Abstract
Background
Contrast-enhanced ultrasound (CEUS) has the potential to improve the imaging of renal blood flow and renal lesional vascularity in real time with high temporal and spatial resolution.
Purpose
This study investigated the clinical significance of real-time CEUS in cases of chronic kidney disease (CKD).
Materials and methods
Included patients were stratified according to their estimated glomerular filtration rate (eGFR): Group I (CKD stage I and II), eGFR ≥ 60 ml/min/1.73 m2; group II (CKD stage III), eGFR of 30 ≤ eGFR < 60 ml/min/1.73 m2; and group III (CKD stage IV and V), eGFR of eGFR < 30 ml/min/1.73 m2. Real-time and dynamic imaging of the renal cortex was performed using CEUS. Several bolus model perfusion and laboratory parameters were compared. The differences in perfusion or laboratory parameters among the groups and correlation between perfusion or laboratory parameters and eGFR were assessed.
Results
Of the 24 patients, 4 were classified into group I, 13 into group II, and 7 into group III. No significant differences were found among the three groups in the perfusion parameter analysis. No parameter was significantly positively correlated with eGFR. In the laboratory parameter analysis, significant differences in several parameters (RBC, BUN, SCr, glucose, TCh, phosphorus, TP, p < 0.05) were detected among the three groups. These parameters significantly correlated with eGFR (correlation coefficient, R = − 0.7625 to 0.6026).
Conclusions
Kidney perfusion parameters in CEUS do not correlate with kidney function in this pilot study.
Keywords: Contrast-enhanced Ultrasound, Chronic Kidney Disease, Perfusion, Microcirculation, Quantitative Evaluation
Chronic kidney disease (CKD) is a common disease with a gradually increasing incidence worldwide [1–3]. It is characterized by a steady decrease in renal function [4]. In other words CKD is defined as a reduced glomerular filtration rate (GFR) increased urinary albumin excretion or both [1, 3].
In CKD, the impairment of kidney structure and function is closely interrelated [5]. As a result, the diagnosis of CKD requires information on both structure and function. In the past, the role of laboratory testing was to provide information on kidney function, whereas imaging predominantly provided structural information [5]. Current imaging modalities, especially ultrasound (US), computed tomography (CT), and magnetic resonance imaging (MRI), provide adequate information on structural changes, but little on functional impairment in CKD [5]. Ideally, the renal imaging modalities should provide detailed information about both structure and function [5]. Functional imaging techniques, such as contrast-enhanced US (CEUS) and several functional MRIs [5], have emerged for this purpose.
CEUS is a safe, effective, and novel imaging technique that has been used for several organs and diseases [6–13]. Recently developed techniques (a combination of CEUS, contrast-specific software, and quantification tools) have been proposed to quantify the blood flow within an organ with CEUS [7, 8, 10, 13–17]. CEUS has the potential to improve the imaging of renal blood flow and renal lesional vascularity in real time, with high temporal and spatial resolution [6, 11, 15, 18–25]. CEUS is a simple technique for detecting the severity of kidney microvascular perfusion deficit [6, 7, 13, 14, 17, 23–26]. However, to the best of our knowledge, only a few previous studies have used CEUS to evaluate CKD [7, 13].
Therefore, the present study aimed to investigate the clinical significance of real-time CEUS by evaluating renal microvascular perfusion in CKD.
Materials and methods
This prospective study was approved by the institutional review board, and written informed consent was obtained from all patients in accordance with the WORLD Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects, 2008.
Patient population
Between May and August 2016, 26 patients with CKD were enrolled. They were diagnosed based on histology or clinical findings in the nephrology division of the internal medicine department. The following exclusion criteria were used: egg allergy, severe heart or pulmonary disease, and pregnancy.
Included patients were separated into three groups according to their estimated GFR (eGFR): Group I, eGFR ≥ 60 ml/min/1.73 m2 (CKD stage I and II); group II, 30 ml/min/1.73 m2 ≤ eGFR < 60 ml/min/1.73 m2 (CKD stage III); and group III, eGFR < 30 ml/min/1.73 m2 (CKD stage IV and V).
Grayscale US examination
A radiologist with more than 15 years’ experience in performing renal US, who was blinded to the current renal functionality, additional imaging findings, or any other clinical information, performed renal US (RS80A; Samsung Medison, Seoul, Korea) using a 1–7-MHz curvilinear transducer probe.
