Abstract
Currently available high-resolution transducers allow a detailed ultrasound (US) assessment of skin tumors. US complements clinical examination, dermoscopy, and biopsy in the initial differential diagnosis, surgical planning, locoregional staging, and follow-up of patients with skin malignancies. It is important for dermatologists, skin surgeons, and US operators to be aware of the US imaging findings and to recognize the clinical scenarios where imaging is indicated in the management of skin cancer. The purpose of this review article is to address the most common indications for US in skin oncology and to provide a comprehensive guide to the gray-scale and color-Doppler findings in cutaneous malignant tumors.
Keywords: Skin ultrasound, Dermatology ultrasound, Skin tumors, Soft tissues, Melanoma
Introduction
Skin tumors are by far the most frequent malignancies of the body. These tumors are classified according to their cells of origin (known or putative). These cells can be located in the epidermis, dermis or skin appendages. Malignant tumors arising from the epidermis include cutaneous melanoma (CM) and non-melanoma cancers, such as basal cell carcinoma (BCC), squamous cell carcinoma (SCC), and Merkel cell carcinoma (MCC). Main malignancies developing from dermal components are atypical fibroxanthoma, dermatofibrosarcoma protuberans (DFSP), angiosarcoma, and Kaposi’s sarcoma (KS). Malignant tumors of skin appendages include sebaceous carcinoma, eccrine porocarcinoma, and microcystic adnexal carcinoma. To all these histotypes, primary and secondary cutaneous lymphomas, as well as skin metastasis, must also be added. CM only represents the 4% of all skin cancers, but it is by far the most important one because of its lethality. BCC and SCC are grouped as epitheliomas and represent up to 95% of skin malignancies (BCC, 3/5 and SCC, 2/5) [1, 2] (Fig. 1). The remaining skin tumors are uncommon but some of them can have a very aggressive behavior.
Fig. 1.
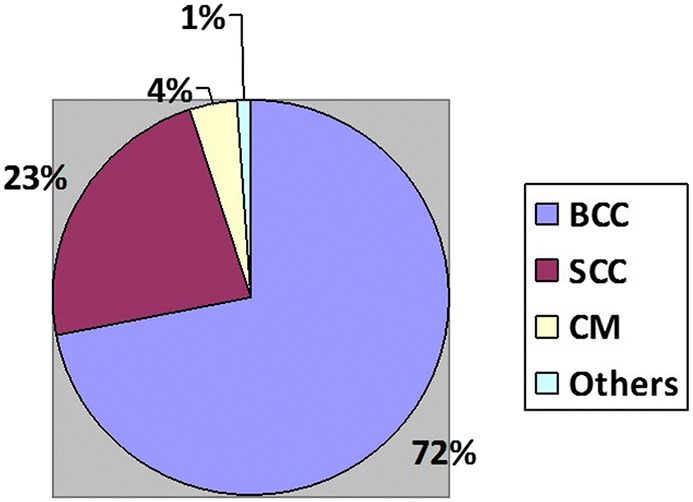
Approximate prevalence of skin cancers. BCC basal cell carcinoma, SCC squamous cell carcinoma, CM cutaneous melanoma
Although clinicians primarily rely on their eyes, a number of instruments have been developed to improve skin tumors assessment. These include digital photography, dermoscopy, reflectance confocal microscopy, optical coherence tomography, and high-frequency ultrasound (US). US is being employed with increasing frequency in the assessment of skin disorders [3–11]. In this article, we review the scanning methodology, the B-mode and Doppler findings, and the practical role of US in patients with skin malignancies.
Scanning methodology
High frequency (≥ 15 MHz), broadband, linear transducers are necessary [3]. Transmission frequency, depth, and time-gain compensation curve are regulated according to the position of the lesion (dermis, superficial hypodermis, deep hypodermis) [4–7]. Beam focus is placed at level of or immediately below the lesion. Gain is regulated to maximize image contrast. Volume acquisition of multiplanar and 3D images can be helpful [3, 8].
Color/power-Doppler mode is set to detect slow flows, which means high Doppler frequency, low pulse repetition frequency, low wall filter, and color gain immediately below the noise threshold [3, 7, 8]. Focus is placed right below the lesion. Spectral analysis of intratumor vessels may be obtained, recording the peak velocity and the resistive index.
