Abstract
Background
FOXO3a has been widely regarded as a tumor suppressor. It also plays a paradoxical role in regulating the cancer stem cells (CSCs), responsible for tumor-initiation, chemo-resistance, and recurrence in various solid tumors, including oral squamous cell carcinoma (OSCC). This study aims to uncover the role of FOXO3a and its importance for a non-canonical pathway of TGFβ in regulating the OSCC stemness.
Methods
We identified FOXO3a expression in OSCC tissues and cell lines using immunohistochemistry and western blot. The correlation between FOXO3a and stemness was evaluated. Stable cell lines with differential expression of FOXO3a were constructed using lentiviruses. The effects of FOXO3a on stem-cell like properties in OSCC was further evaluated in vitro and in vivo. We also explored the effect of TGFβ on FOXO3a with respect to its expression and function.
Findings
Our findings suggest that FOXO3a was widely expressed and negatively correlated with the stemness in OSCC. This regulation can be abolished by TGFβ through phosphorylation, nuclear exclusion, and degradation in the non-Smad pathway. We also observed that non-Smad AKT-FOXO3a axis is essential to regulate stemness of CSCs by TGFβ.
Interpretation
TGFβ induces stemness through non-canonical AKT-FOXO3a axis in OSCC. Our study provides a foundation to understand the mechanism of CSCs and a possible therapeutic target to eliminate CSCs.
Keywords: FOXO3a, TGFβ, Oral squamous cell carcinoma, Stem-cell like properties
Research in context
Evidence before this study
CSC theory states that, analogous to the renewal of normal tissues, tumor growth is similarly fueled by small numbers of CSCs hidden in cancers. CSCs are proposed to be responsible for the poor prognosis of OSCC.
FOXO3a has been widely regarded as a bona fide tumor suppressor because of the ability to induce apoptosis and cell cycle arrest. However, some studies illustrate a more complex supportive role of FOXO3a in cancer. For example, the role of FOXO3a in cancer stem cells remains contradictory.
Added value of this study
By analyzing stable OSCC cell lines with differential expression of FOXO3a, we provide evidence that FOXO3a negatively regulates the stemness of OSCC. Our data further indicates that FOXO3a can be abolished by TGFβ in non-Smad pathway. This non-Smad AKT-FOXO3a axis is crucial to regulate stemness of CSCs by TGFβ.
Implications of all the available evidence
Our study suggests TGFβ induces stemness through non-canonical AKT-FOXO3a axis in OSCC. Moreover, we provide a foundation to understand the mechanism of CSCs and a possible therapeutic target,AKT-FOXO3a, to eliminate CSCs.
1. Introduction
Oral squamous cell carcinoma (OSCC) is an important subset of head and neck cancer. Although there have been advances in the diagnosis and therapy of OSCC in recent years, the overall survival rate is still unsatisfactory [1], [2]. The poor prognosis associated with OSCC has been largely attributed to the high propensity for local recurrence and lymph node metastasis.
Tumors are known to be heterogeneous in nature. Mirroring normal tissue, cancer tissues often consist of a hierarchical organization, with a small group of cancer stem cells (CSCs) at the apex. CSC is a rare sub-population of cells in a tumor with stem cell-like properties, such as self-renewal, drug resistance and tumor initiation [3], [4]. Previous studies have demonstrated that CSCs could be responsible for therapeutic resistance, recurrence and metastasis of cancer, including OSCC [3], [4]. Therefore, understanding the molecular mechanism of CSCs could be beneficial for designing an effective strategy to improve the prognosis of OSCC patients. Several methods, including the side population (SP) assay, have been used in identification and isolation of CSCs. SP assay involves staining the cells using Hoechst 33,342 or Rhodamine 123, followed by FACS analysis. Cells with stem-cell like properties can efflux the dye through their ABC transporters [5].
Forkhead box (FOX) proteins are a highly conserved transcription factor superfamily, characterized by an approximately 100‐residue Forkhead DNA‐binding domain [6]. FOXO3a is a member of FOXO subfamily and involved in a wide range of biological processes, including apoptosis, cell cycle progression, proliferation, DNA repair, anti-oxidative stress responses, longevity, and differentiation [6], [7], [8], [9], [10], [11]. Deregulation of FOXOs’ functions can lead to carcinogenesis. FOXO3a has been widely regarded as a bona fide tumor suppressor due to its ability to induce apoptosis [12], [13], [14] and cell cycle arrest [14], [15]. In recent years, some studies have demonstrated that FOXO3a is not merely a classic tumor suppressor; it plays a more complex supportive role in cancer [8]. Moreover, the role of FOXO3a in CSCs is contradictory. It has been reported that activation of FOXO3a can lead to the elimination of CSCs and stem cell-like properties [16], [17], [18], [19], while other reports suggest the opposite [20], [21], [22].
Our previous study revealed that TGFβ contributes to the regulation of Foxp3+ lymphocytes in Tongue squamous cell carcinoma (TSCC) tumor microenvironment [23]. We observed that TGFβ enhanced the stemness of TSCC cells. TGFβ signaling plays a paradoxical role in the progression of carcinogenesis. On one hand, this pathway induces apoptosis and inhibits proliferation in epithelial cells, which suppresses carcinogenesis. On the other hand, it contributes to tumor progression in advanced tumors by enhancing stemness, invasion and chemoresistance [24]. The canonical pathways of TGFβ depend upon Smad pathway [25]. However, emerging pieces of evidence suggest that TGFβ also crosstalks with other non-Smad pathways, such as PI3K/AKT [26] and MEK/ERK [27], which might regulate FOXO3a functionally.
In this study, we detected the expression of FOXO3a in OSCC tissue specimens and cell lines. Its effects on stem-cell like properties were investigated by overexpression and knockdown in OSCC cell lines. In addition, we also explored the effectiveness of TGFβ on FOXO3a expression and function.
2. Materials and methods
2.1. Patients and tissue samples
One hundred and twenty-four pairs of paraffin-embedded OSCC and adjacent tissues samples were obtained from the Department of Oral and Maxillofacial Surgery, Guanghua Hospital of Stomatology, Sun Yat-sen University from July 2014 to December 2016. Informed consent was obtained from all the study participants. Approval for this research was obtained from the Institutional Research Ethics Committee. The clinical and pathological data associated with the patients were summarized in the supplementary Table S1. None of the patients had undergone chemotherapy or radiotherapy treatments before surgery. The clinicopathological staging was determined according to the 2002 UICC TNM classification system for malignant tumors. Tumor histological stages were determined as per the 2004 WHO classification system.
2.2. Chemicals and reagents
Recombinant human TGFβ was purchased from R&D Systems (Minneapolis, MN). AKT inhibitor MK2206, proteasome inhibitor MG132 and verapamil were purchased from Selleck Chemicals (Houston, TX, USA). Primary antibodies against FOXO3a(AB_2636990, AB_836876), p-FOXO3a (Thr32)(AB_329842), p-FOXO3a (Ser253)(AB_2106674), SOX2 (AB_2799734), ABCG2 (AB_2799211), BMI1 (AB_2799353), CD44(AB_2076465), AKT(AB_10694382), p-AKT(Thr308)(AB_2800089), p-AKT(Ser473)(AB_2797780),ERK1/2 (AB_10693607),p-ERK1/2(Histone H3A (AB_331772) , and tubulin(AB_2799519) were obtained from Cell Signaling Technology (Danvers, MA, USA). Primary antibodies against FOXO3a (Chip grade, AB_298893) and IVL (AB_305656) were purchased from Abcam (Cambridge, MA, USA). All HRP-labeled secondary antibodies were also purchased from Cell Signaling Technology (Danvers, MA, USA). Hoechst 33342 was purchased from Sigma Aldrich (St Louis, MO, USA). Lentiviral particles were designed and synthesized by Hanbio (Shanghai, China). The sequences are listed below.
LV-RNAi1: 5′- GCACAACCTGTCACTGCATAG-3′
LV-RNAi2: 5′- GACTTCCGTTCACGCACCAATTCTA -3′
LV-RNAi3: 5′- GAGAACAAGCCAGCTACCTTCTCTT -3′
Scrambled sequence 5′-TTCTCCGAACGTGTCACGTAA-3′
2.3. Cell lines and cell culture
The human OSCC cell lines SCC15 and SCC25 were obtained from the American Type Culture Collection (ATCC). Cells were cultured in DMEM/F12 (Gibco, Carlsbad, CA, USA) with 10% fetal bovine serum (Gibco, Carlsbad, CA, USA) at 37 °C in 5% CO2 atmosphere.
