Abstract
In infants and children, the spleen is involved in many pathological processes, whether those processes are isolated or related to systemic diseases. Pathology of the pediatric spleen includes congenital anomalies, splenomegaly, trauma, focal lesions, infarction, and tumors. Ultrasonography (US) is a widely available, fast, noninvasive imaging technique to assess the size, shape, and position of the spleen, as well as to define splenic echotexture. US is capable of screening for splenic disorders without the risk of ionizing radiation; it is the initial imaging examination performed to evaluate suspected splenic pathology, providing clinicians with helpful decisional support. US plays an important role in the detection of even very small amounts of hemoperitoneum, a herald of significant abdominal organ injury, in pediatric blunt abdominal trauma. Moreover, contrast-enhanced US may allow early detection of splenic injuries, ideally minimizing children’s risk from radiation exposure. This pictorial essay illustrates the normal ultrasound appearance of the pediatric spleen and the sonographic findings which may guide clinicians to a correct diagnosis of pathologic conditions.
Keywords: Pediatric sonography, Congenital anomalies, Splenomegaly, Focal splenic lesions, Trauma, Tumors
Sommario
In età pediatrica la milza è coinvolta in molti processi patologici, che possono manifestarsi come entità isolate o possono essere secondarie a malattie sistemiche. I quadri patologici splenici includono anomalie congenite, splenomegalia, trauma, lesioni focali, infarti e tumori. L’ecografia è una metodica largamente disponibile, veloce e non invasiva che permette di determinare le dimensioni, la posizione e la morfologia della milza e di caratterizzarne inoltre l’ecotessitura parenchimale. L’esame ecografico permette di identificare precocemente le alterazioni spleniche senza il rischio di radiazioni ionizzanti e viene per questo motivo utilizzato come metodica di imaging iniziale per valutare quadri sospetti di patologia splenica fornendo ai clinici un utile supporto decisionale. Nei traumi addominali chiusi, l’ecografia svolge un ruolo importante nell’identificare quantità persino esigue di emoperitoneo, reperto associato a significative lesioni degli organi addominali. Inoltre, l’utilizzo dell’ecografia con mezzo di contrasto potrebbe permettere di identificare precocemente lesioni traumatiche della milza, e ridurre, idealmente, al minimo le radiazioni nei pazienti pediatrici. Questo pictorial essay illustra il normale aspetto ecografico della milza in età pediatrica ed i reperti ecografici che possono condurre alla corretta diagnosi di processi patologici.
Introduction
The spleen is a semi oval-shaped lymphoreticular organ situated in the left upper abdomen with its long axis obliquely oriented inferiorly, anteriorly, and laterally. The spleen is completely enveloped by the peritoneum, with folds that meet and form the suspensory ligaments of the spleen: the phrenicosplenic, gastrosplenic, splenorenal and splenocolic ligaments. The main clinical indications for evaluating the pediatric spleen with US include congenital anomalies, splenomegaly, trauma, infarction, focal lesions, and malignant tumors.
Ultrasonographic aspects and techniques
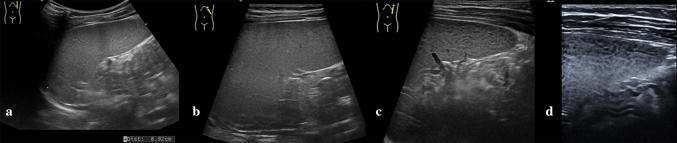
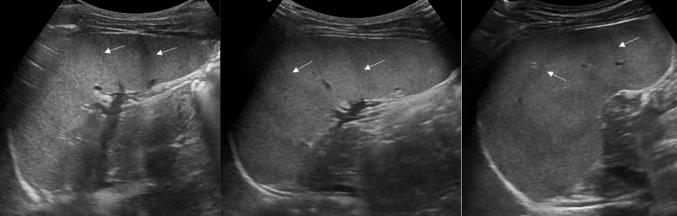
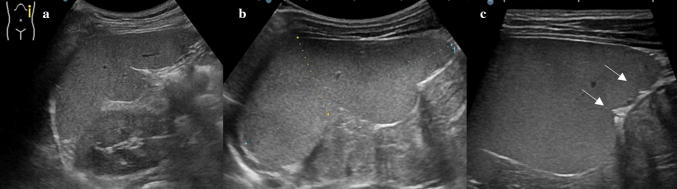
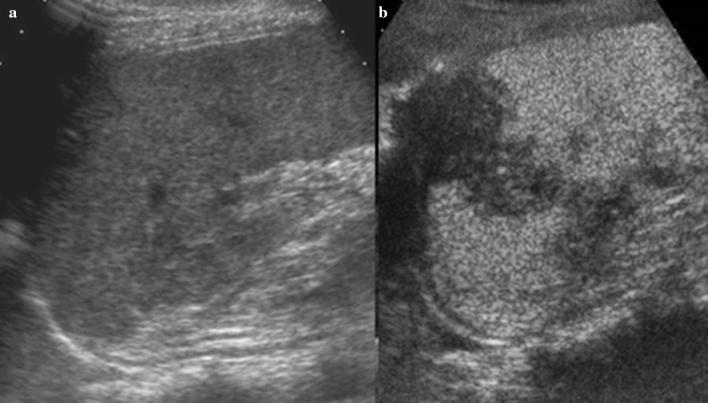