An abundant amount of gel is placed on the lesion. Gel pad spacers may also be employed, although they may compress the thin vessels. The hand pressure applied on the probe is minimized (both for B-mode and Doppler mode) and the little finger can be put below the probe footprint to improve the stability. Lesions are imaged according to longitudinal and transverse planes. Both the maximal width and the vertical thickness at the deepest point of the lesion is measured (electronic zoom may be useful).
Basal cell carcinoma
BCC occurs in the elderly on sun-exposed areas, particularly the face [12]. While BCC is rarely metastatic, large or aggressive, tumors can infiltrate critical anatomic structures [13]. It classically presents as a pearlescent nodule or ulcer. Some lesions may appear as poorly defined plaques or areas of skin induration. Nodular variant, the most common, consists of well-circumscribed nodules of basaloid cells with characteristic peripheral palisading, retraction artifact, and stromal mucin. More infiltrative variants include the micronodular pattern and the morpheic pattern. These infiltrative histologic variants generally require wider surgical margins or Mohs surgery, in contrast with non-infiltrative variants, which may be treated with standard surgical excision [13]. Up to 7% of BCCs are pigmented, mimicking melanoma [12]. For BCC, as well as for SCC and adnexal carcinomas, but not MCC, the current staging system consider T1 for lesions with a larger diameter up to 20 mm, T2 for a 20–39 mm diameter, and T3 for a diameter 40 mm or above [14]. Tumors thicker than 6 mm or tumors invading below the subcutaneous layer are automatically classified as T3, whatever their diameter is [14].
BCC lesions appear as well-defined, oval or slightly irregular, hypoechoic lesions that usually present hyperechoic spots [12, 15, 16]. A high density of hyperechoic spots, representing nests and not calcifications, has been reported to be more frequent in high-risk of recurrence subtypes such as micronodular or morpheaform variants [17]. Low flow arterial and venous vessels are detected within or at the bottom of the lesion. The appearance of tortuous vessels should suggest other tumors [7]. At elastography BCC is usually rather stiff. However, infiltrative BCCs have increased marginal stiffness in comparison with non-infiltrative BCCs (88% vs. 19%) and this finding may help predicting before treating the presence of an infiltrating variant [13] (Fig. 2 and 3).
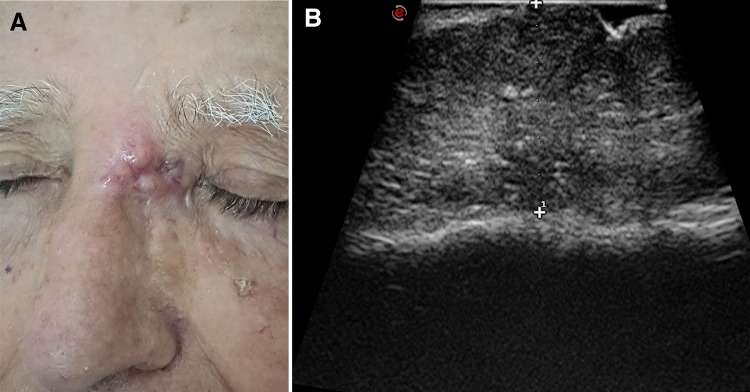
Fig. 2.
Basal cell carcinoma of the nasion. Patient photograph (a). Ultrasound scan with tumor thickness measurement (b). The lesion is hypoechoic and relatively homogeneous, with some hyperechoic spots. It is adherent to the bony cortex
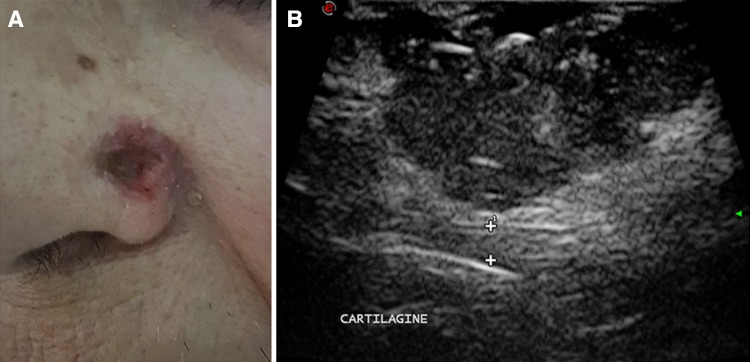
Fig. 3.