Renal length was measured as the maximum pole-to-pole distance on a longitudinal plane. Cortical thickness was determined by measuring the shortest distance between the renal capsule and the base of a medullary pyramid at the level of the mid-kidney [27].
Of the two kidneys, the larger, thicker, and more accessible one was selected as the representative lateralized kidney for further CEUS examination. Doppler study was used to test the renal blood flow.
CEUS examination
An US contrast agent (SonoVue®; Bracco, Milano, Italy) was injected as a bolus into a peripheral vein through an intravenous cannula and using a dedicated syringe. A small volume of microbubble contrast agent (1.5 mL) was administered, followed by flushing with 5-mL saline. The images were collected while the contrast agent was injected. The patients had already been instructed to breathe quietly and to lie down in the supine or contralateral decubitus position.
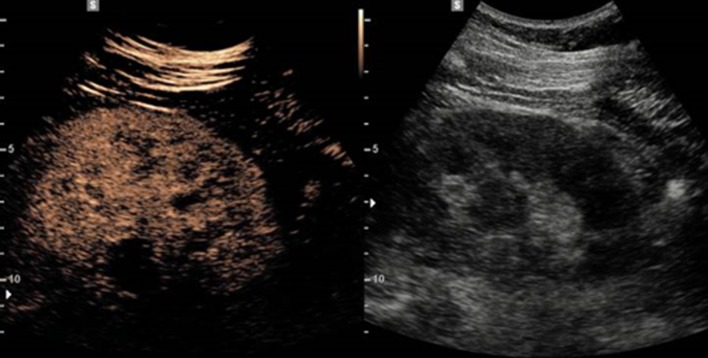
CEUS was performed by the same operator who performed grayscale US, using a contrast-specific mode with a dedicated low mechanical index (MI, acoustic power of US) mode (MI = 0.08). Image depth, focus, gain, and frame rate were optimized at the beginning of each examination and were kept constant during the study. While performing CEUS, we split the US machine screen into two, with the CEUS image displayed on the left and the grayscale US image displayed on the right (Fig. 1). Digital dynamic cine-clips were registered during all CEUS examinations to allow for accurate retrospective evaluation. Static images were also stored. Satisfactory enhancement usually lasted for 2 min in the kidneys.
Fig. 1.
Representative images of CEUS. While performing CEUS, the US machine screen was split into two, with the CEUS image displayed on the left and the grayscale US image displayed on the right. CEUS contrast-enhanced ultrasound, US ultrasound
CEUS analysis and bolus model perfusion parameters
US data sets were exported in a digital imaging and communication in medicine (DICOM) format and analyzed offline using a dedicated software package (VueBox®; Bracco Research, Geneva, Switzerland). The time intensity curve (TIC) was obtained according to quality of fit (QOF); only those with ≥ 85% index were selected for perfusion analysis [7].
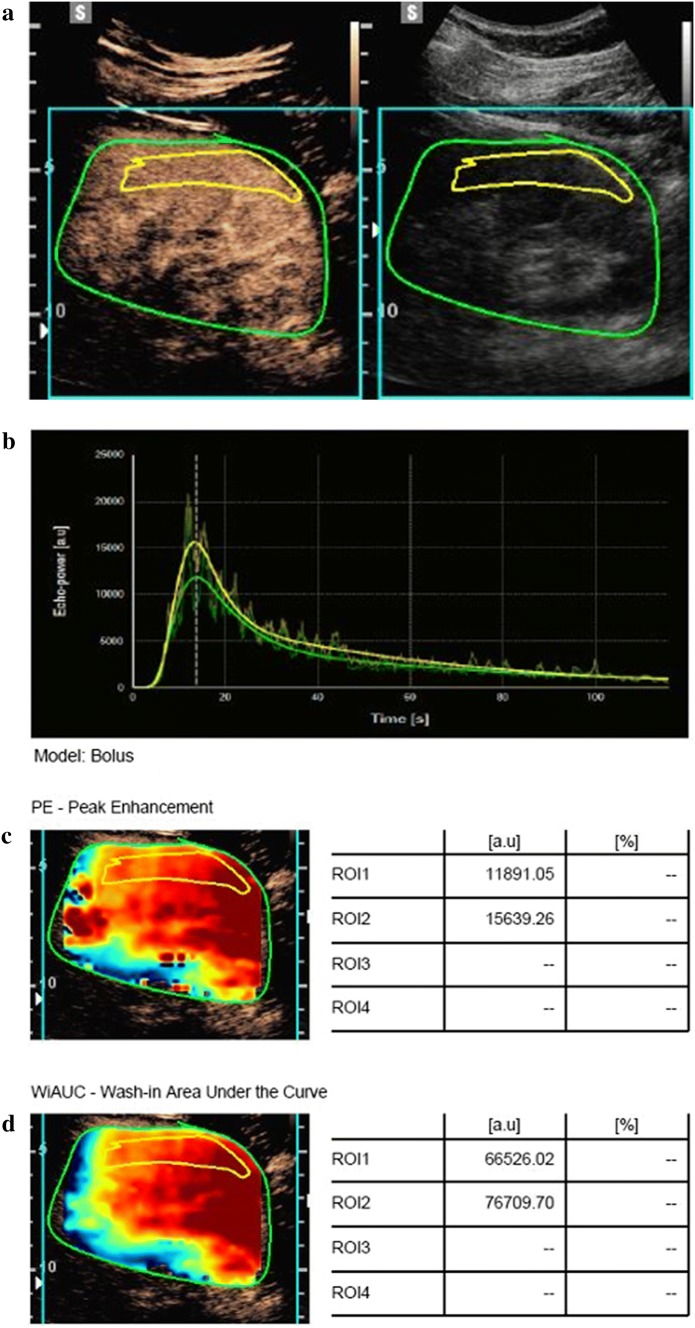
An example of offline analysis is presented in Fig. 2. One region of interest (ROI) was drawn for each sequence. To minimize the influence of local perfusion heterogeneities, this ROI was drawn to enclose the largest visible area of the renal cortex on the surface of the kidney closest to the US probe [17]. The renal cortex that was only intermittently visible because of breathing or other factors was not included in the ROI. The