Abstract
The study was intended to optimise the process variables such as extraction time and solvent concentration to maximize the yield of Murraya koenigii leaf extract and total phenolic content using response surface methodology. The experimental design was conducted for independent factor such as acetone, ethanol, methanol (20–80%) and time (20–100 min). The optimal conditions as the quadratic model were retained through central composite design. All the variables showed significant influence on extract yield and total phenolic content of M. koenigii leaf extract. The optimized conditions of extract were attained as 50% of ethanol, 60% acetone, 80% methanol and further analysed for their DPPH scavenging activity, total phenolic content, flavonoid content, and ferric reducing activity. Extract obtained with 50% ethanol showed highest DPPH scavenging activity and total phenolic content while 60% acetonic extract exhibited highest ferric reducing activity and flavonoid content.
Keywords: Murraya koenigii, Response surface methodology, Flavonoid content, Phenolic content, Ferric reducing power assay
Introduction
Plants are not only restricted to agriculture trade, fibre crop, and traditional food but they also include secondary metabolites which play an important role in therapeutic applications. There is an increasing trend for utilizing the naturally occurring biological compounds capable of inhibiting the free radical cations and oxidation (Brahmi et al. 2015). India is the major producer of medicinal herbs due to which it is called as botanical garden of the world. The herbs are capable of producing desirable low-molecular complex structures viz. secondary metabolites, antioxidant phenolics, flavonoids, to provide defence against oxidative stress from oxidizing agents such as free radicals (Jayapriya and Gricilda 2015). These herbs therefore, can be used for the treatment of chronic and infectious diseases. Herbs as medicines are being used since ancient time for human health care and now it has become a part of human race (Chitra et al. 2011).
Murraya koenigii is one of the medicinal herbs, usually called as kari patta in Indian vernaculars, belongs to Rutaceae family. It is a valuable plant due to its flavour, aroma, and medicinal importance in many food products. The chemical constituents responsible for its aroma are P-caryophyllene, P-gurjunene, O-phellandrene, and P-elemene (Rahman and Gray 2005). Murraya koenigii leaves are rich in carbazole alkaloids and bioactive compounds of which the most important are total phenols, phenolic acids, flavonoids, carotenoids and acridine carbazole (Jain et al. 2012). These compounds interact in such a way to stimulate the pharmaceutical properties, anti-oxidative, cytotoxic, antimicrobial, antibacterial, antifungal, anti-inflammatory, antiulcer, and anticholesterolemic activities (Rahman and Gray 2005). Various studies have been done on every part of M. koenigii plant and recognised for their efficacy in exhibiting antioxidant activity. Its leaves also contain β-carotene, monoterpene, tocopherol, lutein, and alkaloids (Ningappa et al. 2010) which are responsible for biological activity due to their redox potential, reducing agents, oxygen quenchers, and metal-chelators deeds (Tan et al. 2014; Biswas et al. 2012).
Extraction method plays an important role for the extraction of phenolic compounds from herbal plants. The objective of extraction is to maximize the extract yield and bioactive components by using various solvents. Many factors, such as extraction time and temperature, solvent concentration, temperature, pH, feed to solvent ratio, and particle size, show significant influence on extraction of phenolic compounds. The polarities of polyphenols varies from polar to non-polar solvent, thus an extensive role of solvents such as water, aqueous acetone, aqueous methanol, aqueous ethanol have been studied and concentration of each solvents showed the significant influence on phenolic content of the obtained extracts (Dent et al. 2013).
Therefore, this study was focussed on optimization of extraction time, solvent, and solvent concentration for the extraction of M. koenigii leaf extract using response surface methodology (RSM) for the recovery of maximum extract yield and total phenolic content.
Materials and methods
Chemicals
Solvents like deionised water, ethanol, methanol, acetone (Sai scientific, Chennai, Tamilnadu, India), 1,1-diphenyl-2-picrylhydrazil (DPPH), folin–ciocalteau’s reagent, standard gallic acid, standard ascorbic acid, standard quercitin, sodium nitrite, aluminium trichloride, sodium hydroxide, sodium phosphate, sodium bisphosphate, potassium ferricyanide, ferric chloride were purchased form Himedia, Mumbai, India, and trichloroacetic acid, methanol, formic acid, acetonitrile (HPLC grade) were purchased from Merck, Mumbai, India.
Methods
Pre-processing of M. koenigii leaves
Murraya koenigii L. leaves were obtained from Puducherry. It was washed under tap water to eliminate the adhered dust particles, dried in forced conventional tray drier at a temperature of 60 °C for 4 h. After drying, it was ground into grinder (Kenstar model, India) and then sieved through 100 ASTM mesh size sieve, packed in amber glass bottles and stored in refrigerator until use for extraction.
