Abstract
The study of the gastrointestinal tract by imaging, particularly using ultrasound, is a required instrument for diagnosis of acute and chronic gastrointestinal pathologies in pediatric age. Actually, ultrasound plays an increasing role in the evaluation of gastrointestinal tract in neonatal and pediatric patients because of their small body habitus and the presence of less fat tissue in the abdominal wall and peritoneal cavity. Ultrasound has certain advantages, thanks to the new wide-spectrum frequency probes able to assess a detailed study of the morphological aspects and functional characteristics of bowel loops, adding a new dimension to the imaging of this body system. In this paper, we review anatomy, ultrasound technique and sonographic findings of bowel pathology frequently encountered in neonatal and pediatric emergency setting.
Keywords: Ultrasonography, Pediatrics, Emergencies, Gastrointestinal diseases
Sommario
Lo studio ecografico dell’apparato gastrointestinale recentemente è diventato uno strumento indispensabile per la diagnosi di patologie gastrointestinali ad insorgenza acuta o con decorso cronico in età pediatrica. L’ecografia ha un ruolo crescente nello studio del tratto gastrointestinale e specie in ambito pediatrico e neonatale grazie al habitus dei piccoli pazienti ed alla scarsa presenza di adipe interposto. I trasduttori multi-frequenza di ultima generazione forniscono immagini dettagliate, di alta qualità, per lo studio morfologico e funzionale delle anse intestinali, configurando una nuova dimensione degli ultrasuoni nella diagnostica per immagini dell’apparato gastro-intestinale. In questo articolo descriviamo la tecnica di esame e gli aspetti ecografici caratteristici delle più comuni patologie ad insorgenza acuta del tratto gastrointestinale in epoca neonatale e pediatrica.
Introduction
Traditionally, pediatric abdomen examination focuses exclusively on parenchymal organs, putting less stress on the gastrointestinal tract [1]. Actually, ultrasound (US) has become an important diagnostic imaging modality in the evaluation of the gastrointestinal (GI) tract of children adding a new dimension to the imaging of this body system. US observes gastrointestinal dynamics without exposure to ionizing radiation and allows detailed visualization of the mural layers of the bowel wall [2]. New US technologies and methods are able to perform a detailed examination of each section of the digestive system in pediatric age because of their smaller body size and less impaired by gas content and adipose tissues. However, US is most suitable for portions of the GI tract that are not surrounded by or filled with large amount of gas [2].
Complete examination requires experience, time and dedication to carry out a detailed analysis of as many intestinal loops as possible, minding their morphological aspects as well as their functional characteristics [3].
Ultrasonographic aspects and technique
New-generation ultrasonography equipment involves the use of wide-spectrum frequency probes that provide high-quality representation of the examined bowel.
The study of the gastrointestinal tract requires the use of any of the available probes such as convex, micro-convex and linear too, depending on patient’s age and constitution. Specific preparation with water, laxatives, polyethylene glycol solution or anti-meteoric agents is not required; however, in few cases such as in the study of the gallbladder or of the biliary tree, a fasting state of about 3 h in new-born and 5 h in children is recommended, as well as in patients suspected of being affected by hypertrophic pyloric stenosis, a full meal is required to be avoided.
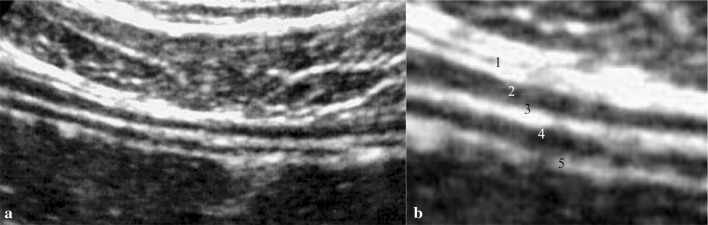
The study of middle abdominal quadrants is not uniformly regulated. However, it is suggested to start the examination with lower-frequency probes, possibly micro-convex to obtain a wider panorama; afterwards, it is possible to switch to high-frequency probes for a detailed analysis of the bowel wall [4]. The examination is delivered on the patient lying supine. Graduated compression, a technique introduced by Puylaert et al. in 1986 [5], is used to improve the visualization of intestinal loop: at first gently, then increasingly firmer pressure is applied from the right iliac fossa through longitudinal, cranial–caudal and caudal–cranial parallel scans covering the whole abdominal area [6]. The intestinal wall structure is substantially the same along the whole intestinal tract. The intestinal wall normally shows 5 alternating hyper- and hypo-echoic layers: serosa, muscularis, sub-mucosa, mucosa and mucosa-luminal content interface (Fig. 1) [7].
Fig. 1.
a Bowel wall layers. b Magnification detail of the wall: (1) Hyper-echoic serous. (2) Hypo-echoic muscular. (3) Hyper-echoic sub-mucous. (4) Hypo-echoic mucous. (5) Hyper-echoic lumen: the hyper-echoic line corresponds to the interface between the intestinal hypo-echoic mucosa and the fluid-filled echo free lumen
Such appearance is constant through the whole canal. An alteration of the normal stratification of the walls could potentially indicate a pathological condition [7].
The intestinal wall should be measured under mild compression from just above an air–mucosal interface to the outside of the outer muscularis layer border, including the whole bowel wall. Under these standardized conditions, the stomach wall thickness measures 3–6 mm, terminal ileum 1–3 mm and colon 0.5–2 mm [7].
Hypertrophic pyloric stenosis
Hypertrophic pyloric stenosis (HPS) is an infantile pathology of unknown etiology. Its incidence varies from 1/300 to 1/1000 live births and is more common among males than females (M/F = 4/1) [8]. It is characterized by the thickening of the circular muscular layer of the pyloric sphincter [9]. Clinical suspects are based on symptomatology and possible presence of a roundish, palpable mass around the epigastric area, commonly known as ‘pyloric olive’ due to its shape.
Vomit, usually defined “explosive” by parents, is the main and worsening symptom, which appears around the III and VI week after birth causing a blockage of impairment of growth curve, constipation and metabolic alkalosis [10].
Currently, the diagnosis of HPS can be performed with US only [11]. The examination is normally delivered with the patient lying supine or in lateral right decubitus through a linear, high-frequency probe. Scans are run from the sub-xiphoid area in right para-median location. To confirm any presence of stenosis, it is necessary to measure the length of the canal and the thickness of the muscular tunic that must be both identified.
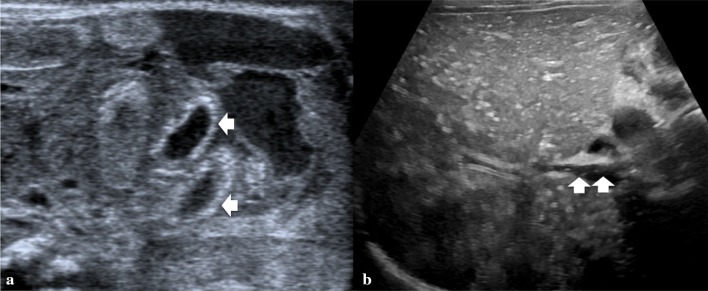
Diagnostic criteria (Fig. 2):
Pyloric canal is 18 mm or longer.
Muscular walls thickness is 4 mm or over.
Fig. 2.
