Abstract
At present there is a growing need for tissue engineering products, including the products of scaffold-technologies. Biopolymer hydrogel scaffolds have a number of advantages and are increasingly being used to provide means of cell transfer for therapeutic treatments and for inducing tissue regeneration. This work presents original hydrogel biopolymer scaffolds based on a blood plasma cryoprecipitate and collagen and formed under conditions of enzymatic hydrolysis. Two differently originated collagens were used for the scaffold formation. During this work the structural and mechanical characteristics of the scaffold were studied. It was found that, depending on the origin of collagen, scaffolds possess differences in their structural and mechanical characteristics. Both types of hydrogel scaffolds have good biocompatibility and provide conditions that maintain the three-dimensional growth of adipose tissue stem cells. Hence, scaffolds based on such a blood plasma cryoprecipitate and collagen have good prospects as cell carriers and can be widely used in regenerative medicine.
Keywords: Biopolymers, Blood plasma cryoprecipitate, Collagen, Hydrogel scaffolds, Stem cells
Graphical abstract
Highlights
-
•
Original hydrogel scaffolds based on a blood plasma cryoprecipitate and collagen.
-
•
Depending on the origin of collagen, scaffolds possess differences in their properties.
-
•
Scaffolds can provide the conditions needed to maintain growth of stem cells.
1. Introduction
Having a combination of properties that imitate the natural extracellular matrix (ECM), hydrogels are widely used in tissue engineering [1,2]. The unique nature of hydrogels results from their self-supporting three-dimensional (3D) viscoelastic network structure that allows both the diffusion and adhesion of water-soluble substances [3]. An important property of hydrogels that facilitates the exchange of oxygen, nutrients and waste products between cells in the conditions of a three-dimensional scaffold structure is their ability to retain a large amount of liquid [4]. A further essential aspect of the functionality of hydrogels is associated with their porous structure. The presence of pores provides the conditions both for cell placement and the development of cell-to-cell contacts [5]. Some biopolymers allow cell encapsulation into the hydrogel structure during its formation and this offers the opportunity to create tissue-like constructions providing for three-dimensional cell growth [[6], [7], [8]]. An undisputable advantage of the majority of biopolymer hydrogels is their biological activity, which determines the possibility for the formation of adhesion complexes between cells and the extracellular matrix. As a result, such biopolymers including fibrinogen/fibrin, collagen, fibronectin, hyaluronic acid, and carrier sequences capable of ligating cell integrins are of special importance [9]. Connecting with the cell integrins they can activate specific intracellular signal pathways which eventually regulate the process of tissue development [10]. Another factor determining the wide use of biopolymer hydrogels is the possibility of creating scaffolds with tissue-like viscoelastic properties [2]. Most tissues do not exhibit linear elasticity due to their main constituents (cells, extracellular matrix, structural proteins and fluids) [11]. The manifestation of a simultaneous viscous and elastic response by hydrogels is also due to the multicomponent nature of these materials. The viscoelastic properties of hydrogels are an integral result of individual characteristics and interactions of the structural component of a material (for example, fibers or filaments), chemical (for example, ionic bonds), fluid and cells [[12], [13], [14]]. There is no doubt that the viscoelastic properties of both artificial and natural ECMs directly affect cell migration, proliferation and differentiation [[15], [16], [17]]. For example, O. Chaudhuri et al. (2016) found out that cells'spreading, proliferation, and osteogenic differentiation of mesenchymal stem cells (MSCs) are all enhanced in cells cultured in gels with faster relaxation [18]. That is why extensive research is devoted to the study of the physicomechanical properties of hydrogels and the development of new methods for assessing these properties [[19], [20], [21], [22], [23]]. Although it is currently difficult to obtain cytocompatible hydrogels with the mechanical properties of native tissues, significant progress has been achieved in the development of materials with custom properties [[24], [25], [26]]. The variability of the properties of hydrogels determines the ability to customize their properties for a particular type of tissue. For example, T. Majumdar et al. (2018) demonstrated a possibility to adjust the properties of a hydrogel to obtain materials mechanically comparable to human cartilage in terms of their ability to dissipate energy [27]. One of the benefits of biopolymer hydrogels is the opportunity for controlling the rate of their biodegradation [28]. This characteristic can provide synchronization of the process of scaffold resorption with respect to the processes involved in the regeneration of specific tissues [29]. It should be mentioned that biopolymer hydrogels can not only degrade but can also be exposed to remodeling by cells [30]. This enhances their regenerative potential and integration into the surrounding tissues during implantation [31]. Thus, hydrogel scaffolds formed on the basis on biopolymers are a promising class of artificial ECMs because of their ability to imitate both the structural and mechanical properties of natural tissues.
Some of the most popular biopolymer materials for the formation of hydrogel scaffolds are fibrinogen/fibrin and collagen. Scaffolds based on these polymers are used for the reconstruction of damaged and lost tissues of skin, cartilage, tendons, heart, muscles, eyes and other organs [32,33]. This wide range of use is associated with the unique properties of these proteins – they allow the creation of three-dimensional structures with biomimetic architecture and functions. The resulting scaffolds have a high level of hydration, porosity and a microfiber structure, providing both a vast surface area for the adhesion of cells as well as the conditions needed to maintain their viability, migration and proliferation [[33], [34], [35]]. The attractiveness of these proteins from the point of view of tissue engineering is the result of the presence of open centers of interaction with cell integrins that remain available after polymerization [36,37]. The fibrin matrix is a unique carrier for proteins, including cell growth factors. Thus, it is possible to provide long-term regulation of cell responses within the scaffold by controlled mass-dependent protein release [38,39]. It is worth mentioning that one of the most readily available sources of fibrinogen/fibrin is blood plasma. In addition to fibrinogen this contains fibronectin and a number of other biologically active substances [40], the presence of which can help create an environment for maintaining the normal activity of cells included in the original bioengineered construction, or those migrating into it from the surrounding tissues. These biologically active components of blood plasma can take an immediate part in the regulation of cell processes through their stimulation of various mechanisms of cell signaling and can also contribute to scaffold vascularization [[41], [42], [43]]. All the above aspects contribute to the value of scaffolds developed using components of blood and collagen in providing required structural, mechanical and biological properties.
However, despite all these benefits of biopolymer hydrogel scaffolds, their application is significantly limited by their poor mechanical properties [13,44]. It is well known that scaffolds do not show complete mechanical equivalence to healthy tissue. However, their rigidity and durability must be sufficient for providing mechanical cell support, the required level of mechanical transduction between the matrix and cells and, as a result, active maintenance of the development of regenerative processes [45]. To improve the mechanical properties of hydrogels supplementary cross-linking is used as well as the addition of non-polymer components that have a reinforcing function, including those that modify the structure of the hydrogel fibers themselves [[46], [47], [48]]. It is also possible to reinforce hydrogels with frames made from synthetic microfibers [49]. The most widespread strategies are those that improve the hydrogel properties by including secondary polymers and various nanostructures into the main hydrogel to create polycomposite hydrogel scaffolds [[50], [51], [52]]. All the above considerations indicate the topicality of developing new hydrogel scaffolds based on natural polymers and of investigations into the effects of various factors (for example, the composite structure, interaction of biopolymers, conditions of co-polymerization of components etc.) on the structural, mechanical and biological properties of the resulting scaffolds.
The objective of our study was to develop a hydrogel scaffold based on a blood plasma cryoprecipitate and collagen, and to evaluate the effects of the use of different origins of collagen on the structural and mechanical properties of the resulting polycomposite scaffolds.
2. Materials and methods
2.1. General operating principles
Peripheral blood of 16 healthy volunteers was used as a plasma source. All donors were inspected by specialists and examined for transmissible infections. The blood from healthy donors was taken in the morning on an empty stomach from the cubital vein into polymer containers (double 450/300 ml, RAVIMED, Poland) with a solution of hemoconservative CPDA-1®. Blood plasma was extracted by centrifugation at 3000 rpm for 20 min at +4°С. After centrifugation, blood plasma was sampled. The obtained blood plasma was frozen and stored at −40 °C.
Fat tissue obtained during cosmetic operations in the Department of Reconstructive and Plastic Surgery at the University Hospital of the Federal State Budgetary Educational Institution of Higher Education « Privolzhsky Research Medical University» of the Ministry of Health of the Russian Federation University was used as initial material for getting mesenchymal stem cells. The material was obtained from three young women, whose average age was 29.3 years (from 20 to 34 years).
The study protocol was approved by the local ethical committee of Federal State Budgetary Educational Institution of Higher Education « Privolzhsky Research Medical University» of the Ministry of Health of the Russian Federation and approved by its Academic Board. Each person included in the study provided voluntary informed consent with the mentioned above manipulations.
All manipulations on extraction and cultivation of cells, with blood and its derivatives, on the formation and cultivation of hydrogel scaffolds were carried out under sterile laminar conditions (class A) in the laboratory of biotechnologies of Federal State Budgetary Educational Institution of Higher Education « Privolzhsky Research Medical University» of the Ministry of Health of the Russian Federation. At all stages of the study the sterility of the used materials and media was controlled. Growth media and hydrogel scaffolds during the process of cells’ cultivation were being regularly monitored for sterility, contamination with mycoplasmas and viruses, and the presence of fungal flora.
2.2. Obtaining cryoprecipitate from blood plasma
The blood plasma of healthy donors frozen at −40 °C was defrosted at +2 °C and centrifuged at +4 °C for 15 min at 1500 rpm to precipitate cryoprecipitate. Then 85% of the supernatant from the initial volume of the frozen plasma was collected and the cryoprecipitate was thermostatically held at 37°С until it had completely re-dissolved. Then the amounts of fibrinogen were compared for the original blood plasma, the blood plasma cryoprecipitate and the supernatant. The initial concentration of fibrinogen in the blood plasma of healthy donors did not differ from the norm and was 2–4 g/l (n = 16). When the cryoprecipitate was obtained from blood plasma, the concentration of fibrinogen in it varied from 7.5 to 19.10 g/l (12.59 ± 0.84 g/l, n = 16). The concentration of fibrinogen in the supernatant varied from 0.33 to 1.7 g/l (1.25 ± 0.10 g/l, n = 16). The cryoprecipitate was standardized by amount of fibrinogen until this reached a final concentration of 6 g/l. For this standardization we used the supernatant (that contained only a low amount of fibrinogen) left after obtaining the initial cryoprecipitate. While carrying out the experiment we used the pool of cryoprecipitate obtained from 16 donors.
2.3. Cell cultures
Adipose stem cells (ASCs) were isolated from the patients' adipose tissue obtained during plastic surgery, after obtaining each patient's signed consent. The cells were extracted using enzymatic processing with collagenase (Sigma-Aldrich, Germany) for an hour at +37°С and cultivated in complete growth medium at absolute humidity, +37°С, and 5% СО2. The complete growth medium had the following composition: α – MEM, 20% fetal bovine serum (FBS), glutamine, penicillin/streptomycin antibiotics. We used media and reagents produced by « Gibco®» (UK), and plastic consumables produced by « Costar» (USA). After reaching a subconfluent monolayer stage (60–70%) the culture was re-plated. Cultures from the third such passage were used for the experiments.