Preparation of M. koenigii leaves extract
10 g of M. koenigii leaf powder was added into 100 mL of deionised water and kept in water bath at an extraction temperature of 80 °C for 1 h (Sablania et al. 2018), followed by filtration using muslin cloth and the obtained extract was centrifuged at 805g for 10 min to obtain the aqueous extract of M. koenigii leaves. The optimization of extraction process variables for M. koenigii leaves was done by using RSM and later the optimized extracts were analysed for their phytochemical properties.
Experimental design
Optimization of process variables was carried out using RSM. It is a commonly used tool for optimization and development of new design for process variables. Central Composite Design (CCD) was opted to define the effect of various solvents, their concentration (ethanol, acetone, and methanol), and time for identification of optimal level to maximize the extract yield and total phenolic content (TPC). RSM was done to obtain the desired level of independent factors using two independent variables (solvent concentration, time of extraction) and their response on extract yield and TPC. The same design was run separately for each solvent at varied extraction time period to acquire the effect of solvent concentration on extraction. CCD resulted in total 13 combinations for each solvent with five replicates at centre points (Table 1). The statistical significance test was done at a significant level of p ≤ 0.05. The significant level was analysed by analysis of variance (ANOVA). The efficiency and reliability of the model was inferred by using F-value and R2 value. The validation of model was done by comparing the experimental and predicted values.
Table 1.
Experimental data for the responses obtained at various experimental runs for extract yield and total phenolic content
| Runs | Factor 1 | Factor 2 | Response for ethanol | Response for methanol | Response for acetone | |||
|---|---|---|---|---|---|---|---|---|
| Solvents (mL) | Time of extraction (min) | Yield (%) | TPC (mg GAE/g) | Yield (%) | TPC (mg GAE/g) | Yield (%) | TPC (mg GAE/g) | |
| 1 | 90.3553 | 60 | 15 | 50.47 | 27 | 25.12 | 19 | 8.254 |
| 2 | 55 | 60 | 21 | 54.35 | 25.21 | 21 | 17.84 | 30.823 |
| 3 | 19.6447 | 60 | 21 | 24.78 | 26 | 19.89 | 17 | 9.885 |
| 4 | 80 | 20 | 20 | 34.78 | 16.5 | 13.4 | 19.55 | 9.432 |
| 5 | 30 | 20 | 24 | 17.15 | 20.14 | 12.75 | 18.067 | 11.098 |
| 6 | 30 | 100 | 20 | 19.12 | 18.89 | 16.2 | 15 | 11.018 |
| 7 | 55 | 60 | 25 | 54.27 | 25 | 21.87 | 17.846 | 31.13 |
| 8 | 55 | 60 | 20 | 52.28 | 26.4 | 21 | 17.846 | 31.23 |
| 9 | 80 | 100 | 16 | 38.87 | 25.38 | 22.87 | 17.28 | 10.1247 |
| 10 | 55 | 60 | 21 | 51.32 | 26 | 21 | 17 | 31.128 |
| 11 | 55 | 60 | 24 | 54.38 | 24 | 22.27 | 18 | 31.178 |
| 12 | 55 | 3.43146 | 21 | 14.21 | 10.27 | 5.23 | 19.26 | 11.287 |
| 13 | 55 | 116.569 | 16 | 19.23 | 18.1 | 15.21 | 16.23 | 11.825 |
Extract yield
The yield of M. koenigii leaves extract was determined by taking weight of the obtained extract per unit weight of the raw material used for extraction (Mohamad et al. 2013).
Total phenolic content
Total phenolic content of optimized extracts was determined by F–C assay according to Shah et al. (2015). In which the calibration curve was plotted by using various concentration from 200 to 1000 μg/mL of gallic acid using 2.5 mL of FC reagent (1:10) and 2.5 mL of sodium bicarbonate (7.5%). The absorbance was noted after 2 h at 725 nm using spectrophotometer (Shimadzu, Japan) and extract was taken as 0.5 mL followed by the same procedure. Total phenolic content of extracts was calculated as mg gallic acid equivalent (mgGAE) per g dry matter of extract.
DPPH scavenging activity
The DPPH scavenging activity was calculated by DPPH assay given by Shah et al. (2015). An aliquot of 0.2–1.0 mL of extract was added into the 1 mL of 0.15 mM DPPH solution and left in dark for 20 min. The reading was noted at 517 nm using a UV spectrophotometer (UV-1800, Shimadzu, Japan). Ascorbic acid was taken as a standard compound for the preparation of standard curve. The DPPH scavenging activity was determined by using the equation given below. The half minimum inhibitory concentration (IC50) value was calculated by fitting the obtained concentration inhibition data in a straight line equation that resulted in 50% reduction in absorbance from that of control.
Where Ac = Absorbance of control, As = Absorbance of extract.