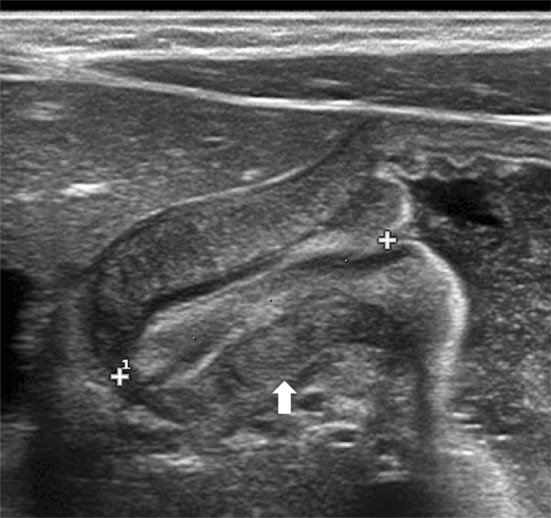
Longitudinal scan shows pyloric muscular wall thickening (arrow). Elongated canal is nearly 2 cm in length (calliper)
Other findings are a slightly curved pyloric canal and a “binary” look of the pyloric mucosa that appears thickened and protuberant in the gastric antrum: the so-called cervix sign (Fig. 3). Extremely useful in the early diagnosis of the pathology is the evaluation of any blockage during the effusion of the gastric content into the duodenum [12].
Fig. 3.
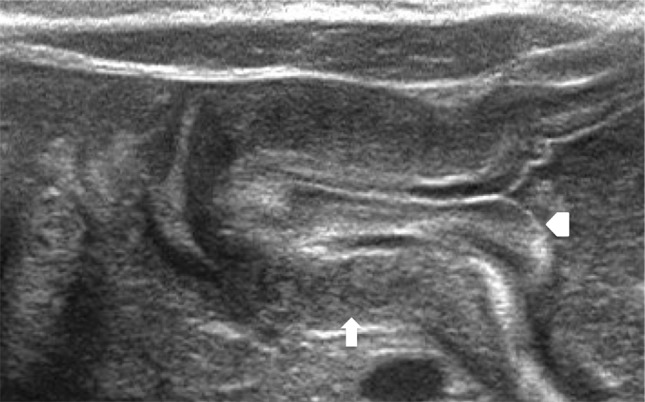
Longitudinal scan shows markedly thickened pyloric muscular wall (arrow) with indentation of the pyloric mucosa (arrowhead) into the fluid-filled antrum (cervix sign)
Malrotation and volvulus
Intestinal malrotation occurs in between 1 in 200 and 1 in 500 live births [13, 14]. However, most patients with malrotation are asymptomatic. Symptomatic malrotation occurs in only 1 in 6000 live births in the first year of life with bilious emesis, episodic pain or symptoms of malabsorption [13, 14]. Rotation and fixation defects of the intestines are the consequence of an abnormal rotating process in the primitive intestines during intra-uterine age. The complex, anti-clockwise rotation of 270° around the superior mesenteric artery (SMA) axis can sometime fail to function properly subsequently leading to “malrotation” [13]. This sometime symptomatic disruption can cause “midgut volvulus”, a real emergency requiring prompt chirurgical intervention [15].
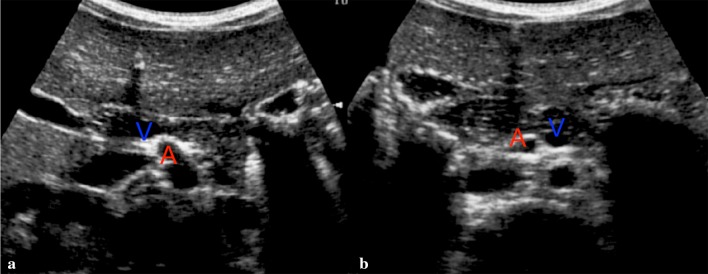
Sonographic examination can raise suspects of malrotation when evidence of alterations in the positioning between the SMA and the superior mesenteric vein (SMV) is found (Fig. 4). Patients are examined whilst lying supine [16, 17]. SMA is identified at its point of origin on the abdominal aorta, while SMV is recognized at its crossing in the portal trunk. On a transversal scan in normal rotating conditions, SMV will be found on the right side of the SMA. Unfortunately, even a normal relation between the vessels cannot completely exclude the presence of such pathology [18–20].
Fig. 4.
Transversal scan on epigastric area. a Normal superior mesenteric vein (V) lies to the right of the superior mesenteric artery (A). b Intestinal malrotation and midgut volvulus: the vein (V) lies to the left of the artery (A)
Complication of intestinal malrotation is medial intestinal volvulus due to a very high mobility of the bowel in the abdominal cavity with an easily rotation around the SMA axis [19–21]. A pathognomonic sign of the disease is the so-called “Whirlpool Sign”, a spiraling of the SMV around the SMA axis (Fig. 5).
Fig. 5.
Transversal scans of the middle abdomen. a B-mode shows a volvulus on the superior mesenteric axis (arrow). b Color Doppler shows a clockwise whirlpool of the superior mesenteric vein (arrowhead) around a volvulus on the mesentery artery axis (arrow)
Appendicitis
Acute appendicitis is the most common cause of abdominal pain that often requires chirurgical intervention in pediatric age [2, 22]. Acute insurgence of pain in the right iliac fossa with abdominal “defence”, light fever, nausea, vomit and increased phlogosis markers are the most common clinical findings.
US is useful in children with ambiguous clinical signs and often can help to suggest or confirm an alternative diagnosis when appendicitis is not found [2, 22].
Furthermore, the “atypical” localization of the appendix can sometimes provoke non-specific and ‘elusive’ signs and symptoms, causing confusions in the clinical picture [23].
The examination has to be performed on the patient lying supine. The advantages of a full bladder have not been univocally established in literature [24, 25]. We prefer to examine the abdominal quadrants involved on an empty bladder.
The employed technique is a gradual compression. Important points of reference are the ileopsoas muscle and the iliac vessels. The appendix runs through the front of such muscle, “crossing” the artery and the iliac vein, almost resting on them.
The visualization of the appendix in normal conditions is not always easy or possible to obtain. Much easier is to display a pathological appendix, frequently combined with indirect signs [26, 27].
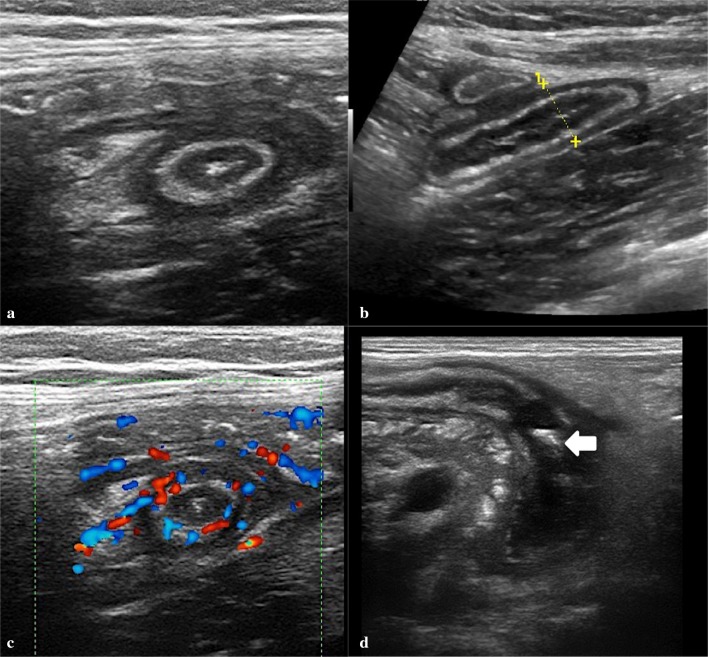
Diagnostic criteria of acute appendix are (Figs. 6, 7) [2, 26]:
Increased diameter: the normal appendix usually measures < 6 mm in diameter. Larger diameters are usually considered pathological; in fact the presence of fecal material in the appendicular lumen can increase its size regardless of any inflammatory phenomena.