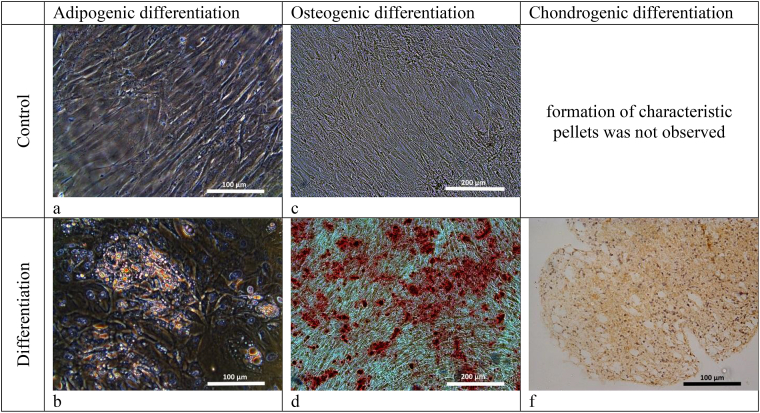
ASCs with confirmed differentiation potential were used in the work Fig. 1. The differentiation potential of cells was evaluated on cultures of the 3rd passage. For differentiation, the Hyman Mesenchymal Stem Cell Functional Identification Kit (R and D systems, USA) was used. As specific dyes, Oil Red (Sigma, USA) was used for staining lipid vacuoles, and alizarin red (Sigma, USA) was used to identify calcium salts in the process of differentiation into osteoblasts. Chondrogenic differentiation was evaluated by the formation of specific pellets in the medium and the deposition of type II collagen, which was determined using polyclonal antibodies to type II collagen (Abcam, ab34712). For histological examination of the balls, the samples were fixed in a solution of neutral 10% formalin. Standard histological examination was performed using an Excelsior ES apparatus (Thermo Scientific, USA). After wiring, paraffin blocks were made using the HistoStar filling station (Thermo Scientific, USA). Sections 4–6 μm thick were obtained on a Microm HM 325 microtome (Thermo Scientific, USA).
Fig. 1.
Differentiation of ASCs. (a, b) Adipogenic differentiation of ASCs, Oil Red stain, (15 days of cultivation; objective lens 20×, eyepiece 10×; phase contrast). (a) control — culture of third passage ASCs without using a differentiating medium, the subconfluent monolayer is formed by typical fibroblast-like cells. In the cells of the control culture, the formation of fat vacuoles is not fixed. (b) the culture of ASCs after cultivation in a differentiating medium. Fat culture vacuoles are clearly defined in culture cells stained with a specific Red Oil dye. The presence of fat vacuoles in the cells indicates the ability of ASC to differentiate in the adipogenic direction; (c, d) Osteogenic differentiation of ASCs. Osteogenic differentiation of ASCs, stained with alizarin red (15 days of cultivation; objective lens 10×, eyepiece 10×, light microscopy). (c) The control culture of ASCs is presented as a subconfluent monolayer formed by morphologically identical spindle-shaped cells, with no calcium deposits. (d) The culture of ASCs after cultivation in a special differentiating medium. The culture is a subconfluent monolayer. Calcium deposits are clearly visualized, which are stained with a differentiating dye, alizarin red. Calcium deposits indicate the ability of ASC to differentiate in the osteogenic direction; (f) Chondrogenic differentiation of ASCs. Staining of type II collagen deposits in the pellet with polyclonal antibodies (Abcam, ab34712; cell nuclei stained with hematoxylin and eosin (Sigma-Aldrich, Germany); objective lens 20×, eyepiece 10×). Ball formation and deposition of type II collagen in them indicates chondrogenic differentiation of ASCs.
Before starting the experiment we defined the cell phenotypes with a panel of the following monoclonal antibodies: CD 90 FITC, CD 105 PE, CD 73 PE, CD 45 PС5, CD 14 PС5, and HLA-DR PС7 (Becton Dickinson, USA) with the corresponding isotypic controls on the BD FACS CANTO II (Becton Dickinson, USA) flow cytometer.
Cell viability prior to introduction into the composite was 98–99%, the immunophenotype of the cells was characteristic to ASC: the cells expressed CD90 +, CD105 +, CD 73+, CD 44 + and did not express CD 45-, CD 14-, HLA DR-, which corresponded to criteria defined by the International Society for Cellular Therapy for Mesenchymal Cells [53].
2.4. The formation of hydrogel scaffold
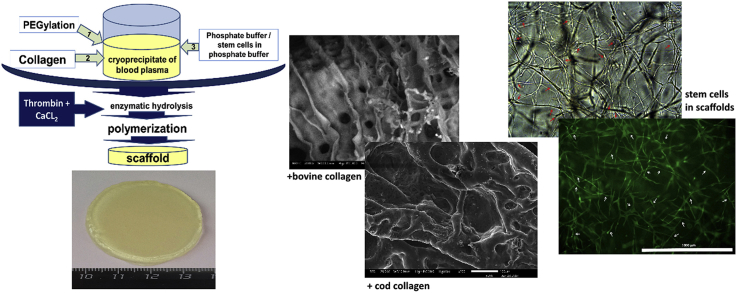
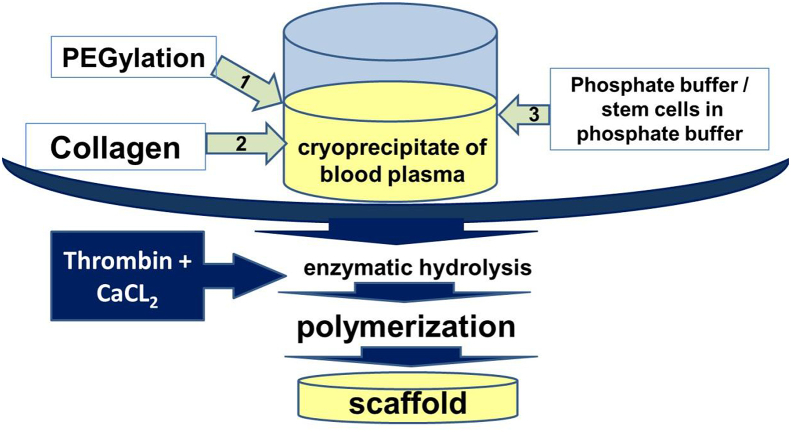
For scaffold formation (Pat.№2653434 The Russian Federation … dated April 11, 2017) we used cryoprecipitate of blood plasma obtained from healthy humans [54]. For PEGylation of the protein part of the cryoprecipitate we used PEG-NHS (Sigma-Aldrich, Germany). To the PEGylated cryoprecipitate we added a 2% solution of acetic collagen (collagen №1 – bovine collagen of type I (Sigma-Aldrich, Germany)); collagen № 2 - cod collagen of type I, collagen isolated from the cod's skin (Gadus macrocephalus) (Pat.№2567171 the Russian Federation … dated October 06, 2014) [55] that had been previously neutralized with sodium hydroxide. To form cell-free scaffolds we added phosphate buffer (PBS) into the composite in a 7:1 ratio. The investigations of the cell-free scaffolds were carried out on the day following their formation. To form cell-containing scaffolds, the cell suspension was injected into the composite to form a concentration of 1.2*105 per 1 ml of the composite. Then the composite was transferred into a 3.5 cm diameter Petri dish that had been preprocessed with silicone. To polymerize the composite, a thrombin-calcium mixture was being introduced into it: 80 IU/ml of human thrombin (Sigma-Aldrich, Germany) in a 1% solution of CaCl2 (Fig. 2).
Fig. 2.
Scaffold formation schematic.
The scaffolds obtained were kept in a mold for 20 min at a temperature of +22–25 °C. During this period, scaffolds were formed, after which each of them was transferred to a plastic Petri dishes of a larger diameter than the prepared scaffolds and poured 5 ml of PBS. Petri dishes with scaffolds were transferred to a CO2 incubator at 37 °C, with a humid atmosphere and 5% CO2 content. Immediately after the formation, the cell scaffolds were flooded with complete growth medium (á – MEM, 20% fetal bovine serum (FBS), glutamine, penicillin/streptomycin antibiotics) and cultured for 6 days with a change of growth medium twice a week.
2.5. Scanning electronic microscopy
The investigation scaffold of structure (3 samples with collagen №1 and 3 samples with collagen №2) was carried out with a JSM-IT300 (JEOL Ltd, Japan) scanning electronic microscope. Samples of dehydrated scaffolds were visualized, the dehydration of the samples being performed in the chamber of the JSM-IT300 under low vacuum.
2.6. Transmission electronic microscopy
The preparation of these samples and their investigation were performed according to standard methods: samples (7 samples with collagen №1 and 7 samples with collagen №2) were fixed in a 2.5% solution of glutaraldehyde in phosphate buffer (рН = 7.4) and in a 1% solution of osmium tetroxide, before being dehydrated in alcohols of ascending concentration (from 50 to 100%) and acetone (100%). Then they were kept in a mixture of 50% embedding medium and 50% of acetone, followed by further embedding in a mixture of Epona with Araldite. After polymerization we obtained ultrathin slices 75–80 nm thick on an UC7 (Leica) ultratome and observed them with a Morgagni 268D (FEI) transmission electronic microscope.
2.7. Characteristics of porosity of scaffold structure
To carry out a comparative characteristic of the porous scaffolds’ structure, we used photomicrographs obtained by electron microscopy (4400×). Micrographs were processed using the ImageJ software package (National Institutes of Health). During this analysis we used a threshold binarisation procedure to identify the areas of interest (the biopolymer part of the scaffold) and the image background (pore lumen). Then the whole image area taken as 100% was scanned and the percentage correlation between the biopolymer area of the scaffold and the pore lumen region in the scaffold structure was calculated.
2.8. Investigation of mechanical characteristics of scaffolds
The mechanic characteristics of the scaffolds (5 samples with collagen №1 and 5 samples with collagen №2) in respect of compression were determined on a universal testing machine, an AG-Xplus-0.5 (Shimadzu, Japan). As prototypes, we used disc-shaped scaffolds with a diamter of 26 mm and a thickness of 2 mm. The thickness of the specimens was determined directly during testing by the distance between the compression plates of the testing machine at the time of reaching a total load of 0.1 N. The initial thickness of the samples was determined directly during testing by the distance between the compression plates of the testing machine at the moment the plate touches the surface of the scaffold while reaching a total load of 0.1 N. The investigations were performed at a squeezing rate of 1 mm/min. We chose compression stress at a strain value of 10% and 50% as the main measured parameters.