Ferric reducing power assay
The optimized extract was taken at various concentrations, mixed with 2.5 mL of phosphate buffer (0.2 M, pH 6.6) followed by the addition of 2.5 mL of potassium ferricyanide (1%), kept at 50 °C for 20 min. Then, 2.5 mL of trichloroacetic acid (10%) was added and the mixture was centrifuged at 805g for 15 min. 2.5 mL of an aliquot was taken from the prepared solution and added into the same amount of deionised water. 0.5 mL of ferric chloride (0.1%) was mixed in it and the absorbance was read at 700 nm using a UV spectrophotometer (Shah et al. 2015).
Total flavonoid content
Total flavonoid content was estimated according to Tan et al. (2014) method with some modifications. 100 μL of extract was mixed with 4 mL of distilled water. 0.3 mL of sodium nitrite (5%) was added and kept for 5 min followed by addition of 0.3 mL of aluminium chloride (10%) and again kept for another 6 min for incubation. Then, 2 mL of sodium hydroxide was added followed by addition of 2.4 mL of distilled water and the absorbance was read at 510 nm using a UV spectrophotometer. Quercetin was used as a standard curve at different concentrations.
Statistical analysis
The Design-Expert Version 10 software (State-Ease Inc., Minneapolis, MN, USA) was used to determine the analysis of variance (ANOVA) for RSM experiments. The statistical analysis of phytochemical analysis was performed in SPSS 20.0 (IBM Corporation, Armonk, NY). The values were expressed as the mean ± standard deviation of triplicate readings and the mean values were separated using Duncan’s multiple range test (p ≤ 0.05).
Results and discussion
Fitting the model and statistical analysis
Central composite design was selected to analyse the linear and interactive effects of independent variables like solvents (acetone, ethanol, and methanol) and time of extraction on extract yields and TPC. The experimental value for each run was listed in Table 1. The analysis of variance and correlation coefficients at confidence level of 95% for extract yields and TPC are shown in Table 2. The quadratic model was significant (p < 0.05) with low residual values and the lack of fitness was insignificant for the response. The coefficient of determination (R2) for acetone, ethanol, and methanol extract was observed as 0.9222, 0.7854, and 0.9829 for extract yields and 0.9999, 0.9973, and 0.9954 for TPC respectively. Furthermore, the adjusted R2 value for the extract yield and TPC was found to be 0.9067, 0.6321, 0.9706, and 0.9998, 0.9954, 0.9922 for acetone, ethanol, and methanol respectively, which were considered adequate to describe the models (Table 2). The coefficient of variance for extract yield and TPC were observed as 2.14%, 0.755 (acetone), 9.32%, 2.97 (ethanol), and 3.90%, 2.65 (methanol) respectively, which showed the greater reliability of the experimental runs.
Table 2.
Analysis of variance (ANOVA) for surface quadratic model
| Source | Ethanol | Methanol | Acetone | |||
|---|---|---|---|---|---|---|
| Yield (%) | TPC (mg GAE/g) | Yield (%) | TPC (mg GAE/g) | Yield (%) | TPC (mg GAE/g) | |
| Model | 0.0271 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 |
| A-Ethanol | 0.0178 | < 0.0001 | 0.1253 | < 0.0001 | 0.0001 | < 0.0001 |
| B-Time | 0.0259 | 0.0041 | 0.0001 | < 0.0001 | < 0.0001 | 0.0099 |
| AB | 1.0000 | 0.3716 | 0.0006 | 0.0004 | – | 0.0269 |
| A2 | 0.0536 | < 0.0001 | 0.1318 | 0.0245 | – | < 0.0001 |
| B2 | 0.0896 | < 0.0001 | < 0.0001 | < 0.0001 | – | < 0.0001 |
| Lack of fit | 0.7346 | 0.9701 | 0.6108 | 0.9113 | 0.6053 | 0.7409 |
| R2 | 0.7854 | 0.9973 | 0.9829 | 0.9954 | 0.9222 | 0.9999 |
| Adjusted R2 | 0.6321 | 0.9954 | 0.9706 | 0.9922 | 0.9067 | 0.9998 |
| Predicted R2 | 0.3671 | 0.9950 | 0.9413 | 0.9900 | 0.8644 | 0.9997 |
| Adeq. precision | 6.1371 | 51.3993 | 27.8275 | 59.8149 | 22.2826 | 242.6480 |
Analysis of response surface on extract yield
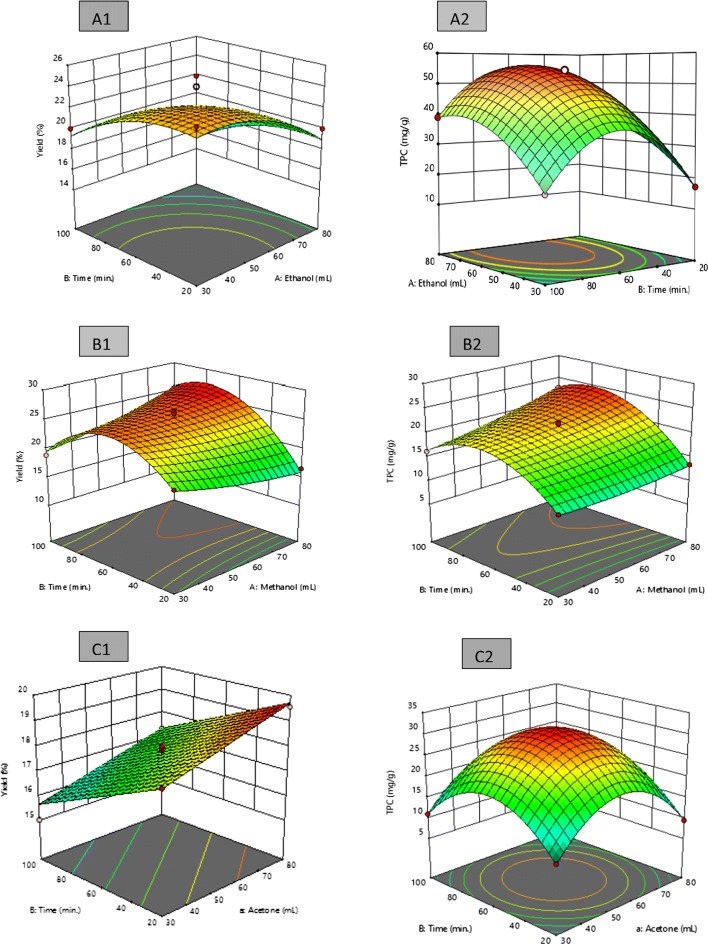