Ulcerated basal cell carcinoma of the nose ala. Patient photograph (a). Ultrasound scan (b). The lesion is hypoechoic and relatively homogeneous, with some hyperechoic spots. The tumor does not infiltrate the cartilage (calipers)
Squamous cell carcinoma
SCC arises on sun-exposed sites of fair-skinned individuals. The head, neck, and dorsal hands are commonly affected. SCC may be associated with local invasiveness, recurrence, and lymph nodes metastasis. US imaging of SCC (primary tumor/surgical scar and regional lymph nodes) is indicated for patients with recurrent tumors, tumors > 2 cm in diameter or > 2 mm in depth, poorly differentiated histology, perineural invasion, lymphovascular invasion, and specific anatomic site (ears, lips, anogenital regions) [2, 18, 19]. Some SCCs present as red patches or plaques, others as hyperkeratotic or ulcerated lesions. The classical form of SCC comprises sheets and nests of relatively large atypical epithelial cells with pleomorphic nuclei and variable numbers of mitotic figures. Poorly differentiated lesions show a greater degree of cytological atypia and are scarcely keratinized. Deep keratinization is present instead in better differentiated tumors. Spindle cell (sarcomatoid) variant also occurs.
At US imaging SCC presents as a heterogeneously hypoechoic lesion with irregular borders, lack of hyperechoic spots, and tend to involve the deeper layers [16]. The low-flow vascular pattern is increased diffusely throughout the entire lesion but particularly at the periphery [7] (Fig. 2 and 4).
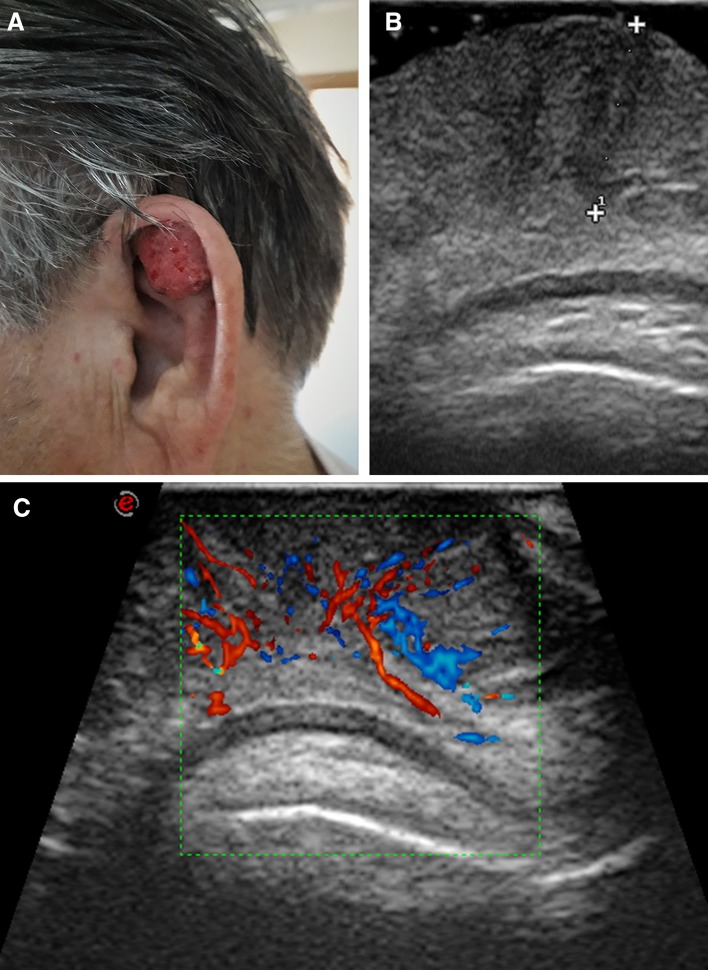
Fig. 4.