2.9. Microscopy
To observe the state of the native cells in the scaffold structures we used both bright-field and phase contrast methods (5 samples with collagen №1 and 5 samples with collagen №2). Microscopy amd videoarchiving were conducted with an inverted Leica DMI 3000 B microscope, equipped with LAZ. V. 3.4 image visualization software.
2.10. Fluorescence microscopy
To visualize the cells and confirm their viability within the scaffold structure (5 samples with collagen №1 and 5 samples with collagen №2) we used for fluorescence microscopy carried out on a Cytation 5 (BioTek, USA) multifunctional imager. To visualize viable cells we used Calcein AM (catalog № 564061, BD having an excitation wavelength of 477 nm and emission wavelength of 525 nm). Coloration was performed in accordance with the manufacturer's protocol. As a control, the cells of the same line and the same passage were used as those used in the formation of the corresponding scaffold, cultivated under standard conditions.
2.11. Quantitative analysis of cells in scaffolds
To characterize the density of distribution of cells in hydrogel scaffolds from the studied samples (3 samples with collagen № 1 and 3 samples with collagen № 2), fragments of 0.64 cm2 were separated using a template. The number of cells was determined by counting the nuclei 3 h after the formation of scaffolds. Scaffolds after separation of the first fragment were cultured under standard conditions. After 72 h, 0.64 cm2 fragments were separated and the number of cell nuclei was counted. To count cell nuclei in scaffolds, the isolated fragments of samples were transferred to a 24-well Black VisiplateTMTC plate (Wallac Oy, Finland). Next, the analysis of the number of cells was carried out according to the method of quantitative analysis of the cellular component of the scaffold (Pat. No. 2675376 of the Russian Federation … from 07/17/2017 [56]). The method includes intravital staining of cell nuclei in a scaffold using Hoechst 3334 (USA), fluorescence microscopy using the Z-stack function (Cytation 5 imager with Gen 5 Imedge software + BioTek, USA). For identification and counting of cells nucleis we used Hoechst 3334 (USA) having an excitation wavelength of 377 nm and emission wavelength of 477 nm. For identification and counting of dead cells, the samples were stained with TO-PROTM3 Ready Flow™ fluorochrome (invitrigen by Thermo Fisher Scientific, USA) having an excitation wavelength of 586 nm and emission wavelength of 647 nm. Coloration was performed in accordance with the manufacturer's protocol. We analyzed 5 microphotographs from each sample taken from different fields of view (magnification: 4× lens, 10× eyepiece) on arbitrary areas in the thickness of the samples. Objects were recorded in areas of 530 μm along the Z axis. Cross-linked Z-stack micrographs were analyzed with the number of cell nuclei counted and the cells recalculated per 1 mm3.
2.12. Investigation of the molecular-mass parameters of the collagen
The molecular-mass characteristics were determined using gel-penetrating chromatography (GPC) on a liquid chromatographer produced by Shimadzu CTO20 A/20AC (Japan) with a LC-Solutions-GPC software module. Separation was conducted on a Tosoh Bioscience TSKgel G3000SWxl column with a pore diameter of 5 μm. As a detector we used an ELSD-LT II low-temperature light-scattering detector. The eluent was a 0.5 M solution of acetic acid used at a flow rate of 0.8 ml/min. For calibration we used narrow dispersion samples of Fluca dextran with molecular masses (MM) ranging from 1000 to 410000 Da.
2.13. Statistical analysis
The results of the investigations were processed with STATISTICA 6.0 using the non-parametric statistics method and a Wilcoxon's paired-comparison test and Spearman correlation analysis (RS).
3. Results
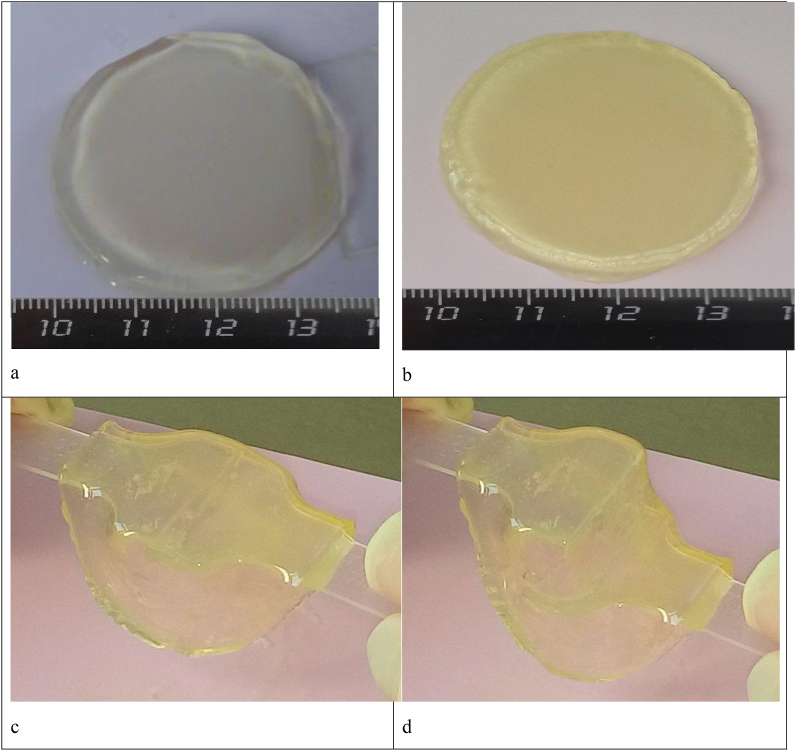
We formed the scaffolds based on the blood plasma cryoprecipitate in accordance with the above methods. Both types of scaffolds with collagen №1 and collagen №2 were transparent hydrogel structures having dimensional stability and elasticity (Fig. 3).
Fig. 3.
The appearance of hydrogel scaffolds immediately after formation with collagen №1 (a) and collagen №2 (b). Manipulations with scaffolds helping to visualize transparency, shape stability and elasticity (c, d).
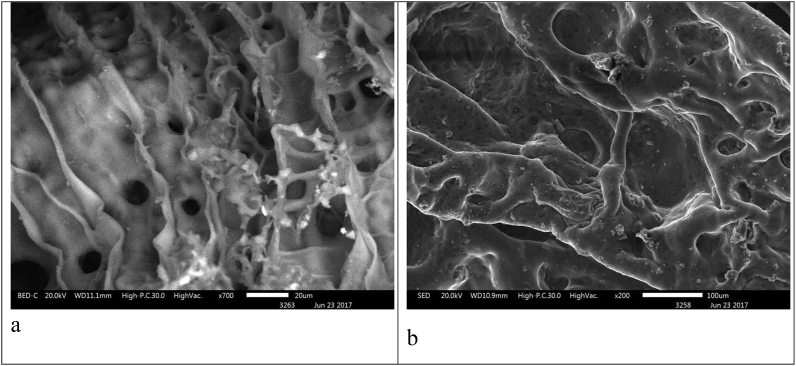
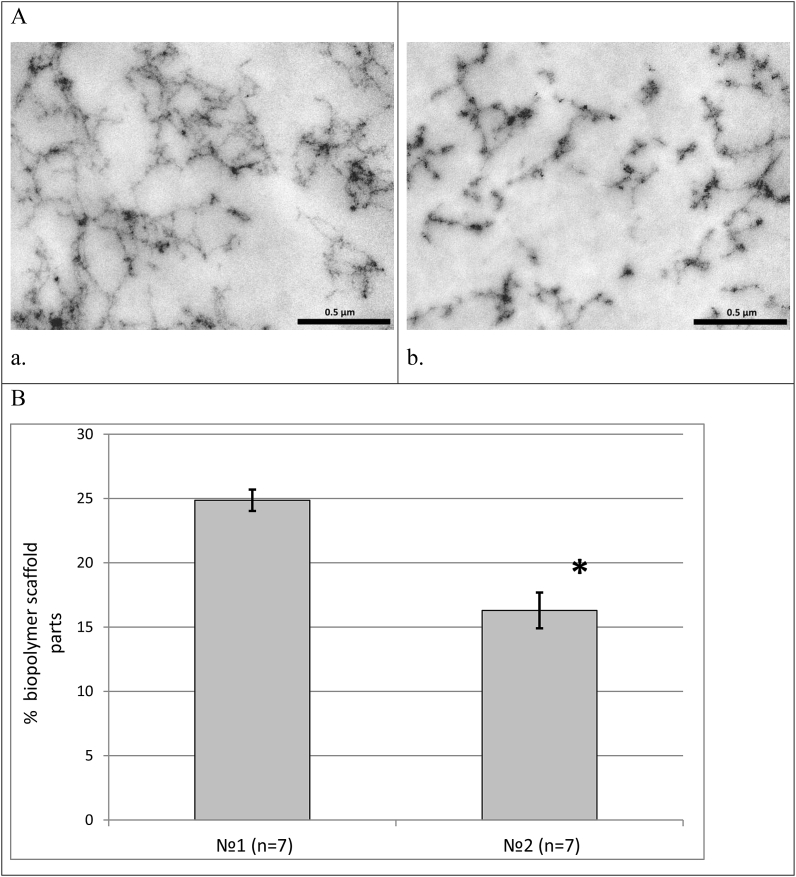
It was found that the internal architecture of scaffolds is characterized by a system of interrelated heterogeneous pores. Scaffolds that included collagen №1 had a more ordered internal structure and a smaller inter-pore distance than scaffolds with collagen №2 (Fig. 4). Fig. 5 shows microphotos of scaffolds with the collagen derived from different sources. Scaffolds with collagen №1 were characterized by a denser structure than scaffolds with collagen №2 (Fig. 5 А).
Fig. 4.
Internal architecture of scaffolds with collagen №1 (a) and collagen №2 (b) shown by scanning electronic microscopy (acellular scaffolds, cross-section).
Fig. 5.
(A) Structure of scaffolds with collagen №1 (a) and collagen №2 (b) shown by transmission electronic microscopy (х44000). Scale represent 5 μm; (B) Comparative characteristics of porosity in scaffolds with different kinds of collagens (in scaffold composition: №1 – is bovine collagen, №2 – sea collagen). Note: * – p < 0.05 – Wilcoxon test.
Analyzing the percentage correlation of the biopolymer part of the scaffold and pore lumen we noted that in scaffolds with collagen №1 this ratio was 1:3. In scaffolds with collagen №2 the ratio of the biopolymer part of a scaffold to pore lumen was 1:5. At the same time the percentage of the biopolymer part of scaffolds with collagen №1 was 1.5 times higher than that in scaffolds with collagen №2 (Fig. 5 B).