software generated a TIC, and this curve was used to generate CEUS-derived parameters: PE: peak enhancement; TTP: time to peak; WiR: wash-in rate, WoR: wash-out rate; WiWoAUC: wash-in and wash-out area under curve; RT: rise time; WiAUC: wash-in area under curve; WoAUC: wash-out area under curve; WiPI: wash-in perfusion index (WiAUC/RT); and FT: fall time. These bolus model-related perfusion parameters were evaluated from fitted TIC using specific software.
Fig. 2.
Representative images of CEUS analysis. a A region of interest was drawn (yellow line) in the largest possible area of the renal cortex close to the US. The green line corresponds to the overall zone (kidney and surrounding tissues). b The software generated a time intensity curve. This curve was used to generate CEUS-derived parameters. c and d Obtained representative parameters are shown. CEUS contrast-enhanced ultrasound, US ultrasound, PE peak enhancement, WiAUC wash-in area under the curve
Laboratory parameters
The laboratory parameters were evaluated from sampled blood and urine according to the discretion of nephrologists within 1 week of CEUS examination. The blood samples were obtained after overnight fasting. The hematocrit (HCT) and red blood cell (RBC) count, as well as the levels of glucose, blood urea nitrogen (BUN), serum creatinine (SCr), uric acid (UA), total cholesterol (TCh), triglyceride (TG), calcium (Ca), phosphorus, total protein (TP), and albumin were analyzed. Urine was collected in the morning for urinary protein (UPr), urinary creatinine (UCr), urinary excretion rate, and UPr/UCr analysis.
Statistical analysis
All statistical analyses were performed using MedCalc 17.6 statistics software (MedCalc Software, Mariakerke, Belgium). The differences in perfusion-related or laboratory parameters among the three groups were assessed with a one-way analysis of variance (ANOVA) test. Pearson correlation analysis was used to determine the relationships between perfusion or laboratory parameters and eGFR. A p value < 0.05 was considered statistically significant.
Results
Patient population
Data from 24 patients met the criteria for perfusion analysis (10 women, 14 men; mean age, 57.2 ± 19.8 years; range 19–86 years). Group I included 4 patients, group II included 13 patients, and group III included 7 patients.
Bolus model perfusion parameters on CEUS
In all groups, the TIC of renal perfusion was an asymmetrical, single-peak curve that obviously had an ascending slope, a peak, and a descending slope. The ascending slope was steep, while the descending slope was flat (Fig. 2) [7].
No significant differences between the three groups were detected in the analysis of perfusion-related parameters (Table 1). No parameters were significantly correlated with eGFR (Table 2).
Table 1.