Ear scapha squamous cell carcinoma. Patient photograph (a). Ultrasound scan (b). The 7-mm thick lesion (calipers) is hypoechoic and relatively homogeneous. There is no infiltration of the cartilage. Color-Doppler scan (c). Discrete vascularization, with vessels mostly arranged vertically, starting from two deep vascular poles
Cutaneous melanoma
CM typically occurs in pale-skinned Caucasians. Rare in childhood, this tumor is predominantly seen in the middle aged and elderly. However, the worldwide incidence of CM is rising, particularly among younger individuals. About 87,000 new cases/year are diagnosed in the USA, with more than 9000 people dying of invasive CM. Overall 5-year survival is 60%, although behavior is variable. Head, back, and lower extremities (particularly in women) are the most common sites [10]. CM is a malignancy of skin melanocytes and fall into four basic categories: superficial spreading CM, lentigo maligna, acral lentiginous CM, and nodular CM. CM presents as pigmented macules or nodules, that can show irregular borders and ulceration. Amelanotic CM account for about 5% of cases. CMs are asymmetrical and poorly circumscribed lesions with architectural disturbance and usually marked cytological atypia. Melanoma cells can be categorized in epithelioid and spindle cells. The current standard for regional lymph nodes staging is represented by the sentinel lymph-node excision biopsy procedure. However, it is important to indicate if histologically involved lymph nodes were clinically occult or detectable [14].
At US melanoma appears as an oval/fusiform, well-defined, homogeneous, and echo-poor lesion [20, 21]. The hyperechoic epidermis line is seen above the tumor, except that for ulcerated CMs. Acoustic transmission is frequently increased. Correlation of the sonographic and histologic thickness has been reported as excellent. However, peritumoral inflammatory infiltrates may be present, falsely increasing the tumor depth measurement. Satellite and in-transit nodules appear as markedly hypoechoic, relatively homogeneous and well demarcate, within the dermis or hypodermis [20–22].
Differently from nevi, CMs are hypervascular, although flow signal can be hard or impossible to detect in very thin lesions. CM tend to show hypervascularity due to their angiogenic capability [20, 21]. The degree of tumoral vascularization correlates with the risk of locoregional of the lymph nodes and with patient prognosis (Fig. 5).
Fig. 5.
Sole melanoma. Patient photograph (a). Ultrasound scan (b). The 3-mm thick tumor is hypoechoic and homogeneous. Directional power-Doppler scan (c). Very intense tumor vascularization
Merkel cell carcinoma
MCC is a relatively rare but increasing neuroendocrine tumor. Mean age at diagnosis is around 70 years, with a moderate prevalence of males. Risk factors include ultraviolet exposure and immunosuppression. The 5-year survival rate is 64% for local disease, 39% for regional nodal involvement, and 18% for distant metastatic disease. Most cases arise in the face, neck, upper extremities, and buttocks [23]. Sentinel lymph-node biopsy is included specifically in the MCC current pN staging system [14].
MCC typically presents as an asymptomatic, rapidly expanding, firm-elastic, pink–purple, dome-shaped nodule. It is a localized, ill-defined dermal proliferation of uniform, small blue cells, arranged in strands and nests.
MCC lesions are located in the dermis and may be oval or dome-shaped. They are hypoechoic, with variable degrees of heterogeneity. Borders are usually ill-defined. Calcifications are absent. A slight dorsal enhancement may be seen, as well as a thickening of the overlying epidermis [23, 24]. Vascularization is typically rich and shows vessels with a chaotic distribution, although a kind of vertical arrangement can be seen. Arterial flows show a mean systolic velocity of 11 cm/s and a mean resistive index of 0.57 [23, 24].
Dermatofibrosarcoma protuberans
DFSP is a rare monoclonal cutaneous fibroblastic sarcoma. It typically arises on the trunk or extremities in the fourth decade and is more common in males. It may be locally aggressive and recurring. The deeply infiltrating growth of DFSP can make tumoral extent challenging to predict based on a clinical examination alone [18]. DSFP starts as a firm dusky plaque and slowly grows into a nodular or multinodular pattern. It is composed of bland monomorphic spindle cells with elongated nuclei, arranged in a storiform pattern, with poorly defined infiltrative tumor margins. About 10% of cases develop a small fibrosarcomatous component, with an associated risk of metastasis. DFSP lesions are located in the subcutaneous fat layer, abutting against the skin, and have a broad base.