The efficiency of extraction method was influenced by various parameters such as temperature, time, and solvent polarity, and their effects can be seen either independent or interactive (Tan et al. 2013). The different solvent concentrations and extraction times showed a significant influence on extract yield and TPC of M. koenigii leaf extract up to a certain level (Fig. 1) followed by a considerable drop in their value with continuously increase in time and solvent concentration. Acetonic extract showed decrease in its extract yield with increase in extraction time period and increased extract yields with increase in concentration of solvent. Extract showed increase in extract yield with increase in concentration of ethanol up to 65% and time period of 74 min followed by decrease in its yield with further increase in time of extraction and solvent concentration. Methanolic extract resulted in increase in its extract yield with increase in time period up to 80 min and then reduces its value whereas the change in solvent concentration did not affect the extract yield. Methanolic extract showed highest extract yield (27.47%) at a concentration of 80% and time period of 74 min followed by ethanol (22.53%) at a concentration of 50% and time period of 60 min and acetone resulted in reduced extract yield (18.30%) at a concentration of 60% and time period of 45 min. These findings were in agreement with the study reported by Ngo et al. (2017) on Salacia chinensis L. where the variation in yield of solid extract can be explained by change in solubility of compounds with samples.
Fig. 1.
Response surface plots for extract yields (%) and total phenolic content (mg GAE/g) as a function of solvent and time, a Ethanol verses extraction time, b methanol verses extraction time, and c acetone verses extraction time
Analysis of response surface on total phenolic content
The 3-dimensional plots of TPC showed a significant effect of time and solvent (Fig. 1) on M. koenigii leaf extracts. Ethanolic extract represented increased TPC with increase in solvent concentration and time of extraction up to a concentration of 55% and time period of 60 min and leads to decrease in TPC with consecutively increase in extraction time period and solvent concentration. Methanol concentration did not show any influence on TPC whereas increase in time of extraction period decreases the TPC of methanolic extract. Furthermore, acetone showed linearly increase in TPC with increased solvent and extraction time period and then mounted down with further increased solvent concentration and extraction time. High solvent concentration (> 60%) reveals lower TPC due to higher polarity at higher concentration which reduces the extraction of polyphenols whereas absolute solvent do not report higher extraction of phenolics as compared to aqueous solvent at a concentration of 50% and time period of 60 min (Premi and Sharma 2017).
Optimization
Optimization of process variables (time of extraction and solvent concentration) for the recovery of high extract yield and TPC of M. koenigii leaf extract was carried out using response surface methodology. The optimization was applied to a selected range of time of extraction (20–100 min) and solvents rate ethanol/methanol/acetone (20–80%). The variables showed a significant effect on extract yield and TPC as shown by their respective R2 values. The experimental runs obtained for each combination was done and responses were noted (Table 1). The most desirable combinations were obtained as 60% acetone, 50% ethanol and 80% of methanol at an extraction time of 45 min, 60 min, and 74 min with desirability of 0.811, 0.845, and 0.993 respectively (Table 3). At these optimized conditions, the predicted values for extract yields and TPC were observed to be 22.53%, 18.30%, 27.47%, and 62.250 mg GAE/g dried weight, 29.042 mg GAE/g dried weight, 24.85 mg GAE/g dried weight for ethanolic, acetonic, and methanolic extracts respectively. The measured responses had proximity to the predicted ones. Hence, the adequacy of the model was thus reconfirmed. The most desirable combinations were further analysed for their phytochemical properties.