2.4. Overexpression, knockdown and mutation of FOXO3a in OSCC cell lines
To down-regulate expression of endogenous FOXO3a, SCC15 and SCC25 cells were transfected with the vector containing FOXO3a shRNA lentiviral particles following the manufacturer's protocol (Hanbio, Shanghai, China). Stable clones were selected after 2 µg/ml puromycin treatment and were named SCC15-FOXO3a-shRNA and SCC25-FOXO3a-shRNA.
For overexpressing FOXO3a, lentiviruses containing the human FOXO3a full-length sequence were used to transfect SCC15 and SCC25 cell lines. An empty vector was also transfected into these two cell lines to produce negative controls. These stable cell lines were named SCC15-FOXO3a-WT and SCC25-FOXO3a-WT.
A vector containing a mutant version of FOXO3a, in which the three AKT phosphorylation sites (Ser253, Thr32, Thr315) were mutated to alanine, was also transfected into SCC15 and SCC25 cells. These stable cell lines were named SCC15-FOXO3a-A3 and SCC25-FOXO3a-A3.
2.5. Western blotting
Cells were washed with ice-cold PBS. Total protein was extracted using the RIPA cell lysis buffer (CWBIO, Beijing, China) containing proteinase and phosphatase inhibitors (Roche, Indianapolis, IN, USA). Nuclear and cytoplasmic extraction was performed following the standard protocol of NE-PER Nuclear and Cytoplasmic Extraction Reagents (Thermo-Pierce, Rockford, USA). Equal amounts of protein samples were loaded onto a 10% sodium dodecyl sulfate-polyacrylamide gel and separated by electrophoresis (SDS-PAGE). Protein was transferred from the gel onto polyvinylidene fluoride (PVDF) membranes (Millipore, Billerica, MA, USA). After blocking, membranes were incubated with specific primary antibodies overnight at 4 °C. After PBS washes, the membranes were incubated with the corresponding secondary antibodies for 1 h. Target protein bands were detected by enhanced chemiluminescence (ECL; Immobilon Western HRP substrate; Millipore, Billerica, MA, USA) and visualized using the GeneGnome XRQ bio-imaging system (Syngene, Cambridge, UK).
2.6. Quantitative real-time PCR
Total RNA was isolated from cells using TRIzol reagent (Thermo-Pierce, Rockford, USA) following the manufacturer's protocol. A total of 1000 ng RNA was converted into cDNA using PrimeScriptTMRT Master Mix (TaKaRa, Shiga, Japan). After a 2-fold dilution, cDNA was subjected to PCR amplification using LightCycler 480 SYBR Green I Master mix (Roche, Indianapolis, IN, USA) in a LightCyclerⓇ 480 Instrument (Roche). GAPDH was used as a reference gene for qRT-PCR analysis. The sequence of the primers used for PCR is provided in Table S2.
2.7. RNA sequencing
To investigate the transcriptome after overexpression of FOXO3a, total RNA was isolated from cells using TRIzol reagent. RNA-seq was performed by BGI (Shenzhen, China). Gene set enrichment analysis (GSEA) was used to analyze the differential gene expression [28], [29]. Accession numbers for raw data have been listed in Table S3. The gene sets of cancer stem cells were obtained from the Molecular Signatures Database (MSigDB).
2.8. Immunohistochemistry
For the immunohistochemistry assay, 4 µm-thick slides were de-waxed and rehydrated. For antigen retrieval, the slides were heated in citrate buffer (pH 6.0) in a pressure-cooker for 3 min and cooled naturally to room temperature. Endogenous peroxidase was blocked by incubating the slides with a nonspecific-staining blocker (Gene Tech, Shanghai, China) for 15 min. Serial sections were incubated with primary antibody against FOXO3a (1:300) or SOX2 (1:200) overnight at 4 °C. The staining procedure was performed using a highly sensitive streptavidin-biotin-peroxidase detection system for the primary antibody (Gene Tech, Shanghai, China), and the counterstaining procedure was performed with hematoxylin.
2.9. Immunofluorescence
Cells were fixed with 4% formaldehyde and then permeabilized with 0.2% Triton X-100 for 10 min at room temperature. After blocking, cells were incubated with primary antibodies against FOXO3a (1:1000) overnight at 4 °C, and then with the corresponding secondary antibodies for 1 h 4′, 6-Diamidino-2-phenylindole (DAPI) was used to stain nuclei. Images were taken using an Axio Image.Z2 microscope (ZEISS, Shanghai, China).
2.10. Sphere formation assay
For the sphere formation assays, 1000 cells/well were plated in ultra-low attachment 6-well plates (Corning, Steuben, NY, USA). The DMEM/F12 was supplemented with 2% B27, 10 ng/mL rh-bFGF, and 20 ng/mL rh-EGF (Life Technologies, Carlsbad, CA, USA). Spheres larger than 25 µm in diameter in each well were calculated on the 14th day as the primary generation, using an inverted microscope (Zeiss, Shanghai, China). The primary spheres were harvested and dissociated to single cells with AccutaseⓇ (Thermo-Pierce, Rockford, USA) for the secondary sphere formation. Sphere formation efficiency was calculated by dividing the number of spheres larger than 25 µm by the total number of plated cells.
2.11. Flow cytometry analysis for side population
Cells were prepared at a concentration of 1 × 106/ml and stained with 2.5 µg/mL Hoechst 33,342 in F12 medium containing 2% FBS at 37 °C for 90 min. Control cells were treated with 50 µg/ml verapamil inhibiting the ABC transporters combined with Hoechst 33,342. After incubation, cells were washed and resuspended in PBS with 2% BSA. The cells were analyzed for examination and isolation of side population using FACSAria Fusion (BD Bioscience, Franklin Lakes, NJ, USA).
2.12. Drug resistance assays
For the drug resistance assays, 2 × 103 cells per well were plated in 96-well plates (Corning, Steuben, NY, USA). Cells were exposed to different concentrations of cisplatin (0, 1, 2, 4, 8 or 16 µM) for 48 h. Then, 10 µL of CCK8 solution was added to each well, followed by a 2 h incubation at 37 °C. The reaction was quantitatively measured in Infinite M200 (Tecan, Switzerland) at a wavelength of 450 nm.
2.13. ChIP-PCR assay
ChIP assays were performed using a Magna Chip A/G Chromatin Immunoprecipitation Kit (Merck Millipore, Darmstadt, Germany) following the manufacturer's protocol. Briefly, cells were cross-linked with formaldehyde and sonicated to shear the DNA into fragments of 200–500 bp. After pre-cleaning, for each sample, 1% of the supernatant was used as the input loading control (positive control), whereas the remaining chromatin supernatant was incubated with FOXO3a antibodies or normal rabbit IgG (negative control) for chromatin immunoprecipitation. Protein-chromatin complexes were eluted to recover free DNA and then analyzed using quantitative PCR analysis. PCR products were separated by electrophoresis on a 1.5% agarose gel and detected with ethidium bromide. Primer sequences for ChIP assay are listed in Table S2.
2.14. Xenograft mouse models of OSCC
Animal experiments were approved by the Institutional Research Ethics Committee of Sun Yat-sen University (Project License 2017-036QX). Four-week-old female BALB/c-nu mice were randomly divided into different groups. All mice had free access to water, environmental enrichment, maintenance diet, and were exposed to a 12-h light/dark cycle, at 22 °C and 55% humidity. Approximately 104, 105 or 106 cells of SCC15-FOXO3a-shRNA and SCC25-FOXO3a-shRNA cell lines or their controls were injected subcutaneously into the axilla of BALB/c-nu mice. Tumor growth was monitored every 3 days. Tumor volume was calculated using the formula: 0.2618 × L × W × (L + W), where W and L represent the average width and length of the tumor [30]. The total tumor volume was measured on the 28th day.
2.15. Statistical analysis
Statistical analysis was performed using the Student's t-test, one-way analysis of variance (ANOVA), chi-square test, Wilcoxon rank-sum test or Extreme Limiting Dilution Analysis (ELDA) [26] using the SPSS 20.0 software (SPSS Inc, Chicago, IL, USA). The p-value < 0.05 was considered as statistically significant.