US demonstrates an ill-defined hypoechoic pattern in the surface and hyperechogenicity in the deep part. This tumor commonly invades fascial or muscular layers [7, 25]. Hypercellular DSFP are rather homogeneous while tumors with large fibrous tissue components show a mixed appearance with echoic areas. The margin can be focally lobulated or may show irregular pseudopodia-like projections to the fascial or muscle layers. Flow signals can be detected throughout the entire tumor or only at its peripheral portions [25] (Fig. 6).
Fig. 6.
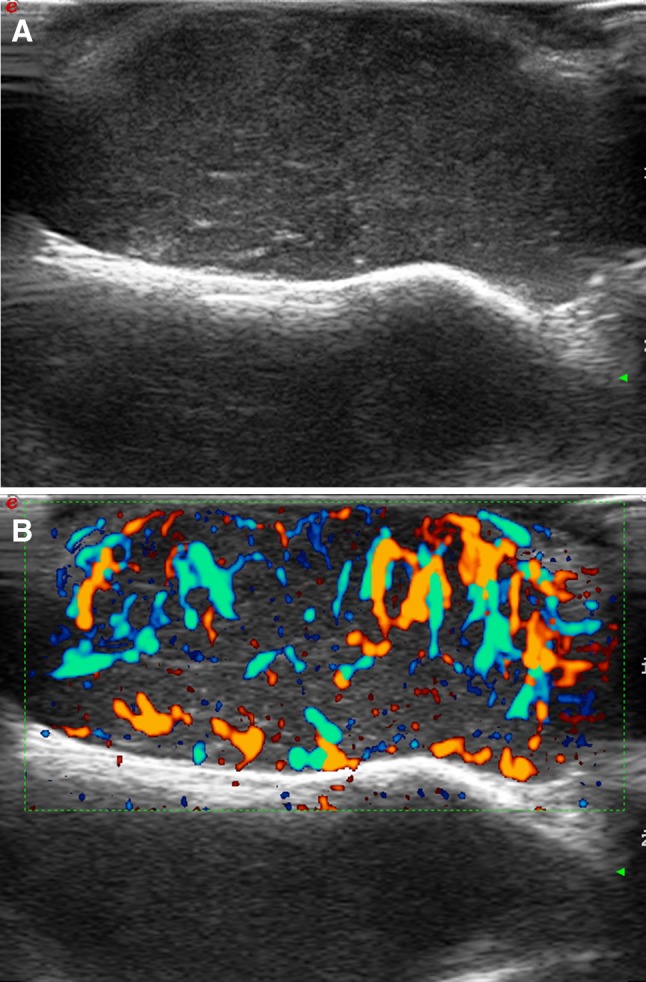
Dermatofibrosarcoma protuberans of the head (frontal region), local recurrence. Ultrasound scan (a). Thick, homogeneous, and ill-defined lesion, adherent to the bone surface. Directional power-Doppler scan (b). Strong, irregular vascularization at power-Doppler imaging
Kaposi sarcoma
KS is a low-grade mesenchymal tumor involving the blood and lymphatic vessels. It is associated with HIV infection but may also occur endemically (Jewish and Mediterranean African populations) and in transplant recipients. KS lesions are usually identified in the lower leg, except in the case of AIDS-related KS where the lesions mainly affect the face and trunk [26]. KS presents as asymptomatic purple patches, later developing into nodules or plaques. Histology demonstrates a proliferation of endothelial-lined slit-like vascular channels and spindle cells.
KS lesions appear as solid and hypoechoic lesions, located in the dermis but sometimes extending to the subcutaneous [27]. Nodules are usually rather homogeneous, with well-defined, frequently lobulated, borders. Plaques are less homogeneous, showing irregular edges and acicular morphology. Infiltration of deeper structures is possible [26]. The intralesional vascularization is more marked at the lower pole in nodules and poor or absent vascularization in plaques. AIDS-KS lesions may show a greater vascularization than classic KS ones [26] (Fig. 7).
Fig. 7.