Incompressibility: appendix does not appear reduced under compression-probe visualization.
Adipose hyper-echogenicity sign (AHS): it is the result of the spreading of the inflammatory process over the appendicular walls, with a progressive involvement of the surrounding peritoneal adipose cellular tissue. AHS can easily be found in the peri-appendicular fat, often among the lymph nodes. It is an useful sign, especially in minimally enlarged appendices.
Fig. 6.
Appendicitis on transverse scans of the right lower quadrant. a, b Thickened appendix at the base and middle of viscera. c Hyperemia of the appendicular wall at color Doppler. d Fluid-filled tip appendix with echogenic fecaliths inside (arrow)
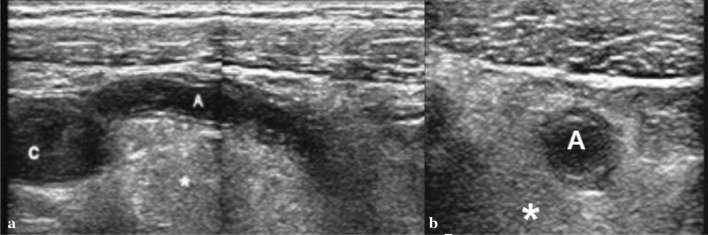
Fig. 7.
Enlarged appendix with adipose hyper-echogenicity sign (*) of the peritoneum. a Longitudinal scan. b Transverse scans. Appendix (A). Cecum (C)
The appendix is also hyper-vascular at color Doppler study in acute non-perforated setting [26, 28, 29]. Fecaliths can often be identified, as echogenic foci with posterior acoustic shadowing [26, 29]. Tracing the entire appendix is important to prevent false-negative diagnoses. Inflammatory changes may be limited to the tip of the appendix, which is seen as a tubular structure ending in a blind pouch [28]. If inflammation is advanced and suppurate appendicitis has evolved, heterogeneous echogenicity and hyperemic changes in the peri-appendiceal tissue may be present [28] Gangrenous changes are suggested by the presence of increasing appendicular dilatation and loss of the echogenic sub mucosal layer with a lack of vascularity at color Doppler study [28].
The involved complications are often a consequence of organic perforation with fluid potentially evolving into an abscess able to cause “smashing” of the appendix [28, 29]. In such cases, the cecal appendix is no longer structurally visualized because, with perforation, the appendix becomes decompressed [30]. During the first 3 years of life, appendicitis is rare and often complicated by sudden perforation. Therefore, during early US examination, we could find a pseudo-tumour mass as a result of perforated appendicitis, whose nature and etiology are often hard to identify [31].
Intussusception
Intussusception is the penetration of a portion of the bowel loops, either the ileus or colon, toward the distal segment of the digestive tube. The most affected age range is 6–48 months (about 90%) [32–34]. Depending on the affected portion of the bowel, we can find two major forms of intussusception [35]:
ileo-cecal (about 90%);
ileo-ileal (about 10%).
The most common clinical sign is recurrent paroxysmal abdominal pain, sometimes associated with vomit and precocious anorexia [32, 33]. Often, in clinical history, previous episodes of infection are discovered in the upper respiratory tract along with gastroenteritis [34].
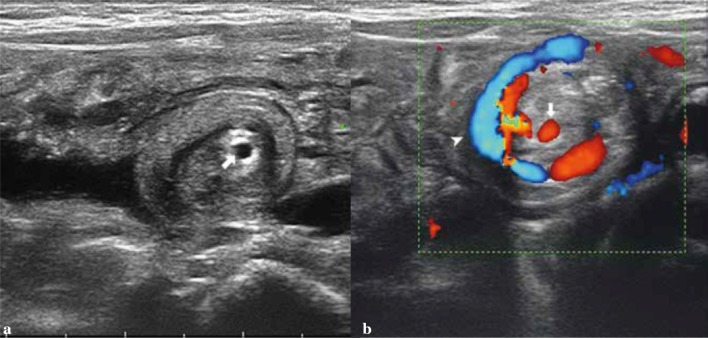
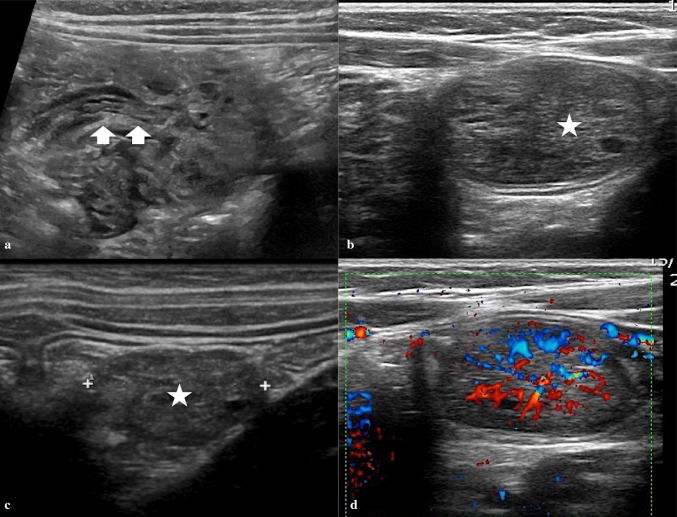
On US, intestinal intussusception appears as an “intestinal mass” whose anterior–posterior diameter ranges between 20 and 50 mm in relation to the affected portion. Transversal scans show a peculiar “concentric rings” structure inside the mass (Fig. 8). This structure appears as an oval hypo-echoic mass with bright central echoes on the longitudinal scans as well as hypo-echoic “doughnut” or with “target” configuration on the transverse scans (Fig. 9) [34, 35].
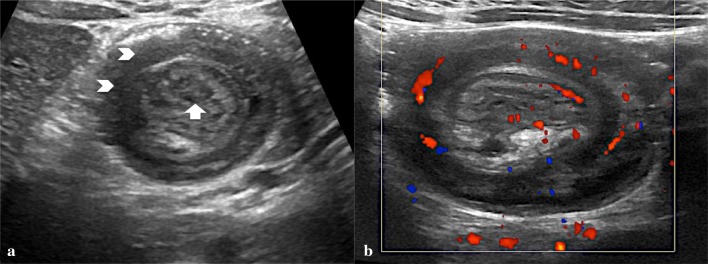
Fig. 8.
a Linear probe shows the concentric rings of the oedematous intussuscepted (arrowheads), with echogenic fat and mesenteric lymph nodes (arrow). b Color Doppler ultrasound demonstrates substantial blood flow within the wall of the intussuscepted
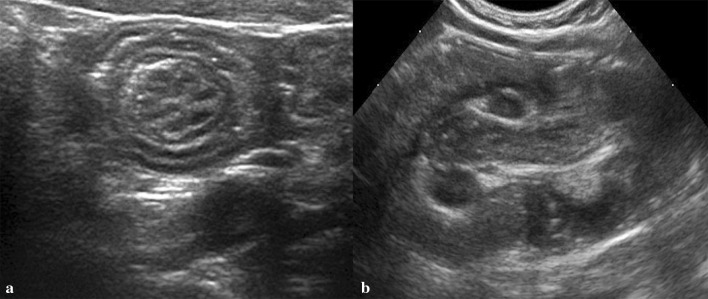
Fig. 9.