3. Results
3.1. FOXO3a is negatively correlated with the stemness of OSCC
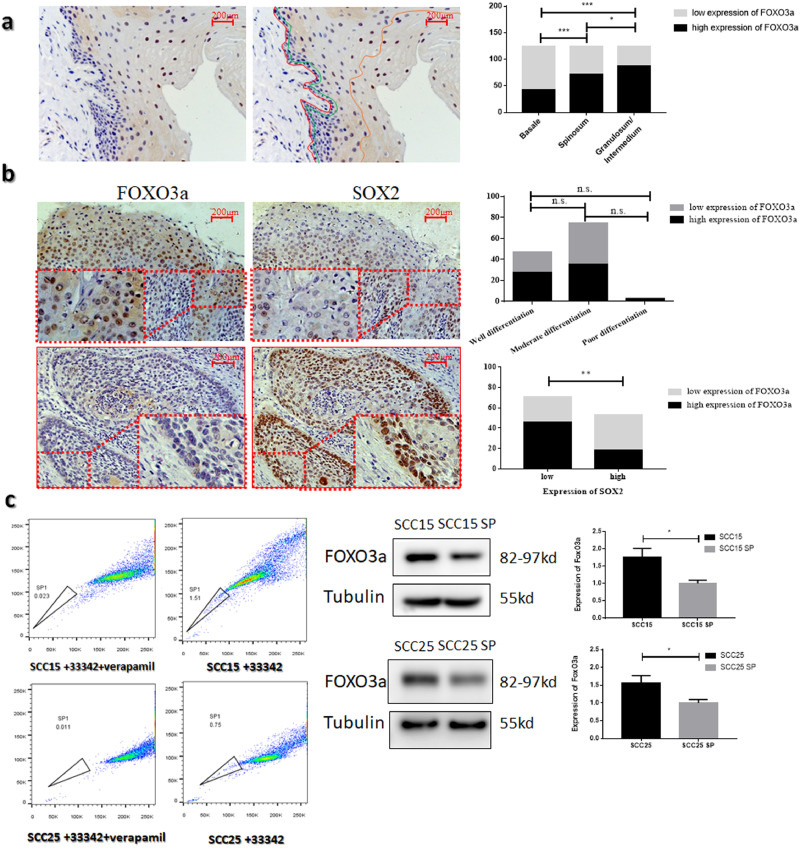
Based on the results of immunohistochemistry, 124 OSCC patients were divided into low-expression and high-expression groups. The expression levels of FOXO3a were not related to age, gender, histological grade, and T or N stage (Table 1). As shown in Fig 1a, in the hierarchy of pericarcinous tissue, the expression of FOXO3a increased from stratum basal to stratum granulosum/intermedium (p < 0.001, Wilcoxon rank-sum test). These results indicate that FOXO3a expression can be positively correlated with the differentiation level of keratinocytes. Moreover, the expression of SOX2 was negatively related to the expression of FOXO3a in OSCC (Fig 1b Table 1, p = 0.001, chi-square test). SOX2 is one of the markers of cancer stem cells (CSC) in OSCC. We also isolated the CSCs by flow cytometry. This subgroup appears on the left side of a FACS analysis panel, which refers to SP [5].The expression of FOXO3a was significantly decreased in the side population in SCC15 and SCC25 (Fig 1c, p < 0.05, Student's t-test).
Table 1.
Correlation of FOXO3a expression with clinicopathological variables of OSCC patients.
| Characteristic | case | FOXO3a expression |
|||
|---|---|---|---|---|---|
| Low | High | p-value | |||
| Ages | ≤50 | 59 | 26 | 33 | 0.375 |
| >50 | 65 | 34 | 31 | ||
| Gender | Male | 90 | 46 | 44 | 0.421 |
| Female | 34 | 14 | 20 | ||
| Histological grade | Well | 47 | 20 | 27 | 0.484 |
| Moderate | 74 | 39 | 35 | ||
| Poor | 3 | 1 | 2 | ||
| Size (T stage) | T1-T2 | 80 | 37 | 43 | 0.576 |
| T3-T4 | 44 | 23 | 21 | ||
| LN metastasis (N Stage) | N0 | 71 | 31 | 40 | 0.276 |
| N1-N3 | 53 | 29 | 24 | ||
| Expression of SOX2 | Low | 71 | 25 | 46 | 0.001* |
| High | 53 | 35 | 18 | ||
Statistical analysis was performed by Chi-square test, p< 0.05 was considered significant.
Fig. 1.
FOXO3a is negatively correlated with the stemness of OSCC (a) Immunohistochemistry images of FOXO3a in pericarcinous epithelium (n = 124). The red line represents basement membrane. The layers from the red line to the green line represent the stratum basale. The layers from the green line to the orange line represent stratum spinosum. The layers from the orange line to the surface represent stratum granulosum/intermedium (b) Representative immunohistochemistry images of FOXO3a(left) and SOX2(right) expression in paraffin-embedded samples from OSCC tissue (n = 124) (c) The expression of FOXO3a in the side population of SCC15 and SCC25.
3.2. FOXO3a negatively regulates the stemness of OSCC
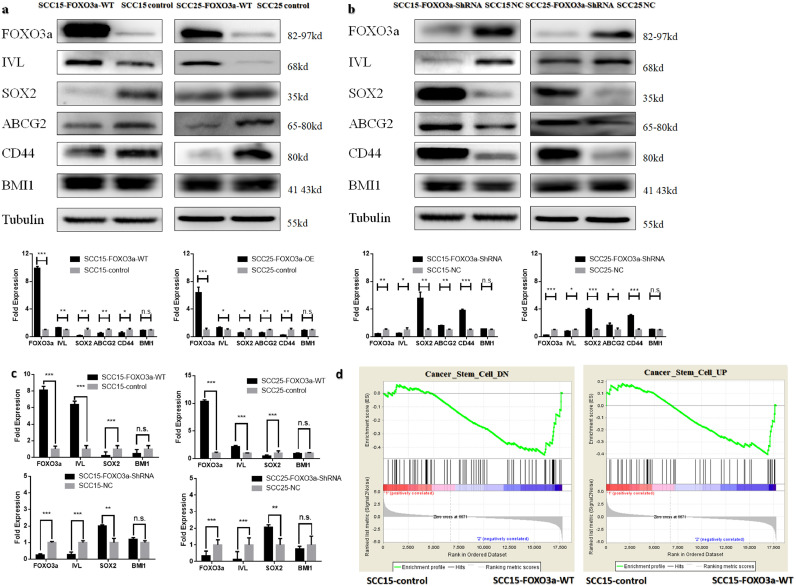
To further investigate the relationship between FOXO3a and stemness in OSCC, stable cell lines with differential expression of FOXO3a were constructed (Fig. 2a–c Fig S1a). Our findings suggest that the expression level of FOXO3a is negatively correlated with stemness markers including SOX2, ABCG2, CD44 and positively related to differentiation marker IVL (Fig. 2a,b, p-value < 0.05, Student's t-test). Surprisingly, the protein level of BMI1 , another stemness marker, was not affected by the up- or down-regulation of FOXO3a (Fig. 2a, b, p-value > 0.05, Student's t-test). The results of qRT-PCR show a similar pattern at the mRNA level (Fig. 2c). We also performed the transcriptome analysis after overexpression of FOXO3a in SCC15. GSEA show that FOXO3a expression was positively associated with the genes down-regulated in cancer stem cells (Fig. 2d, |NES|= 1.51, p-value = 0.009, FDR q-val = 0.027). However, FOXO3a expression was not associated with the genes up-regulated in cancer stem cells (Fig. 2d, p-value > 0.05, Student's t-test). Since FOXO3a is a transcription factor, we used ChIP-PCR assay to test the interaction between FOXO3a and SOX2 in SCC15 and SCC25 cells. Results suggest that FOXO3a can bind to the promoter region of SOX2 (Supplementary Fig. S1b), indicating that FOXO3a regulates SOX2 expression through direct interaction on genetic level.
Fig. 2.