Ultrasonography (US) is the first imaging method used to assess and measure the spleen in the examination of neonates and children [1, 2]. The easy method of scanning the spleen is to place patients in the supine or slightly right lateral decubitus position and place the probe on the posterior axillary line in one of the lower left intercostal spaces. Longitudinal and transverse dimensions are measured using a coronal or oblique coronal view that includes the hilum. The length of a normal spleen depends on age and body size [3, 4]. Normal ranges of pediatric spleen dimensions determined by US are available in the literature (Table 1) [5]. The normal appearance of splenic parenchyma is homogeneous and uniform, with mid- to low-level fine tissue echotexture, and relatively hypervascular on color Doppler imaging. More recently, some authors have focused on the distinctive features of normal splenic echotexture in children. It has been proven that splenic echotexture can appear rather heterogeneous when high-frequency transducers are used (Fig. 1). In particular, high-frequency transducer US can depict nodules (“reticulonodular patterns”) in the splenic parenchyma of children between the ages of 1 and 5, which likely represent white-pulp lymphoid follicles [6]. Another recent study documented an ultrasound pattern of bands of hypoechogenicity distributed throughout the spleen, which resembles the zebra spleen pattern in the arterial phase of enhancement during CT and MRI (Fig. 2). This “zebra pattern” was found in 5% of the children evaluated in the study and they had no evidence of splenic abnormalities [7]. During US, these patterns should be considered to be normal findings, and not be misinterpreted as pathology.
Table 1.
Mean values of splenic size (mm) in children according to age (month) [5]
| Age versus longitudinal dimensions of spleen | |
|---|---|
| Age range (month) | Longitudinal dimensions (mm) |
| 1–3 | 53 |
| 4–6 | 59 |
| 7–9 | 63 |
| 12–30 | 70 |
| 36–59 | 75 |
| 60–83 | 84 |
| 84–107 | 85 |
| 108–131 | 86 |
| 132–155 | 97 |
| 156–179 | 101 |
| 180–200 | 101 |
Fig. 1.
Normal splenic appearances at transducers of different frequencies. Gray-scale images acquired with: a a convex array at 5.0 MHz b a linear array in trapezoidal format at 7.5 MHz c a linear array at 9.0 MHz and d at 14.0 MHz
Fig. 2.
The zebra pattern of splenic parenchyma. Longitudinal sonograms of spleens from different patients, obtained with curved-array transducer, show bands of hypoechogenicity (white arrows)
Congenital anomalies
The fetal spleen is divided into lobules, which normally fuse into one organ before birth. The incomplete or missing fusion of the primordial splenic buds may lead to congenital splenic variations such as accessory spleens, polysplenia, septations, lobulations and notches [8].
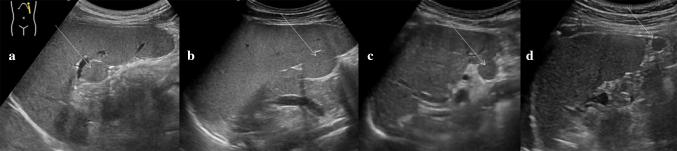
Accessory spleens are frequently observed (appearing in 10–30% of the population) and consist of small round or oval masses of normal splenic tissue separated from the main body of the spleen, with an analogous echotexture. These spleens range from one to six in number, with sizes varying from a few millimeters to several centimeters. Accessory spleens are usually found near the splenic hilum, but they may also be detected along the course of the splenic vessels or in the pancreatic tail (Fig. 3) [9]. Accessory spleens acquire a clinical significance in patients who have undergone previous splenectomies for hematological disease. In these patients, residual accessory splenic tissue can increase significantly, resulting in compensatory hypertrophy (Fig. 4) [10].
Fig. 3.
Accessory spleens. Coronal oblique ultrasound images, from different patients, show small ovoid nodules (white arrows) a, b near the splenic hilum and c, d at the lower splenic pole
Fig. 4.
Hypertrophied accessory spleen a Gray scale and b color Doppler images show a compensatory hypertrophy of an accessory spleen secondary to a previous splenectomy
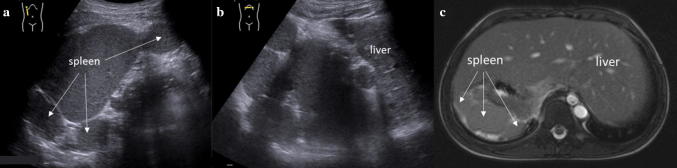
Polysplenia is a congenital syndrome characterized by multiple, similarly-sized splenic nodules, which can be located in the right or left upper quadrants, with no large single splenic organ (Fig. 5). It is part of the spectrum of anomalies classified as heterotaxy or cardiosplenic syndromes, and is usually diagnosed in early childhood because of cardiac anomalies [11]. Polysplenia should not be confused with splenosis, which occurs in cases of splenic trauma or splenectomy, with subsequent autotransplantation of splenic tissue in the peritoneal cavity, or even in other unusual locations, including the thoracic cavity.