Ileo-cecal intussusception. a Transverse scan shows concentric rings structure inside the mass (target sign). b Longitudinal scan shows an oval hypo-echoic mass with bright central echoes
Currently, the diagnosis of intussusception can be performed with US alone [36]. US have proven a very high grade of diagnostic accuracy in the ileo-cecal intussusception, with a sensitivity and specificity virtually of about 100% in experienced hands [37]. For this reason, actually enema is used just for therapeutic treatment. Furthermore, US can also be used to monitor the attempt to hydrostatic reduction with physiological saline. The role of the US in predicting the reducibility of invagination by enema is controversial. According to some, the US can predict the reducibility of the intussuscepted bowel loop by enema reduction [28]. Findings such as reduced vascular flow (seen during a color Doppler study), trapped peritoneal fluid within the intussuscepted, thickened outer wall (> 10 mm), lymph nodes larger than 1 cm within the intussusception have shown some correlation with decreased success of enema reductions [28, 37, 38]. However, some authors have not found any relations between the reduction rate and these above-described findings [37].
Ischemia, intestinal perforation and peritonitis are all possible serious consequences; in these cases, the color Doppler study can be useful to identify a reduction or absence of vascular signal in the intestinal walls. According to some authors, this finding may increase the risk of perforation during enema study although not everyone considers them as certain contraindications to the hydrostatic reduction [37, 38]. The presence of intramural or subserosal gas indicates a risk of bowel necrosis and perforation. The latter is established as contraindications for enema reduction [28].
Meckel’s diverticulum
Meckel’s diverticulum (MD) is one of the most common malformations of gastrointestinal tract associated with the incomplete obstruction of the omphalomesenteric duct that connects yolk sac and primitive gut during foetal period [39].
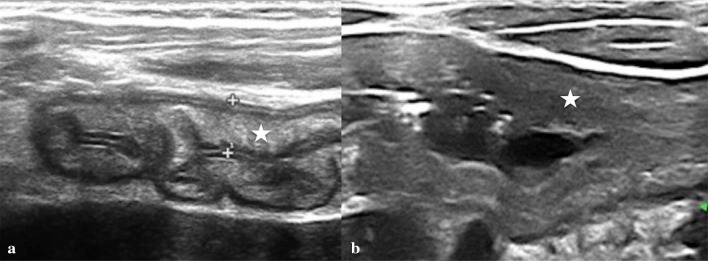
It is a true diverticulum containing all layers of the bowel wall. Its incidence is between 0.3 and 1.2% with no differences in sex [40]. Generally, MD ranges from 1 to 10 cm in length and is found within 100 cm from the ileo-cecal valve on anti-mesenteric border of the ileum. It frequently contains heterotopic tissue; gastric mucosa accounts for 50%, more rarely pancreatic, biliary and colonic mucosa. Although MD is usually asymptomatic and is discovered as an incidental finding on surgery, can present with complications associated with it. The risk of complications ranges from 4 to 25% and occur more in pediatric age, especially in children younger than 2 years of age. Common complications are bleeding associated with peptic ulceration from heterotopic gastric mucosa located within the diverticulum, intestinal obstruction, due to banding, diverticulitis or tumour formation [41]. MD can be also a lead point for intussusception and an extremely rare complication is the axial torsion around the narrow base that easily causes necrosis and perforation. Diagnosis of symptomatic MD is very difficult in patients with the symptoms other than bleeding. The most common and non-invasive method for the preoperative diagnosis of MD is technetium-99 m pertechnetate scan because of tracer’s propensity to concentrate in ectopic gastric mucosa. It has a sensitivity of 80–90%, a specificity of 95% in children [42]. US is routinely used to study children who present with symptoms of acute abdomen, especially on the right lower quadrant pain. On US, inflamed MD can appear as a cystic or tubular structure with thickened walls, sometimes displaying the characteristic alternating hyper-echoic and hypo-echoic bands of intestinal wall (Fig. 10). Otherwise they could be found be found the signs of inflammation and its complications such as a complex cystic structure with surrounding hyper-echogenic fatty mesentery with or without abscesses or perforation as well as the signs of the obstruction or intussusception [41, 42].
Fig. 10.
Inflamed Meckel’s diverticulum. a Enlarged tubular mass with thickened walls. b, c Increase hyperemia of the wall at color Doppler
Enteric duplication cyst complication
Enteric duplication cyst is an uncommon congenital abnormality that can occur anywhere in the alimentary tract from mouth to anus [43, 44]. Most commonly, the duplication cyst occurs in the small intestine, especially in the distal ileum, often on the mesenteric side of the lumen. Duplication can be cystic or tubular with fluid content and consists of an inner lining of GI epithelium and an outer layer of smooth muscle. The tubular variant, infrequent, is in direct connection with the bowel lumen, instead of the spherical form [43]. The origin of the cysts is unclear, but the theory of an abnormal recanalization of the gut lumen during embryogenesis is the most accredited. The clinical presentation of an enteric duplication depends on location, relative mass effect and complications relating to secretions of the involved epithelium [43, 44].
Patients with small bowel duplication cysts can present with a variety of symptoms including vomiting and abdominal pain. Duodenal cyst can cause other complications such as pancreatitis, infection, weight loss, and GI bleeding from ulceration of the ectopic gastric mucosa within the cyst; jejune duplication cyst can cause abdominal bloating, constipation, intussusception, volvulus, and partial small bowel obstruction; ileal duplication cyst may be asymptomatic or present with abdominal pain, small bowel obstruction, a palpable abdominal mass, or hematochezia [44]. Rarely, malignant transformation can occur in the setting of gastric mucosa heterotopia within the duplication cyst. Because of its potential complications, an early diagnosis has to be achieved when possible. Diagnosis is made with US and/or contrast studies, which may show a filling defect. US can detect a cystic lesion with internal echoes or debris and a quite characteristic appearance of double-layered wall, with the inner hyper-echoic mucosa and outer hypo-echoic muscle layer defined as a “gut signature” (Fig. 11). This is a specific but not always persistent sign such as in complicated forms by inflammation; in the latter case, the layers may be obscured, lessening the specificity. Nonetheless, the demonstration of a cystic mass adjacent to the bowel should prompt the consideration of a duplication cyst. CT and MRI may be used to define the location and size of the duplication and complicated cysts. Treatment is usually surgical excision [43, 45].
Fig. 11.
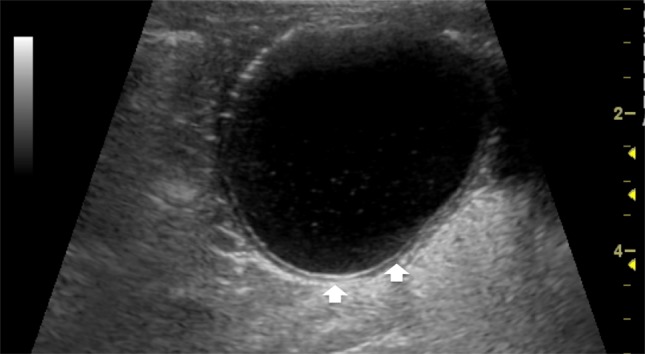
Ileal duplication cyst with a thick, double-layered wall (arrows)
Colon polyp
Polyps of the intestinal tract are frequent in the pediatric population. Juvenile polyps are most common lesions in children with age from 3 to 10 years, uncommon before 2 years of age [46]. These lesions measure from 1 to 3 cm in size, often are pedunculated and confined to the recto-sigmoid colon. However, through the increased use of colonoscopy, we have learned that between 50 and 60% of patients have more than one polyp and about 25% of patients have polyps in the cecum or ascending colon [47].
Usually these lesions are isolated, few in number and benign, but are described familial polyposis syndrome associated with the development of numerous polyps with an increased risk of malignant transformation [46, 47].
The most frequent presenting symptoms of colonic polyps are painless intestinal bleeding, mucous-purulent stools and abdominal pain. In some patients, we can find a microcytic, hypochromic anemia from chronic bleeding or a colonic intussusception if the polyp extrudes sufficiently into the lumen propelling distally by peristalsis and traction.
