAD, and AD (Amperometric Detector)). Watnabe et al. (2000) developed a fast analysis MEKC method employing a high molecular mass surfactant. However, this method is highly dependent on the commercial availability of the micelle-forming agents. Sun et al. (2002) used the CE method together with AD for the determination of curcuminoids in turmeric samples. They successfully separated and estimated the curcuminoids under optimized conditions. Based on the pattern of curcuminoids, Lechtenberg et al. (2004) differentiated C. domestic and C. xanthorrhiza verities using CE-PAD method. Maráková et al. (2011) also used the same method with cyclodextrin as a complexing agent. This modification resulted in successful separation of curcuminoids and reduced the absorption of curcuminoids on capillary wall. Li et al. (2014a) observed the degradation of curcuminoids when they come in contact with alkaline buffer which is generally used in CE and MEKC methods. To avoid this problem Nhujak et al. (2006) tried acidic buffer (pH 2.5) in MEEKC method, although alkaline degradation is avoided they found that solubility of curcuminoids is poor in acidic and neutral buffers. Similarly, Li et al. (2014a) used the same method with electrically charged liquid as an oil phase which showed protective effects on analytes during analysis. In CE analysis, detectable optical path-length usually is very small (in micrometers), and for this reason, the sensitivity of this method is low if traditional absorbance detectors are used (Swinney and Bornhop 2000). To overcome this problem, Wu et al. (2018)developed the MEKC method coupled with LINF (laser-induced native fluorescence) detection to improve the sensitivity of the CE method. As per their results, LINF detector can sense the lowest level (LOD for C: 4.1; DMC: 2.6; BDMC: 0.4 ng/ml) curcuminoids among other CE detectors.
Fig. 7.
General schematic view of a capillary electrophoresis system with detector and control unit
Biosensors
Biosensor is a compact analytical device consisting of a biological receptor and a physicochemical transducer (Lu et al. 2017). They can facilitate rapid analysis and offer the potential for real-time monitoring and portability. Spectrofluorimetry and Electrochemical detection methods are the two extensively studied biosensor methods for determination of curcumin in various sample matrices which are summarized in Table 3.
Table 3.
Spectrofluorimetry and electrochemical methods for determination of curcumin
| Sample | Method type | Limit of detection | References |
|---|---|---|---|
| Spectrofluorimetric methods | |||
| Curry powder | Fluorimetric Method using the Enhancement of Mixed Micelle | 0.017 ng/mL | Wang et al. (2006) |
| Drug sample | Fluorescent carbon dots | 44.8 ng/mL | Shi et al. (2015) |
| Urine samples and | Nitrogen-doped carbon dots as fluorescent probe | 31.24 ng/mL | Zhang et al. (2015) |
| Urine | Boron and nitrogen co-doped carbon dots | 23.94 ng/mL | Bian et al. (2018) |
| Drinking water and the food samples | A nitrogen and phosphorus dual-doped carbon dots (NP-C dots) | 21.37 ng/mL | Liu et al. (2018a) |
| Food matrix | Nitrogen and chlorine dual-doped carbon nanodots | 14.00 ng/mL | Hu et al. (2019) |
| Electrochemical methods | |||
| Curcumin standard | Voltammetry-Glassy carbon electrode modified by carbon nanotubes | 1.84 ng/mL | Daneshgar et al. (2009) |
| Multicomponent spices | Voltammetry- glassy carbon electrode | 1.51 µg/mL | Ziyatdinova et al. (2012) |
| Curcumin standard | Cyclic Voltammetry- poly-Acid chrome blue K (poly- ACBK) film is synthesized on the surface of glassy carbon electrode (GCE) | 15.10 ng/mL | Peng et al. (2012) |
| Spice powder | Adsorptive stripping voltammetry- Carbon-Screen Printed Electrodes | 1.80 µg/mL | Wray et al. (2012) |
| Turmeric rhizomes | Voltammetric method- Graphene on Glassy carbon electrode | 11.05 ng/mL | Li et al. (2014c) |
| Turmeric extractive | Voltammetric method- Electrochemically reduced Graphene oxide | 36.84 ng/mL | Zhang et al. (2016) |
| Curcumin in plasma | Ru@Au nanoparticle decorated nitrogen and sulfur- functionalized reduced graphene oxide nanomaterials | 0.073 pg/mL | Kotan et al. (2016) |
Spectrofluorimetry
Spectrofluorimetry is attracting researchers with unique advantages, such as simple and fast analysis, high sensitivity and economical (Zhang et al. 2003). Fluorescent nanomaterials especially, carbon dots (CDs) have been widely used as chem-probe and biosensor due to its numerous advantages (Xu et al. 2016). Although fluorescent CDs have many advantages, their fluorescence quantum yield is low. Nowadays, to improve the optical properties of CDs, doping with heteroatoms such as nitrogen has been tried. However, the results are not promising compared to pure CDs (Ju and Chen 2014). Bian et al. (2018) synthesized boron and nitrogen co-doped CDs (BNCDs) by using microwave heating and citric acid monohydrate as carbon source. They proved florescence intensity BNCDs could be considerably quenched by curcumin. Using this property, they successfully estimated curcumin content in liquid samples. Liu et al. (2018a) synthesized double doped CDs with nitrogen and phosphorus and glucose as the carbon source. They tested the new sensor for detection of low levels of curcumin in aqueous solution which was able to detect very low levels of curcumin (21.37 ng/mL), which was the lowest detectable limit using spectrofluorimetry method till now.