FOXO3a negatively regulates the stemness of OSCC. (a) Western blotting analysis of FOXO3a, stemness markers SOX2, ABCG2, CD44, BMI1 and differentiation marker IVL expression following FOXO3a overexpression in SCC15 and SCC25. β-tubulin was used as a protein loading control (b) Western blotting analysis of FOXO3a, stemness markers SOX2, ABCG2, CD44, BMI1 and differentiation marker IVL expression following shRNA knock down of FOXO3a in SCC15 and SCC25. β-tubulin was used as a protein loading control. (c) qRT-PCR analysis of FOXO3a, stemness markers SOX2, BMI1 and differentiation marker IVL expression following FOXO3a overexpression or knock down in SCC15 and SCC25. GAPDH was used as protein loading control. (d) GSEA to analyze the differential gene expression following down regulating FOXO3a in SCC15. Results are the mean ± SEM of three independent experiments and analyzed by GSEA, Student's t-test. * p < 0.05 ** p < 0.01 *** p < 0.001.
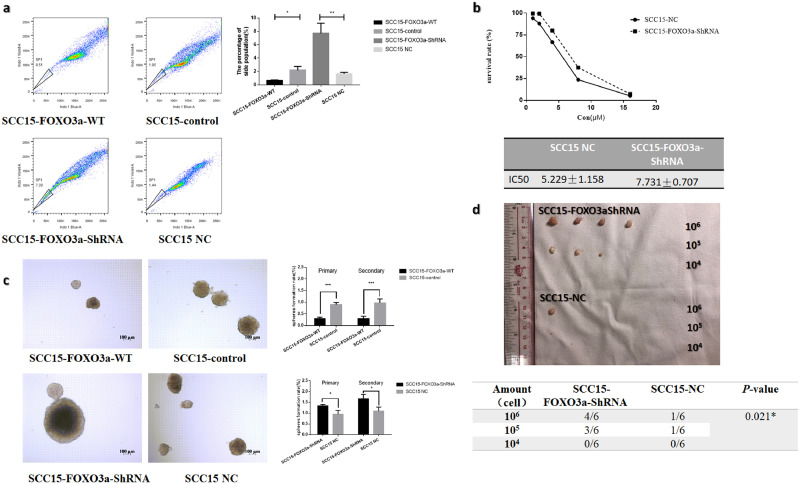
Then we investigated whether FOXO3a can alter the stem cell-like properties of OSCC cells, including the abilities of self-renewal, drug resistance and tumor initiation. It was observed that the side population was expanded with FOXO3a down-regulation in SCC15 and vice-versa (Fig. 3a, p-value < 0.05, Student's t-test). Similar results were observed within SCC25 cells (Supplementary Fig S1c). The drug resistance assays showed that decreased FOXO3a can enhance the chemoresistance to cisplatin in both SCC15 (Fig 3b, IC50: SCC15-NC 5.229 ± 1.158 µM; SCC15-FOXO3a-ShRNA7.731 ± 0.707) and SCC25 cells (Fig S1d, IC50: SCC25-NC 3.885 ± 0.863; SCC25-FOXO3a-ShRNA5.799 ± 0.453). Subsequently, serial sphere-forming assay suggests that overexpression of FOXO3a can significantly reduce the sphere formation ability (Fig. 3c, Supplementary FigS1e), whereas the FOXO3a-knocked-down group displays an increase in the sphere-forming ability (Fig. 3c, Supplementary FigS1e). These results indicate that overexpression of FOXO3a has an inhibitory effect on the self-renewal capacity. To further prove this, a mouse xenograft model was used to determine the tumorigenesis ability of these cell lines in vivo. FOXO3a knock-down group showed a higher tumor formation rate with SCC15 cells (Fig. 3d, p-value < 0.05, ELDA). A similar tendency was observed in SCC25 cells, though it was statistically insignificant (Supplementary Fig. S3f, p = 0.186, ELDA). Moreover, FOXO3a knock-down group had higher average tumor volume than the control group in both cell lines (Fig. 3d, Supplementary Fig. S1E, Table S4, p-value < 0.05, Student's t-test). Overall these findings suggest that FOXO3a is negative regulator of the stemness of OSCC.
Fig. 3.
FOXO3a alter the stem cell-like properties in SCC15 (a) The percentage of side population among the stable cell lines with differential expression of FOXO3a in SCC15. (b)The chemoresistance against cisplatin of the stable cell lines with decreased FOXO3a in SCC15. (c) Serial sphere forming assay assessing the self-renewal ability of SCC15 following knock-down or overexpression of FOXO3a in SCC15. (d) Decreasing numbers (106, 105, 104) of SCC15-FOXO3a-shRNA or their controls were injected subcutaneously into the axilla of BALB/c-nu mice to compare the tumorigenesis ability (n = 6/group). Results are mean ± SEM of three independent experiments and analyzed by ELDA, Student's t-test. * p < 0.05 ** p < 0.01 ***p < 0.001.
3.3. TGFβ enhances the stemness of OSCC cells
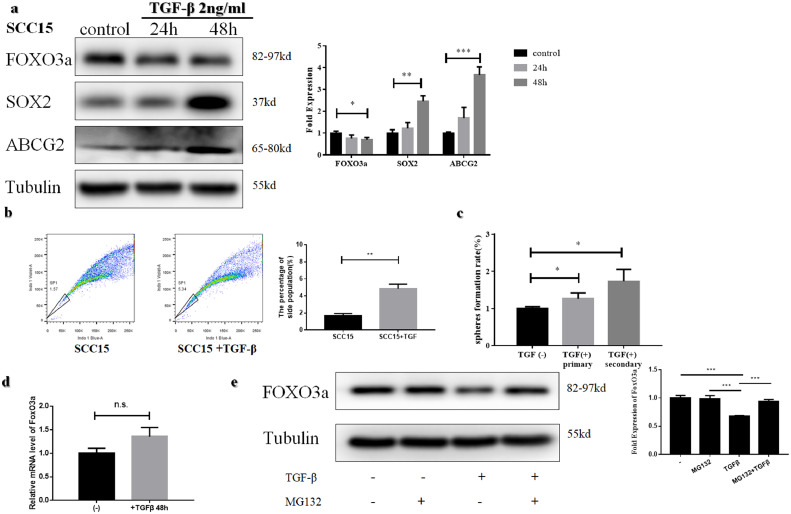
TGFβ is a well-researched cytokine involved in inducing stemness [31], [32], [33], [34], [35]. Therefore, we tested its efficacy in enhancing stemness in OSCC cell lines with TGFβ (2.0 ng/ml). The expression of SOX2 and ABCG2 increased in a time-dependent manner (Fig. 4a, Supplementary Fig. S2a). The side population percentage increased after treatment with TGFβ for 48 h in both SCC15(Fig. 4b) and SCC25 cells (Supplementary Fig. S2b). Moreover, the sphere-forming assay showed a higher rate after treatment with 2.0 ng/ml TGFβ (Fig 4c, Supplementary Fig. S2c). These results indicated that TGFβ can also enhance the stemness of OSCC cells.
Fig. 4.
TGFβ enhances the stemness and decreases FOXO3a level of SCC15 cells (a) Western blotting assessment of FOXO3a, SOX2 and ABCG2 in SCC15 after treatment with TGFβ(2 ng/ml) (b) The percentage of side population increased after treatment of TGFβ(2 ng/ml) for 48 h in SCC15 (c)The self-renewal ability was enhanced after treatment of TGFβ(2 ng/ml) for 48 h in SCC15. (d)No significant difference was observed in mRNA level between TGFβ(2 ng/ml) treated group and control in SCC15. (e) Reduction of FOXO3a can be rescued with pretreatment with proteasome inhibitor MG132 (10 µM) before TGFβ (2 ng/ml) treatment in SCC15.