Thigh metastases from Kaposi sarcoma. Patient photograph (a). Ultrasound scans, taken at 13.5 MHz (b) and at 22 MHz (c). The latter image shows the two hypodermis, hypoechoic nodules with a superior detail compared to the former
Primary cutaneous lymphoma
Primary cutaneous lymphoma (PCL) is rare and must be distinguished from secondary skin involvement by systemic lymphoma. Mycosis fungoides is a neoplastic proliferation of T-cells, which accounts for approximately half of PCLs. Patch, plaque and tumor stages of disease are recognized. Mycosis fungoides shows atypical, cerebriform lymphocytes with a surrounding halo infiltrating the epidermis, arranged individually or in small aggregates. Cutaneous B cell lymphoma comprises a heterogeneous group of tumors, often presenting as infiltrated plaques and irregular infiltration of lymphoma cells between dermal collagen bundles.
Lymphoma cell varieties have mixed echo patterns. The echo poor areas may be mistaken for fluid collections or cysts [7, 28, 29]. Although rarely, a subcutaneous panniculitis-like pattern can also be seen, particularly in T-lymphoma. This pattern may sometime mimic cellulitis (panniculitis) [28]. Vascularity of cutaneous lymphomas is variable, ranging from the less common hypovascular pattern to the more common hypervascular one [28, 29]. A vertical disposition of the vessels may be seen. The resistive index is relatively low.
Role in US patients with skin cancer
Although the majority of skin tumors can be treated without additional information provided by imaging studies, large or aggressive high-risk cancers or those that compromise relevant anatomic structures necessitate additional information to optimize management [17, 18]. US examination allows a fast and accurate assessment of the tumoral diameters, including thickness, as well as provides the extent of involvement of deeper layers and improves the performance of loco-regional staging [4–6, 14, 17, 30]. US-based categorization of epitheliomas into superficial (thickness < 5 mm) and deep (thickness ≥ 5 mm) may help to decide for non-surgical treatments, such as brachytherapy [31]. Appropriate pre-surgical assessment of tumor extent can help reducing the extent of surgical defects. On the other side, infiltration of relevant anatomic structures, including fascia, muscles, cartilages, and bone cortex, can be effectively recognized using US and help the surgeon for choosing a more aggressive approach. The presence of a firm, fixed tumor mass or focal tenderness over a bony or cartilaginous margin is suspicious for invasion, and an US imaging is indicated in these cases [11]. Particularly in the head, where the subcutaneous layer is very thin, there is risk of bony involvement and US is an effective alternative to CT and MRI in establishing the presence of calvarium infiltration. Moreover, an US-guided pre-operative mapping of the lesion and the detection of relevant neurovascular structures close to the lesion can decrease the surgical risk and improve the cosmetic prognosis of the patient [1, 8, 30]. Non-palpable, satellite lesions may also be detected. Pre-surgical information provided by US allows improving the surgical planning, which can help to decrease the time spent on surgery and to establish the site and size of the excision and identify the optimal technique for surgical closure. Moreover, post-surgical surveillance of high-risk cases allows an early detection of local or nodal recurrence [30]. It is, however, our experience that, with some notable exceptions, US is underemployed by dermatologists and surgeons in the assessment of skin tumors and that more efforts are needed to make spread this US application worldwide.
Acknowledgements
The authors are grateful to Alessandra Trocino, librarian at the National Cancer Institute Fondazione Pascale of Naples, Italy, for the bibliographic assistance.