Fig. 5.
Situs inversus with polysplenia. a Longitudinal sonogram of the right upper quadrant showing multiple right-sided splenic masses (white arrows). b Transverse ultrasound of the left upper quadrant demonstrating a left-sided liver. c Axial T2-weighted magnetic resonance image shows situs inversus with multiple splenic masses in the right upper quadrant (white arrows)
Lobulation of the spleen may persist after birth, with resulting normal variation in spleen shape (Fig. 6). The persisting lobule may extend medially, anterior to the upper pole of the left kidney, and can be marked by deep clefts and notches which may reach 2–3 cm in depth [12]. Although these variations occur frequently, an accurate sonographic evaluation is mandatory to rule out a splenic lesion, which may display echogenicity similar to the adjacent parenchyma.
Fig. 6.
Splenic lobulation. Longitudinal ultrasound images, from different patients, show a, b lobulations along the medial part of the spleen and c two clefts at the lower splenic pole (white arrows)
Splenomegaly
The definition and grading of splenomegaly in pediatric patients are not standardized [13]. Enlargement of splenic size is a non-specific finding, representing the manifestation of several and different disease processes. Echostructure of the increased splenic parenchyma presents a variable spectrum ranging from completely homogeneous patterns to a diffuse alteration of its echotexture secondary to many etiologies [14].
The most common causes of splenomegaly in children include infectious processes (viral, bacterial, fungal, protozoal and parasitic); portal hypertension; hemoglobinopathies and hemolytic anemias; myeloproliferative disorders (e.g. leukemia); lymphomas; and storage disorders such as Gaucher disease and amyloidosis [15].
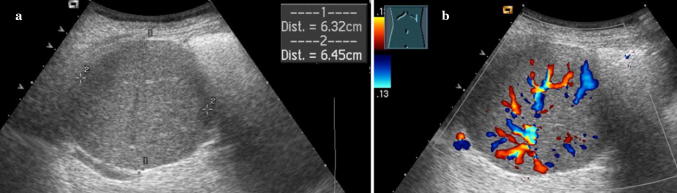
US is a very useful method to examine an enlarged spleen, allowing measurement of the splenic length with scans parallel to the long axis, according to age-specific maximal values, and allowing characterization of the morphological features of splenic parenchyma, like the presence of agglomerations, bulky masses or possible vascular ectasia (Fig. 7). Moreover, US and color Doppler evaluation may reveal extra-splenic manifestations of disease and detect additional findings suggestive of a specific diagnosis, such as portal hypertension, which shows hepatic parenchymal heterogeneity and dilated portosystemic collateral vessels, or lymphoma, with the presence of enlarged retroperitoneal and mesenteric lymph nodes.
Fig. 7.
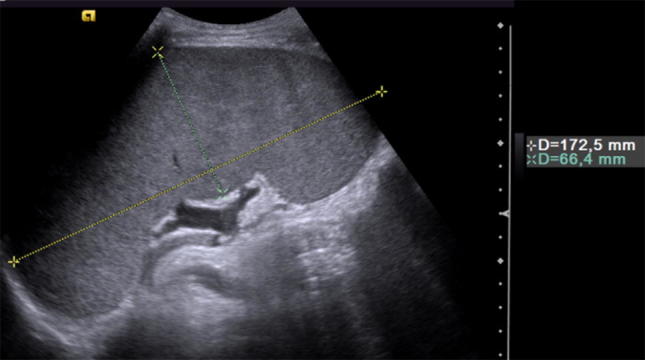
Splenomegaly. Coronal oblique ultrasound image demonstrating the sonographic measurement of longitudinal and transverse dimensions of an enlarged spleen
Trauma
The spleen is one of the most frequently injured organs in abdominal trauma, among both children and adults. While the spleen is partially protected by the rib cage in adults, it is bigger in children, protruding from the ribcage, and is often involved in blunt abdominal trauma, with 25% of trauma cases appearing as both isolated and multi-organ lesions. In children, blunt abdominal trauma is commonly related to motor vehicle accidents, falls, athletic injuries and iatrogenic injury during surgery [16]. The range of splenic injuries includes lacerations, intraparenchymal and subcapsular hematomas, infarction due to vascular compromise and splenic rupture.
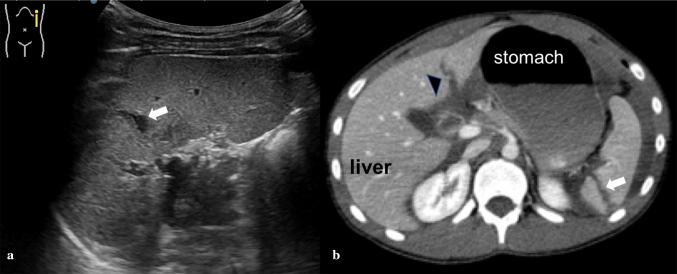
Lacerations appear as linear or branched hypoechoic areas, and may also extend to the splenic borders, with capsular rupture and resultant hemoperitoneum (Fig. 8). Lacerations can sometimes be difficult to detect on US and rupture of the splenic capsule can sometimes be delayed (i.e. second-time rupture). Moreover, lacerations can be associated with hematomas contained by an intact capsule, referred to as a sub-capsular hematoma. A laceration that extends through two surfaces of the spleen is referred to as a splenic rupture and is often accompanied by hemoperitoneum [17].