Electrochemical analysis
The reductive properties of curcumin are due to its ability to rather easily donate electrons; therefore, curcumin can be determined by electrochemical methods. The electrochemical analytical technique has the advantages of simplicity and high sensitivity. However, the major drawback of this method is lack of inherent specificity. Therefore, development of proper separation systems can overcome the above said obstacle. Wray et al. (2012) successfully separated curcumin by chelating it with nickel (II) using the molecular functionality of diketone which contributed for its chelation with nickel (II). Through acidification of the precipitate, recovery of the curcumin from the nickel, the complex is achieved simply and quantitatively. Li et al. (2014c) designed a sensitive graphene-modified glassy carbon electrode for determination of low levels of curcumin. The currents measured in this method presented a good linear relationship with curcumin concentrations in the range of 5.0 × 10−8 to 3.0 × 10−6 mol/L, with a low detection limit of 3.0 × 10−8 mol/L. They also did a sensitivity test by spiking various possible interferences and proved that the proposed method had a reasonable selectivity. Dey et al. (2018) fabricated graphene oxide electrode and reduced graphene oxide modified on glassy carbon electrode respectively. The reported limit of detection using this sensor is 0.9 pM/mL which is the lowest to date for any plant-based component using any bio sensor method. However, these methods can only determine the concentration of curcumin only. The major limitation of these methods is that reported information is not available about other curcuminoids content. Comparison of different analytical methods based on detection limit of curcumin was given in Table 4.
Table 4.
Comparison of different analytical methods based on detection limit of curcumin
| Method | Lowest detection limit | References |
|---|---|---|
| Near-infrared spectroscopy | 10 ng/mL | Tanaka et al. (2008) |
| HPLC-fluorescence method | 15 ng/ml | Zhang et al. (2009) |
| UPLC | 40.66 pg/mL | Cheng et al. (2010) |
| UV–VIS spectrophotometric method | 39 ng/mL | Pundarikakshudu and Dave (2010) |
| LC–MS/MS | 1 ng/mL | Vijaya Saradhi et al. (2010) |
| HPTLC | 49 ng/mL | Gantait et al. (2011) |
| HPLC-electrochemical detection | 76.62 ng/mL | Long et al. (2014) |
| Reversed phase-HPLC phenyl column | 0.3 ng/mL | Ali et al. (2014) |
| micellar electrokinetic chromatography | 4.1 ng/mL | Wu et al. (2018) |
| Ru@Au nanoparticle decorated nitrogen and sulfur- functionalized reduced graphene oxide nanomaterials | 0.073 pg/mL | Kotan et al. (2016) |
Conclusion
Many research reports on turmeric have revealed that its bioactive compounds called curcuminoids have an exceptionally wide range of beneficial health properties. Due to these properties, many foods, pharmaceutical, and cosmetic industries are using curcuminoids as an ingredient in food formulations. Therefore, monitoring these bioactive components during processing and storage is critical for its quality control in turmeric powder and processed products.
Spectrophotometric is the simple method for curcumin estimation, but it is useful only when individual curcuminoids concentration is not an important quality parameter.
Infrared spectrometry method is rapid, nondestructive and best suits for quality control in industries.
Separation of curcuminoids with TLC method before quantification allows identification of low levels on curcuminoids without any interference with other compounds. However, mobile phase optimization, the broadness of spots, plate-to-plate variation, is major limitations for TLC and HPTLC methods.
The highest number of reports is available for estimation of curcuminoids using HPLC. Nevertheless, these methods are highly dependent on sample matrix type.
For determining very low levels of curcuminoids in any sample matrices, LC–MS/MS is the best choice.