Table 3.
Optimized conditions for the extraction of Murraya koenigii leaf extracts
| Solvents | Solvent ratio (%) | Time of extraction (min) |
|---|---|---|
| Ethanol | 50 | 60 |
| Methanol | 80 | 74 |
| Acetone | 60 | 45 |
Total phenolic content
Solvent concentration plays an important role for the extraction of polyphenols, and flavonoids from various plants extract. The results showed that extracting solvent had a significant effect on total phenolic content (TPC) (Table 4). Ethanolic extract (50%) showed highest TPC value (63.055 mg GAE/g dried weight) followed by water extract (32.495 mg GAE/g dried weight), 60% acetonic extract (28.915 mg GAE/g dried weight), and 80% methanolic extract (24.620 mg GAE/g dried weight). These findings confirmed that solvents play an important role for the extraction of phenolic compounds. The highest TPC value for ethanolic extract could be due to the polarity and solubility of the extracting compounds in comparison to the other solvents (Premi and Sharma 2017). The polarity of acetone, ethanol, and methanol is 0.355, 0.654, and 0.762 respectively. Acetone is more useful for the extraction of polyphenols associated with protein matrix owing to its better cell wall degradation ability for releasing polyphenols whereas ethanol and methanol are more effective for extracting polyphenols linked with fibrous protein (Al-Farsi and Lee 2008). Hence, ethanolic extract showed significantly better antioxidant activity due to its higher phenolic content in comparison to other extracts. These findings were supported by the previous study in which Ngo et al. (2017) suggested that 50% ethanol, acetone and methanol is the best solvent for the extraction of TPC from Salacia chinensis L. Ningappa et al. (2008) observed that the total phenolic content in plant extracts was associated with the improved antioxidant activity.
Table 4.
Phytochemical analysis of optimized extracts
| Extracts | Flavonoid content (μg/g) | Total phenolic content (mg GAE/g) | DPPH IC50 value (µg/mL) |
|---|---|---|---|
| Water | 2.535 ± 0.07c | 32.495 ± 0.02b | 3.033 ± 0.05c |
| 50% Ethanol | 5.760 ± 0.12b | 63.055 ± 0.20a | 1.556 ± 0.09d |
| 80% Methanol | 1.950 ± 0.01d | 24.620 ± 0.18d | 3.259 ± 0.12b |
| 60% Acetone | 6.440 ± 0.05a | 28.915 ± 0.07c | 3.765 ± 0.06a |
All the values are mean ± standard deviation for the obtained triplicate readings. Means with different superscript in same column show significant difference at p ≤ 0.05
DPPH scavenging activity
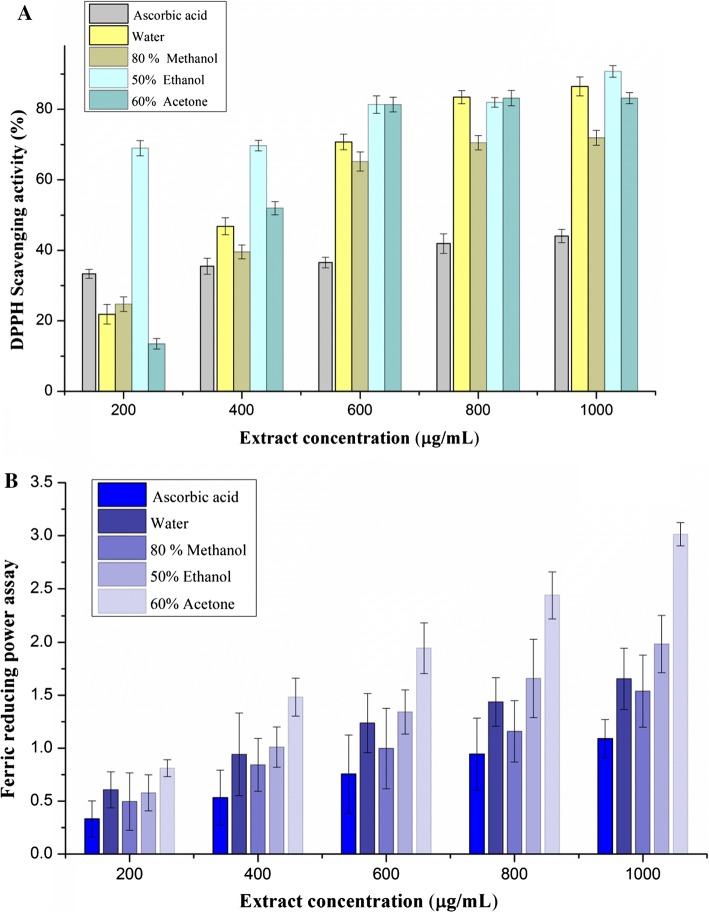
In DPPH scavenging activity, the antioxidants from M. koenigii leaf extracts were able to reduce the stable DPPH radical to the yellow coloured diphenyl picryl hydrazine. Murraya koenigii leaf extract with various solvents exhibited significant difference in their DPPH scavenging activity in a concentration dependent manner (Fig. 2) which is responsible for the antioxidant activity also. Ethanolic extract and ascorbic acid showed highest DPPH scavenging activity as compared to acetonic and methanolic extract. The IC50 values of ethanolic, ascorbic acid, methanolic, and acetonic extract were found to be 1.556 µg/mL, 3.132 µg/mL, 3.259 µg/mL, and 3.765 µg/mL respectively (Table 4). Higher antioxidant activity of ethanolic extract could be due to acidic pH, polarity of the solvent, and reducing ability of the extracted phenolics into the solvent (Ramos et al. 2017). Biswas et al. (2012) reported that an increase in antioxidant activity by decreasing the pH value of the medium. Fu et al. (2016) suggested that aqueous solvent had high extraction efficiency in sweet potato leaves extracts which leads to higher antioxidant activity and total phenols. It can also be related to the presence of catechin, myricetin, isoquercitrin, rutin which act as strong antioxidants. Other extracts showed significant difference in their antioxidant activity