Parameters of renal microvascular perfusion according to renal function
| Parameter | Group I: n = 4 | Group II: n = 13 | Group III: n = 7 | P value |
|---|---|---|---|---|
| PE | 9617.56 ± 7639.83 | 23354.72 ± 22595.42 | 9340.56 ± 4628.62 | 0.177 |
| TTP | 14.11 ± 14.55 | 9.35 ± 9.01 | 8.75 ± 4.18 | 0.606 |
| WiR | 3602.26 ± 3983.62 | 16106.08 ± 23208.84 | 3698.69 ± 3995.93 | 0.254 |
| WoR | 718.72 ± 527.30 | 4240.57 ± 8591.73 | 1392.84 ± 733.37 | 0.520 |
| WiWoAUC | 126007.01 ± 91293.11 | 265341.07 ± 217280.35 | 105934.40 ± 50621.02 | 0.115 |
| RT | 9.65 ± 9.64 | 6.63 ± 5.94 | 5.83 ± 2.37 | 0.580 |
| WiAUC | 32585.56 ± 19142.77 | 68319.16 ± 47756.60 | 32617.31 ± 16169.53 | 0.094 |
| WoAUC | 93421.44 ± 72287.26 | 197021.91 ± 174436.63 | 73317.10 ± 35868.88 | 0.135 |
| WiPI | 6432.35 ± 5344.25 | 15533.57 ± 14543.14 | 6011.64 ± 2998.24 | 0.153 |
| FT | 22.62 ± 18.36 | 19.40 ± 21.03 | 13.24 ± 5.87 | 0.654 |
*Data are presented as mean ± SD
PE peak enhancement, TTP time to peak, WiR wash-in rate, WoR wash-out rate, WiWoAUC wash-in and wash-out area under curve, RT rise time, WiAUC wash-in area under curve, WoAUC wash-out area under curve, WiPI wash-in perfusion index (WiAUC/RT), FT fall time
Table 2.
Correlation between eGFR and parameters of renal microvascular perfusion
| Parameter | Correlation coefficient r | P value |
|---|---|---|
| PE | 0.01454 | 0.9463 |
| TTP | 0.1026 | 0.6334 |
| WiR | − 0.03806 | 0.8599 |
| WoR | − 0.1037 | 0.6298 |
| WiWoAUC | 0.1667 | 0.4362 |
| RT | 0.1542 | 0.4718 |
| WiAUC | 0.1056 | 0.6233 |
| WoAUC | 0.1800 | 0.3999 |
| WiPI | 0.03116 | 0.8851 |
| FT | 0.1983 | 0.3530 |
PE Peak Enhancement, TTP time to peak, WiR wash-in rate, WoR wash-out rate, WiWoAUC wash-in and wash-out area under curve, RT rise time, WiAUC wash-in area under curve, WoAUC wash-out area under curve, WiPI wash-in perfusion index (WiAUC/RT), FT fall time
Laboratory parameters
Analysis of laboratory parameters revealed significant differences in RBC, BUN, and levels of SCr, glucose, TCh, phosphorus, and TP (p < 0.05) between patients in the different groups (Table 3). Additionally, significant correlations were detected between eGFR and several laboratory parameters (HCT, RBC, and levels of BUN, SCr, glucose, TCh, phosphorus, and TP, p < 0.05) (Table 4).
Table 3.