Spectrofluorimetry and Electrochemical methods have been gaining attention because of their advantages. However, like the spectrophotometric method, these methods cannot give the relative composition of each curcuminoids.
Overall, researchers are working hard to improve the efficiency of instrumental analytical methods by focusing on sample preparation procedures, which allows a single analytical method to cover a wide range of sample matrices, and give low levels of detection.
Acknowledgements
The authors appreciate R Sudarshan Reddy (Research Scholar, Agricultural and Food Engineering Department, IIT Kharagpur, India) for providing valuable suggestions.
Compliance with ethical standards
Conflict of interest
All authors declare no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ali I, Haque A, Saleem K. Separation and identification of curcuminoids in turmeric powder by HPLC using phenyl column. Anal Methods. 2014;6:2526–2536. doi: 10.1039/c3ay41987h. [DOI] [Google Scholar]
- Ansari MJ, Ahmad S, Kohli K, et al. Stability-indicating HPTLC determination of curcumin in bulk drug and pharmaceutical formulations. J Pharm Biomed Anal. 2005;39:132–138. doi: 10.1016/j.jpba.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Attimarad M, Mueen Ahmed KK, Aldhubaib BE, Harsha S. High-performance thin layer chromatography: a powerful analytical technique in pharmaceutical drug discovery. Pharm Methods. 2011;2:71–75. doi: 10.4103/2229-4708.84436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avula B, Wang Y-H, Khan IA. Quantitative determination of curcuminoids from the roots of Curcuma longa, Curcuma species and dietary supplements using an UPLC-UV-MS method. J Chromatogr Sep Tech. 2012;03:3–8. doi: 10.4172/2157-7064.1000120. [DOI] [Google Scholar]
- Baghel US, Nagar AS, Pannu M, et al. HPLC and HPTLC methods for simultaneous estimation of quercetin and curcumin in polyherbal formulation. Indian J Pharm Sci. 2017;79:197–203. doi: 10.4172/pharmaceutical-sciences.1000217. [DOI] [Google Scholar]
- Bian W, Wang X, Wang Y, et al. Boron and nitrogen co-doped carbon dots as a sensitive fluorescent probe for the detection of curcumin. Luminescence. 2018;33:174–180. doi: 10.1002/bio.3390. [DOI] [PubMed] [Google Scholar]
- Chen W, Fan-Havard P, Yee LD, et al. A liquid chromatography–tandem mass spectrometric method for quantification of curcumin-O-glucuronide and curcumin in human plasma. J Chromatogr B. 2012;900:89–93. doi: 10.1016/J.JCHROMB.2012.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Weijun K, Yun L, et al. Development and validation of UPLC method for quality control of Curcuma longa Linn.: fast simultaneous quantitation of three curcuminoids. J Pharm Biomed Anal. 2010;53:43–49. doi: 10.1016/j.jpba.2010.03.021. [DOI] [PubMed] [Google Scholar]
- Daneshgar P, Norouzi P, Moosavi-Movahedi AA, et al. Fabrication of carbon nanotube and dysprosium nanowire modified electrodes as a sensor for determination of curcumin. J Appl Electrochem. 2009;39:1983–1992. doi: 10.1007/s10800-009-9908-0. [DOI] [Google Scholar]
- Dave HN, Mashru RC, Thakkar AR. Simultaneous determination of salbutamol sulphate, bromhexine hydrochloride and etofylline in pharmaceutical formulations with the use of four rapid derivative spectrophotometric methods. Anal Chim Acta. 2007;597:113–120. doi: 10.1016/j.aca.2007.06.035. [DOI] [PubMed] [Google Scholar]
- Dey N, Devasena T, Sivalingam T. A Comparative evaluation of graphene oxide based materials for Electrochemical non-enzymatic sensing of curcumin. Mater Res Express. 2018;5:025406. doi: 10.1088/2053-1591/aaaa78. [DOI] [Google Scholar]
- Erpina E, Rafi M, Darusman LK, et al. Simultaneous quantification of curcuminoids and xanthorrhizol in Curcuma xanthorrhiza by high-performance liquid chromatography. J Liq Chromatogr Relat Technol. 2017;40:635–639. doi: 10.1080/10826076.2017.1343729. [DOI] [Google Scholar]