which can be related to their chemical composition and phenolic content. Phenols and other similar compounds found to be responsible to quench the free radicals and inhibit the chain reactions during oxidation (Shahidi and Zhong 2015). Moreover, ethanolic extract showed more DPPH scavenging activity than ascorbic acid (positive control) which could be due to overlapped spectra of compounds like anthocyaninidin, flavonoids, and kaempferol indicating a higher scavenging activity. Choudhary et al. (2012) observed the highest radical scavenging activity in Tricosanthes cucumerena leaf extract as compared to BHT. The free radical scavenging activity is also attributed to high reactivity and hydroxyl substituents of the antioxidant.
Fig. 2.
DPPH scavenging activity (a) and ferric reducing activity (b) of optimized extracts
Ferric reducing power assay
Ferric reducing power assay serves as an important parameter for antioxidant activity which shows various uncontrolled effects on human body. Free radicals (oxidized intermediate) then will be allowed to react with extract of herbs, spices, condiments, and lead to the formation of more stable compounds to terminate the free radical chain reactions. Ferric reducing power and antioxidant activity can be interrelated owing to the presence of reductones and breakdown of free radical chains respectively (Ningappa et al. 2008). Reducing power determined through the reduction of Fe(III) to Fe(II) where the yellow colour test sample turns into perls prussian blue colour and reduces the ferric cyanide complex to ferrous form. In this study, ferric reducing power activity was observed to be improved by increasing the concentration of extract (Fig. 2). The reducing activity could be associated with loss of electron from the extract followed by hydrogen transfer to the reactive free radicals and leads to stabilization of free radicals and terminates the chain reaction (Muthukumar et al. 2014). Acetonic extract showed more reducing power (2.814) than ascorbic acid (1.899) at a concentration of 1 mg/mL. This might be allied to the presence of maximum flavonoid content in acetonic extract which acts as a strong antioxidant (Trabelsi et al. 2012). Wang et al. (2003) observed that natural antioxidants are involved in dissolution of free radical reactions and improve the reducing activity.
Total flavonoid content
Various solvents showed significant effect on total flavonoid content of M. koenigii leaf extracts (Table 4). 60% acetonic extract showed the highest flavonoid content (6.440 µg/g dried weight) followed by 50% ethanol (5.760 µg/g dried weight), water (2.535 µg/g dried weight) and 80% methanol (1.950 µg/g dried weight) due to its ability to denature the polyphenol oxidase and more efficiently degradation of cell wall thus promoting the release of more flavonoids into extract (Othman et al. 2014). Methanolic extract exhibited reduced flavonoid content which could be due to its less solubility and difference in the polarities of the extracted compounds into various solvents (Tan et al. 2013). Thus, acetone was found to be more appropriate solvent for the extraction of flavonoids due to the presence of methoxylated and free aglycones in plants such as luteolin, kaempferol, quercetin which are less soluble in water (Chebil et al. 2007). French marigolds showed more flavonoid content in acetonic extract in comparison to ethanolic and methanolic extract (Munhoza et al. 2014). Several studies reported that extraction of bioactive compounds such as flavonoids, tannins, phenolic compounds was improved in aqueous solvent in comparison to absolute solvent (Meneses et al. 2013). The results were supported by previous studies on Silacia chinensis L. and Macadamia tetraphylla skin waste where Ngo et al. (2017) and Dailey and Vuong (2015) reported that solvents had a significant effect on flavonoid content.