Laboratory parameters according to renal function
| Parameter | Group I: n = 4 | Group II: n = 13 | Group III: n = 7 | P value |
|---|---|---|---|---|
| HCT (%) | 37.95 ± 9.76 | 36.8 ± 6.81 | 29.96 ± 7.25 | 0.130 |
| RBC (× 1012/L) | 4.36 ± 1.10 | 4.01 ± 0.74 | 3.12 ± 0.71 | 0.036★ |
| BUN (mg/dL) | 16.75 ± 4.35 | 23.38 ± 5.82 | 64.29 ± 24.79 | < 0.001★▼ |
| SCr (mg/dL) | 0.94 ± 0.17 | 1.45 ± 0.30 | 4.60 ± 2.1 | < 0.001★▼ |
| UA (mg/dL) | 5.78 ± 1.85 | 6.66 ± 1.66 | 5.54 ± 1.67 | 0.356 |
| Glucose (mg/dL) | 232 ± 167.97 | 125.54 ± 44.61 | 95.86 ± 9.96 | 0.020★ |
| TCh (mg/dL) | 249.25 ± 92.77 | 163.54 ± 38.55 | 134.14 ± 27.89 | 0.003★▲ |
| TG (mg/dL) | 140 ± 57.3 | 225.38 ± 183.87 | 104.71 ± 46.38 | 0.194 |
| Ca (mg/dL) | 8.68 ± 0.43 | 8.83 ± 0.49 | 8.54 ± 1.06 | 0.702 |
| Phosphorus (mg/dL) | 3.4 ± 0.45 | 3.68 ± 0.36 | 5.17 ± 1.64 | 0.006★▼ |
| TP (g/dL) | 5.85 ± 0.76 | 6.74 ± 0.39 | 7.03 ± 0.35 | 0.002★▲ |
| Albumin (g/dL) | 3.38 ± 0.87 | 3.82 ± 0.48 | 4.09 ± 0.34 | 0.121 |
| UPr (mg/dL) | 253.8 ± 250.47 | 250.38 ± 302.23 | 70.7 ± 35.37 | 0.346 |
| UCr (mg/dL) | 89.64 ± 54.36 | 128.12 ± 77.81 | 67.33 ± 26.5 | 0.171 |
| UPr/UCr | 4492.89 ± 4447.47 | 2238.11 ± 2474.84 | 1079.75 ± 366.33 | 0.150 |
Groups I and II versus group III: ▼P < 0.05, group I versus groups II and III: ▲P < 0.05
HCT: hematocrit, RBC: red blood cell, BUN: blood urea nitrogen, SCr: serum creatinine, UA: uric acid, TCh: total cholesterol, TG: triglyceride, Ca: calcium, TP: total protein, UPr: urinary protein, UCr: urinary creatinine, UPr/UCr: urinary excretion rate
*Data are presented as mean ± SD. ★P < 0.05
Table 4.
Correlation between eGFR and laboratory parameters
| Parameter | Correlation coefficient r | P value |
|---|---|---|
| HCT | 0.4454 | 0.0332★ |
| RBC | 0.5518 | 0.0063★ |
| BUN | − 0.7625★ | < 0.0001★ |
| SCr | − 0.7442 | < 0.0001★ |
| UA | 0.06862 | 0.7557 |
| Glucose | 0.4547 | 0.0256★ |
| TCh | 0.6026★ | 0.0018★ |
| TG | 0.1981 | 0.3535 |
| Ca | 0.1373 | 0.5322 |
| Phosphorus | − 0.6046 | 0.0022★ |
| TP | − 0.5923 | 0.0029★ |
| Albumin | − 0.3208 | 0.1355 |
| UPr | 0.1912 | 0.3820 |
| UCr | 0.1910 | 0.3827 |
| UPr/UCr | − 0.4134 | 0.0558 |
HCT hematocrit, RBC red blood cell, BUN blood urea nitrogen, SCr serum creatinine, UA uric acid, TCh total cholesterol, TG triglyceride, Ca calcium, TP total protein, UPr urinary protein, UCr urinary creatinine, UPr/UCr urinary excretion rate
★P < 0.05
Discussion
CEUS has shown encouraging potential for non-invasive assessment of tissue and organ health, as well as the assessment of tumor response to therapy. Indeed, the most common application of CEUS is blood perfusion imaging, and CEUS has demonstrated promising results in assessing the presence and perfusion of neoplasms of the heart, liver, kidney, spleen, pancreas, as well as other organs and tissues [25, 28, 29]. It is a relatively novel method for non-invasive quantification of circulation of different sonographically accessible parenchymatous organs [10]. The kidney serves as a good model for measurements of organ blood flow, since it is highly vascularized, readily imaged with US, and typically has a single feeder artery [25]. Thus, the kidney enhances quickly and intensively [19]. Additionally, CEUS is a unique method of perfusion imaging that is safe and non-invasive in the presence of renal insufficiency, since contrast-enhanced MRI and CT techniques are contraindicated in this population [6, 25]. According to the European Federation of Societies for Ultrasound in Medicine and Biology (EFSUMB) guidelines, imaging with CEUS should be considered in every patient with renal function impairment [6]. Indeed, CEUS can be performed during the same examination session as color Doppler US, thus acting as first-line and problem-solving imaging modality at the same time [6].
The pathophysiology of CKD or AKI involves tubular injury, inflammatory processes and changes in renal microvascular perfusion, which result in a generalized or localized impairment of oxygen and nutrient delivery to, and waste product removal from, cells of the kidney [23]. The change in renal microvascular perfusion is the principal process underlying CKD progression, as well as renal fibrosis.