- Gantait A, Barman T, Mukherjee PK. Validated method for estimation of curcumin in turmeric powder. Indian J Tradit Knowl. 2011;10:247–250. [Google Scholar]
- Green CE, Hibbert SL, Bailey-Shaw YA, et al. Extraction, processing, and storage effects on curcuminoids and oleoresin yields from Curcuma longa L. grown in Jamaica. J Agric Food Chem. 2008;56:3664–3670. doi: 10.1021/jf073105v. [DOI] [PubMed] [Google Scholar]
- He X-G, Lin L-Z, Lian L-Z, Lindenmaier M. Liquid chromatography–electrospray mass spectrometric analysis of curcuminoids and sesquiterpenoids in turmeric (Curcuma longa) J Chromatogr A. 1998;818:127–132. doi: 10.1016/S0021-9673(98)00540-8. [DOI] [Google Scholar]
- Hu Q, Gao L, Rao S, et al. Nitrogen and chlorine dual-doped carbon nanodots for determination of curcumin in food matrix via inner filter effect. Food Chem. 2019;280:195–202. doi: 10.1016/J.FOODCHEM.2018.12.050. [DOI] [PubMed] [Google Scholar]
- Hwang K-W, Son D, Jo H-W, et al. Levels of curcuminoid and essential oil compositions in turmerics (Curcuma longa L.) grown in Korea. Appl Biol Chem. 2016;59:209–215. doi: 10.1007/s13765-016-0156-9. [DOI] [Google Scholar]
- Inoue K, Nomura C, Ito S, et al. Purification of curcumin, demethoxycurcumin, and bisdemethoxycurcumin by high-speed countercurrent chromatography. J Agric Food Chem. 2008;56:9328–9336. doi: 10.1021/jf801815n. [DOI] [PubMed] [Google Scholar]
- Jadhav B-K, Mahadik K-R, Paradkar A-R. Development and validation of improved reversed phase-HPLC method for simultaneous determination of curcumin, demethoxycurcumin and bis-demethoxycurcumin. Chromatographia. 2007;65:483–488. doi: 10.1365/s10337-006-0164-8. [DOI] [Google Scholar]
- Janben A, Gole T. Thin-layer chromatographic determination of curcumine (turmeric) in spices. Chromatographia. 1984;18:546–549. doi: 10.1007/BF02265692. [DOI] [Google Scholar]
- Jangle RD, Thorat BN. Reversed-phase high-performance liquid chromatography method for analysis of curcuminoids and curcuminoid-loaded liposome formulation. Indian J Pharm Sci. 2013;75:60–66. doi: 10.4103/0250-474X.117435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaprakasha GK, Rao LJM, Sakariah KK. Improved HPLC method for the determination of curcumin, demethoxycurcumin, and bisdemethoxycurcumin. J Agric Food Chem. 2002;50:3668–3672. doi: 10.1021/jf025506a. [DOI] [PubMed] [Google Scholar]
- Jayaprakasha Jagan Mohan, Rao L, Sakariah KK. Chemistry and biological activities of C. longa. Trends Food Sci Technol. 2005;16:533–548. doi: 10.1016/j.tifs.2005.08.006. [DOI] [Google Scholar]
- Jerkovich AD, Mellors JS, Thompson JW, Jorgenson JW. Linear velocity surge caused by mobile-phase compression as a source of band broadening in isocratic ultrahigh-pressure liquid chromatography. Anal Chem. 2005;77:6292–6299. doi: 10.1021/ac0504924. [DOI] [PubMed] [Google Scholar]
- Jia S, Du Z, Song C, et al. Identification and characterization of curcuminoids in turmeric using ultra-high performance liquid chromatography-quadrupole time of flight tandem mass spectrometry. J Chromatogr A. 2017;1521:110–122. doi: 10.1016/j.chroma.2017.09.032. [DOI] [PubMed] [Google Scholar]
- Jiang H, Somogyi Á, Jacobsen NE, et al. Analysis of curcuminoids by positive and negative electrospray ionization and tandem mass spectrometry. Rapid Commun Mass Spectrom. 2006;20:1001–1012. doi: 10.1002/rcm.2401. [DOI] [PubMed] [Google Scholar]
- Jiang H, Timmermann BN, Gang DR. Use of liquid chromatography–electrospray ionization tandem mass spectrometry to identify diarylheptanoids in turmeric (Curcuma longa L.) rhizome. J Chromatogr A. 2006;1111:21–31. doi: 10.1016/J.CHROMA.2006.01.103. [DOI] [PubMed] [Google Scholar]