Conclusion
The optimization of extraction process parameters such as solvent concentration and time of extraction, for M. koenigii leaf extract was successfully done by response surface methodology. It was found that solvents and time of extraction played a significant role for the extraction of M. koenigii leaf extracts. Acetone, ethanol, methanol resulted in extract yields of 18.30%, 22.53%, 27.47% at a time period (45, 60, 74 min) and concentration (60%, 50%, 80%) respectively. The results were correlated with dependent variables and exhibited significant model with higher R2 values. The optimized extracts were analysed for their phytochemical analysis in which ethanolic extract presented higher DPPH scavenging activity and total phenolic content whereas acetonic extract resulted in higher ferric reducing activity and flavonoid content. The phenolic and flavonoid content seem to be responsible for the antioxidant activity of the extracts. The study demonstrates the potential of M. koenigii leaf extract as a rich source of phenolic components and a strong antioxidant. Hence, it can be used extensively in our food systems for the prevention of many diseases.
Acknowledgements
The author would like to acknowledge the Department of Food Science and Technology, Pondicherry University, Pondicherry, for providing amenities. The author would also be grateful to UGC for providing Senior Research Fellowship.
Compliance with ethical standards
Conflict of interest
The authors declared no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Al-Farsi MA, Lee CY. Optimization of phenolics and dietary fibre extraction from date seeds. Food Chem. 2008;108:977–985. doi: 10.1016/j.foodchem.2007.12.009. [DOI] [PubMed] [Google Scholar]
- Biswas AK, Chatli MK, Sahoo J. Antioxidant potential of curry (Murraya koenigii L.) and mint (Mentha spicata) leaf extracts and their effect on colour and oxidative stability of raw ground pork meat during refrigeration storage. Food Chem. 2012;133:467–472. doi: 10.1016/j.foodchem.2012.01.073. [DOI] [PubMed] [Google Scholar]
- Brahmi F, Mechri B, Dhibi M, Hammami M. Effect of growth stage and solvent extract on the antioxidant potential of olive leaves. J Plant Sci. 2015;3:1–7. [Google Scholar]
- Chebil Humeau C, Anthoni J, Dehez F, Engasser JM, Ghoul M. Solubility of flavonoids in organic solvents. J Chem Eng Data. 2007;52:1552–1556. doi: 10.1021/je7001094. [DOI] [Google Scholar]
- Chitra M, Muga V, Dhanarasu S, Al-hazimi AM. Screening of phytochemical and in vitro activity of Euphorbia hirta L. J Chem Pharm Res. 2011;3(6):110–114. [Google Scholar]
- Choudhary S, Tanwer BS, Vijayvergia R. Total phenolics, flavonoids and antioxidant activity of Tricosanthes cucumerena Linn. J Drug Invent Today. 2012;4(5):368–370. [Google Scholar]
- Dailey A, Vuong QV (2015) Effect of extraction solvents on recovery of bioactive compounds and antioxidant properties from macadamia (Macadamia tetraphylla) skin waste. Cogent Food Agric 1(1). Article ID 1115646
- Dent M, Uzelac VD, Peni M, Brncic M, Bosiljkov T, Levaj B. The effect of extraction solvents, temperature, and time on the composition and mass fraction of polyphenols in Dalmatian wild sage (Salvia officinalis L.) extracts. J Food Technol Biotechnol. 2013;51(1):84–91. [Google Scholar]
- Fu Z, Tu Z, Zhang L, Wang H, Wen Q, Huang T. Antioxidant activities and polyphenols of sweet potato (Ipomoea batatas L.) leaves extracted with solvents of various polarities. Food Biosci. 2016;15:11–18. doi: 10.1016/j.fbio.2016.04.004. [DOI] [Google Scholar]
- Jain V, Momin M, Laddha K. Murraya Koenigii: an updated review. Int Ayurv Herb Med. 2012;2(4):607–627. [Google Scholar]
- Jayapriya G, Gricilda SF. Phytochemical analysis and antimicrobial efficacy of Rhinacanthus nasutus (l) Linn. J Pharma Phytochem. 2015;3(6):83–86. [Google Scholar]
- Meneses NGT, Martins S, Teixeira JA, Mussatto SI. Influence of extraction solvents on the recovery of antioxidant phenolic compounds from Brewer’s spent grains. Sep Purif Technol. 2013;108:152–158. doi: 10.1016/j.seppur.2013.02.015. [DOI] [Google Scholar]