By studying the mechanical properties of scaffolds we noted that when the compression stress was 0.1 N, the registered thickness of the scaffolds with collagen №1 was 6% higher than with collagen №2. Compression stress at a strain value 10% did not differ for the scaffolds with the different kinds of collagens. Neither did the compression force show statistically significant differences between the samples with collagen №1 (0.16 ± 0.02 N) and the samples with collagen №2 (0.15 ± 0.03 N). To reach a strain value 50% of the scaffold samples with the different kinds of collagens we needed different compression forces (samples with collagen №1: 2.88 ± 0.28 N; samples with collagen №2: 1.76 ± 0.08 N). The compression stress at a strain value 50% of scaffold samples with collagen №1 was 1.6 times higher than the same parameter for scaffold samples with collagen №2 (Table 1).
Table 1.
Characteristics of scaffold mechanical properties.
| Origin of a collagen kind as a part of scaffold | Compression stress at a strain value 10%, kPa | Compression stress at a strain value 50%, kPa | The thickness of the scaffold |
|---|---|---|---|
| Collagen №1 (n = 5) | 0.50 ± 0.06 | 9.18 ± 0.90 | 1.86 ± 0.09 |
| Collagen №2 (n = 5) | 0.49 ± 0.05 | 5.61 ± 0.26 * | 1.75 ± 0.05* |
Note: * – p < 0.05 – Wilcoxon test.
The samples of initial collagens №1 and №2 were characterized according to their molecular-mass parameters (Table 2). It was noted that the parameters of collagens №1 and №2 were considerably different. The values of molecular mass (MM) exemplified by weight – average MM (Mw) were more than 3 times higher for collagen №1 compared to collagen №2. At the same time the polydispersion coefficients (Мn/Мw) of the analyzed collagens were rather similar. It should be mentioned that the values of polydispersion coefficient, which were greater than 1 in both cases, testify to partial hydrolysis of the collagen macromolecules during the process of extraction and storage.
Table 2.
Characteristics of molecular-mass collagen properties.
| Origin of a collagen kind | Мw (kDa) | Мn/Мw | Amount of high-molecular collagen in the sample (%) |
|---|---|---|---|
| Collagen № 1 (n = 5) | 947.2 | 1.4 | 93 |
| Collagen № 2 (n = 5) | 234.9 | 1.3 | 97 |
Note: Мw – average molecular weight, Мn/Мw – coefficient of polydispersion.
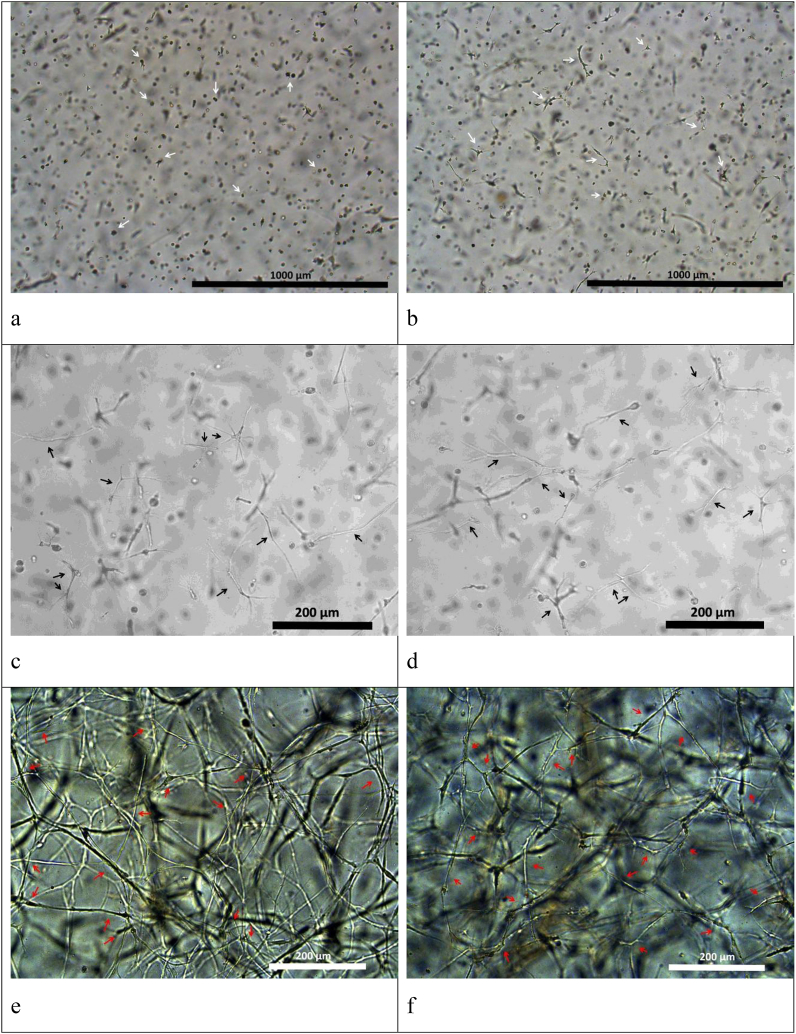
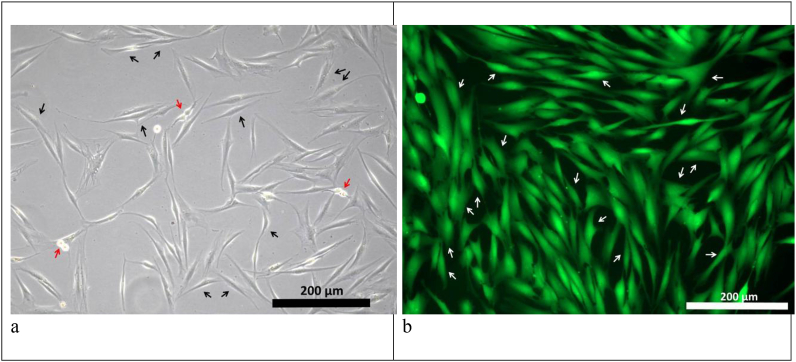
The potential of hydrogels based on cryoprecipitate used with collagens №1 and №2 as cell scaffolds was demonstrated during the encapsulation of stem cells obtained from human adipose tissue (ASCs) into the hydrogel structure in vitro. Cell cultivation for 6 days within the hydrogel scaffolds showed that they provided conditions contributing to the maintenance of both viability and typical ASC morphology. ASCs encapsulated into such scaffolds showed active matrix-cell adhesion and, within 24 h, began to form multiple processes (Fig. 6 a, b), similar to control culture cells (Fig. 8 a). As the cultivation proceeded we observed a dynamic three-dimensional cell growth with the formation of multiple processes and intercellular contacts (Fig. 6 c, d). On Day 6 in the scaffolds with collagen №1 and scaffolds with collagen №2 we noted the formation and development of cell networks (Fig. 6 g, h). The viability of cells cultivated in scaffolds was proved by staining with Calcein AM (Fig. 7 a, b). The cells of the control culture on the 6th day of cultivation formed a confluent monolayer and were characterized by high viability (Fig. 8 b).
Fig. 6.
ASC culture in scaffolds with collagen №1 (a, c, e) and collagen №2 (b, d, f) – light microscopy. (a, b) – 24 h after scaffold formation: beginning of cell process formation. Scale represent 1000 μm; (c, d) – Day 3 of cultivation: three-dimensional cell growth with the formation of multiple processes and intercellular contacts. Scale represent 200 μm; (e, f) – Day 6 of cultivation, cellular network. Scale represent 200 μm. The arrows indicate some of the ASCs.
Fig. 8.
ASCs culture cultivated under standard conditions (seeding density 10 thousand/cm2). (a) - 24 h of cultivation: cells adhere to the surface of the culture plastic and form cell processes (black arrows indicate some of the adhered ASCs; red arrows indicate dividing ASCs); (b) - 3 days of cultivation: morphologically identical spindle-shaped cells form a subconfluent monolayer, which is typical for two-dimensional cultivation. The specific fluorescent dye Calcein AM (green) stains viable cells (some of the ASCs stained with Calcein AM are marked with arrows).
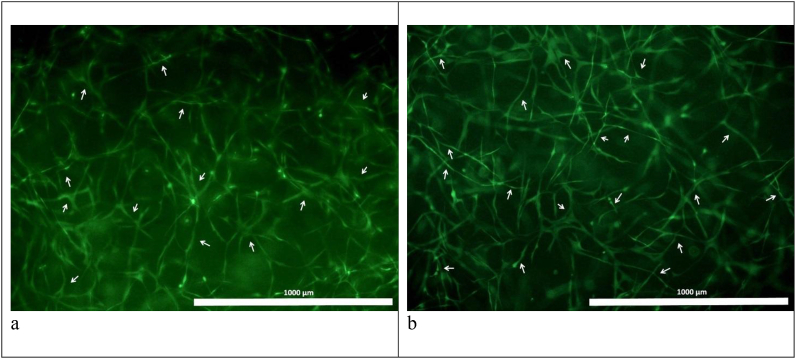
Fig. 7.
Characteristics of the viability of cells cultivated in scaffolds with collagen №1 (a) and collagen №2 (b). Scale represent 1000 μm. Day 6 of cultivation, fluorescence microscopy: with the specific fluorescent dye Calcein AM (green) – all the viable cells are stained. The arrows indicate some of the ASCs.
While carrying out a quantitative analysis of cells in the structure of scaffolds 3 h after their formation, it was found that the cells’ density in scaffolds with collagen № 1 was 40% higher than in scaffolds with collagen № 2. While cultivating within 72 h, the number of cells in scaffolds increased by 1.23 times, which indicated proliferative activity of cells (Table 3). The number of dead cells identified by staining with the specific dye TO-PROTM3 Ready Flow™ in the samples can be described as missing or single in the field of view. There were no statistically significant differences in the number of dead cells in scaffolds with collagen № 1 and scaffolds with collagen № 2.
Table 3.
Quantitative analysis of the cells in scaffolds.
| Origin of a collagen kind | 3 h |
72 h |
||
|---|---|---|---|---|
| Total number of cell nuclei in 1 mm3 scaffold | The number of dead cells' nuclei in 1 mm3 scaffold | Total number of cell nuclei in 1 mm3 scaffold | The number of dead cells' nuclei in 1 mm3 scaffold | |
| Collagen №1 | 200.98 ± 5.61 | 0.73 ± 0.23 | 246.74 ± 19.89 ● | 1.39 ± 0.27 |
| Collagen №2 | 143.08 ± 5.45 * | 0.81 ± 0.29 | 176.70 ± 9.26 * ● | 1.25 ± 0.35 |
Note: * - p < 0.05 - comparison with “Collagen № 1”; ● - p < 0.05 - comparison with “3 h”; Wilcoxon test.
An analysis of the correlation of the percentage of the biopolymer part of scaffolds and the number of cells in 1 mm3 of scaffolds 3 h after their formation confirmed the presence of a positive correlation between the analyzed parameters (RS = 0.65; P = 0.008712).