Fig. 8.
Splenic trauma. a Longitudinal sonogram of the spleen following blunt trauma demonstrates an intraparenchymal laceration that presents with a linear hypoechoic area (white arrow). b Axial computed tomography after contrast confirms the splenic lesion (white arrow) and shows a left hepatic lobe laceration (black-headed arrow)
Hematomas appear as hyperechoic oval areas in the initial stages and become progressively hypoechoic over time. The use of color Doppler US can raise lesion conspicuity by showing focal avascular regions in the splenic parenchyma. It is crucial to exclude splenic hematoma in traumatized patients with clinical signs of acute bleeding [18].
Although rare, infarction may occur following damage to a segmental arterial branch. The resulting infarcted areas are typically wedge-shaped, with the apexes towards the hilum of the spleen, and with no vascular flow within. Infarcts can develop singly or can be multiple and associated with other splenic injuries.
Hemoperitoneum linked to splenic injuries is most frequent when the hilum region is involved. It usually courses along the left paracolic gutter, into the rectouterine space in girls and the rectovesical space in boys.
Often, the most evident indicator of splenic lesions is the presence of hypo-anechoic fluid collection in the subcapsular or perisplenic space (Fig. 9). Furthermore, parenchymal lesions can sometimes be very difficult to recognize, especially when perisplenic fluid is not reported. CEUS can help overcome these limits, with a sensitivity to diagnoses of splenic laceration, rupture, fracture or active bleeding which is significantly higher than that displayed by B-mode or Doppler US, and almost equivalent to that of CT scans (Fig. 10) [19]. Although the use of CEUS for children is considered off-label, it represents an ideal application in diagnosis and follow-up of splenic trauma [20, 21].
Fig. 9.
Splenic trauma. a Gray scale and b e-flow technique images show a mild heterogeneity of the splenic lower pole (thin white arrows) associated with the c presence of splenic capsular rupture (thick white arrows) and resultant perisplenic hematoma
Fig. 10.
CEUS in splenic trauma. a Longitudinal sonogram following blunt trauma demonstrates an area of ill-defined altered echogenicity at the middle of the spleen. b CEUS clearly demonstrates a hypoechoic lesion extending at the surface, suggestive of splenic laceration
Infarction
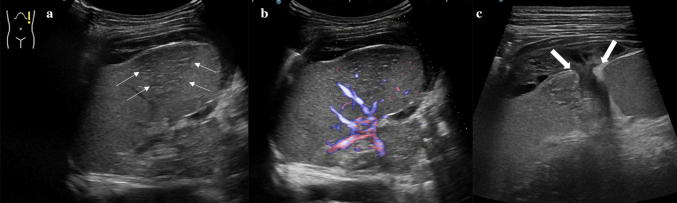
Splenic infarction is a common finding in children suffering from anemia, particularly in those with sickle cell disease or sickle thalassemia [22]. Other well-documented causes include hematological malignancy, vasculitis, cardiac emboli, portal hypertension, and infiltrative disorders. Infectious mononucleosis also increases the risk for splenic infarction, especially in children with hematologic disorders [23]. Infarctions of the spleen appear as subcapsular and triangular hypoechoic segmental lesions (due to edema and necrosis), with the bases parallel to the splenic periphery and the apexes pointing towards the hilum. On color Doppler US, the affected areas show no blood flow (Fig. 11). Torsion of the spleen, due to anomalies in splenic development (e.g. accessory spleen) and splenic stability (e.g. wandering spleen), represents an additional cause of splenic infarction which rarely involves children.
Fig. 11.
Splenic infarction. a Longitudinal gray scale and b color Doppler images of the spleen showing a subcapsular hypoechoic segmental lesion, which is clearly demonstrated b as an area of absent color flow consistent with a splenic infarction
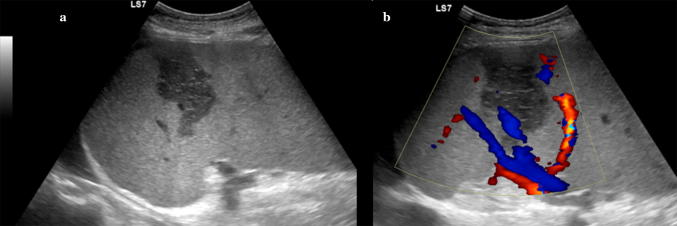
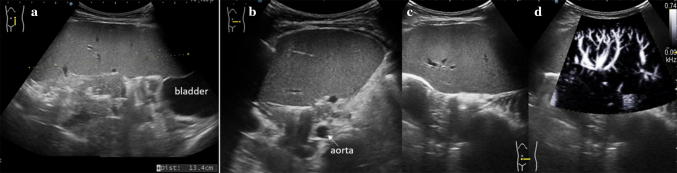
Wandering spleen results from absence or laxity of the splenic supporting ligaments, which allow the spleen to be inferiorly displaced from its normal anatomical location and suspended by a long vascular pedicle (Fig. 12). In children, a common and dangerous complication is the torsion of the spleen around its pedicle, which may lead to an acute surgical abdomen [24]. When a wandering spleen is suspected, US examination plays a primary role in confirming the diagnosis and avoiding possibly life-threatening complications requiring immediate surgery [25].