With this theoretical background, we aimed to investigate the clinical significance of CEUS by evaluating renal microvascular perfusion in CKD. To our knowledge, only a few previous studies have used CEUS to evaluate CKD [7, 13].
Our results for patients with CKD investigated with CEUS show that no significant differences existed among the three groups (Table 1) and perfusion parameters were not correlated with eGFR (Table 2). These results mean that the time intensity curve and enhancement pattern are similar regardless of kidney function, making it difficult to diagnose CKD through CEUS, but explaining why CEUS can characterize dynamic enhancement patterns of renal lesions in patients with CKD. In this study, however, the images of renal cortex microvascular beds in patients with CKD were rapidly and clearly displayed on CEUS. Microvascular perfusion changes in CKD increase hemodynamic impedance of renal microcirculation, reduce renal perfusion, and lead to ischemia; therefore, fewer contrast microbubbles enter the renal parenchyma. The highest number of perfusion images (24/26) detected in the kidney cortex tissue was clearly observed in this study. Two patients’ data did not meet the criteria (QOF ≥ 85% for analysis of perfusion), as those patients failed to breathe quietly. All data obtained, including those of the two participants mentioned above, were easily recorded and quantified. None of the study participants reported any side effect of CEUS.
Treatment in the earlier stages of CKD is effective in slowing the progression toward renal failure [3]. In this study, significant differences in several laboratory parameters (RBC, BUN, SCr, glucose, TCh, phosphorus, and TP levels, p < 0.05) and significant correlations of these laboratory parameters with eGFR (correlation coefficient, R = − 0.7625 to 0.6026) were found among patients in the three groups. These results were in keeping with traditional markers of CKD. However, traditional markers of CKD, such as SCr, BUN, and UPr, have been known to be insensitive and might result in extensive time lapses when successful interventions could be applied [3, 30]. Currently, no CKD marker could satisfy the requirement of progression prediction and early detection. As sensitive laboratory markers are missing, renal biopsy is currently the best method to assess the severity of renal fibrosis and other pathologic changes. However, renal biopsy is invasive, susceptible to sampling errors, and impractical for longitudinal monitoring [3, 31]. Therefore, a critical need to develop non-invasive and reproducible alternatives to renal biopsy has emerged. CEUS may be a powerful candidate for this use.
Several limitations need to be considered while interpreting our data. First, this study included a relatively small number of patients. This small number might have not reflected the full spectrum of data in patients with CKD. Second, the data in this study were not compared with those of normal, healthy volunteers as controls. Finally, other factors, such as interstitial fibrosis, in addition to microvascular perfusion, may have contributed to the pathophysiology of CKD. A larger cohort of patients with CKD and healthy volunteers is required to further analyze obtained correlations and determine whether this novel method might provide substantial information. This study was performed to be a pilot and feasibility study and to justify further investigations.
In conclusion, the use of CEUS to diagnose CKD is not clinically significant. Nonetheless, we believe that this study makes a useful contribution to the literature. It is one of the few studies that have used CEUS to evaluate CKD. In this study, we have demonstrated that CEUS can be used to easily and effectively detect, quantify, and monitor renal microvascular perfusion in patients with CKD. With additional trials in a larger population, CEUS may serve a viable alternative to current evaluations of CKD.