4. Discussion
The base for the formation of hydrogel scaffolds was a cryoprecipitate of blood plasma. It is known that such blood plasma cryoprecipitates contain fibrinogen, fibronectin, factor XIII, fibrinolysis inhibitors, and circulating molecules of cell adhesion in a considerably higher concentration than standard blood plasma [57,58]. Fibrinogen is the main biopolymer that takes part in the formation of a scaffold structure according to the method described by ourselves. The concentration of fibrinogen in the cryoprecipitate varied and substantially exceeded its concentration in blood plasma. That is why for unification of the technique for obtaining scaffolds the concentration of fibrinogen was standardized to 6 g/l. Previous studies had indicated that this concentration of fibrinogen is optimal for scaffold formation. Such use of blood plasma cryoprecipitate provides an opportunity not only to increase the amount of fibrinogen but also to reach the required concentration. It should be noted that the methods for obtaining the cryoprecipitate, involving further standardization according to fibrinogen concentration as used here, does not have any negative impact on the polymerization ability of the fibrinogen and does not require any additional reagents.
It is known that the formation of a fibrin network is a physiological process resulting from the interaction of fibrinogen of the blood plasma and thrombin. Thrombin splits the amino-terminal ends of the α- and β-chains of fibrinogen and results in the formation of febrinopeptides A and B – fibrin-monomers. By spontaneous polymerization these fibrin-monomers self-organize to form fibrin protofibrils and then a fibrin network [59,60]. The self-assembly of the fibrin fibers depends on the concentration of fibrinogen, thrombin and calcium as well as on other proteins [61]. Depending on this, the network can possess various different densities and thicknesses of fibrils. For instance, high concentrations of thrombin contribute to the formation of thin bundles of thin fibril fibers packed in a dense network. A lower thrombin concentration in relation to the same amount of fibrinogen leads to a greater average size of the bundles of fibers and to increased porosity of the fibrin matrix [62]. When the thickness of the fibrin fibers is changed, their morphology is also changed. J.W. Weisel and R.I. Litvinov [63] showed that thick fibers have fewer side branches compared to thin ones. The data we obtained with scanning electron microscopy showed that the structure of the scaffolds was characterized by the presence of rather thick interconnected fibers and a developed system of pores. Scaffolds with collagen №1 showed a more organized structure of the fibers compared to the structures with collagen №2. At the same time the internal structure of the resulting scaffold differed from fibrin-only hydrogels in which the structure is, as a rule, formed by the cross-linking of poorly-ordered fibers [64]. The formation of the internal architecture of the scaffolds under study can be immediately affected by other components included in the composite. Thus, it is known that PEGylation of PEG-NHS protein leads to the cross-linking of protein molecules and an increase in the thickness of the fibrin fibers [65,66]. Blood plasma cryoprecipitate also contains fibronectin and factor XIII. Fibronectin can modify a three-dimensional structure of the fibrin matrix by regulating the thickness and density of the fibrin fibers. This is connected with the ability of fibronectin to covalently and non-covalently bind with the fibrin matrix forming high-molecular multimers [67]. Factor XIII catalyzes the formation of γ-glutamyl-ε-lysyl amide-bonds between the fibrin monomers and hence stabilizes its polymers, increasing the durability of the fibrin matrix [59,68].
The composite, our scaffolds are formed from, includes type I collagen. In our work we used collagen derived from two different sources – bovine and cod collagen (marine). Bovine collagen is typically widely used in scaffold-technologies and is valued as a base for the formation of mono- and poly-composite scaffolds [36,69,70]. Sea collagen from fish scales, skin and bones is also used as a material for scaffolds. According to the reference data, based on a structural analysis of the protein, fish have type I collagen equivalent to the type I collagen of mammals and birds [71,72]. In the sphere of tissue engineering sea collagen is popular as a material having both good bioactivity and biocompatibility, low antigenicity, and high biodegradability [[73], [74], [75]].
We found no data on the effect of collagen's origin on the structural characteristics and mechanical properties of scaffolds in the reference materials. There is also very little information on the effect of the collagen's origin on the characteristics of fibrin-collagen scaffolds produced applying technology that uses hydrolytic enzymes affecting both the structure-forming proteins. That is why it was of interest to investigate whether the source of collagen could influence the properties of the scaffolds obtained by co-polymerization of fibrinogen that originated from blood plasma cryoprecipitate.
According to our findings scaffolds with collagen №1 had a more elastic structure than scaffolds with collagen №2. This resulted in higher compression stress at a strain value 50% of scaffolds with collagen №1 compared to scaffolds with collagen №2. It should be mentioned that during the formation of scaffolds all the components except the source of collagen were identical and used at the same concentrations and ratios. Thus, the identified differences can be explained by the peculiarities of the internal architecture of the scaffolds formed with the collagen derived from different sources. We know of works that indicate that the density of scaffold structures can determine their module of elasticity to a great extent [76]. F. You et al. [77] also demostrated that the mechanical properties of hydrogel scaffolds depend on their porosity and other structural characteristics. Our own studies show that the scaffolds that included collagen №1 have a higher percentage of the biopolymer component in relation to pore lumen compared to scaffolds wth collagen №2. It can be concluded that the differences in porosity of the scaffold structures containing the different collagens can have a direct effect on their elasticity. This also explains the difference in the thickness of scaffolds with different collagens even though they were formed in similar conditions. The initial thickness of the scaffolds was measured at the moment the instrument plate touched the surface of the scaffold when the total load was 0.1 N. Thus, under the same conditions, the measured value was higher for those samples that had a denser structure with lower porosity — for scaffolds with collagen №1.
The identified peculiarities of the internal structure of the scaffolds can be explained both by the initially different molecular-mass characteristics of collagens №1 and №2 and by the difference in the products of enzymatic hydrolysis of the collagens used. The investigation of the molecular-mass characteristics of collagens №1 and №2 showed that they were different in molecular mass and in their polydispersion coefficients. According to the reference data, collagens of different origin are different in their composition and sequence of amino acids [75,78,79]. During scaffold formation, thrombin was added to the composite where fibrinogen had already been present in the blood plasma cryoprecipitate and collagen. Hence, the thrombin interacted with both the proteins of the blood plasma cryoprecipitate and with the collagen. It is supposed that the products of the enzymatic hydrolysis of collagen take part in the formation of the resulting scaffolds. The enzyme, which is in our case, thrombin, hydrolyzes a peptide bond between specific amino acids [80], and so the sites for this will be controlled by the composition and sequence of the amino acids in collagen macromolecule that, in our scaffolds are different by nature of the collagen source [[81], [82], [83]]. A.R. Vidal et al. [84] showed that hydrolysates obtained with pepsin from differently originated collagen have different molecular characteristics and functional activity. M. Blanco et al. [85] identified cross-species peculiarities during their investigation of the products of enzymatic hydrolysis of type I collagen isolated from the skins of some species of sea fish. It is obvious and admissible that there is a difference in the products of enzymatic hydrolysis of collagens №1 and №2 under the effect of thrombin. The products of enzymatic hydrolysis have a lower molecular mass than the initial collagen molecule and are used like fibrinogen/fibrin to form scaffolds. Thus, the different molecular mass molecules formed after enzymatic hydrolysis of collagens №1 and №2 result in the formation of different internal structures of the scaffold and, consequently result in differences in the parameters and properties. It should be noted that during the formation of scaffolds the amount of fibrinogen in the composite is 22 times higher than the amount of collagen. This demonstrates the significance of the effect that the origin of collagen has on the structure formation even in this ratio, as it works by co-polymerization with the fibrinogen. Thus, when developing polycomposite scaffolds for tissue engineering, special attention should be paid to the molecular-mass characteristics of the biopolymers to be used. This is especially important if, in the process of scaffold formation, enzymatic hydrolysis and simultaneous polymerization of the hydrolysates of the various types of biological molecules are involved.
The combination of several polymers during scaffold formation is a promising approach that allows modification of their structural, mechanical and other properties. In particular, the combination of collagen and fibrin gives such combined hydrogels mechanical characteristics that differ from the mechanical characteristics of those using purely collagen or purely fibrin materials. As a rule, this is caused by the interaction of the microfibrils of both structural proteins [86,87]. At the same time the structure of the majority of current polycomposite scaffolds is represented by several separate interpenetrating polymer networks that are not connected with each other by covalent bonds [88,89]. It is a more attractive idea to use several structure-forming components with different properties that form a unified structure having similar cross-linking mechanisms when preparing scaffolds. Such similar cross-linking mechanisms will simplify the process of scaffold preparation and allow the concurrent formation of a unified structure from the different components. Whereas, if the components have different mechanisms of cross-linking, this can lead to a multistage and more complicated process of scaffold formation. The scanning electron microscope data characterizing the internal architecture of the scaffolds presented by us confirm a unified structure. The specific properties of the scaffold structures with collagens №1 and №2 are caused only by differences in the products of the enzymatic hydrolysis of the collagens taking part in the formation of that unified structure. This enables us to state that the products of the enzymatic hydrolysis of collagen and fibrinogen are covalently bound. Thus, our suggested technique for the formation of hydrogel scaffolds from composites including blood plasma cryoprecipitate and collagen allows the production of polycomposite scaffolds with a unified structure requiring a minimal number of process stages.
At the present time there is no doubt in high potential of mesenchymal stem cells (MSCs) in regenerative medicine and tissue engineering [[90], [91], [92], [93]]. Review by S.M. Willerth and S.E. Sakiyama-Elbert (2019) demonstrates the dynamic development of the direction of creating tissue-engineering constructs and cell delivery systems based on biomaterials in combination with stem cells [94]. MSCs are often used for in vitro biocompatibility testing of new materials developed for regenerative medicine and tissue engineering [95,96], including hydrogels [97]. To demonstrate the principal possibility of using the studied hydrogels in the future as scaffolds for culturing and delivering cells, human adipose tissue ASCs were encapsulated in the structure of scaffolds.
It was shown that the conditions for the formation of scaffolds and the composition of the composite allow cells to be encapsulated in the hydrogel structure without negatively affecting their viability, morphology, and proliferation. Mesenchymal stem cells encapsulated in hydrogel scaffolds are known to exhibit three-dimensional growth [98,99]. Adipose tissue stem cells cultured in the scaffolds we presented had the corresponding morphological characteristics, showed active three-dimensional growth and proliferative activity. There is no doubt that successful cellular events are directly associated with a scaffold structure that provides both mechanical support and appropriate conditions for the placement and interaction of cells in its system of pores. This agrees with the reference data on the effect of scaffold microstructure on cell adhesion, migration and proliferation [100,101].