Fig. 12.
Wandering spleen. Ultrasound images of the spleen obtained in the right lateral decubitus position. a Longitudinal and b, c transverse views of the lower abdomen and pelvis demonstrating the displaced enlarged spleen in the pelvis, extending behind the bladder. d Interrogation with the e-flow technique shows normal blood flow within the enlarged spleen
Torsion of an accessory spleen with resultant splenic infarction is a very rare condition that should also be considered in the differential diagnosis of acute abdomen in children [26].
Focal lesions
Focal lesions in the spleen are rare in comparison with those located in other solid abdominal organs. US can be very helpful in finding and characterizing focal splenic lesions. However, because the US appearance of disease in the spleen is rather nonspecific, a full knowledge of the patient’s history and associated symptoms is important in narrowing down the differential diagnosis [27].
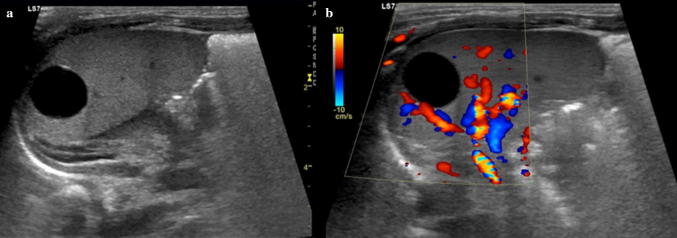
Splenic cysts are the most common lesions in the pediatric spleen and can be congenital (primary or true cysts) or acquired (secondary or pseudocysts). Histopathologically, congenital cysts, also known as “epidermoid cysts,” have an epithelial lining, whereas acquired cysts do not. Acquired cysts may be secondary to infection (i.e. echinococcosis), traumatic contusion, or previous splenic infarction. Through US, cysts appear as well-defined, spherical, anechoic lesions, with posterior acoustic enhancement, and absent on color Doppler interrogation (Fig. 13) [28].
Fig. 13.
Splenic cyst in a neonate. a Gray scale and b color Doppler images of the spleen show a round, sharply marginated anechoic cyst with no internal flow
Splenic abscesses are rarely seen in children, with a 0.14–0.7% occurrence reported [29]. They have a non-specific clinical presentation, and a delayed diagnosis is associated with high mortality, even in the present era of easily accessible antibiotics. Splenic abscesses can be the result of hematogenous spread of infection, an immunocompromised state, hematological disorders, malignancy, trauma, acquired immune deficiency syndrome (AIDS), and diabetes. Affected children present with non-specific high grade fever, vomiting and abdominal, left shoulder, chest, or flank pain. Splenic abscesses may be pyogenic, parasitic, fungal or tuberculous. A pyogenic splenic abscess appears as ill-defined hypoechoic lesions with internal septations and debris. Additional findings include intralesional gas bubbles, visible as foci of increased echogenicity.
Splenic calcifications are not a common pediatric finding. US identifies them as single or multiple echogenic foci, with or without acoustic shadowing [30]. The most likely etiologies include granulomatous infections (e.g. histoplasmosis, tuberculosis), chronical granulomatous diseases (e.g. Langerhans cell histiocytosis) and vascular anomalies (e.g. vascular and lymphatic malformations).
Splenic venous malformations are low-flow congenital lesions composed of abnormally dilated vascular channels lined by a single endothelial layer and filled with red blood cells. Lesions may be solitary or associated with a syndrome, such as Klippel–Trenaunay–Weber or Beckwith–Wiedemann syndrome [31]. Their sonographic appearance is extremely polymorphous. Lesions may be well-defined and heterogeneously hyperechoic with sporadic calcifications or multiple cysts, depending on the size of the vascular channels. Doppler US may show intralesional venous waveforms, or a pattern of no flow.
The term “hemangioma” has been applied to a wide variety of anomalies, which are now classified as distinct pathological entities according to the International Society for the Study of Vascular Anomalies (ISSVA) system [32]. Assuming the criteria outlined in the ISSVA guidelines, it has been found that several lesions, reported in the literature as hemangiomas, seem to correspond to venous vascular malformations [33].
Lymphatic malformations are congenital malformations of the lymphatic system consisting of endothelial-lined spaces of various sizes filled with lymph and separated by fibrous bands. They may be unilocular or multilocular. Sonographically, lymphatic malformations commonly appear as multilocular, subcapsular, hypoechoic cyst-like lesions containing intralocular echogenic debris and hyperechoic septations. Calcifications can be seen in the walls or septations. The lesions are relatively avascular on Doppler sonography, although arteries and veins can be detected within the septa [34].