3.4. TGFβ treatment results in phosphorylation, nuclear exclusion and degradation of FOXO3a
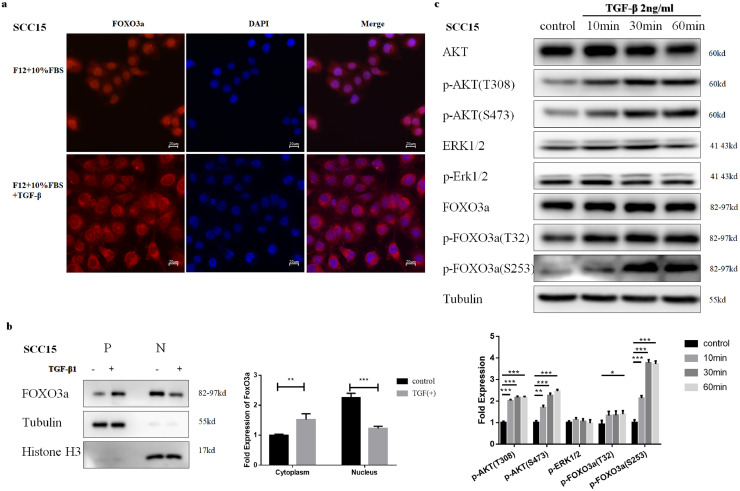
We observed a significant reduction of FOXO3a 48 h after the treatment with TGFβ (Fig. 4a, Supplementary Fig. S2a, p-value < 0.05, Student's t-test). There was no difference at the mRNA level between TGFβ treated group and control (Fig. 4d, Supplementary Fig. S2d, p-value > 0.05, Student's t-test), indicating that the reduction of FOXO3a occurs post-transcriptionally. Since FOXO3a is known to be degraded via the proteasome [7], we tested whether the FOXO3a reduction was due to increased proteasomal degradation. Results suggest that reduction of FOXO3a can be rescued by pretreating cells with a proteasome inhibitor MG132 (10 µM) prior to the TGFβ treatment (Fig. 4e, Supplementary Fig. S2e). These results suggest that long TGFβ treatment results in proteasomal degradation of FOXO3a.
We also investigated the effect of short TGFβ treatment on FOXO3a. Immunofluorescence demonstrated that under normal conditions in the presence of 10% FBS, FOXO3a can be localized in both cytoplasm and the nucleus. However, nuclear exclusion of FOXO3a in SCC15 cells was observed 4-hour after the TGFβ treatment FOXO3a (Fig. 5a and b). We observed similar translocation with SCC25 cells (Supplementary Fig. S3 a, b). FOXO3a phosphorylation at Ser253 and Thr32, at 10 min after the treatment with TGFβ (Fig. 5c, Supplementary Fig. S3c) was also noted. These results suggested that short TGFβ treatment can also lead to phosphorylation and nuclear exclusion of FOXO3a. Overall, TGFβ treatment can result in phosphorylation, nuclear exclusion, and degradation of FOXO3a.
Fig. 5.
TGFβ treatment results in phosphorylation, nuclear exclusion and degradation of FOXO3a in SCC15. (a) Immunofluorescence of FOXO3a localization after 4 h TGFβ (2 ng/ml) treatment in SCC15. (b) Nuclear and cytoplasmic protein extracts were subjected to Western blotting analysis after treatment with TGFβ (2 ng/ml) . β-tubulin and Histone H3 were used as a protein loading control. (c) After short TGFβ (2 ng/ml; 10 min,30 min and 60 min) treatment, FOXO3a was phosphorylated at Ser253 and Thr32, along with Akt phosphorylation at Ser473 and Thr308 in SCC15. No significant difference of p-ERK1/2 was observed. Results are the mean ± SEM of three independent experiments and analyzed by Student's t-test. * p < 0.05 ** p < 0.01 ***p < 0.001.
3.5. AKT is essential for phosphorylation, nuclear exclusion and degradation of FOXO3a by TGFβ
Post-transcriptional phosphorylation by kinases is considered a primary cause for translocation of FOXO3a [6], [7], [8]. Therefore, we investigated upstream targets, such as AKT and ERK1/2, which might be the reason for the nuclear exclusion of FOXO3a. Our results suggest that AKT, rather than ERK1/2, was phosphorylated and activated by TGFβ (Fig. 5c, Supplementary Fig. S3c). We also observed AKT phosphorylation at Thr308 and Ser 473, 10 min after TGFβ treatment, corresponding with the phosphorylation of FOXO3a.
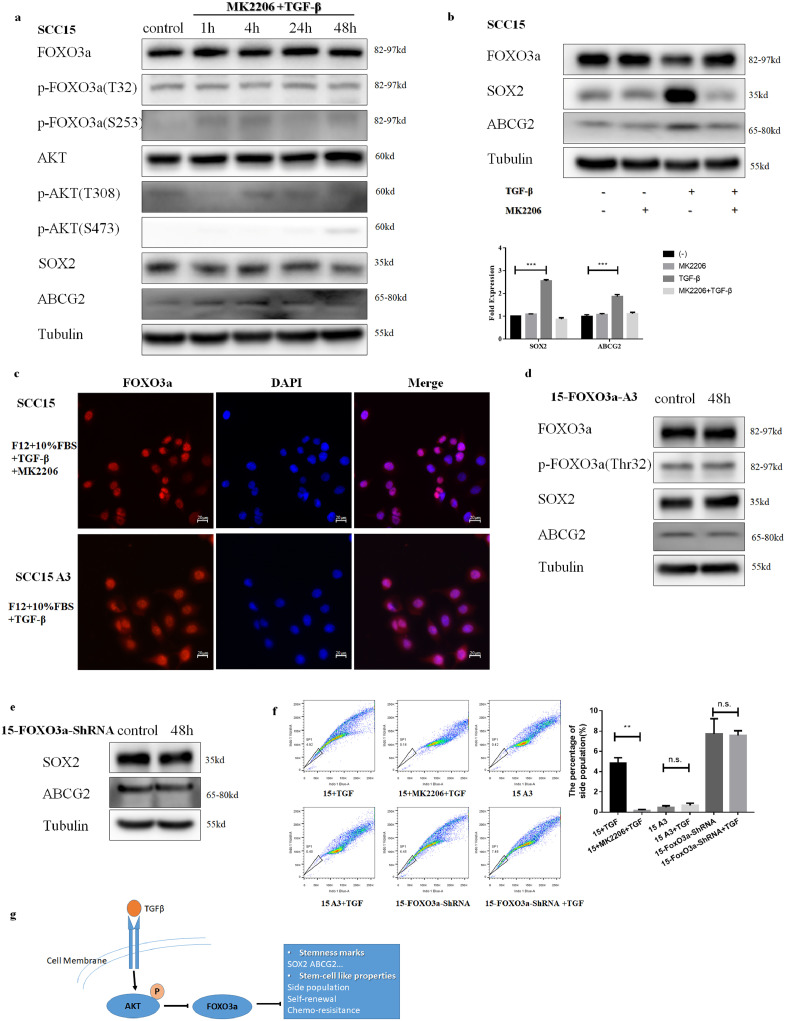
Further, to verify the importance of AKT, we used an inhibitor MK2206 (8 µM) to block its function. After pretreating with MK2206, the phosphorylation of FOXO3a was inhibited (Fig. 6a, Supplementary Fig. S4a). Moreover, MK2206 rescued the nuclear exclusion and degradation of FOXO3a upon TGFβ stimulation (Fig. 6b and c Supplementary Fig. S4 b and c). We also observed that upon mutation of all three AKT phosphorylation sites (Ser253, Thr32, Thr315) in FOXO3a, TGFβ stimulation does not influence the phosphorylation, localization, and level of FOXO3a. (Fig. 6c and d, Supplementary Fig. S4 c and d)
Fig. 6.
AKT is essential for phosphorylation, nuclear exclusion and degradation of FOXO3a by TGFβ, while AKT-FOXO3a axis plays a crucial role in TGFβ induced stemness in SCC15. (a) After pretreating with MK2206(8 µM) for 30 min, treatment with TGFβ (2 ng/ml) failed to induce phosphorylation, degradation of FOXO3a and upregulation of SOX2, ABCG2 in SCC15. β-tubulin was used as a protein loading control. (b) After pretreating with MK2206(8 µM) for 30 min, treatment with TGFβ (2 ng/ml) failed to induce degradation of FOXO3a and upregulation of SOX2, ABCG2 in SCC15. β-tubulin was used as a protein loading control. (c) Immunofluorescence showed cytoplasmic translocation of FOXO3a was inhibited with pre-treatment of MK2206(8 µM) or mutation at three AKT phosphorylation sites of FOXO3a in SCC15. (d) Stable cell line 15-FOXO3a-A3 was treated with 2 ng/ml TGFβ for 48 h and was analyzed using antibodies to SOX2, ABCG2. β-tubulin was used as a protein loading control. (e) Stable cell line 15-FOXO3a-shRNA was treated with 2 ng/ml TGFβ for 48 h and was analyzed using antibodies to p-FOXO3a(T32), SOX2, ABCG2. β-tubulin was used as a protein loading control. (f) No expansion of side population was observed with 2 ng/ml TGFβ for 48 h after pre-treatment of MK2206(8 µM), knock-down or mutation at three AKT phosphorylation sites of FOXO3a in SCC15. (g) FOXO3a is widely expressed and negatively correlated with the stemness in OSCC. This regulation can be abolished by TGFβ through phosphorylation, nuclear exclusion, and degradation in the non-Smad pathway. This non-Smad AKT-FOXO3a axis is essential to regulate stemness of CSCs by TGFβ. Results are the mean ± SEM of three independent experiments and analyzed by Student's t-test. * p < 0.05 ** p < 0.01 ***p < 0.001.