While conducting a quantitative analysis of cells in the structure of scaffolds 3 h later after their formation, it was found that the number of cells per 1 mm3 in scaffolds with collagen № 1 is more than in scaffolds with collagen № 2. Taking that into account the fact that in the formation of scaffolds in both cases, we used the same cell concentration, we can assume that the differences obtained by counting the cells in the formed scaffolds are associated with differences in their internal architectonics. So, scaffolds with collagen № 1 had a denser structure than scaffolds with collagen № 2. The number of cells per volume unit in scaffolds with collagen № 1 was greater than in scaffolds with collagen № 2. Thus, initially the same number of cells in the composite during the formation of scaffolds, apparently, was redistributed relative to the formed structure. This is confirmed by the revealed correlation relationship between the percentage of the biopolymer part of scaffolds and the number of cells in 1 mm3 of formed hydrogels. The number of cells in scaffolds increased after 72 h of cultivation, which indicates the maintenance of proliferative activity by cells. At the same time, the increase in the number of cells in scaffolds with collagen № 1 and collagen № 2 was comparable. The latter, suggests that the revealed differences in the structural characteristics of the studied scaffolds did not significantly affect the proliferative activity of cells.
Not only the structure of scaffolds, but also their hydrophilic nature could facilitate the maintenance of viability and proliferative activity, facilitating the exchange of nutrients and waste products. Cellular events taking place within scaffolds can be significantly influenced not only by their structure, but also the properties of the biologically active substances included in their composition. Thus, fibrinogen/fibrin molecules have sequences that interact with integrin cell receptors and therefore affect cell processes [37,102,103]. The blood plasma that is the basis of the composite of the presented scaffold contains all the amino acids that are needed for cellular growth and proliferation [104], as well as fibronectin which is one of the key proteins of the intercellular matrix [105]. Evaluation of the comparative characteristics of cell growth and activity in scaffolds with different collagens and therefore with differing structures is an aspect to be investigated further.
5. Conclusion
We have presented a hydrogel scaffold based on fibrinogen/fibrin derived from a blood plasma cryoprecipitate and collagen co-polymerized by enzymatic hydrolysis. We conducted a study of the structural and mechanical characteristics of scaffolds formed from collagen derived from two different sources where the rest of the composite components and technology used were identical. We found that the structural and mechanical properties of these scaffolds do depend on the origin of collagen. The resulting differences can be connected with the specificity of the molecular-mass characteristics of the products of enzymatic hydrolysis of the collagens used. Undoubtedly, the internal architectonics of the obtained scaffolds directly determined their mechanical properties and cells’ distribution density.
The data presented demonstrate that hydrogel scaffolds have good biocompatibility and provide conditions that support the three-dimensional growth of ASCs. The enzymatic hydrolysis reactions underlying the technology of scaffold formation occur readily in physiological conditions and are highly biocompatible. Their application allows the encapsulation of cells into hydrogel structures at the stage of scaffold formation without damaging them. In our view, hydrogel scaffolds based on blood plasma cryoprecipitates and collagen can be a good choice for cell cultivation and their delivery in the future.
Acknowledgments
The study on the determination of the structural (scanning electronic microscopy) and mechanical characteristics of scaffolds was performed with equipment at the Center for Continuing Education “New Materials and Resource-Saving Technologies” (Scientific Research Institute of Chemistry of N.I. Lobachevsky State University of Nizhny Novgorod, Nizhny Novgorod, Russia).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
References
- 1.Naahidi S., Jafari M., Logan M., Wang Y., Yuan Y., Bae H., Dixon B., Chen P. Biocompatibility of hydrogel-based scaffolds for tissue engineering applications. Biotechnol. Adv. 2017;35:530–544. doi: 10.1016/j.biotechadv.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 2.Zhu J. Biomimetic hydrogels as scaffolds for tissue engineering. J. Biochips Tissue Chips. 2012:2. 1000e119. [Google Scholar]
- 3.Chirani N., Yahia L., Gritsch L., Motta F.L., Chirani S., Faré S. History and applications of hydrogels. J. Biomed. Sci. 2015;4:1–23. [Google Scholar]
- 4.Lee K.Y., Mooney D.J. Hydrogels for tissue engineering. Chem. Rev. 2001;101:1869–1879. doi: 10.1021/cr000108x. [DOI] [PubMed] [Google Scholar]
- 5.De France K.J., Xu F., Hoare T. Structured macroporous hydrogels: progress, challenges, and opportunities. Adv. Healthc. Mater. 2018;7:1–17. doi: 10.1002/adhm.201700927. [DOI] [PubMed] [Google Scholar]
- 6.Włodarczyk-Biegun M.K., Farbod K., Werten M.W.T., Slingerland C.J., De Wolf F.A., Van Den Beucken J.J.J.P., Leeuwenburgh S.C.G., Cohen Stuart M.A., Kamperman M. Fibrous hydrogels for cell encapsulation: a modular and supramolecular approach. PLoS One. 2016;11 doi: 10.1371/journal.pone.0155625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gwon K., Kim E., Tae G. Heparin-hyaluronic acid hydrogel in support of cellular activities of 3D encapsulated adipose derived stem cells. Acta Biomater. 2017;49:284–295. doi: 10.1016/j.actbio.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Hunt N.C., Grover L.M. Cell encapsulation using biopolymer gels for regenerative medicine. Biotechnol. Lett. 2010;32:733–742. doi: 10.1007/s10529-010-0221-0. [DOI] [PubMed] [Google Scholar]
- 9.Velema J., Kaplan D. Biopolymer-based biomaterials as scaffolds for tissue engineering. Adv. Biochem. Eng. Biotechnol. 2006;102:187–238. doi: 10.1007/10_013. [DOI] [PubMed] [Google Scholar]
- 10.Kim S.H., Turnbull J., Guimond S. Extracellular matrix and cell signalling: the dynamic cooperation of integrin, proteoglycan and growth factor receptor. J. Endocrinol. 2011;209:139–151. doi: 10.1530/JOE-10-0377. [DOI] [PubMed] [Google Scholar]
- 11.Sasaki N. Viscoelastic Properties Ofbiological Material. 2012. [DOI]
- 12.Bartnikowski M., Wellard R.M., Woodruff M., Klein T. Tailoring hydrogel viscoelasticity with physical and chemical crosslinking. Polymers. 2015;7:2650–2669. [Google Scholar]
- 13.Oyen M.L. Mechanical characterisation of hydrogel materials. Int. Mater. Rev. 2014;59:44–59. [Google Scholar]
- 14.Toohey K.S., Kalyanam S., Palaniappan J., Insana M.F. Indentation analysis of biphasic viscoelastic hydrogels. Mech. Mater. 2016;92:175–184. doi: 10.1016/j.mechmat.2015.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chaudhuri O. Viscoelastic hydrogels for 3D cell culture. Biomater. Sci. 2017;5:1480–1490. doi: 10.1039/c7bm00261k. [DOI] [PubMed] [Google Scholar]
- 16.Kim H.N., Choi N. Consideration of the mechanical properties of hydrogels for brain tissue engineering and brain-on-a-chip. Biochip J. 2019;13:8–19. [Google Scholar]
- 17.Eddhahak A., Zidi M. Influence of viscoelastic properties of an hyaluronic acid-based hydrogel on viability of mesenchymal stem cells. Bio Med. Mater. Eng. 2015;26:103–114. doi: 10.3233/BME-151557. [DOI] [PubMed] [Google Scholar]
- 18.Chaudhuri O., Gu L., Klumpers D., Darnell M., Bencherif S.A., Weaver J.C., Huebsch N., Lee H.P., Lippens E., Duda G.N., Mooney D.J. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat. Mater. 2016;15:326–334. doi: 10.1038/nmat4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kocen R., Gasik M., Gantar A., Novak S. Viscoelastic behaviour of hydrogel-based composites for tissue engineering under mechanical load. Biomed. Mater. 2017;12 doi: 10.1088/1748-605X/aa5b00. 025004. [DOI] [PubMed] [Google Scholar]
- 20.Calhoun M.A., Bentil S.A., Elliott E., Otero J.J., Winter J.O., Dupaix R.B. Beyond linear elastic modulus: viscoelastic models for brain and brain mimetic hydrogels. ACS Biomater. Sci. Eng. 2019;5:3964–3973. doi: 10.1021/acsbiomaterials.8b01390. [DOI] [PubMed] [Google Scholar]
- 21.Czerner M., Fellay L.S., Suárez M.P., Frontini P.M., Fasce L.A. Determination of elastic modulus of gelatin gels by indentation experiments. Procedia Mater. Sci. 2015;8:287–296. [Google Scholar]
- 22.Mattei G., Cacopardo L., Ahluwalia A. Micro-mechanical viscoelastic properties of crosslinked hydrogels using the nano-epsilon dot method. Materials. 2017;10:889. doi: 10.3390/ma10080889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tummala G.K., Bachi I., Mihranyan A. Role of solvent on structure, viscoelasticity, and mechanical compressibility in nanocellulose-reinforced poly(vinyl alcohol) hydrogels. J. Appl. Polym. Sci. 2019;136:1–8. [Google Scholar]
- 24.Li X., Sun Q., Li Q., Kawazoe N., Chen G. Functional hydrogels with tunable structures and properties for tissue engineering applications. Front. Chem. 2018;6:499. doi: 10.3389/fchem.2018.00499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar A., Rao K.M., Han S.S. Mechanically viscoelastic nanoreinforced hybrid hydrogels composed of polyacrylamide, sodium carboxymethylcellulose, graphene oxide, and cellulose nanocrystals. Carbohydr. Polym. 2018;193:228–238. doi: 10.1016/j.carbpol.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 26.Hui E., Gimeno K.I., Guan G., Caliari S.R. Spatial control of viscoelasticity in phototunable hyaluronic acid hydrogels. BioRxiv. 2019:646778. doi: 10.1021/acs.biomac.9b00965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cooke M.E., Lawless B.M., Bellier F., Hughes E.A.B., Grover L.M., Jones S.W., Cox S.C. Formulation and viscoelasticity of mineralised hydrogels for use in bone-cartilage interfacial reconstruction. J. Mech. Behav. Biomed. Mater. 2018;80:33–41. doi: 10.1016/j.jmbbm.2018.01.016. [DOI] [PubMed] [Google Scholar]