Splenic hamartomas are rare, benign, malformed lesions composed of lymphoid tissue and disorganized, congested splenic sinuses (red pulp). They have been associated with Beckwith–Wiedemann syndrome and tuberous sclerosis [35]. On US, lesions usually appear as well-circumscribed, homogeneous masses, but some may be heterogeneous with cystic changes. Occasionally, they appear isoechoic or hyperechoic to the normal spleen parenchyma. Color Doppler interrogation of these lesions demonstrates increased blood flow.
Littoral cell angioma (LCA) is a rare benign vascular tumor arising from littoral cells lining the splenic red pulp sinuses. It usually affects adults, rarely children. Although LCAs are benign tumors, some malignant features have been reported [36]. Their sonographic appearance is variable, though it may include an enlarged, heterogeneous spleen without definite lesions, as well as multiple lesions of various sizes, which may be isoechoic, hypoechoic, or hyperechoic to surrounding splenic parenchyma [34].
Malignant tumors
The most common malignant splenic involvement is caused by lymphoma and leukemia. They are difficult to detect through US because they usually manifest as diffuse splenic infiltration, only rarely as focal lesions.
Leukemia, the most common childhood cancer, can involve the spleen during the active stages of the disease, or during remission. US evaluation usually shows a generalized increase in the size of the organ.
Lymphoma is the most common malignant tumor involving the spleen and accounts for 10–15% of all childhood cancers. In patients of all ages, splenic involvement occurs in about 35% of Hodgkin’s disease cases, and in about 15% of non-Hodgkin’s lymphoma cases [37]. Lymphomatous involvement can be present in an enlarged or normal-sized spleen. It may also manifest as mild–moderate splenomegaly with no tumor within. Conversely, significantly enlarged spleens are more likely to be involved in lymphoma. Sonographic appearances of lymphoma in children include homogeneous splenomegaly, solitary masses, and multiple nodular masses. Lymphomatous masses are generally hypoechoic and avascular on Doppler imaging. Associated lymphadenopathy can be detected in the splenic hilum, mesentery, and retroperitoneum.
Angiosarcoma is a very rare malignant tumor which represents the most common non-hematolymphoid malignant tumor of the spleen [38]. It can occur at any age and is rarely seen in children. Metastatic disease is frequent and involves the liver, lungs, bones, and lymphatic system [39]. US usually reveal a complex mass with a heterogeneous echotexture and Doppler imaging generally finds an increased flow in the solid hyperechoic regions of the tumor [34].
Conclusions
US plays a crucial role in the primary evaluation of pediatric splenic pathology. A knowledge of the sonographic features of splenic variations and disorders is needed to avoid diagnostic misinterpretations and to guide clinicians to the next appropriate imaging study.
Certain limitations to splenic sonography persist in differential diagnosis of the focal lesions because of the equivocal and nonspecific appearance of these various lesions. The sonographic appearances of the various lesions often overlap and pose a dilemma for the radiologist and clinician. Computed tomography, magnetic resonance imaging, or tissue sampling may be necessary to make a conclusive diagnosis.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, and its later amendments.
Human and animal rights
This article does not contain any studies with human or animal subjects performed by any of the authors.
Informed consent
Additional informed consent was obtained from all the patients for which identifying information is not included in this article.
References
- 1.Kahramaner Z, Erdemir A, Arik B, Bilgili G, Tekin M, Genc Y. Reference ranges of liver and spleen dimensions in term infants: sonographic measurements. J Med Ultrason (2001) 2015;42(1):77–81. doi: 10.1007/s10396-014-0578-0. [DOI] [PubMed] [Google Scholar]
- 2.Rosenberg HK, Markowitz RI, Kolberg H, Park C, Hubbard A, Bellah RD. Normal splenic size in infants and children: sonographic measurements. AJR Am J Roentgenol. 1991;157(1):119–121. doi: 10.2214/ajr.157.1.2048509. [DOI] [PubMed] [Google Scholar]
- 3.Megremis SD, Vlachonikolis IG, Tsilimigaki AM. Spleen length in childhood with US: normal values based on age, sex, and somatometric parameters. Radiology. 2004;231(1):129–134. doi: 10.1148/radiol.2311020963. [DOI] [PubMed] [Google Scholar]