3.6. AKT-FOXO3a axis plays a crucial role in TGFβ induced stemness
Previous results have suggested that FOXO3a inhibits the stemness. Therefore, in the next step, we checked if TGFβ exposure can enhance the stemness in OSCC through down-regulation of FOXO3a.SCC15-FOXO3a-shRNA and SCC25-FOXO3a-shRNA cell lines were treated with TGFβ for 48 h, and no additional upregulation of SOX2 or ABCG2 was observed (Fig. 6e, Supplementary Fig. S4e). No change in the side population was observed either (Fig. 6f, Supplementary Fig. S4f). Further, blocking AKT with 8 µM MK2206 or mutating FOXO3aat Ser253, Thr32, Thr315 inhibits the up-regulation of SOX2, ABCG2 and the expansion of side population by TGFβ in OSCC (Fig. 6a, b, d, Supplementary Fig. S4 a, b, d). These findings are supportive of the fact that AKT-FOXO3a axis plays a crucial role in TGFβ induced stemness in OSCC.
4. Discussion
CSC theory states that, analogous to the renewal of normal tissues, tumor growth is similarly fueled by small numbers of CSCs hidden in cancers [3]. CSCs are proposed to be responsible for the poor prognosis of OSCC [37], [38]. Todoroki et al. reported CD44v3+/CD24−cells possess cancer stem cell-like properties in OSCC. OSCC patients with CD44v3+/CD24− showed poor overall survival [37]. Linge A showed that low CSC marker expression and low hypoxia identify good prognosis subgroups in HPV(-) HNSCC after postoperative radio-chemotherapy [38]. Exploring the molecular mechanism of CSCs might help to improve the prognosis of OSCC patients. In our preliminary study, by detecting FOXO3a in OSCC specimens and cell lines, we revealed a possible negative relationship between FOXO3a and CSCs. This conclusion is supported by the following observations:(1) in pericarcinous epithelium, FOXO3a is absent or low in the stratum basal where epithelial stem cells are housed [39] (2) the expression of FOXO3a is increased as epithelial cells differentiate in the pericarcinous epithelium (3) the expression of SOX2, a well-known stemness marker [40], [41], [42], was negatively related with the expression of FOXO3a (4) the expression of FOXO3a was significantly decreased in the population of CSCs.
FOXO3a has been reported as a suppressor of stem-cell like properties in various solid tumors, such as lung [16], breast [17], colon [18] and nervous system [19]. However, in recent years some studies provided contradicting evidence. FOXO3a plays a crucial role in the expression of stemness marker CD44 in pancreatic cancer [21]. FOXO3/PGC-1β signaling axis was found to be essential for stem-cell like properties in pancreatic ductal adenocarcinoma [22]. The role of FOXO3a in regulating stemness in OSCC is unexplored. In our study, stemness markers were negatively regulated by FOXO3a, while differentiation marker IVL was positively regulated. GSEA revealed that FOXO3a expression was positively associated with down-regulated genes in CSCs. The CSCs are characterized by the overexpression of specific surface markers such as CD44 [21], [22], [37], which was repressed by FOXO3a in our study. Transcriptional regulation of SOX2 is important for induction of stem-cell like properties including self-renewal [40], [41] and chemoresistance [42]. As SOX2 was repressed by FOXO3a, we also observed that the abilities of self-renewal and chemoresistance were negatively regulated. Our study suggests that SOX2 could be a direct target negatively regulated by FOXO3a in OSCC. Up-regulation of ABC transporters is another feature of CSCs, which can lead to chemoresistance [43]. Side population (SP) assay evaluates the activity of ABC transporters and identifies putative CSCs by efflux of fluorescent dyes, such as Hoechst 33,342 and Rhodamine 123, which are exported by ABCG2 and ABCB1 transporters, respectively [5]. Our data suggest that chemoresistance, as well as the side population, were reduced by FOXO3a and it is coupled with downregulation of ABCG2 transporters. Dubrovska et al. reported that shRNA knockdown of FOXO3a can lead to an increase in the tumorigenic ability [44]. In our study, we observed a similar phenomenon, but in SCC25 cells the difference was not significant. CSCs have a much stronger ability for tumorigenesis. Even only 100 cancer stem cells can induce tumor formation in nude mice [45]. But in our experiments, even a group of 10,000 cells failed to induce a tumor. This might also be due to limited tumorigenesis ability of SCC15 and SCC25 cell lines. Our future studies will involve validation in other OSCC cell lines. We also observed that not all stemness markers were upregulated when FOXO3a levels were modulated. It was only sufficient to induce stem cell-like properties and expansion of CSCs partially. As stem-cell like properties are one of the main reasons for bad prognosis, high expression of FOXO3a can provide a better outcome as the suppressor of stemness in OSCC.
TGFβ plays a complex dual role in the tumor microenvironment, converting itself from suppressor to promoter with cancer progression [24]. Previous studies have suggested that TGFβ contributes to CSCs in various cancer. Consistent with these findings [31], [32], [33], [34], we also observed that TGFβ can enhance stem-cell-like traits and expanded CSCs in OSCC.
TGFβ mainly acts through the canonical Smad pathway [24]. Blockage of this pathway effectively attenuates the stem-like properties and eliminates CSCs subpopulation in breast cancer [46], [47]. TGFβ signaling is also integrated into the complex intracellular signaling network through crosstalk with other pathways [25].Cross-talk was observed between TGFβ and NF-κB signal pathway through TAK1 and SMAD7 in a subset of head and neck cancers [48]. Wu et al. reported that TGFβ1-induced CK17 could enhance the CSC characteristics and facilitate the epithelial-mesenchymal transition (EMT) of cervical cancer through ERK1/2-MZF1 pathways [31]. Matsumoto et al. had demonstrated that TGFβ-mediated LEFTY/AKT/GSK-3β/Snail axis regulates EMT and stem-cell like properties in ovarian clear cell carcinomas [33]. Taken together, the canonical pathway as well as crosstalk of TGFβ, both are important factors to regulate the cancer stemness. Our results suggest that TGFβ induced the activation of AKT by phosphorylation at Ser473 and Thr308 rather than ERK1/2 in OSCC. The increased activity of AKT after treatment of TGFβ has been widely reported [33], [49], [50], [51], [52], [53]. However, the precise mechanism of crosstalk between TGFβ and AKT is still unclear. Yi et al. had shown that both TβRII and TβRI are directly involved in the activation of AKT by interacting with the p85 subunit of PI3K [52]. In pancreatic adenocarcinoma cells, TGFβ has been shown to transcriptionally downregulate PTEN and repressing the PI3K/AKT pathway [53]. Recently, it has been reported that TGFβ can activate PI3K and AKT via TRAF6,which poly-ubiquinates the PI3K regulatory subunit p85α and promote complex formation between TβRI and p85α [51]. The molecular mechanism underlying the crosstalk between TGFβ and AKT-FOXO3a remains to be further explored.
Phosphorylation is an important and complex way to regulate the function of FOXO3a [6], [7], [8]. In one hand, activation of a number of different protein kinases by insulin/growth factor signaling, including AKT, SGK, ERK1/2, CK1 and IKK, induces translocation of FOXO3a into cytoplasm [6], [7], [8], [36]. In the other hand, under oxidant/nutrient stress, several upstream regulators, such as JNK, MST1, AMPK and LMTK3, promotes nuclear localization and transcriptional activity of FOXO3a [6], [7], [8], [36]. Different kinases phosphorylate FOXO3a at specific sites. AKT and GSK phosphorylates FOXO3a at Thr32, Ser 253 and Ser315 [54], while ERK1/2 phosphorylates FOXO3a at Ser294,Ser344 and Ser425 [55]. Moreover, kinases such as AKT, ERK1/2, IKK, can be also activated by cross-talk of TGFβ. After translocation into cytoplasm, FOXO3a is further degraded through proteasome, which can be inhibited by MG132 [8]. In our study, activated AKT resulted in subsequent phosphorylation, translocation to the cytoplasm and finally degradation via the proteasome of FOXO3a. Also, function of FOXO3a is disturbed by phosphorylation at Thr32 and Ser253. Downstream targets as well as stem-cell like properties regulated by FOXO3a, are further affected. We further investigated whether AKT-FOXO3a axis plays a major role in TGFβ-induced stem-cell like properties. OSCC cells pretreated with AKT inhibitor MK2206, or with lower expression levels of FOXO3a, or with a mutation on AKT binding sites, FOXO3a showed little enhancement in stemness markers or expansion of the side population after treatment with TGFβ. This further suggests that AKT-FOXO3a axis does play a crucial regulatory role in TGFβ-induced stemness. The role of TGFβ and this non-Smad axis in vivo will be investigated in our future study.