- Ju J, Chen W. Synthesis of highly fluorescent nitrogen-doped graphene quantum dots for sensitive, label-free detection of Fe(III) in aqueous media. Biosens Bioelectron. 2014;58:219–225. doi: 10.1016/J.BIOS.2014.02.061. [DOI] [PubMed] [Google Scholar]
- Jupille TH, Perry JA. High-performance thin-layer chromatography: a review of principles, practice, and potential. C R C Crit Rev Anal Chem. 1977;6:325–359. doi: 10.1080/10408347708542695. [DOI] [Google Scholar]
- Kadam PV, Bhingare CL, Nikam RY, Pawar SA. Development and validation of UV Spectrophotometric method for the estimation of curcumin in cream formulation. Pharm Methods. 2013;4:43–45. doi: 10.1016/j.phme.2013.08.002. [DOI] [Google Scholar]
- Kar S, Tudu B, Bag AK, Bandyopadhyay R. Application of near-infrared spectroscopy for the detection of metanil yellow in turmeric powder. Food Anal Methods. 2018;11:1291–1302. doi: 10.1007/s12161-017-1106-9. [DOI] [Google Scholar]
- Kasemsumran S, Apiwatanapiwat W, Suttiwijitpukdee N, et al. Evaluation of Fourier transform-near infraredspectroscopic measurements for the quantification of curcumin in turmeric herbal medicines. J Near Infrared Spectrosc. 2014;22:113–120. doi: 10.1255/jnirs.1103. [DOI] [Google Scholar]
- Khurana A, Ho C-T. High performance liquid chromatographic analysis of curcuminoids and their photo-oxidative decomposition compounds in Curcuma longa L. J Liq Chromatogr. 1988;11:2295–2304. doi: 10.1080/01483918808067200. [DOI] [Google Scholar]
- Kim Y-J, Lee HJ, Shin H-S, Shin Y. Near-infrared reflectance spectroscopy as a rapid and non-destructive analysis tool for curcuminoids in turmeric. Phytochem Anal. 2014;25:445–452. doi: 10.1002/pca.2514. [DOI] [PubMed] [Google Scholar]
- Kotan G, Kardaş F, Yokuş ÖA, et al. A novel determination of curcumin via Ru@Au nanoparticle decorated nitrogen and sulfur-functionalized reduced graphene oxide nanomaterials. Anal Methods. 2016;8:401–408. doi: 10.1039/C5AY02950C. [DOI] [Google Scholar]
- Kunati SR, Yang S, William BM, Xu Y. An LC–MS/MS method for simultaneous determination of curcumin, curcumin glucuronide and curcumin sulfate in a phase II clinical trial. J Pharm Biomed Anal. 2018;156:189–198. doi: 10.1016/J.JPBA.2018.04.034. [DOI] [PubMed] [Google Scholar]
- Lechtenberg M, Quandt B, Nahrstedt A. Quantitative determination of curcuminoids in curcuma rhizomes and rapid differentiation ofCurcuma domestica Val. and Curcuma xanthorrhiza Roxb. by capillary electrophoresis. Phytochem Anal. 2004;15:152–158. doi: 10.1002/pca.759. [DOI] [PubMed] [Google Scholar]
- Lee JH, Choung M-G. Determination of curcuminoid colouring principles in commercial foods by HPLC. Food Chem. 2011;124:1217–1222. doi: 10.1016/J.FOODCHEM.2010.07.049. [DOI] [Google Scholar]
- Li R, Xiang C, Ye M, et al. Qualitative and quantitative analysis of curcuminoids in herbal medicines derived from Curcuma species. Food Chem. 2011;126:1890–1895. doi: 10.1016/j.foodchem.2010.12.014. [DOI] [PubMed] [Google Scholar]
- Li F, Liu R, Yang F, et al. Determination of three curcuminoids in Curcuma longa by microemulsion electrokinetic chromatography with protective effects on the analytes. Anal Methods. 2014;6:2566–2571. doi: 10.1039/C3AY42106F. [DOI] [Google Scholar]
- Li H-X, Zhang H-L, Zhang N, et al. Isolation of three curcuminoids for stability and simultaneous determination of only using one single standard substance in turmeric colour principles by HPLC with ternary gradient system. LWT Food Sci Technol. 2014;57:446–451. doi: 10.1016/J.LWT.2013.11.020. [DOI] [Google Scholar]
- Li K, Li Y, Yang L, et al. The electrochemical characterization of curcumin and its selective detection in Curcuma using a graphene-modified electrode. Anal Methods. 2014;6:7801–7808. doi: 10.1039/C4AY01492H. [DOI] [Google Scholar]
- Liu Y, Gong X, Dong W, et al. Nitrogen and phosphorus dual-doped carbon dots as a label-free sensor for Curcumin determination in real sample and cellular imaging. Talanta. 2018;183:61–69. doi: 10.1016/J.TALANTA.2018.02.060. [DOI] [PubMed] [Google Scholar]