In adult patients, the diagnosis of intestinal polyp is generally made with endoscopy or intestinal contrast studies. Colonoscopy is the gold standard for diagnosis and possible treatment in the patient with persistent or recurrent bleeding, abdominal pain or other symptoms [46, 47]. Biopsies also confirm the type of polyp and can help to determine if there is a need for additional studies like genetic tests or screenings or special follow-ups.
Although US is not intended to replace endoscopy in the diagnostic work-up of intestinal polyps in young patients, it plays an important role in the detection of intestinal polyps. A specific diagnosis is possible in many cases, as most polyps have a characteristic appearance [48, 49].
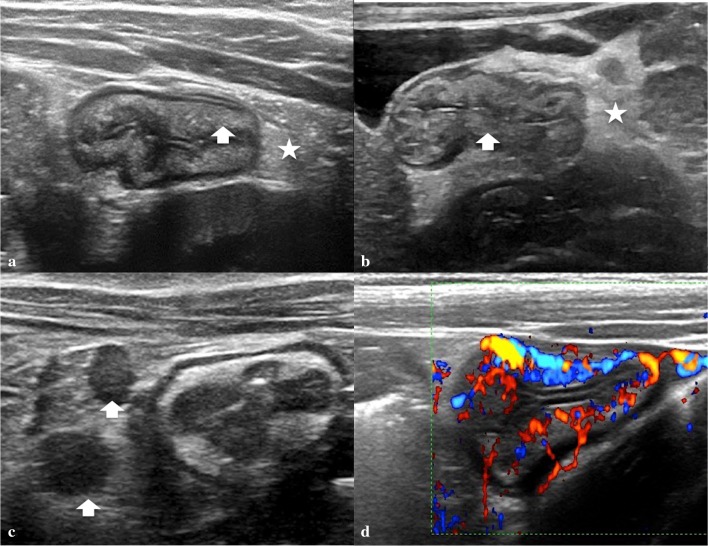
At US imaging, a juvenile polyp appears as a rounded hypo-echoic nodule located within the colon lumen and with a peripheral hyper-echoic layer, sometimes containing small cysts, attached to the intestinal wall through a peduncle (Fig. 12). Color Doppler shows arterial and venous blood flow within the polyp and peduncle [48–50]. These US findings are fairly specific and allow differentiation between juvenile polyps and normal intestinal loops containing stools and inflammatory pseudo-polyps. Intestinal loops containing stools usually appear hyper-echoic, whereas the intestinal walls appear hypo-echoic and there is no internal flow signal at color Doppler [48, 51]. Inflammatory pseudo-polyps are more echoic, there is no vascularization within the pedicle, and they are located in areas with intestinal wall thickening [50, 52].
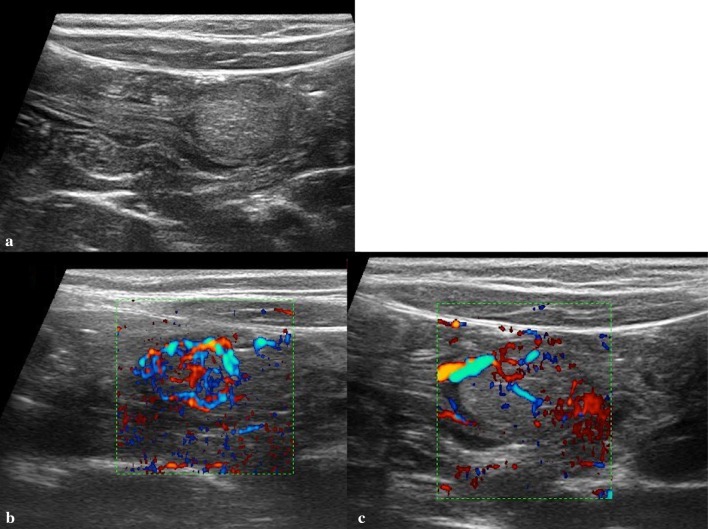
Fig. 12.
Giant colon polyp. a Longitudinal scan shows the peduncle (arrows). b, c Transverse scan shows the head of the polyp (star). d Color Doppler shows blood flow into the polyp mass
There is no mention in the literature about the sensitivity of US in the diagnosis of intestinal polyps [48]. Operator dependency, obscuration by gas and/or stool, and deep location in the abdomen or pelvis may account for the lack of visualization. Nevertheless, a negative sonogram does not exclude the presence of an intestinal polyp and reinforces the rationale of our current practice that colonoscopy should be performed in all children with clinical suspicion of a polyp [48].
Necrotizing enterocolitis
Necrotizing enterocolitis (NEC) is one of the most common gastrointestinal emergencies in the new-born [53]. It is an inflammatory process, characterized by ischemic necrosis of the intestinal mucosa, invasion of enteric gas-forming organisms, diffusion of gas into the intestinal wall and portal venous system and complicated by perforation, peritonitis and death [54].
Although it affects term infant also, NEC is more frequent in preterm, particularly in very low birth weight infants (VLBW) with BW < 1500 g and gestational age (GA) < 32 weeks. The incidence of NEC in preterm infants with GA < 32 weeks varies globally from 2 to 7% across different neonatal intensive care units (NICU) [55].
Clinical presentation of NEC in new-born ranges from a sudden change in feeding tolerance, associated with abdominal distension, vomiting, diarrhoea, rectal bleeding to nonspecific systemic findings, that include apnoea, respiratory failure, lethargy or temperature instability.
Since the NEC clinical signs, both early and late, are often non-specific, as well as laboratory tests, imaging plays an important role in the timing of diagnosis. While plain abdominal X-ray examination remains the main and most used modality in the evaluation and monitoring of the NEC, US in recent years is playing an increasingly important role for making an early diagnosis of NEC in the different stages. US has certain advantages over conventional X-ray examination, because it provides real-time images of the abdominal structures, able to assess the presence and validity of peristalsis of the bowel loops, minimal amounts of fluid in the peritoneal cavity, not detectable with standard X-ray, the thickness and the perfusion of the intestinal wall and pneumatosis [56]. Pneumatosis is a pathognomonic sign of NEC that appears at US as multiple hyper-echoic spots limited to some continuous wall portions or with a circumferential pattern in one or more loops affected with or without portal pneumatosis represented by hyper-echoic spots, irregularly distributed in the liver parenchyma (Fig. 13) [56].
Fig. 13.
a Intramural gas (pneumatosis) creates an echogenic ring (arrows). b Widespread echogenic bubbles of gas in the portal vein system (arrows)
Inflammatory bowel disease
Inflammatory bowel disease (IBD) is a generic term including a series of acute and non-acute diseases not requiring surgical treatment, ranging from the most common and often self-limited affections (e.g., enteritis, mesenteric lymphadenitis) to the rare debilitating and/or chronic ones (e.g. purpura, chronic inflammatory diseases) [57, 58]. These diseases can be very different from each other sharing similar semeiological elements. Diagnosis of IBD in pediatric patients can be challenging due to presentation with atypical symptoms and/or extra intestinal manifestations (e.g., short stature, chronic anemia, unexplained fever, arthritis, mouth ulcers) [59]. There is currently no single diagnostic test for IBD, and the diagnosis is based on a combination of history, physical examination, endoscopic appearance, histologic findings in gastrointestinal biopsies, the presence of serum and fecal inflammatory markers, and typical imaging results too [59]. The course of the disease is unpredictable, exacerbations and phases of remissions can alternate, and no single parameter alone can at present reliably define the disease activity or prognosis [59]. In the ESPGHAN Revised Porto Criteria for the Diagnosis of Inflammatory Bowel Disease in Children and Adolescents [59, 60], US has been described as a non invasive, and widespread patients with suspected IBD due to its major advantages of low costs and lack of radiation exposure [59]. US investigation is very sensitive to the detection minimal alterations in the bowel wall [61, 62]. High-resolution linear array transducers allow direct and detailed evaluation of the intestinal wall, and areas of intestinal wall thickening can often be identified [2].