In summary, we demonstrated that FOXO3a plays an important role in negatively regulating stemness. TGFβ results in phosphorylation, nuclear exclusion, and degradation of FOXO3a by activating AKT. We further illustrated that the non-Smad AKT-FOXO3a axis is essential to regulate the stemness of CSCs (Fig. 6g). Our study provides a foundation that can help to understand the mechanism of CSCs and a possible therapeutic target to eliminate CSCs in OSCC.
Funding sources
The study was supported by grants from National Natural Science Foundation of China (No. 81302367). The funders did not play a role in manuscript design, data collection, data analysis, data interpretation or writing of the manuscript.
CRediT authorship contribution statement
Kan Li: Data curation, Formal analysis, Writing - review & editing. Le Yang: Data curation. Jingyuan Li: Data curation. Chenyu Guan: Data curation. Sien Zhang: Formal analysis. Xiaomei Lao: Formal analysis. Daiqiao Ouyang: Data curation. Guangsen Zheng: Data curation. Siyong Gao: Data curation. Dikan Wang: Data curation. Yujie Liang: Conceptualization, Writing - review & editing. Guiqing Liao: Conceptualization, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no potential conflict of interest to disclose.
Acknowledgements
None.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ebiom.2019.09.027.
Contributor Information
Yujie Liang, Email: yujie0350@126.com.
Guiqing Liao, Email: drliaoguiqing@hotmail.com.
Appendix. Supplementary materials
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 2.Zeng H., Chen W., Chen J., Chen J., Zheng R., Zhang S. Changing cancer survival in China during 2003–15: a pooled analysis of 17 population-based cancer registries. Lancet Glob Health. 2018;6(5):e555–e567. doi: 10.1016/S2214-109X(18)30127-X. [DOI] [PubMed] [Google Scholar]
- 3.Batlle E., Clevers H. Cancer stem cells revisited. Nat Med. 2017;23(10):1124–1134. doi: 10.1038/nm.4409. [DOI] [PubMed] [Google Scholar]
- 4.Prasetyanti P.R., Medema J.P. Intra-tumor heterogeneity from a cancer stem cell perspective. Mol Cancer. 2017;16(1):41. doi: 10.1186/s12943-017-0600-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abbaszadegan M.R., Bagheri V., Razavi M.S., Momtazi A.A., Sahebkar A., Gholamin M. Isolation, identification, and characterization of cancer stem cells: a review. J Cell Physiol. 2017;232(8):2008–2018. doi: 10.1002/jcp.25759. [DOI] [PubMed] [Google Scholar]
- 6.Coomans D.B.A., Demoulin J.B. FOXO transcription factors in cancer development and therapy. Cell Mol Life Sci. 2016;73(6):1159–1172. doi: 10.1007/s00018-015-2112-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Y., Ao X., Ding W., Ponnusamy M., Wu W., Hao X. Critical role of FOXO3a in carcinogenesis. Mol Cancer. 2018;17(1):104. doi: 10.1186/s12943-018-0856-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hornsveld M., Dansen T.B., Derksen P.W., Burgering B.M.T. Re-evaluating the role of FOXOs in cancer. Semin Cancer Biol. 2017;50:90–100. doi: 10.1016/j.semcancer.2017.11.017. [DOI] [PubMed] [Google Scholar]
- 9.Link W., Fernandez-Marcos P.J. FOXO transcription factors at the interface of metabolism and cancer. Int J Cancer. 2017;141(12):2379–2391. doi: 10.1002/ijc.30840. [DOI] [PubMed] [Google Scholar]
- 10.Eijkelenboom A., Burgering B.M. FOXOs: signalling integrators for homeostasis maintenance. Nat Rev Mol Cell Biol. 2013;14(2):83–97. doi: 10.1038/nrm3507. [DOI] [PubMed] [Google Scholar]
- 11.Martins R., Lithgow G.J., Link W. Long live FOXO: unraveling the role of FOXO proteins in aging and longevity. Aging Cell. 2016;15(2):196–207. doi: 10.1111/acel.12427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qiang W., Sui F., Ma J., Li X., Ren X., Shao Y. Proteasome inhibitor MG132 induces thyroid cancer cell apoptosis by modulating the activity of transcription factor FOXO3a. Endocrine. 2017;56(1):98–108. doi: 10.1007/s12020-017-1256-y. [DOI] [PubMed] [Google Scholar]
- 13.Lin L., Ding D., Jiang Y., Li Y., Li S. MEK inhibitors induce apoptosis via FOXO3a-dependent puma induction in colorectal cancer cells. Oncogenesis. 2018;7(9):67. doi: 10.1038/s41389-018-0078-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shrestha A., Nepal S., Kim M.J., Chang J.H., Kim S.H., Jeong G.S. Critical role of AMPK/FOXO3a axis in globular Adiponectin-induced cell cycle arrest and apoptosis in cancer cells. J Cell Physiol. 2016;231(2):357–369. doi: 10.1002/jcp.25080. [DOI] [PubMed] [Google Scholar]
- 15.Li F., Dong X., Lin P., Jiang J. Regulation of AKT/foxo3a/SKP2 axis is critically involved in Berberine-induced cell cycle arrest in hepatocellular carcinoma cells. Int J Mol Sci. 2018;19(2):327. doi: 10.3390/ijms19020327. pii: E327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chiu C., Chang Y., Kuo K., Shen Y., Liu C., Yu Y. NF-κB-driven suppression of FOXO3a contributes to EGFR mutation-independent gefitinib resistance. Proc Natl Acad Sci USA. 2016;113(18):E2526–E2535. doi: 10.1073/pnas.1522612113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu H., Song Y., Qiu H., Liu Y., Luo K., Yi Y. Downregulation of FOXO3a by DNMT1 promotes breast cancer stem cell properties and tumorigenesis. Cell Death Differ. 2019 doi: 10.1038/s41418-019-0389-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prabhu V.V., Allen J.E., Dicker D.T., El-Deiry W.S. Small-molecule ONC201/TIC10 targets Chemotherapy-resistant colorectal cancer stem – like cells in an AKT/FOXO3a/trail-dependent manner. Cancer Res. 2015;75(7):1423–1432. doi: 10.1158/0008-5472.CAN-13-3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sato A., Okada M., Shibuya K., Watanabe E., Seino S., Narita Y. Pivotal role for ROS activation of p38 MAPK in the control of differentiation and tumor-initiating capacity of glioma-initiating cells. Stem Cell Res. 2014;12(1):119–131. doi: 10.1016/j.scr.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 20.Touil Y., Zuliani T., Wolowczuk I., Kuranda K., Prochazkova J., Andrieux J. The PI3K/AKT signaling pathway controls the quiescence of the low-Rhodamine123-retention cell compartment enriched for melanoma stem cell activity. Stem Cells. 2013;31(4):641–651. doi: 10.1002/stem.1333. [DOI] [PubMed] [Google Scholar]
- 21.Kumazoe M., Takai M., Bae J., Hiroi S., Huang Y., Takamatsu K. FOXO3 is essential for CD44 expression in pancreatic cancer cells. Oocogene. 2017;36(19):2643–2654. doi: 10.1038/onc.2016.426. [DOI] [PubMed] [Google Scholar]
- 22.Kumazoe M., Takai M., Hiroi S., Takeuchi C., Kadomatsu M., Nojiri T. The FOXO3/PGC-1beta signaling axis is essential for cancer stem cell properties of pancreatic ductal adenocarcinoma. J Biol Chem. 2017;292(26):10813–10823. doi: 10.1074/jbc.M116.772111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li K., Huang S.H., Lao X.M., Yang L., Liao G.Q., Liang Y.J. Interaction of cancer cell-derived FOXp3 and tumor microenvironment in human tongue squamous cell carcinoma. Exp Cell Res. 2018;370(2):643–652. doi: 10.1016/j.yexcr.2018.07.029. [DOI] [PubMed] [Google Scholar]