- Liu Y, Siard M, Adams A, et al. Simultaneous quantification of free curcuminoids and their metabolites in equine plasma by LC-ESI–MS/MS. J Pharm Biomed Anal. 2018;154:31–39. doi: 10.1016/j.jpba.2018.03.014. [DOI] [PubMed] [Google Scholar]
- Long Y, Zhang W, Wang F, Chen Z. Simultaneous determination of three curcuminoids in Curcuma longa L. by high performance liquid chromatography coupled with electrochemical detection. J Pharm Anal. 2014;4:325–330. doi: 10.1016/J.JPHA.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Xia Y, Liu G, et al. A review of methods for detecting melamine in food samples. Crit Rev Anal Chem. 2017;47:51–66. doi: 10.1080/10408347.2016.1176889. [DOI] [PubMed] [Google Scholar]
- Malasoni R, Srivastava A, Pandey RR, et al. Development and validation of improved HPLC method for the quantitative determination of curcuminoids in herbal medicament. J Sci Ind Res (India) 2013;72:88–91. [Google Scholar]
- Maráková K, Mikuš P, Pieštanský J, Havránek E. Determination of curcuminoids in substances and dosage forms by cyclodextrin-mediated capillary electrophoresis with diode array detection. Chem Pap. 2011;65:398–405. doi: 10.2478/s11696-011-0043-0. [DOI] [Google Scholar]
- Meng F-C, Zhou Y-Q, Ren D, et al (2018) Turmeric: a review of its chemical composition, quality control, bioactivity, and pharmaceutical application. In: Natural and artificial flavoring agents and food dyes. Elsevier Inc., pp 299–350
- Mudge E, Chan M, Venkataraman S, Brown PN. Curcuminoids in turmeric roots and supplements: method optimization and validation. Food Anal Methods. 2016;9:1428–1435. doi: 10.1007/s12161-015-0326-0. [DOI] [Google Scholar]
- Naidu MM, Shyamala BN, Manjunatha JR, et al. Simple HPLC method for resolution of curcuminoids with antioxidant potential. J Food Sci. 2009;74:C312–C318. doi: 10.1111/j.1750-3841.2009.01124.x. [DOI] [PubMed] [Google Scholar]
- Nelson KM, Dahlin JL, Bisson J, et al. The essential medicinal chemistry of curcumin. J Med Chem. 2017;60:1620–1637. doi: 10.1021/acs.jmedchem.6b00975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nhujak T, Saisuwan W, Srisa-art M, Petsom A. Microemulsion electrokinetic chromatography for separation and analysis of curcuminoids in turmeric samples. J Sep Sci. 2006;29:666–676. doi: 10.1002/jssc.200500333. [DOI] [PubMed] [Google Scholar]
- Osorio-Tobón JF, Carvalho PIN, Barbero GF, et al. Fast analysis of curcuminoids from turmeric (Curcuma longa L.) by high-performance liquid chromatography using a fused-core column. Food Chem. 2016;200:167–174. doi: 10.1016/J.FOODCHEM.2016.01.021. [DOI] [PubMed] [Google Scholar]
- Paramasivam M, Poi R, Banerjee H, Bandyopadhyay A. High-performance thin layer chromatographic method for quantitative determination of curcuminoids in Curcuma longa germplasm. Food Chem. 2009;113:640–644. doi: 10.1016/j.foodchem.2008.07.051. [DOI] [Google Scholar]
- Pathania V, Gupta AP, Singh B. Improved HPTLC method for determination of curcuminoids from Curcuma longa. J Liq Chromatogr Relat Technol. 2006;29:877–887. doi: 10.1080/10826070500531417. [DOI] [Google Scholar]
- Peng J, Nong K, Cen L. Electropolymerization of Acid chrome blue K on glassy carbon electrode for the determination of curcumin. J Chinese Chem Soc. 2012;59:1415–1420. doi: 10.1002/jccs.201200085. [DOI] [Google Scholar]
- Peram MR, Jalalpure SS, Joshi SA, et al. Single robust RP-HPLC analytical method for quantification of curcuminoids in commercial turmeric products, Ayurvedic medicines, and nanovesicular systems. J Liq Chromatogr Relat Technol. 2017;40:487–498. doi: 10.1080/10826076.2017.1329742. [DOI] [Google Scholar]
- Phattanawasin P, Sotanaphun U, Sriphong L. Validated TLC-image analysis method for simultaneous quantification of curcuminoids in Curcuma longa. Chromatographia. 2009;69:397–400. doi: 10.1365/s10337-008-0893-y. [DOI] [Google Scholar]