Acknowledgements
This study was supported by a grant from Samsung Medison Medical Systems. We appreciate the assistance in the working the study of Yunjung Lee (Bracco Imaging Korea).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Levey AS, de Jong PE, Coresh J, El Nahas M, Astor BC, Matsushita K, Gansevoort RT, Kasiske BL, Eckardt KU. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int. 2011;80(1):17–28. doi: 10.1038/ki.2010.483. [DOI] [PubMed] [Google Scholar]
- 2.Jha V, Garcia-Garcia G, Iseki K, Li Z, Naicker S, Plattner B, Saran R, Wang AY, Yang CW. Chronic kidney disease: global dimension and perspectives. Lancet. 2013;382(9888):260–272. doi: 10.1016/S0140-6736(13)60687-X. [DOI] [PubMed] [Google Scholar]
- 3.Wang L, Xia P, Lv K, Han J, Dai Q, Li XM, Chen LM, Jiang YX. Assessment of renal tissue elasticity by acoustic radiation force impulse quantification with histopathological correlation: preliminary experience in chronic kidney disease. Eur Radiol. 2014;24(7):1694–1699. doi: 10.1007/s00330-014-3162-5. [DOI] [PubMed] [Google Scholar]
- 4.Remer EM, Papanicolaou N, Casalino DD, Bishoff JT, Blaufox MD, Coursey CA, Dighe M, Eberhardt SC, Goldfarb S, Harvin HJ, Heilbrun ME, Leyendecker JR, Nikolaidis P, Oto A, Preminger GM, Raman SS, Sheth S, Vikram R, Weinfeld RM. ACR Appropriateness Criteria (®) on renal failure. Am J Med. 2014;127(11):1041–1048. doi: 10.1016/j.amjmed.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 5.Herget-Rosenthal S. Imaging techniques in the management of chronic kidney disease: current developments and future perspectives. Semin Nephrol. 2011;31(3):283–290. doi: 10.1016/j.semnephrol.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 6.Girometti R, Stocca T, Serena E, Granata A, Bertolotto M. Impact of contrast-enhanced ultrasound in patients with renal function impairment. World J Radiol. 2017;9(1):10–16. doi: 10.4329/wjr.v9.i1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma F, Cang Y, Zhao B, Liu Y, Wang C, Liu B, Wu T, Song Y, Peng A. Contrast-enhanced ultrasound with SonoVue could accurately assess the renal microvascular perfusion in diabetic kidney damage. Nephrol Dial Transplant. 2012;27(7):2891–2898. doi: 10.1093/ndt/gfr789. [DOI] [PubMed] [Google Scholar]
- 8.Cantisani V, Wilson SR. CEUS: where are we in 2015? Eur J Radiol. 2015;84(9):1621–1622. doi: 10.1016/j.ejrad.2015.05.028. [DOI] [PubMed] [Google Scholar]
- 9.Malhi H, Grant EG, Duddalwar V. Contrast-enhanced ultrasound of the liver and kidney. Radiol Clin North Am. 2014;52(6):1177–1190. doi: 10.1016/j.rcl.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 10.Frohlich E, Muller R, Cui XW, Schreiber-Dietrich D, Dietrich CF. Dynamic contrast-enhanced ultrasound for quantification of tissue perfusion. J Ultrasound Med. 2015;34(2):179–196. doi: 10.7863/ultra.34.2.179. [DOI] [PubMed] [Google Scholar]
- 11.Denham SL, Alexander LF, Robbin ML. Contrast-Enhanced Ultrasound: practical review for the assessment of hepatic and renal lesions. Ultrasound Q. 2016;32(2):116–125. doi: 10.1097/RUQ.0000000000000182. [DOI] [PubMed] [Google Scholar]
- 12.Nolsoe CP, Lorentzen T. International guidelines for contrast-enhanced ultrasonography: ultrasound imaging in the new millennium. Ultrasonography. 2016;35(2):89–103. doi: 10.14366/usg.15057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsuruoka K, Yasuda T, Koitabashi K, Yazawa M, Shimazaki M, Sakurada T, Shirai S, Shibagaki Y, Kimura K, Tsujimoto F. Evaluation of renal microcirculation by contrast-enhanced ultrasound with Sonazoid as a contrast agent. Int Heart J. 2010;51(3):176–182. doi: 10.1536/ihj.51.176. [DOI] [PubMed] [Google Scholar]
- 14.Schneider AG, Hofmann L, Wuerzner G, Glatz N, Maillard M, Meuwly JY, Eggimann P, Burnier M, Vogt B. Renal perfusion evaluation with contrast-enhanced ultrasonography. Nephrol Dial, Transplant. 2012;27(2):674–681. doi: 10.1093/ndt/gfr345. [DOI] [PubMed] [Google Scholar]