- 28.Bitar K., Zakhem E. Biomedical engineering and computational biology design strategies of biodegradable scaffolds for tissue regeneration. Biomed. Eng. Comput. Biol. 2014;6:13–20. doi: 10.4137/BECB.S10961. (RECEIVED) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Annor A.H., Tang M.E., Pui C.L., Ebersole G.C., Frisella M.M., Matthews B.D., Deeken C.R. Effect of enzymatic degradation on the mechanical properties of biological scaffold materials. Surg. Endosc. 2012;26:2767–2778. doi: 10.1007/s00464-012-2277-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pang Y., Ucuzian A.A., Matsumura A., Brey E.M., Gassman A.A., Husak V.A., Greisler H.P. Biomaterials the temporal and spatial dynamics of microscale collagen scaffold remodeling by smooth muscle cells. Biomaterials. 2009;30:2023–2031. doi: 10.1016/j.biomaterials.2008.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ahearne M. Introduction to cell − hydrogel mechanosensing. Interface Focus. 2014;4:20130038. doi: 10.1098/rsfs.2013.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De la Puente P., Ludeña D. Cell culture in autologous fibrin scaffolds for applications in tissue engineering. Exp. Cell Res. 2014;322:1–11. doi: 10.1016/j.yexcr.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 33.Dong C., Lv Y. Application of collagen scaffold in tissue engineering: recent advances and new perspectives. Polymers. 2016;8:42. doi: 10.3390/polym8020042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rajangam T., An S.S.A. Fibrinogen and fibrin based micro and nano scaffolds incorporated with drugs, proteins, cells and genes for therapeutic biomedical applications. Int. J. Nanomed. 2013;8:3641–3662. doi: 10.2147/IJN.S43945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Noori A., Ashrafi S.J., Vaez-Ghaemi R., Hatamian-Zaremi A., Webster T.J. A review of fibrin and fibrin composites for bone tissue engineering. Int. J. Nanomed. 2017;12:4937–4961. doi: 10.2147/IJN.S124671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davidenko N., Schuster C.F., Bax D.V., Farndale R.W., Hamaia S., Best S.M., Cameron R.E. Evaluation of cell binding to collagen and gelatin: a study of the effect of 2D and 3D architecture and surface chemistry. J. Mater. Sci. Mater. Med. 2016;27:148. doi: 10.1007/s10856-016-5763-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laurens N., Engelse M.A., Jungerius C., Löwik C.W., van Hinsbergh V.W., Koolwijk P. Single and combined effects of alphavbeta3- and alpha5beta1-integrins on capillary tube formation in a human fibrinous matrix. Angiogenesis. 2009;12:275–285. doi: 10.1007/s10456-009-9150-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martino M.M., Briquez P.S., Ranga A., Lutolf M.P., Hubbell J.A. Heparin-binding domain of fibrin(ogen) binds growth factors and promotes tissue repair when incorporated within a synthetic matrix. Proc. Natl. Acad. Sci. U.S.A. 2013;110:4563–4568. doi: 10.1073/pnas.1221602110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hadjipanayi E., Kuhn P.H., Moog P., Bauer A.T., Kuekrek H., Mirzoyan L., Hummel A., Kirchhoff K., Salgin B., Isenburg S., Dornseifer U., Ninkovic M., Machens H.G., Schilling A.F. The fibrin matrix regulates angiogenic responses within the hemostatic microenvironment through biochemical control. PLoS One. 2015;10 doi: 10.1371/journal.pone.0135618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schaller J., Gerber S., Kämpfer U., Lejon Crottet S., Trachsel C. Wiley; 2008. Human Blood Plasma Proteins: Structure and Function. [Google Scholar]
- 41.Chung E., Rytlewski J.A., Merchant A.G., Dhada K.S., Lewis E.W., Suggs L.J. Fibrin-based 3D matrices induce angiogenic behavior of adipose-derived stem cells. Acta Biomater. 2015;17:78–88. doi: 10.1016/j.actbio.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hielscher A., Ellis K., Qiu C., Porterfield J., Gerecht S. Fibronectin deposition participates in extracellular matrix assembly and vascular morphogenesis. PLoS One. 2016;11 doi: 10.1371/journal.pone.0147600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Starke R.D., Ferraro F., Paschalaki K.E., Dryden N.H., McKinnon T.A.J., Sutton R.E., Payne E.M., Haskard D.O., Hughes A.D., Cutler D.F., Laffan M.A., Randi A.M. Endothelial von Willebrand factor regulates angiogenesis. Blood. 2011;117:1071–1080. doi: 10.1182/blood-2010-01-264507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang Q., Zou Y., Arno M.C., Chen S., Wang T., Gao J., Dove A.P., Du J. Hydrogel scaffolds for differentiation of adipose-derived stem cells. Chem. Soc. Rev. 2017;46:6255–6275. doi: 10.1039/c6cs00052e. [DOI] [PubMed] [Google Scholar]
- 45.Robles-Vazquez O., Orozco-Avila I., Sánchez Díaz J.C., Hernandez E. An overview of mechanical tests for polymeric biomaterial scaffolds used in tissue engineering. J. Res. Updates Polym. Sci. 2016;4:168–178. [Google Scholar]
- 46.Kuhn A.I., Müller M., Knigge S., Glasmacher B. Novel blood protein based scaffolds for cardiovascular tissue engineering. Curr. Dir. Biomed. Eng. 2016;2:5–9. [Google Scholar]
- 47.Enea D., Henson F., Kew S., Wardale J., Getgood A., Brooks R., Rushton N. Extruded collagen fibres for tissue engineering applications: effect of crosslinking method on mechanical and biological properties. J. Mater. Sci. Mater. Med. 2011;22:1569–1578. doi: 10.1007/s10856-011-4336-1. [DOI] [PubMed] [Google Scholar]
- 48.Rana D., Kumar T.S.S., Ramalingam M. Cell-laden hydrogels for tissue engineering. J. Biomater. Tissue Eng. 2014;4:507–535. [Google Scholar]
- 49.Castilho M., Hochleitner G., Wilson W., Van Rietbergen B., Dalton P.D., Groll J., Malda J., Ito K. Mechanical behavior of a soft hydrogel reinforced with three-dimensional printed microfibre scaffolds. Sci. Rep. 2018;8:1245. doi: 10.1038/s41598-018-19502-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Annabi N., Tamayol A., Uquillas J.A., Akbari M., Bertassoni L.E., Cha C., Camci-Unal G., Dokmeci M.R., Peppas A., Khademhosseini N.A. 25th anniversary article: rational design and applications of hydrogels in regenerative medicine. Adv. Mater. 2014;26:85–124. doi: 10.1002/adma.201303233. (Fluid) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Raucci M.G., Demitri C., Soriente A., Fasolino I., Sannino A., Ambrosio L. Gelatin/nano-hydroxyapatite hydrogel scaffold prepared by sol-gel technology as filler to repair bone defects. J. Biomed. Mater. Res. A. 2018;106 doi: 10.1002/jbm.a.36395. 2007–2019. [DOI] [PubMed] [Google Scholar]
- 52.Sumayya A.S., Muraleedhara Kurup G. Marine macromolecules cross-linked hydrogel scaffolds as physiochemically and biologically favorable entities for tissue engineering applications. J. Biomater. Sci. Polym. Ed. 2017;28:807–825. doi: 10.1080/09205063.2017.1303119. [DOI] [PubMed] [Google Scholar]
- 53.Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F.C., Krause D.S., Deans R.J., Keating A., Prockop D.J., Horwitz E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement, Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 54.M.N. Egorikhina, G.Y. Levin, I.N. Charykova, D.Y. Alejnik, S.L. N., Patent. 2653434 RU, Int. Cl. C12N 5/00. Method for creating a bioresorbable cellular scaffold based on fibrin of blood plasma, Bull. (Arch. Am. Art). 13 (2018).
- 55.L.L. Semenycheva, M. V. Astanina, J.L. Kuznetsova, N.B. Valetova, E. V. Geras’kina, T.O. A., Patent. 2567171 Int. Cl. C08H 1/06, A23J 1/04 Method for production of acetic dispersion of high molecular fish collagen, Bull. (Arch. Am. Art). vol 31 (2015).
- 56.Egorikhina M.N., Charykova I.N., Alejnik D.Y. 2018. Patent №2675376 the Russian Federation, Int. Cl. G01N 33/52 Method of Quantitative Analysis of Cellular Components of Scaffold. Application: 2017125696, 17.07.2017, Bull. [Google Scholar]
- 57.Sparrow R.L., Simpson R.J., Greening D.W. A protocol for the preparation of cryoprecipitate and cryo-depleted plasma for proteomic studies. Methods Mol. Biol. 2017;1619:23–30. doi: 10.1007/978-1-4939-7057-5_2. [DOI] [PubMed] [Google Scholar]
- 58.Nascimento B., Goodnough L.T., Levy J.H. Cryoprecipitate therapy. Br. J. Anaesth. 2014;113:922–934. doi: 10.1093/bja/aeu158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Falvo M.R., Gorkun O.V., Lord S.T. The molecular origins of the mechanical properties of fibrin. Biophys. Chem. 2010;152:15–20. doi: 10.1016/j.bpc.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mosesson M.W. Fibrinogen and fibrin structure and functions. J. Thromb. Haemost. 2005;3:1894–1904. doi: 10.1111/j.1538-7836.2005.01365.x. [DOI] [PubMed] [Google Scholar]
- 61.Weisel J.W., Nagaswami C. Computer modeling of fibrin polymerization kinetics correlated with electron microscope and turbidity observations: clot structure and assembly are kinetically controlled. Biophys. J. 1992;63:111–128. doi: 10.1016/S0006-3495(92)81594-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Blombäck B., Bark N. Fibrinopeptides and fibrin gel structure. Biophys. Chem. 2004;112:147–151. doi: 10.1016/j.bpc.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 63.Weisel J.W., Litvinov R.I. Mechanisms of fibrin polymerization and clinical implications. Blood. 2013;121:1712–1719. doi: 10.1182/blood-2012-09-306639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Janmey P.A., Winer J.P., Weisel J.W. Fibrin gels and their clinical and bioengineering applications. J. R. Soc. Interface. 2009;6:1–10. doi: 10.1098/rsif.2008.0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bryant S.J., Durand K.L., Anseth K.S. Manipulations in hydrogel chemistry control photoencapsulated chondrocyte behavior and their extracellular matrix production. J. Biomed. Mater. Res. A. 2003;67:1430–1436. doi: 10.1002/jbm.a.20003. [DOI] [PubMed] [Google Scholar]
- 66.Shpichka A.I., Koroleva A.V., Deiwick A., Timashev P.S., Semenova E.F., Moiseeva I.Y., Konoplyannikov M.A., Chichkov B.N. Evaluation of the vasculogenic potential of hydrogels based on modified fibrin. Cell Tissue Biol. 2017;11:81–87. [PubMed] [Google Scholar]