- Pitt JJ. Principles and applications of liquid chromatography–mass spectrometry in clinical biochemistry. Clin Biochem Rev. 2009;30:19–34. [PMC free article] [PubMed] [Google Scholar]
- Prasad S, Tyagi AK, Aggarwal BB. Recent developments in delivery, bioavailability, absorption and metabolism of curcumin: the golden pigment from golden spice. Cancer Res Treat. 2014;46:2–18. doi: 10.4143/crt.2014.46.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priyadarsini KI. The chemistry of curcumin: from extraction to therapeutic agent. Molecules. 2014;19:20091–20112. doi: 10.3390/molecules191220091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pundarikakshudu K, Dave HN. Simultaneous determination of curcumin and berberine in their pure form and from the combined extracts of Curcuma longa and Berberis aristata. Int J Appl Sci Eng. 2010;8:19–26. [Google Scholar]
- Rao DS, Sekhara NC, Satyanarayana MN, Srinivasan M. Effect of curcumin on serum and liver cholesterol levels in the rat. J Nutr. 1970;100:1307–1315. doi: 10.1093/jn/100.11.1307. [DOI] [PubMed] [Google Scholar]
- Roggo Y, Chalus P, Maurer L, et al. A review of near infrared spectroscopy and chemometrics in pharmaceutical technologies. J Pharm Biomed Anal. 2007;44:683–700. doi: 10.1016/J.JPBA.2007.03.023. [DOI] [PubMed] [Google Scholar]
- Rohman A. Mini review analysis of curcuminoids in food and pharmaceutical products. Int Food Res J. 2012;19:19–27. [Google Scholar]
- Scotter MJ. Synthesis and chemical characterisation of curcuminoid colouring principles for their potential use as HPLC standards for the determination of curcumin colour in foods. LWT Food Sci Technol. 2009;42:1345–1351. doi: 10.1016/J.LWT.2009.03.014. [DOI] [Google Scholar]
- Shi Y, Li C, Liu S, et al. Facile synthesis of fluorescent carbon dots for determination of curcumin based on fluorescence resonance energy transfer. RSC Adv. 2015;5:64790–64796. doi: 10.1039/C5RA13404H. [DOI] [Google Scholar]
- Sotanaphun U, Phattanawasin P, Sriphong L. Application of Scion image software to the simultaneous determination of curcuminoids in turmeric (Curcuma longa) Phytochem Anal. 2009;20:19–23. doi: 10.1002/pca.1086. [DOI] [PubMed] [Google Scholar]
- Sun X, Gao C, Cao W, et al. Capillary electrophoresis with amperometric detection of curcumin in Chinese herbal medicine pretreated by solid-phase extraction. J Chromatogr A. 2002;962:117–125. doi: 10.1016/S0021-9673(02)00509-5. [DOI] [PubMed] [Google Scholar]
- Swinney K, Bornhop DJ. Electrophoresis. Hoboken: Wiley-Blackwell; 2000. Detection in capillary electrophoresis; pp. 1239–1250. [DOI] [PubMed] [Google Scholar]
- Syed HK, Bin Liew K, Loh GOK, Peh KK. Stability indicating HPLC–UV method for detection of curcumin in Curcuma longa extract and emulsion formulation. Food Chem. 2015;170:321–326. doi: 10.1016/J.FOODCHEM.2014.08.066. [DOI] [PubMed] [Google Scholar]
- Taha MN, Krawinkel MB, Morlock GE. High-performance thin-layer chromatography linked with (bio)assays and mass spectrometry—A suited method for discovery and quantification of bioactive components? Exemplarily shown for turmeric and milk thistle extracts. J Chromatogr A. 2015;1394:137–147. doi: 10.1016/J.CHROMA.2015.03.029. [DOI] [PubMed] [Google Scholar]
- Taibon J, Sturm S, Seger C, et al. Quantitative assessment of destruxins from strawberry and maize in the lower parts per billion range: combination of a QuEChERS-based extraction protocol with a fast and selective UHPLC-QTOF-MS assay. J Agric Food Chem. 2015;63:5707–5713. doi: 10.1021/acs.jafc.5b01562. [DOI] [PubMed] [Google Scholar]
- Tan S, Rupasinghe TWT, Tull DL, et al. Liquid–liquid extraction and liquid chromatography–mass spectrometry detection of curcuminoids from bacterial culture medium. J Chromatogr B. 2015;988:116–120. doi: 10.1016/j.jchromb.2015.02.024. [DOI] [PubMed] [Google Scholar]