- 15.McArthur C, Baxter GM. Current and potential renal applications of contrast-enhanced ultrasound. Clin Radiol. 2012;67(9):909–922. doi: 10.1016/j.crad.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 16.Greis C. Quantitative evaluation of microvascular blood flow by contrast-enhanced ultrasound (CEUS) Clin Hemorheol Microcirc. 2011;49(1–4):137–149. doi: 10.3233/CH-2011-1464. [DOI] [PubMed] [Google Scholar]
- 17.Schneider AG, Goodwin MD, Schelleman A, Bailey M, Johnson L, Bellomo R. Contrast-enhanced ultrasonography to evaluate changes in renal cortical microcirculation induced by noradrenaline: a pilot study. Crit Care. 2014;18(6):653. doi: 10.1186/s13054-014-0653-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Setola SV, Catalano O, Sandomenico F, Siani A. Contrast-enhanced sonography of the kidney. Abdom Imaging. 2007;32(1):21–28. doi: 10.1007/s00261-006-9001-7. [DOI] [PubMed] [Google Scholar]
- 19.Nilsson A. Contrast-enhanced ultrasound of the kidneys. Eur Radiol. 2004;14(Suppl 8):P104–P109. [PubMed] [Google Scholar]
- 20.Prakash A, Tan GJ, Wansaicheong GK. Contrast enhanced ultrasound of kidneys. Pictorial essay. Med Ultrason. 2011;13(2):150–156. [PubMed] [Google Scholar]
- 21.Bertolotto M, Martegani A, Aiani L, Zappetti R, Cernic S, Cova MA. Value of contrast-enhanced ultrasonography for detecting renal infarcts proven by contrast enhanced CT. A feasibility study . Eur Radiol. 2008;18(2):376–383. doi: 10.1007/s00330-007-0747-2. [DOI] [PubMed] [Google Scholar]
- 22.Ascenti G, Mazziotti S, Zimbaro G, Settineri N, Magno C, Melloni D, Caruso R, Scribano E. Complex cystic renal masses: characterization with contrast-enhanced US. Radiology. 2007;243(1):158–165. doi: 10.1148/radiol.2431051924. [DOI] [PubMed] [Google Scholar]
- 23.Fischer K, Meral FC, Zhang Y, Vangel MG, Jolesz FA, Ichimura T, Bonventre JV. High-resolution renal perfusion mapping using contrast-enhanced ultrasonography in ischemia-reperfusion injury monitors changes in renal microperfusion. Kidney Int. 2016;89(6):1388–1398. doi: 10.1016/j.kint.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kleinert S, Roll P, Baumgaertner C, Himsel A, Mueller A, Fleck M, Feuchtenberger M, Jenett M, Tony HP. Renal perfusion in scleroderma patients assessed by microbubble-based contrast-enhanced ultrasound. Open Rheumatol J. 2012;6:50–53. doi: 10.2174/1874312901206010050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kogan P, Johnson KA, Feingold S, Garrett N, Guracar I, Arendshorst WJ, Dayton PA. Validation of dynamic contrast-enhanced ultrasound in rodent kidneys as an absolute quantitative method for measuring blood perfusion. Ultrasound Med Biol. 2011;37(6):900–908. doi: 10.1016/j.ultrasmedbio.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schneider AG, Schelleman A, Goodwin MD, Bailey M, Eastwood GM, Bellomo R. Contrast-enhanced ultrasound evaluation of the renal microcirculation response to terlipressin in hepato-renal syndrome: a preliminary report. Ren Fail. 2015;37(1):175–179. doi: 10.3109/0886022X.2014.977140. [DOI] [PubMed] [Google Scholar]
- 27.Moghazi S, Jones E, Schroepple J, Arya K, McClellan W, Hennigar RA, O’Neill WC. Correlation of renal histopathology with sonographic findings. Kidney Int. 2005;67(4):1515–1520. doi: 10.1111/j.1523-1755.2005.00230.x. [DOI] [PubMed] [Google Scholar]
- 28.Wilson SR, Burns PN. Microbubble-enhanced US in body imaging: what role? Radiology. 2010;257(1):24–39. doi: 10.1148/radiol.10091210. [DOI] [PubMed] [Google Scholar]
- 29.Cosgrove D, Lassau N. Imaging of perfusion using ultrasound. Eur J Nucl Med Mol Imaging. 2010;37(Suppl 1):S65–S85. doi: 10.1007/s00259-010-1537-7. [DOI] [PubMed] [Google Scholar]
- 30.Levey AS, Cattran D, Friedman A, Miller WG, Sedor J, Tuttle K, Kasiske B, Hostetter T. Proteinuria as a surrogate outcome in CKD: report of a scientific workshop sponsored by the National Kidney Foundation and the US Food and Drug Administration. Am J Kidney Dis. 2009;54(2):205–226. doi: 10.1053/j.ajkd.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 31.Bosmans JL, Ysebaert DK, Verpooten GA. Chronic allograft nephropathy: what have we learned from protocol biopsies? Transplantation. 2008;85(7 Suppl):S38–S41. doi: 10.1097/TP.0b013e318169c5d0. [DOI] [PubMed] [Google Scholar]