- 67.Ramanathan A., Karuri N. Fibronectin alters the rate of formation and structure of the fibrin matrix. Biochem. Biophys. Res. Commun. 2014;443:395–399. doi: 10.1016/j.bbrc.2013.11.090. [DOI] [PubMed] [Google Scholar]
- 68.Collet J.P., Shuman H., Ledger R.E., Lee S., Weisel J.W. The elasticity of an individual fibrin fiber in a clot. Proc. Natl. Acad. Sci. U.S.A. 2005;102:9133–9137. doi: 10.1073/pnas.0504120102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chan E.C., Kuo S.M., Kong A.M., Morrison W.A., Dusting G.J., Mitchell G.M., Lim S.Y., Liu G.S. Three dimensional collagen scaffold promotes intrinsic vascularisation for tissue engineering applications. PLoS One. 2016;11 doi: 10.1371/journal.pone.0149799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhong S.P., Zhang Y.Z., Lim C.T. Tissue scaffolds for skin wound healing and dermal reconstruction. Wiley Interdiscip. Rev. Nanomedicine Nanobiotechnology. 2010;2:510–525. doi: 10.1002/wnan.100. [DOI] [PubMed] [Google Scholar]
- 71.Saito M., Takenouchi Y., Kunisaki N., Kimura S. Complete primary structure of rainbow trout type I collagen consisting of alpha 1(I)alpha 2(I)alpha 3(I) heterotrimers. Eur. J. Biochem. 2001;268:2817–2827. doi: 10.1046/j.1432-1327.2001.02160.x. doi:ejb2160 [pii] [DOI] [PubMed] [Google Scholar]
- 72.Muralidharan N., Jeya Shakila R., Sukumar D., Jeyasekaran G. Skin, bone and muscle collagen extraction from the trash fish, leather jacket (Odonus Niger) and their characterization. J. Food Sci. Technol. 2013;50:1106–1113. doi: 10.1007/s13197-011-0440-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cho J.K., Jin Y.G., Rha S.J., Kim S.J., Hwang J.H. Biochemical characteristics of four marine fish skins in Korea. Food Chem. 2014;159:200–207. doi: 10.1016/j.foodchem.2014.03.012. of four marine fish skins in Korea, Food Chem. 159 (2014) 200–207. doi:10.1016/j.foodchem.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 74.Kittiphattanabawon P., Benjakul S., Visessanguan W., Shahidi F. Isolation and properties of acid- and pepsin-soluble collagen from the skin of blacktip shark (Carcharhinus limbatus) Eur. Food Res. Technol. 2010;230:475–483. [Google Scholar]
- 75.Subhan F., Ikram M., Shehzad A., Ghafoor A. Marine collagen: an emerging player in biomedical applications. J. Food Sci. Technol. 2015;52:4703–4707. doi: 10.1007/s13197-014-1652-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kundanati L., Singh S.K., Mandal B.B., Murthy T.G., Gundiah N., Pugno N.M. Fabrication and mechanical characterization of hydrogel infused network silk scaffolds. Int. J. Mol. Sci. 2016;17:1631. doi: 10.3390/ijms17101631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.You F., Wu X., Chen X. 3D printing of porous alginate/gelatin hydrogel scaffolds and their mechanical property characterization. Int. J. Polym. Mater. Polym. Biomater. 2016;66:299–306. [Google Scholar]
- 78.Mahmoodani F., Ardekani V.S., Fern S.S., Yusop S.M., Babji A.S. Optimization of extraction and physicochemical properties of gelatin from pangasius catfish (pangasius sutchi) skin. Sains Malays. 2014;43:995–1002. [Google Scholar]
- 79.Antipova L.V., Storublevtsev S.A. Comparative properties of collagenic proteins of fish and animal origin. Bull. Vor. State Univ. Ser. Chem. Biol. Pharm. 2016;4:37–41. [Google Scholar]
- 80.Binnie C.G., Lord S.T. The fibrinogen sequences that interact with thrombin. Blood. 1993;81:3186–3192. [PubMed] [Google Scholar]
- 81.Gojkovic Z., Marova I., Matouskova P., Obruca S., Miloslav P. Use of ultrasonic spectroscopy and viscosimetry for the characterization of chicken skin collagen in comparison with collagens from other animal tissues. Prep. Biochem. Biotechnol. 2014;44:761–771. doi: 10.1080/10826068.2013.867869. [DOI] [PubMed] [Google Scholar]
- 82.Raja Mohd Hafidz R.N., Yaakob C.M., Amin I., Noorfaizan A. Chemical and functional properties of bovine and porcine skin gelatin. Int. Food Res. J. 2011;817:813–817. http://ifrj.upm.edu.my/18 (02) 2011/(48) IFRJ-2010-159.pdf [Google Scholar]
- 83.Wu G.P., Wang X.M., Lin L.P., Chen S.H., Wu Q.Q. Isolation and characterization of pepsin-solubilized collagen from the skin of black carp (mylopharyngdon piceus) Adv. Biosci. Biotechnol. 2014;5:642–650. [Google Scholar]
- 84.Vidal A.R., Cechin C.F., Cansian R.L., Mello R.O., Schmidt M.M., Demiate I.M., Kempka A.P., Dornelles R.C.P. Enzymatic hydrolysis (Pepsin) assisted by ultrasound in the functional properties of hydrolyzates from different collagens. Ciência Rural. 2018;48 [Google Scholar]
- 85.Blanco M., Vázquez J.A., Pérez-Martín R.I., Sotelo C.G. Hydrolysates of fish skin collagen: an opportunity for valorizing fish industry byproducts. Mar. Drugs. 2017;15:131. doi: 10.3390/md15050131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rowe S.L., Stegemann J.P. Interpenetrating collagen-fibrin composite matrices with varying protein contents and ratios. Biomacromolecules. 2006;7:2942–2948. doi: 10.1021/bm0602233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Brougham C.M., Levingstone T.J., Jockenhoevel S., Flanagan T.C., O'Brien F.J. Incorporation of fibrin into a collagen-glycosaminoglycan matrix results in a scaffold with improved mechanical properties and enhanced capacity to resist cell-mediated contraction. Acta Biomater. 2015;26:205–214. doi: 10.1016/j.actbio.2015.08.022. [DOI] [PubMed] [Google Scholar]
- 88.Weinstein-Oppenheimer C.R., Brown D.I., Coloma R., Morales P., Reyna-Jeldes M., Díaz M.J., Sánchez E., Acevedo C.A. Design of a hybrid biomaterial for tissue engineering: biopolymer-scaffold integrated with an autologous hydrogel carrying mesenchymal stem-cells. Mater. Sci. Eng. C. 2017;79:821–830. doi: 10.1016/j.msec.2017.05.116. [DOI] [PubMed] [Google Scholar]
- 89.Vega S.L., Kwon M.Y., Burdick J.A. Recent advances in hydrogels for cartilage tissue engineering. Eur. Cells Mater. 2017;33:59–75. doi: 10.22203/eCM.v033a05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Maranda E., Rodriguez-Menocal L., Badiavas E. Role of mesenchymal stem cells in dermal repair in burns and diabetic wounds. Curr. Stem Cell Res. Ther. 2017;12:61–70. doi: 10.2174/1574888x11666160714115926. [DOI] [PubMed] [Google Scholar]
- 91.Drela K., Stanaszek L., Nowakowski A., Kuczynska Z., Lukomska B. Experimental strategies of mesenchymal stem cell propagation: adverse events and potential risk of functional changes. Stem Cell. Int. 2019;2019:1–10. doi: 10.1155/2019/7012692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Choi J.R., Yong K.W., Nam H.Y. Current status and perspectives of human mesenchymal stem cell therapy. Stem Cell. Int. 2019;2019:4762634. doi: 10.1155/2019/4762634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fitzsimmons R.E.B., Mazurek M.S., Soos A., Simmons C.A. Mesenchymal stromal/stem cells in regenerative medicine and tissue engineering. Stem Cell. Int. 2018;2018:4762634. doi: 10.1155/2018/8031718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Willerth S.M., Sakiyama-Elbert S.E. Combining stem cells and biomaterial scaffolds for constructing tissues and cell delivery. J. StemJournal. 2019;1:1–25. [PubMed] [Google Scholar]
- 95.Achatz F.P., Kujat R., Pfeifer C.G., Koch M., Nerlich M., Angele P., Zellner J. In vitro testing of scaffolds for mesenchymal stem cell-based meniscus tissue engineering-Introducing a new biocompatibility scoring system. Materials. 2016;9 doi: 10.3390/ma9040276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Baba Ismail Y.M., Wimpenny I., Bretcanu O., Dalgarno K.W., El Haj A.J. In vitro biocompatibility of SiCHA nanopowders on human mesenchymal stem cells. J. Eng. Sci. 2018;14:35–46. [Google Scholar]
- 97.Benning L., Gutzweiler L., Tröndle K., Riba J., Zengerle R., Koltay P., Zimmermann S., Stark G.B., Finkenzeller G. Cytocompatibility testing of hydrogels toward bioprinting of mesenchymal stem cells. J. Biomed. Mater. Res. A. 2017;105:3231–3241. doi: 10.1002/jbm.a.36179. [DOI] [PubMed] [Google Scholar]
- 98.Fan C., Wang D.-A. Macroporous hydrogel scaffolds for three-dimensional cell culture and tissue engineering. Tissue Eng. B Rev. 2017;23:451–461. doi: 10.1089/ten.TEB.2016.0465. [DOI] [PubMed] [Google Scholar]
- 99.Caliari S.R., Burdick J.A. A practical guide to hydrogels for cell culture. Nat. Methods. 2016;13:405–414. doi: 10.1038/nmeth.3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sadeghi-Ataabadi M., Mostafavi-pour Z., Vojdani Z., Sani M., Latifi M., Talaei-Khozani T. Fabrication and characterization of platelet-rich plasma scaffolds for tissue engineering applications. Mater. Sci. Eng. C. 2017;71:372–380. doi: 10.1016/j.msec.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 101.Loh Q.L., Choong C. Three-dimensional scaffolds for tissue engineering applications: role of porosity and pore size. Tissue Eng. B Rev. 2013;19:485–502. doi: 10.1089/ten.teb.2012.0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Clark R.A.F., Tonnesen M.G., Gailit J., Cheresh D.A. Transient functional expression of alphaVbeta3 on vascular cells during wound repair. Am. J. Pathol. 1996;148:1407–1421. [PMC free article] [PubMed] [Google Scholar]
- 103.Knight C.G., Morton L.F., Peachey A.R., Tuckwell D.S., Farndale R.W., Barnes M.J. The collagen-binding a-domains of integrins α1/β1 and α2/β1 recognize the same specific amino acid sequence, GFOGER, in native (triple- helical) collagens. J. Biol. Chem. 2000;275:35–40. doi: 10.1074/jbc.275.1.35. [DOI] [PubMed] [Google Scholar]
- 104.Schmidt J.A., Rinaldi S., Scalbert A., Ferrari P., Achaintre D., Gunter M.J., Appleby P.N., Key T.J., Travis R.C. Plasma concentrations and intakes of amino acids in male meat-eaters, fish-eaters, vegetarians and vegans: a cross-sectional analysis in the EPIC-Oxford cohort. Eur. J. Clin. Nutr. 2016;70:306–312. doi: 10.1038/ejcn.2015.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.To W.S., Midwood K.S. Plasma and cellular fibronectin: distinct and independent functions during tissue repair. Fibrogenesis Tissue Repair. 2011;4:21. doi: 10.1186/1755-1536-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]