- Mohamad M, Ali MW, Ripin A, Ahmad A. Effect of extraction process parameters on the yield of bioactive compounds from the roots of Eurycoma longifolia. J Teknol. 2013;60:51–57. [Google Scholar]
- Munhoza VM, Longhini R, Souza JRP, Eneri JAC, Mello EV, Lopes GC, Joao CPM. Extraction of flavonoids from Tagetes patula: process optimization and screening for biological activity. Rev Bras Farmacogn. 2014;24:576–583. doi: 10.1016/j.bjp.2014.10.001. [DOI] [Google Scholar]
- Muthukumar M, Naveena BM, Vaithiyanathan S, Sen AR, Sureshkumar K. Effect of incorporation of Moringa oleifera leaves extract on quality of ground pork patties. J Food Sci Technol. 2014;51(11):3172–3180. doi: 10.1007/s13197-012-0831-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngo TV, Scarlett CJ, Bowyer MC, Ngo PD, Vuong QV. Impact of different extraction solvents on bioactive compounds and antioxidant capacity from the root of Salacia chinensis L. J Food Qual. 2017 doi: 10.1155/2017/9305047. [DOI] [Google Scholar]
- Ningappa MB, Dinesha R, Srinivas L. Antioxidant and free radical scavenging activities of polyphenol-enriched curry leaf (Murraya koenigii L.) extracts. Food Chem. 2008;106:720–728. doi: 10.1016/j.foodchem.2007.06.057. [DOI] [Google Scholar]
- Ningappa MB, Dhananjaya BL, Dinesha R, Harsha R, Srinivas L. Potent antibacterial property of APC protein from curry leaves (Murraya koenigii L.) Food Chem. 2010;118:747–750. doi: 10.1016/j.foodchem.2009.05.059. [DOI] [Google Scholar]
- Othman A, Mukhtar NJ, Ismail NS, Chang SK. Phenolics, flavonoids content and antioxidant activities of 4 Malaysian herbal plants. Int Food Res J. 2014;21(2):759–766. [Google Scholar]
- Premi MS, Sharma HK. Effect of extraction conditions on the bioactive compounds from Moringa oleifera (PKM 1) seeds and their identification using LC–MS. J Food Meas Charact. 2017;11:213–225. doi: 10.1007/s11694-016-9388-y. [DOI] [Google Scholar]
- Rahman MM, Gray AI. A benzoisofuranone derivative and carbazole alkaloids from Murraya koenigii and their antimicrobial activity. Phytochemistry. 2005;66:1601–1606. doi: 10.1016/j.phytochem.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Ramos LR, Santos JS, Daguer H, Valese AC, Cruz AG, Granato D. Analytical optimization of a phenolic-rich herbal extract and supplementation in fermented milk containing sweet potato pulp. Food Chem. 2017;221:950–958. doi: 10.1016/j.foodchem.2016.11.069. [DOI] [PubMed] [Google Scholar]
- Sablania V, Bosco SJD, Rohilla S, Shah MA. Microencapsulation of Murraya koenigii L. leaf extract using spray drying. J Food Meas Charact. 2018;12(2):892–901. doi: 10.1007/s11694-017-9704-1. [DOI] [Google Scholar]
- Shah MA, Bosco SJD, Mir SA. Effect of Moringa oleifera leaf extract on the physicochemical properties of modified atmosphere packaged raw beef. Food Packag Shelf Life. 2015;3:31–38. doi: 10.1016/j.fpsl.2014.10.001. [DOI] [Google Scholar]
- Shahidi F, Zhong Y. Measurement of antioxidant activity. J Funct Foods. 2015;18:757–781. doi: 10.1016/j.jff.2015.01.047. [DOI] [Google Scholar]
- Tan MC, Tan CP, Ho CW. Effects of extraction solvent system, time and temperature on total phenolic content of henna (Lawsonia inermis) stems. Int Food Res J. 2013;20(6):3117–3123. [Google Scholar]
- Tan SP, Parks SE, Stathopoulos CE, Roach PD. Extraction of flavonoids from bitter melon. J Food Nutr Sci. 2014;5:458–465. [Google Scholar]
- Trabelsi N, Oueslati S, Falleh H, Waffo-Téguo P, Papastamoulis Y, Mérillon JM, Abdelly C, Ksouri R. Isolation of powerful antioxidants from the medicinal halophyte Limoniastrum guyonianum. Food Chem. 2012;135:1419–1424. doi: 10.1016/j.foodchem.2012.05.120. [DOI] [PubMed] [Google Scholar]
- Wang L, Yen JH, Ling HL, Wu MJ. Antioxidant effect of methanol extracts from lotus plumule and blossom (Nelumbo nucifera Gertn.) J Food Drug Anal. 2003;11:60–66. [Google Scholar]