- 4.Safak AA, Simsek E, Bahcebasi T. Sonographic assessment of the normal limits and percentile curves of liver, spleen, and kidney dimensions in healthy school-aged children. J Ultrasound Med. 2005;24(10):1359–1364. doi: 10.7863/jum.2005.24.10.1359. [DOI] [PubMed] [Google Scholar]
- 5.Konus OL, Ozdemir A, Akkaya A, Erbas G, Celik H, Isik S. Normal liver, spleen, and kidney dimensions in neonates, infants, and children: evaluation with sonography. AJR Am J Roentgenol. 1998;171(6):1693–1698. doi: 10.2214/ajr.171.6.9843315. [DOI] [PubMed] [Google Scholar]
- 6.Doria AS, Daneman A, Moineddin R, Smith CR, Mohanta A, Clarke J, Kellenberger CJ. High-frequency sonographic patterns of the spleen in children. Radiology. 2006;240(3):821–827. doi: 10.1148/radiol.2403050529. [DOI] [PubMed] [Google Scholar]
- 7.Kuint RC, Daneman A, Navarro OM, Oates A. Sonographic bands of hypoechogenicity in the spleen in children: zebra spleen. AJR Am J Roentgenol. 2016;207(3):648–652. doi: 10.2214/AJR.16.16401. [DOI] [PubMed] [Google Scholar]
- 8.Gayer G, Hertz M, Strauss S, Zissin R. Congenital anomalies of the spleen. Semin Ultrasound CT MR. 2006;27(5):358–369. doi: 10.1053/j.sult.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 9.Paterson A, Frush DP, Donnelly LF, Foss JN, O’Hara SM, Bisset GS., 3rd A pattern-oriented approach to splenic imaging in infants and children. Radiographics. 1999;19(6):1465–1485. doi: 10.1148/radiographics.19.6.g99no231465. [DOI] [PubMed] [Google Scholar]
- 10.Back SJ, Maya CL, Khwaja A. Ultrasound of congenital and inherited disorders of the pediatric hepatobiliary system, pancreas and spleen. Pediatr Radiol. 2017;47(9):1069–1078. doi: 10.1007/s00247-017-3869-y. [DOI] [PubMed] [Google Scholar]
- 11.Yildiz AE, Ariyurek MO, Karcaaltincaba M. Splenic anomalies of shape, size, and location: pictorial essay. ScientificWorldJournal. 2013;2013:321810. doi: 10.1155/2013/321810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Varga I, Babala J, Kachlik D. Anatomic variations of the spleen: current state of terminology, classification, and embryological background. Surg Radiol Anat. 2018;40(1):21–29. doi: 10.1007/s00276-017-1893-0. [DOI] [PubMed] [Google Scholar]
- 13.Pelizzo G, Guazzotti M, Klersy C, Nakib G, Costanzo F, Andreatta E, Bassotti G, Calcaterra V. Spleen size evaluation in children: time to define splenomegaly for pediatric surgeons and pediatricians. PLoS ONE. 2018;13(8):e0202741. doi: 10.1371/journal.pone.0202741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robertson F, Leander P, Ekberg O. Radiology of the spleen. Eur Radiol. 2001;11(1):80–95. doi: 10.1007/s003300000528. [DOI] [PubMed] [Google Scholar]
- 15.Hilmes MA, Strouse PJ. The pediatric spleen. Semin Ultrasound CT MR. 2007;28(1):3–11. doi: 10.1053/j.sult.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 16.Miele V, Piccolo CL, Trinci M, Galluzzo M, Ianniello S, Brunese L. Diagnostic imaging of blunt abdominal trauma in pediatric patients. Radiol Med. 2016;121(5):409–430. doi: 10.1007/s11547-016-0637-2. [DOI] [PubMed] [Google Scholar]
- 17.Doody O, Lyburn D, Geoghegan T, Govender P, Munk PL, Torreggiani WC. Blunt trauma to the spleen: ultrasonographic findings. Clin Radiol. 2005;60(9):968–976. doi: 10.1016/j.crad.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 18.Lynn KN, Werder GM, Callaghan RM, Sullivan AN, Jafri ZH, Bloom DA. Pediatric blunt splenic trauma: a comprehensive review. Pediatr Radiol. 2009;39(9):904–916. doi: 10.1007/s00247-009-1336-0. [DOI] [PubMed] [Google Scholar]
- 19.Menichini G, Sessa B, Trinci M, Galluzzo M, Miele V. Accuracy of contrast-enhanced ultrasound (CEUS) in the identification and characterization of traumatic solid organ lesions in children: a retrospective comparison with baseline US and CE-MDCT. Radiol Med. 2015;120(11):989–1001. doi: 10.1007/s11547-015-0535-z. [DOI] [PubMed] [Google Scholar]
- 20.Esposito F, Di Serafino M, Sgambati P, Mercogliano F, Tarantino L, Vallone G, Oresta P. Ultrasound contrast media in paediatric patients: is it an off-label use? Regulatory requirements and radiologist’s liability. Radiol Med. 2012;117(1):148–159. doi: 10.1007/s11547-011-0718-1. [DOI] [PubMed] [Google Scholar]
- 21.Sidhu PS, Cantisani V, Deganello A, Dietrich CF, Duran C, Franke D, Harkanyi Z, Kosiak W, Miele V, Ntoulia A, Piskunowicz M, Sellars ME, Gilja OH. Role of contrast-enhanced ultrasound (CEUS) in paediatric practice: an EFSUMB position statement. Ultraschall Med. 2017;38(1):33–43. doi: 10.1055/s-0042-110394. [DOI] [PubMed] [Google Scholar]