- Tanaka Ken, Kuba Yosiaki, Sasaki Tetsuro, Hiwatashi Fumiko, Komatsu Katsuko. Quantitation of Curcuminoids in Curcuma Rhizome by Near-infrared Spectroscopic Analysis. Journal of Agricultural and Food Chemistry. 2008;56(19):8787–8792. doi: 10.1021/jf801338e. [DOI] [PubMed] [Google Scholar]
- Taylor SJ, McDowell IJ. Determination of the curcuminoid pigments in turmeric (Curcuma-Domestica Val) by reversed-phase high-performance liquid-chromatography. Chromatographia. 1992;34:73–77. doi: 10.1007/bf02290463. [DOI] [Google Scholar]
- Tonnesen HH, Karlsen J. High-performance liquid chromatography of curcumin and related compounds. J Chromatogr A. 1983;259:367–371. doi: 10.1016/S0021-9673(01)88022-5. [DOI] [Google Scholar]
- Vijaya Saradhi UVR, Ling Y, Wang J, et al. A liquid chromatography–tandem mass spectrometric method for quantification of curcuminoids in cell medium and mouse plasma. J Chromatogr B. 2010;878:3045–3051. doi: 10.1016/J.JCHROMB.2010.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Wu X, Wang F, et al. The sensitive fluorimetric method for the determination of curcumin using the enhancement of mixed micelle. J Fluoresc. 2006;16:53–59. doi: 10.1007/s10895-005-0025-0. [DOI] [PubMed] [Google Scholar]
- Watnabe T, Mazumder TK, Yamamoto A, et al. Separation and determination of curcuminoids in turmeric samples by miceller electrokinetic chromatography with a high molecular mass surfactant. Nippon SHOKUHIN KAGAKU KOGAKU KAISHI. 2000;47:780–786. doi: 10.3136/nskkk.47.780. [DOI] [Google Scholar]
- Wichitnithad W, Jongaroonngamsang N, Pummangura S, Rojsitthisak P. A simple isocratic HPLC method for the simultaneous determination of curcuminoids in commercial turmeric extracts. Phytochem Anal. 2009;20:314–319. doi: 10.1002/pca.1129. [DOI] [PubMed] [Google Scholar]
- Wray DM, Batchelor-McAuley C, Compton RG. Selective curcuminoid separation and detection via nickel complexation and adsorptive stripping voltammetry. Electroanalysis. 2012;24:2244–2248. doi: 10.1002/elan.201200560. [DOI] [Google Scholar]
- Wu C, Wang W, Quan F, et al. Sensitive analysis of curcuminoids via micellar electrokinetic chromatography with laser-induced native fluorescence detection and mixed micelles-induced fluorescence synergism. J Chromatogr A. 2018;1564:207–213. doi: 10.1016/j.chroma.2018.06.012. [DOI] [PubMed] [Google Scholar]
- Xu Q, Kuang T, Liu Y, et al. Heteroatom-doped carbon dots: synthesis, characterization, properties, photoluminescence mechanism and biological applications. J Mater Chem B. 2016;4:7204–7219. doi: 10.1039/C6TB02131J. [DOI] [PubMed] [Google Scholar]
- Zhang SZ, Xie JW, Liu CS. Microenvironmental properties and chiral discrimination abilities of bile salt micelles by fluorescence probe technique. Anal Chem. 2003;75:91–97. doi: 10.1021/ac020373d. [DOI] [PubMed] [Google Scholar]
- Zhang J, Jinnai S, Ikeda R, et al. A simple HPLC-fluorescence method for quantitation of curcuminoids and its application to turmeric products. Anal Sci. 2009;25:385–388. doi: 10.2116/analsci.25.385. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Zhang C, Li Z, et al. Nitrogen-doped carbon dots as fluorescent probe for detection of curcumin based on the inner filter effect. RSC Adv. 2015;5:95054–95060. doi: 10.1039/C5RA18176C. [DOI] [Google Scholar]
- Zhang D, Ouyang X, Ma J, et al. Electrochemical behavior and voltammetric determination of curcumin at electrochemically reduced graphene oxide modified glassy carbon electrode. Electroanalysis. 2016;28:749–756. doi: 10.1002/elan.201500494. [DOI] [Google Scholar]
- Ziyatdinova GK, Nizamova AM, Budnikov HC. Voltammetric determination of curcumin in spices. J Anal Chem. 2012;67:591–594. doi: 10.1134/S1061934812040132. [DOI] [Google Scholar]