US imaging of inflammatory bowel is often unspecific as well as many different bowel diseases appear remarkably similar on US. For this reason, US examination could be useful for diagnosis only if combined with clinical evaluation and laboratory tests [63].
Among the many factors highlighted on a scan (bowel loop mobility, lumen content, fluid in abdomen), the thickening of the wall is by far the most relevant. It needs to be classified according to its entity [64].
Mild: 3–5 mm.
Moderate: 6–9 mm.
Severe: > 9 mm.
And mostly fall under two categories [65].
Layered.
Not-layered.
Layered thickening: characterized by mucosal inflammation with indirect involvement of the sub mucous, which appears hyper-echoic and clearly defined with organized structure (Fig. 14a).
Fig. 14.
Longitudinal scan of terminal ileum. a Stratified thickening wall (calliper) with relative prevalence of the sub-mucous (star). b Un-stratified thickening wall (star) without definition of the layers
Non-layered thickening: characterized by phlogosis and infiltration of the sub-mucous with disorganized structure—hence no longer reflective (Fig. 14b).
It has been proposed that the kind of thickening may suggest a pathology or relative staging [64, 65]. When a condition of layered thickening is found, the first step is the evaluation of the following possible diseases:
at the ileum level: infectious ileitis and ileo-colitis (e.g., Campylobacter or Salmonella); early Crohn disease (Fig. 15).
at the colon level: gram-induced germ infectious colitis (e.g., Salmonella, Shigella, E. coli); early chronic intestinal infectious diseases (CIID).
Fig. 15.
Crohn’s disease in the active phase of the terminal ileum. a, b Stratified thickening wall with relative prevalence of the sub-mucous (arrow) and mesenteric oedema (star). c Reactive lymph node (arrow). d Color Doppler imaging reveals hyperemia of the terminal ileum wall
In case of not-layered thickening, we need to consider the following:
at the ileum level: Schonlein-Henoch Purpura; advanced Crohn disease; tubercular ileitis; protein-losing enteropathy;
at the colon level: ischemic colitis prodromal of hemolytic uremic syndrome (HUS); advanced inflammatory bowel disease (IBD); pseudomembranous colitis; neutropenia, colitis.
US is useful for the detection of IBD in pediatric patients by evaluating the bowel wall thickening and surrounding structures. As stated in current guidelines, color Doppler imaging increases the sensitivity and specificity of US and estimates the disease activity showing the hyperemia both of the bowel wall and adjacent mesentery [59, 60]. Peri-intestinal inflammatory reaction, the extent and localization of involved bowel segments and the presence of extra luminal complications such as fistula, abscesses, and ileus can be assessed using US [59].
Conclusion
US is the modality of choice for the initial evaluation of acute abdominal pain in pediatric patients because of their small body habitus and the presence of less fat tissue in the abdominal wall and peritoneal cavity [28]. Proper selection of the transducers, optimal positioning and the use of graded compression techniques improve the visualization of pathological bowel loops. A complete examination of the gastrointestinal tract requires experience, time and dedication to carry out a detailed analysis of as many intestinal loops as possible, minding their morphological aspects as well as their functional characteristics [3, 28]. US can diagnose several diseases that cause abdominal pain and can differentiate among various medical and surgical problems in pediatric patients helping to an earlier diagnosis. However, when nonspecific, US can provide quickly information about the intestinal wall diseases adding complementary information to lab tests and clinical amnestic elements [28, 66].
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, and its late amendments. Additional informed consent was obtained from all patients for which identifying information is not included in this article.
Human and animal rights
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gale HI, Gee MS, Westra SJ, Nimkin K. Abdominal ultrasonography of the paediatric gastrointestinal tract. World J Radiol. 2016;8(7):656–667. doi: 10.4329/wjr.v8.i7.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.John SD, Hollingsworth C. The pediatric gastrointestinal tract. In: Rumack C, editor. Diagnostic ultrasound. 4. Missouri: Elsevier Mosby; 2011. [Google Scholar]
- 3.Lobo ML, Roque M. Gastrointestinal ultrasound in neonates, infants and children. Eur J Radiol. 2014;83(9):1592–1600. doi: 10.1016/j.ejrad.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 4.Cogley JR, O’Connor SC, Houshyar R, Al Dulaimy K. Emergent paediatric US: what every radiologist should know. Radiographics. 2012;32(3):651–665. doi: 10.1148/rg.323115111. [DOI] [PubMed] [Google Scholar]
- 5.Puylaert JB. Mesenteric adenitis and acute terminal ileitis: US evaluation using graded compression. Radiology. 1986;161(3):691–695. doi: 10.1148/radiology.161.3.3538138. [DOI] [PubMed] [Google Scholar]
- 6.Arys B, Mandelstam S, Rao P, Kernicks S, Kumbla S. Sonography of the pediatric gastrointestinal system. Ultrasound Q. 2014;30(2):101–117. doi: 10.1097/ruq.0b013e3182a38dcc. [DOI] [PubMed] [Google Scholar]
- 7.Atkinson NSS, Bryant RV, Dong Y, Maaser C, Kucharzik T, Maconi G, Asthana AK, Blaivas M, Goudie A, Gilja OH, Nuernberg D, Schreiber-Dietrich D, Dietrich CF. How to perform gastrointestinal ultrasound: anatomy and normal findings. World J Gastroenterol. 2017;23(38):6931–6941. doi: 10.3748/wjg.v23.i38.6931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Markowitz RI. Olive without a cause: the story of infantile hypertrophic pyloric stenosis. Pediatr Radiol. 2014;44(2):202–211. doi: 10.1007/s00247-013-2834-7. [DOI] [PubMed] [Google Scholar]
- 9.Helton K, Strife J, Warner B, Byczkowski T, Donovan E. The impact of a clinical guideline on imaging children with hypertrophic pyloric stenosis. Pediatr Radiol. 2004;34(9):733–736. doi: 10.1007/s00247-004-1255-z. [DOI] [PubMed] [Google Scholar]
- 10.Hernanz-Schulman M. Pyloric stenosis: role of imaging. Pediatr Radiol. 2009;39(S2):134–139. doi: 10.1007/s00247-008-1106-4. [DOI] [PubMed] [Google Scholar]
- 11.Costa Dias S, Swinson S, Torrão H, Gonçalves L, Kurochka S, Vaz CP, Mendes V. Hypertrophic pyloric stenosis: tips and tricks for ultrasound diagnosis. Insights Imaging. 2012;3(3):247–250. doi: 10.1007/s13244-012-0168-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hernanz-Schulman M, Zhu Y, Stein SM, Heller RM, Bethel LA. Hypertrophic pyloric stenosis in infants: US evaluation of vascularity of the pyloric canal. Radiology. 2003;229(2):389–393. doi: 10.1148/radiol.2292021303. [DOI] [PubMed] [Google Scholar]