Abbreviations
- CM
Cutaneous melanoma
- BCC
Basal cell carcinoma
- DFSP
Dermatofibrosarcoma protuberans
- KS
Kaposi sarcoma
- MCC
Merkel cell carcinoma
- PCL
Primary cutaneous lymphoma
- SCC
Squamous cell carcinoma
- US
Ultrasound
Funding
The authors declare that they did not receive any funding for this study.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This is a review article. The images included are from current clinical practice. Anyway, all procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
This is a review article. The images included are from current clinical practice. For retrospective chart reviews, the authors obtained the consent requirement to be waived.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bard RL. High-frequency ultrasound examination in the diagnosis of skin cancer. Dermatol Clin. 2017;35:505–511. doi: 10.1016/j.det.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 2.Que SKT, Zwald FO, Schmults CD. Cutaneous squamous cell carcinoma: incidence, risk factors, diagnosis, and staging. J Am Acad Dermatol. 2018;78:237–247. doi: 10.1016/j.jaad.2017.08.059. [DOI] [PubMed] [Google Scholar]
- 3.Wortsman X, Alfageme F, Roustan G, Arias-Santiago S, Martorell A, Catalano O, Scotto di Santolo M, Zarchi K, Bouer M, Gonzalez C, Bard R, Mandava A, Gaitini D. Guidelines for performing dermatologic ultrasound examinations by the DERMUS Group. J Ultrasound Med. 2016;35:577–580. doi: 10.7863/ultra.15.06046. [DOI] [PubMed] [Google Scholar]
- 4.Alfageme Roldán F. Handbook of skin ultrasound. Charleston: CreateSpace Independent Publishing Platform; 2013. [Google Scholar]
- 5.Alfageme Roldán F. Ultrasound skin imaging. Actas Dermosifiliogr. 2014;105:891–899. doi: 10.1016/j.ad.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 6.Kleinerman R, Whang TB, Bard RL, Marmur ES. Ultrasound in dermatology: principles and applications. J Am Acad Dermatol. 2012;67:478–487. doi: 10.1016/j.jaad.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 7.Wortsman X, Jemec GBE. Dermatologic ultrasound with clinical and histologic correlations. 1. New York: Springer; 2013. [Google Scholar]
- 8.Wortsman X. Common applications of dermatologic sonography. J Ultrasound Med. 2012;31:97–111. doi: 10.7863/jum.2012.31.1.97. [DOI] [PubMed] [Google Scholar]
- 9.Esposito F, Ferrara D, Di Serafino M, et al. Classification and ultrasound findings of vascular anomalies in pediatric age: the essential. J Ultrasound. 2018 doi: 10.1007/s40477-018-0342-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aluja Jaramillo F, Quiasúa Mejía DC, Martínez Ordúz HM, et al. Nail unit ultrasound: a complete guide of the nail diseases. J Ultrasound. 2017;20:181. doi: 10.1007/s40477-017-0253-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elia F, Paolino G, Donati M, et al. Ultrasound pattern of a rare skin disease: multiple miliary osteoma cutis. J Ultrasound. 2016;19:145. doi: 10.1007/s40477-015-0184-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wortsman X. Sonography of facial cutaneous basal cell carcinoma: a first-line imaging technique. J Ultrasound Med. 2013;32:567–572. doi: 10.7863/jum.2013.32.4.567. [DOI] [PubMed] [Google Scholar]
- 13.Alfageme F, Salgüero I, Nájera L, Suarez ML, Roustan G. Increased marginal stiffness differentiates infiltrative from noninfiltrative cutaneous basal cell carcinomas in the facial area: a prospective study. J Ultrasound Med. 2018;1:1. doi: 10.1002/jum.14880. [DOI] [PubMed] [Google Scholar]
- 14.Keohane SG, Proby CM, Newlands C, Motley RJ, Nasr I, Mohd Mustapa MF, British Association of Dermatologists (Squamous and Basal Cell Carcinoma Guideline Development Groups), Slater DN; Royal College of Pathologists (Skin Cancer Lead) The new 8th edition of TNM staging and its implications for skin cancer: a review by the British Association of Dermatologists and the Royal College of Pathologists, U.K. Br J Dermatol. 2018;179(4):824–828. doi: 10.1111/bjd.16892. [DOI] [PubMed] [Google Scholar]