- 22.Gale HI, Bobbitt CA, Setty BN, Sprinz PG, Doros G, Williams DD, Morrison TC, Kalajian TA, Tu P, Mundluru SN, Castro-Aragon I. Expected sonographic appearance of the spleen in children and young adults with sickle cell disease: an update. J Ultrasound Med. 2016;35(8):1735–1745. doi: 10.7863/ultra.15.09023. [DOI] [PubMed] [Google Scholar]
- 23.Breuer C, Janssen G, Laws HJ, Schaper J, Mayatepek E, Schroten H, Tenenbaum T. Splenic infarction in a patient hereditary spherocytosis, protein C deficiency and acute infectious mononucleosis. Eur J Pediatr. 2008;167(12):1449–1452. doi: 10.1007/s00431-008-0781-3. [DOI] [PubMed] [Google Scholar]
- 24.Lombardi R, Menchini L, Corneli T, Magistrelli A, Accinni A, Monti L, Toma P. Wandering spleen in children: a report of 3 cases and a brief literature review underlining the importance of diagnostic imaging. Pediatr Radiol. 2014;44(3):279–288. doi: 10.1007/s00247-013-2851-6. [DOI] [PubMed] [Google Scholar]
- 25.Di Crosta I, Inserra A, Gil CP, Pisani M, Ponticelli A. Abdominal pain and wandering spleen in young children: the importance of an early diagnosis. J Pediatr Surg. 2009;44(7):1446–1449. doi: 10.1016/j.jpedsurg.2009.02.062. [DOI] [PubMed] [Google Scholar]
- 26.Perez Fontan FJ, Soler R, Santos M, Facio I. Accessory spleen torsion: US, CT and MR findings. Eur Radiol. 2001;11(3):509–512. doi: 10.1007/s003300000547. [DOI] [PubMed] [Google Scholar]
- 27.Caremani M, Occhini U, Caremani A, Tacconi D, Lapini L, Accorsi A, Mazzarelli C. Focal splenic lesions: US findings. J Ultrasound. 2013;16(2):65–74. doi: 10.1007/s40477-013-0014-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miele V, Galluzzo M, Cortese A, Bellussi A, Valenti M. Diagnostic imaging of splenic cysts in children. Radiol Med. 1998;95(1–2):62–65. [PubMed] [Google Scholar]
- 29.Faruque AV, Qazi SH, Arshad M, Anwar N. Isolated splenic abscess in children, role of splenic preservation. Pediatr Surg Int. 2013;29(8):787–790. doi: 10.1007/s00383-013-3336-2. [DOI] [PubMed] [Google Scholar]
- 30.Chen MJ, Huang MJ, Chang WH, Wang TE, Wang HY, Chu CH, Lin SC, Shih SC. Ultrasonography of splenic abnormalities. World J Gastroenterol. 2005;11(26):4061–4066. doi: 10.3748/wjg.v11.i26.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Warshauer DM, Hall HL. Solitary splenic lesions. Semin Ultrasound CT MR. 2006;27(5):370–388. doi: 10.1053/j.sult.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 32.Lowe LH, Marchant TC, Rivard DC, Scherbel AJ. Vascular malformations: classification and terminology the radiologist needs to know. Semin Roentgenol. 2012;47(2):106–117. doi: 10.1053/j.ro.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 33.Kollipara R, Dinneen L, Rentas KE, Saettele MR, Patel SA, Rivard DC, Lowe LH. Current classification and terminology of pediatric vascular anomalies. AJR Am J Roentgenol. 2013;201(5):1124–1135. doi: 10.2214/AJR.12.10517. [DOI] [PubMed] [Google Scholar]
- 34.Abbott RM, Levy AD, Aguilera NS, Gorospe L, Thompson WM. From the archives of the AFIP: primary vascular neoplasms of the spleen: radiologic-pathologic correlation. Radiographics. 2004;24(4):1137–1163. doi: 10.1148/rg.244045006. [DOI] [PubMed] [Google Scholar]
- 35.Zhang LF, Tou JF, Wang X, Gu WZ, Ma XH, Qin Q. Splenic hamartomas in two children. World J Surg Oncol. 2014;12:180. doi: 10.1186/1477-7819-12-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ertan G, Tekes A, Mitchell S, Keefer J, Huisman TA. Pediatric littoral cell angioma of the spleen: multimodality imaging including diffusion-weighted imaging. Pediatr Radiol. 2009;39(10):1105–1109. doi: 10.1007/s00247-009-1339-x. [DOI] [PubMed] [Google Scholar]
- 37.Toma P, Granata C, Rossi A, Garaventa A. Multimodality imaging of Hodgkin disease and non-Hodgkin lymphomas in children. Radiographics. 2007;27(5):1335–1354. doi: 10.1148/rg.275065157. [DOI] [PubMed] [Google Scholar]
- 38.Thompson WM, Levy AD, Aguilera NS, Gorospe L, Abbott RM. Angiosarcoma of the spleen: imaging characteristics in 12 patients. Radiology. 2005;235(1):106–115. doi: 10.1148/radiol.2351040308. [DOI] [PubMed] [Google Scholar]
- 39.Ricci ZJ, Mazzariol FS, Flusberg M, Chernyak V, Oh SK, Kaul B, Stein MW, Rozenblit AM. Improving diagnosis of atraumatic splenic lesions, part II: benign neoplasms/nonneoplastic mass-like lesions. Clin Imaging. 2016;40(4):691–704. doi: 10.1016/j.clinimag.2016.02.002. [DOI] [PubMed] [Google Scholar]