- 13.Warner B, Oldham KT, Colombani PM. Malrotation. In: Foglia RP, editor. Surgery of infants and children: scientific principles and practice. Philadelphia: Lippincott Williams and Wilkins; 1997. p. 1229. [Google Scholar]
- 14.Dilley AV, Pereira J, Shi EC, Adams S, Kern IB, Currie B. The radiologist says malrotation: does the surgeon operate? Pediatr Surg Int. 2000;16(1–2):45–49. doi: 10.1007/s003830050012. [DOI] [PubMed] [Google Scholar]
- 15.Shalaby MS, Kuti K, Walker G. Intestinal malrotation and volvulus in infants and children. BMJ. 2013;26:347. doi: 10.1136/bmj.f6949. [DOI] [PubMed] [Google Scholar]
- 16.Marine MB, Karmazyn B. Imaging of malrotation in the neonate. Semin Ultrasound CT MR. 2014;35(6):555–570. doi: 10.1053/j.sult.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 17.Chandramohan A, Gibikote S, Saxena AK. US as a primary tool in the work-up of malrotation. Pediatr Radiol. 2010;40(11):1844–1845. doi: 10.1007/s00247-010-1795-3. [DOI] [PubMed] [Google Scholar]
- 18.Garel C, Blouet M, Belloy F, Petit T, Pelage J-P. Diagnosis of paediatric gastric, small-bowel and colonic volvulus. Pediatr Radiol. 2015;46(1):130–138. doi: 10.1007/s00247-015-3445-2. [DOI] [PubMed] [Google Scholar]
- 19.Esposito F, Vitale V, Noviello D, Di Serafino M, Vallone G, Salvatore M, Oresta P. Ultrasonographic diagnosis of midgut volvulus with malrotation in children: 7-years single center experience. J Pediatr Gastroenterol Nutr. 2014;59(6):786–788. doi: 10.1097/mpg.0000000000000505. [DOI] [PubMed] [Google Scholar]
- 20.Esposito F, Vitale V, Noviello D, Di Serafino M, Vallone G, Salvatore M, Oresta P. Authors’ response. J Pediatr Gastroenterol Nutr. 2015;60(3):e25. doi: 10.1097/mpg.0000000000000643. [DOI] [PubMed] [Google Scholar]
- 21.Esposito F, Di Serafino M, Vitale V, Aragione N, et al. Malrotazione e volvolo: può bastare la sola ecografia? Il giornale italiano di Radiologia Medica. 2017;4:1073–1077. doi: 10.17376/girm_4-6-11122017-15. [DOI] [Google Scholar]
- 22.Di Serafino M, Severino R, Gullotto C, Listanti F, Scarano E. Idiopathic renal infarction mimicking appendicitis. Case Rep Emerg Med. 2017;2017:8087315. doi: 10.1155/2017/8087315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coyne SM, Zhang B, Trout AT. Does appendiceal diameter change with age? A sonographic study. AJR Am J Roentgenol. 2014;203(5):1120–1126. doi: 10.2214/ajr.13.12205. [DOI] [PubMed] [Google Scholar]
- 24.Linam LE, Munden M. Sonography as the first line of evaluation in children with suspected acute appendicitis. J Ultrasound Med. 2012;31(8):1153–1157. doi: 10.7863/jum.2012.31.8.1153. [DOI] [PubMed] [Google Scholar]
- 25.Binkovitz LA, Unsdorfer KM, Thapa P, Kolbe AB, Hull NC, Zingula SN, Thomas KB, Homme JL. Pediatric appendiceal ultrasound: accuracy, determinacy and clinical outcomes. Pediatr Radiol. 2015;45(13):1934–1944. doi: 10.1007/s00247-015-3432-7. [DOI] [PubMed] [Google Scholar]
- 26.Trout AT, Towbin AJ, Fierke SR, Zhang B, Larson DB. Appendiceal diameter as a predictor of appendicitis in children: improved diagnosis with three diagnostic categories derived from a logistic predictive model. Eur Radiol. 2015;25(8):2231–2238. doi: 10.1007/s00330-015-3639-x. [DOI] [PubMed] [Google Scholar]
- 27.Xu Y, Jeffrey RB, Shin LK, DiMaio MA, Olcott EW. Color doppler imaging of the appendix: criteria to improve specificity for appendicitis in the borderline-size appendix. J Ultrasound Med. 2016;35(10):2129–2138. doi: 10.7863/ultra.15.11064. [DOI] [PubMed] [Google Scholar]
- 28.Hwang JY. Emergency ultrasonography of the gastrointestinal tract of children. Ultrasonography. 2017;36(3):204–221. doi: 10.14366/usg.16052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tulin-Silver S, Babb J, Pinkney L, Strubel N, Lala S, Milla SS, Tomita S, Fefferman NR. The challenging ultrasound diagnosis of perforated appendicitis in children: constellations of sonographic findings improve specificity. Pediatr Radiol. 2015;45(6):820–830. doi: 10.1007/s00247-014-3232-5. [DOI] [PubMed] [Google Scholar]
- 30.Trout AT, Sanchez R, Ladino-Torres MF, Pai DR, Strouse PJ. A critical evaluation of US for the diagnosis of paediatric acute appendicitis in a real-life setting: how can we improve the diagnostic value of sonography? Pediatr Radiol. 2012;42(7):813–823. doi: 10.1007/s00247-012-2358-6. [DOI] [PubMed] [Google Scholar]
- 31.Doria AS. Optimizing the role of imaging in appendicitis. Pediatr Radiol. 2009;39(S2):144–148. doi: 10.1007/s00247-008-1105-5. [DOI] [PubMed] [Google Scholar]
- 32.Stein-Wexler R, O’Connor R, Daldrup-Link H, Wootton-Gorges SL. Current methods for reducing intussusception: survey results Pediatr Radiol. 2015;45(5):667–674. doi: 10.1007/s00247-014-3214-7. [DOI] [PubMed] [Google Scholar]
- 33.Esposito F, Ambrosio C, De Fronzo S, Panico MR, D’Aprano M, Giugliano AM, et al. Fluoroscopy-guided hydrostatic reduction of intussusception in infancy: role of pharmacological premedication. Radiol Med. 2015;120(6):549–556. doi: 10.1007/s11547-014-0486-9. [DOI] [PubMed] [Google Scholar]
- 34.Applegate KE. Clinically suspected intussusception in children: evidence-based review and self-assessment module. Am J Roentgenol. 2005;185(3 Suppl):S175–S183. doi: 10.2214/ajr.185.3_supplement.0185s175. [DOI] [PubMed] [Google Scholar]
- 35.Menke J, Kahl F. Sonography-guided hydrostatic reduction of ileocolic intussusception in children: analysis of failure and success in consecutive patients presenting timely to the hospital. Eur J Pediatr. 2015;174(3):307–316. doi: 10.1007/s00431-014-2394-3. [DOI] [PubMed] [Google Scholar]
- 36.Vazquez JL, Ortiz M, Doniz MC, Montero M, Del Campo VM. External manual reduction of paediatric idiopathic ileocolic intussusception with US assistance: a new, standardised, effective and safe manoeuvre. Pediatr Radiol. 2012;42(10):1197–1204. doi: 10.1007/s00247-012-2424-0. [DOI] [PubMed] [Google Scholar]
- 37.Daneman A, Navarro O. Intussusception. Part 1: a review of diagnostic approaches. Pediatr Radiol. 2003;23(2):79–85. doi: 10.1007/s00247-002-0832-2. [DOI] [PubMed] [Google Scholar]
- 38.Ilivitzki A, Shtark LG, Arish K, Engel A. Deep sedation during pneumatic reduction of intussusception. Pediatr Radiol. 2012;42(5):562–565. doi: 10.1007/s00247-012-2398-y. [DOI] [PubMed] [Google Scholar]