- 15.Bobadilla F, Wortsman X, Muñoz C, Segovia L, Espinoza M, Jemec GB. Pre-surgical high resolution ultrasound of facial basal cell carcinoma: correlation with histology. Cancer Imaging. 2008;8:163–172. doi: 10.1102/1470-7330.2008.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.MacFarlane D, Shah K, Wysong A, Wortsman X, Humphreys TR. The role of imaging in the management of patients with nonmelanoma skin cancer: diagnostic modalities and applications. J Am Acad Dermatol. 2017;76:579–588. doi: 10.1016/j.jaad.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 17.Wortsman X, Vergara P, Castro A, Saavedra D, Bobadilla F, Sazunic I, Zemelman V, Wortsman J. Ultrasound as predictor of histologic subtypes linked to recurrence in basal cell carcinoma of the skin. J Eur Acad Dermatol Venereol. 2015;29:702–707. doi: 10.1111/jdv.12660. [DOI] [PubMed] [Google Scholar]
- 18.Humphreys TR, Shah K, Wysong A, Lexa F, MacFarlane D. The role of imaging in the management of patients with nonmelanoma skin cancer: when is imaging necessary? J Am Acad Dermatol. 2017;76:591–607. doi: 10.1016/j.jaad.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 19.Que SKT, Zwald FO, Schmults CD. Cutaneous squamous cell carcinoma: management of advanced and high-stage tumors. J Am Acad Dermatol. 2018;78:249–261. doi: 10.1016/j.jaad.2017.08.058. [DOI] [PubMed] [Google Scholar]
- 20.Catalano O, Setola SV, Vallone P, Mattace Raso M, Gallipoli D’Errico A. Sonography for locoregional staging and follow-up of cutaneous melanoma: how we do it. J Ultrasound Med. 2010;29:791–802. doi: 10.7863/jum.2010.29.5.791. [DOI] [PubMed] [Google Scholar]
- 21.Catalano O, Caracò C, Mozzillo N, Siani A. Locoregional spread of cutaneous melanoma: sonography findings. AJR. 2010;194:735–745. doi: 10.2214/AJR.09.2422. [DOI] [PubMed] [Google Scholar]
- 22.Corvino A, Corvino F, Catalano O, Sandomenico F, Petrillo A. The tail and the string sign: new sonographic features of subcutaneous melanoma metastasis. Ultrasound Med Biol. 2017;43:370–374. doi: 10.1016/j.ultrasmedbio.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 23.Catalano O, Alfageme Roldán F, Scotto di Santolo M, Solivetti FM, Wortsman X. Color Doppler sonography of Merkel cell carcinoma. J Ultrasound Med. 2018;37:285–292. doi: 10.1002/jum.14329. [DOI] [PubMed] [Google Scholar]
- 24.Hernández-Aragüés I, Vázquez-Osorio I, Alfageme F, Ciudad-Blanco C, Casas-Férnandez L, Rodríguez-Blanco MI, Suárez-Fernández R. Skin ultrasound features of Merkel cell carcinoma. J Eur Acad Dermatol Venereol. 2017;31:e315–e318. doi: 10.1111/jdv.14102. [DOI] [PubMed] [Google Scholar]
- 25.Shin YR, Kim JY, Sung MS, Jung JH. Sonographic findings of dermatofibrosarcoma protuberans with pathologic correlation. J Ultrasound Med. 2008;27:269–274. doi: 10.7863/jum.2008.27.2.269. [DOI] [PubMed] [Google Scholar]
- 26.Solivetti FM, Elia F, Latini A, Cota C, Cordiali-Fei P, di Carlo A. AIDS-Kaposi Sarcoma and Classic Kaposi Sarcoma: are different ultrasound patterns related to different variants? J Exp Clin Cancer Res. 2011;30:40. doi: 10.1186/1756-9966-30-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carrascosa R, Alfageme F, Roustán G, Suarez MD. Skin ultrasound in Kaposi sarcoma. Actas Dermosifiliogr. 2016;107:e19–e22. doi: 10.1016/j.ad.2015.05.019. [DOI] [PubMed] [Google Scholar]
- 28.Chiou HJ, Chou YH, Chiou SY, Chen WM, Chen W, Wang HK, Chang CY. High-resolution ultrasonography of primary peripheral soft tissue lymphoma. J Ultrasound Med. 2005;24:77–86. doi: 10.7863/jum.2005.24.1.77. [DOI] [PubMed] [Google Scholar]
- 29.Mandava A, Koppula V, Wortsman X, Catalano O, Alfageme F. The clinical value of imaging in primary cutaneous lymphomas: role of high resolution ultrasound and PET-CT. Br J Radiol. 2019;92:20180904. doi: 10.1259/bjr.20180904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hung EHY, Griffith JF, Ng AWH, Lee RKL, Lau DTY, Leung JCS. Ultrasound of musculoskeletal soft-tissue tumors superficial to the investing fascia. AJR. 2014;202:W532–W540. doi: 10.2214/AJR.13.11457. [DOI] [PubMed] [Google Scholar]
- 31.Goyal U, Kim Y, Tiwari HA, Witte R, Stea B. A pilot study of ultrasound-guided electronic brachytherapy for skin cancer. J Contemp Brachyther. 2015;7:374–380. doi: 10.5114/jcb.2015.55538. [DOI] [PMC free article] [PubMed] [Google Scholar]