- 39.Mackey WC, Dineen P. A fifty year experience with Meckel’s Diverticulum. Surg Gynecol Obstet. 1983;156:56–64. [PubMed] [Google Scholar]
- 40.Rattan KR, Singh J, Dalal P, Rattan A. Meckel’s diverticulum in children: our 12-year experience. Afr J Paediatr Surg. 2016;13(4):170–174. doi: 10.4103/0189-6725.194671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gambling TC, Glenn J, Herring D, McKinney WB. Bowel obstruction caused by a Meckel’s diverticulum enterolith: a case report and review of the literature. Curr Surg. 2003;60(1):63–64. doi: 10.1016/S0149-7944(02)00650-5. [DOI] [PubMed] [Google Scholar]
- 42.Sagar J, Kumar V, Shah DK. Meckel’s diverticulum: a systematic review. J R Soc Med. 2006;99(10):501–505. doi: 10.1177/014107680609901011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Di Serafino M, Mercogliano C, Vallone G. Ultrasound evaluation of the enteric duplication cyst: the gut signature. J Ultrasound. 2015;19(2):131–133. doi: 10.1007/s40477-015-0188-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gleason CA, Sandra EJ. (2018) Gastrointestinal system. In: Avery’s diseases of the newborn. 10.1016/C2013-0-00320-9
- 45.Di Serafino M, Severino R, Mercogliano C, Lisanti F, De Martino C, Rocca R, Abate R, Salata M, Vallone G, Maroscia D. A complicated ileal duplication cyst in a young adult: the value of the “Gut Signature”. Open J Radiol. 2016;6:100–104. doi: 10.4236/ojrad.2016.62015. [DOI] [Google Scholar]
- 46.Adolph VR, Bernabe K. Polyps in children. Clin Colon Rectal Surg. 2008;21(4):280–285. doi: 10.1055/s-0028-1089943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.de Ridder L, Lingen AV, Taminiau J, Benninga M. Rectal bleeding in children: endoscopic evaluation revisitied. Eur J Gastroenterol Hepatol. 2007;19:317–320. doi: 10.1097/MEG.0b013e328080caa6. [DOI] [PubMed] [Google Scholar]
- 48.Parra DA, Navarro OM. Sonographic diagnosis of intestinal polyps in children. Pediatr Radiol. 2008;38:680–684. doi: 10.1007/s00247-008-0812-2. [DOI] [PubMed] [Google Scholar]
- 49.Vitale V, Di Serafino M, Mercogliano C, Vallone G. Giant colon polyp in a child with suspected inflammatory bowel disease: US finding. J Ultrasound. 2014;19(1):53–55. doi: 10.1007/s40477-014-0091-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baldisserotto M, Spolidoro JV, Bahu Mda G. Graded compression sonography of the colon in the diagnosis of polyps in paediatric patients. AJR Am J Roentgenol. 2002;179(1):201–205. doi: 10.2214/ajr.179.1.1790201. [DOI] [PubMed] [Google Scholar]
- 51.Drose Julia A. Sonographic abdominal anatomy. In: Sanders RC, Winter T, editors. Clinical sonography-a practical guide. 4. Hagerstown: Lippincott Williams and Wilkins; 2007. p. 51. [Google Scholar]
- 52.Giardiello FM, Hamilton SR, Kern SE, Offerhaus GJ, Green PA, Celano P, Krush AJ, et al. Colorectal neoplasia in juvenile polyposis or juvenile polyps. Arch Dis Child. 1991;66:971–975. doi: 10.1136/adc.66.8.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Neu J, Walker WA. Necrotizing enterocolitis. N Engl J Med. 2011;364(3):255. doi: 10.1056/NEJMra1005408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ballance WA, Dahms BB, Shenker N, Kliegman RM. Pathology of neonatal necrotizing enterocolitis: a ten-year experience. J Pediatr. 1990;117(1 Pt 2):S6. doi: 10.1016/S0022-3476(05)81124-2. [DOI] [PubMed] [Google Scholar]
- 55.Battersby C, Santhalingam T, Costeloe K, Modi N. Incidence of neonatal necrotising enterocolitis in high-income countries: a systematic review. Arch Dis Child Fetal Neonatal Ed. 2018;103(2):F182. doi: 10.1136/archdischild-2017-313880. [DOI] [PubMed] [Google Scholar]
- 56.Esposito F, Mamone R, Di Serafino M, Mercogliano C, Vitale V, Vallone G, Oresta P. Diagnostic imaging features of necrotizing enterocolitis: a narrative review. Quant Imaging Med Surg. 2017;7(3):336–344. doi: 10.21037/qims.2017.03.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Casciani E, De Vincentiis C, Polettini E, Masselli G, Di Nardo G, Civitelli F, Cucchiara S, Gualdi GF. Imaging of the small bowel: Crohn’s disease in paediatric patients. World J Radiol. 2014;6(6):313–328. doi: 10.4329/wjr.v6.i6.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chiorean L, Schreiber-Dietrich D, Braden B, Cui X, Dietrich CF. Transabdominal ultrasound for standardized measurement of bowel wall thickness in normal children and those with Crohn’s disease. Med Ultrason. 2014;16(4):319–324. doi: 10.11152/mu.201.3.2066.164.dsd2. [DOI] [PubMed] [Google Scholar]
- 59.Chiorean L, Schreiber-Dietrich D, Braden B, Cui XW, Buchhorn R, Chang JM, Dietrich CF. Ultrasonographic imaging of inflammatory bowel disease in pediatric patients. World J Gastroenterol. 2015;21(17):5231–5241. doi: 10.3748/wjg.v21.i17.5231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Levine A, Koletzko S, Turner D, Escher JC, Cucchiara S, de Ridder L, Kolho KL, Veres G, Russell RK, Paerregaard A. ESPGHAN revised porto criteria for the diagnosis of inflammatory bowel disease in children and adolescents. J Pediatr Gastroenterol Nutr. 2014;58:795–806. doi: 10.1097/MPG.0000000000000239. [DOI] [PubMed] [Google Scholar]
- 61.Duigenan S, Gee MS. Imaging of paediatric patients with inflammatory bowel disease. AJR Am J Roentgenol. 2012;199(4):907–915. doi: 10.2214/AJR.11.7966. [DOI] [PubMed] [Google Scholar]
- 62.Anupindi SA, Halverson M, Khwaja A, Jeckovic M, Wang X, Bellah RD. Common and uncommon applications of bowel ultrasound with pathologic correlation in children. AJR Am J Roentgenol. 2014;202(5):946–959. doi: 10.2214/ajr.13.11661. [DOI] [PubMed] [Google Scholar]
- 63.Chiorean L, Schreiber-Dietrich D, Braden B, Cui X-W, Buchhorn R, Chang J-M, et al. Ultrasonographic imaging of inflammatory bowel disease in paediatric patients. World J Gastroenterol. 2015;21(17):5231–5241. doi: 10.3748/wjg.v21.i17.5231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Biko DM, Rosenbaum DG, Anupindi SA. Ultrasound features of paediatric Crohn disease: a guide for case interpretation. Pediatr Radiol. 2015;45(10):1557–1566. doi: 10.1007/s00247-015-3351-7. [DOI] [PubMed] [Google Scholar]
- 65.Frisoli JK, Desser TS, Jeffrey RB. Thickened sub mucosal layer: a sonographic sign of acute gastrointestinal abnormality representing sub mucosal edema or hemorrhage. ARRS Executive Council Award II. American Roentgen Ray Society. AJR Am J Roentgenol. 2000;175(6):1595–1599. doi: 10.2214/ajr.175.6.1751595. [DOI] [PubMed] [Google Scholar]
- 66.Chaubal N, Dighe M, Shah M, Chaubal J. Sonography of the gastrointestinal tract. J Ultrasound Med. 2006;25(1):87–97. doi: 10.7863/jum.2006.25.1.87. [DOI] [PubMed] [Google Scholar]