- 24.Seoane J., Gomis R.R. TGF-beta family signaling in tumor suppression and cancer progression. Cold Spring Harb Perspect Biol. 2017;9(12) doi: 10.1101/cshperspect.a022277. pii: a022277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luo K. Signaling cross talk between TGF-beta/Smad and other signaling pathways. Cold Spring Harb Perspect Biol. 2017;9(1) doi: 10.1101/cshperspect.a022137. pii: a022137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guillen D.N., Sanz-Pamplona R., Berdiel-Acer M., Cimas F.J., Garcia E., Goncalves-Ribeiro S. Noncanonical TGFbeta pathway relieves the blockade of IL1beta/TGFbeta-Mediated crosstalk between tumor and stroma: TGFBR1 and TAK1 inhibition in colorectal cancer. Clin Cancer Res. 2019;25(14):4466–4479. doi: 10.1158/1078-0432.CCR-18-3957. [DOI] [PubMed] [Google Scholar]
- 27.Principe D.R., Diaz A.M., Torres C., Mangan R.J., DeCant B., McKinney R. TGFbeta engages MEK/ERK to differentially regulate benign and malignant pancreas cell function. Oncogene. 2017;36(30):4336–4348. doi: 10.1038/onc.2016.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Subramanian A., Tamayo P., Mootha V.K., Mukherjee S., Ebert B.L., Gillette M.A. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102(43):15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mootha V.K., Lindgren C.M., Eriksson K.F., Subramanian A., Sihag S., Lehar J. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. 2003;34(3):267–273. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- 30.Han X.G., Du L., Qiao H., Tu B., Wang Y.G., Qin A. CXCR1 knockdown improves the sensitivity of osteosarcoma to cisplatin. Cancer Lett. 2015;369(2):405–415. doi: 10.1016/j.canlet.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 31.Wu L., Han L., Zhou C., Wei W., Chen X., Yi H. TGF-beta1-induced CK17 enhances cancer stem cell-like properties rather than EMT in promoting cervical cancer metastasis via the ERK1/2-MZF1 signaling pathway. FEBS J. 2017;284(18):3000–3017. doi: 10.1111/febs.14162. [DOI] [PubMed] [Google Scholar]
- 32.Fu Y., Zhang P., Nan H., Lu Y., Zhao J., Yang M. LncRNA CASC11 promotes TGF-beta1, increases cancer cell stemness and predicts postoperative survival in small cell lung cancer. Gene. 2019;704:91–96. doi: 10.1016/j.gene.2019.04.019. [DOI] [PubMed] [Google Scholar]
- 33.Matsumoto T., Yokoi A., Hashimura M., Oguri Y., Akiya M., Saegusa M. TGF-beta-mediated LEFTY/Akt/GSK-3beta/Snail axis modulates epithelial-mesenchymal transition and cancer stem cell properties in ovarian clear cell carcinomas. Mol Carcinog. 2018;57(8):957–967. doi: 10.1002/mc.22816. [DOI] [PubMed] [Google Scholar]
- 34.Liu F., Kong X., Lv L., Gao J. TGF-beta1 acts through miR-155 to down-regulate TP53INP1 in promoting epithelial-mesenchymal transition and cancer stem cell phenotypes. Cancer Lett. 2015;359(2):288–298. doi: 10.1016/j.canlet.2015.01.030. [DOI] [PubMed] [Google Scholar]
- 35.Watabe T., Miyazono K. Roles of TGF-beta family signaling in stem cell renewal and differentiation. Cell Res. 2009;19(1):103–115. doi: 10.1038/cr.2008.323. [DOI] [PubMed] [Google Scholar]
- 36.Wang X., Hu S., Liu L. Phosphorylation and acetylation modifications of FOXO3a: independently or synergistically? Oncol Lett. 2017;13(5):2867–2872. doi: 10.3892/ol.2017.5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Todoroki K., Ogasawara S., Akiba J., Nakayama M., Naito Y., Seki N. CD44v3+/CD24- cells possess cancer stem cell-like properties in human oral squamous cell carcinoma. Int J Oncol. 2016;48(1):99–109. doi: 10.3892/ijo.2015.3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Linge A., Lock S., Gudziol V., Nowak A., Lohaus F., von Neubeck C. Low cancer stem cell marker expression and low hypoxia identify good prognosis subgroups in HPV(-) HNSCC after postoperative radiochemotherapy: a multicenter study of the DKTK-ROG. Clin Cancer Res. 2016;22(11):2639–2649. doi: 10.1158/1078-0432.CCR-15-1990. [DOI] [PubMed] [Google Scholar]
- 39.Papagerakis S., Pannone G., Zheng L., About I., Taqi N., Nguyen N.P. Oral epithelial stem cells - implications in normal development and cancer metastasis. Exp Cell Res. 2014;325(2):111–129. doi: 10.1016/j.yexcr.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu L., Liu C., Zhang Q., Shen J., Zhang H., Shan J. SIRT1-mediated transcriptional regulation of SOX2 is important for self-renewal of liver cancer stem cells. Hepatology. 2016;64(3):814–827. doi: 10.1002/hep.28690. [DOI] [PubMed] [Google Scholar]
- 41.Kim M., Jang K., Miller P., Picon-Ruiz M., Yeasky T.M., El-Ashry D. VEGFA links self-renewal and metastasis by inducing SOX2 to repress miR-452, driving slug. Oncogene. 2017;36(36) doi: 10.1038/onc.2017.4. 5199-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chou M., Hu F., Yu C., Yu C. Sox2 expression involvement in the oncogenicity and radiochemoresistance of oral cancer stem cells. Oral Oncol. 2015;51(1):31–39. doi: 10.1016/j.oraloncology.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 43.Begicevic R.R., Falasca M. ABC transporters in cancer stem cells: beyond chemoresistance. Int J Mol Sci. 2017;18(11) doi: 10.3390/ijms18112362. pii: E2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dubrovska A., Kim S., Salamone R.J., Walker J.R., Maira S.M., Garcia-Echeverria C. The role of PTEN/AKT/PI3K signaling in the maintenance and viability of prostate cancer stem-like cell populations. Proc Natl Acad Sci USA. 2009;106(1):268–273. doi: 10.1073/pnas.0810956106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Al-Hajj M., Wicha M.S., Benito-Hernandez A., Morrison S.J., Clarke M.F. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100(7):3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chihara Y., Shimoda M., Hori A., Ohara A., Naoi Y., Ikeda J.I. A small-molecule inhibitor of SMAD3 attenuates resistance to anti-HER2 drugs in HER2-positive breast cancer cells. Breast Cancer Res Treat. 2017;166(1):55–68. doi: 10.1007/s10549-017-4382-6. [DOI] [PubMed] [Google Scholar]
- 47.Bhola N.E., Balko J.M., Dugger T.C., Kuba M.G., Sanchez V., Sanders M. TGF-beta inhibition enhances chemotherapy action against triple-negative breast cancer. J Clin Investig. 2013;123(3):1348–1358. doi: 10.1172/JCI65416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Freudlsperger C., Bian Y., Contag W.S., Burnett J., Coupar J., Yang X. TGF-beta and NF-kB signal pathway cross-talk is mediated through TAK1 and SMAD7 in a subset of head and neck cancers. Oncogene. 2013;32(12):1549–1559. doi: 10.1038/onc.2012.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Madne T.H., Dockrell M. TGFbeta1-mediated PI3K/AKT and p38 MAP kinase dependent alternative splicing of fibronectin extra domain a in human podocyte culture. Cell Mol Biol. 2018;64(5):127–135. [PubMed] [Google Scholar]
- 50.Rodriguez-Garcia A., Samso P., Fontova P., Simon-Molas H., Manzano A., Castano E. TGF-beta1 targets SMAD, p38 MAPK, and PI3K/Akt signaling pathways to induce PFKFB3 gene expression and glycolysis in glioblastoma cells. FEBS J. 2017;284(20):3437–3454. doi: 10.1111/febs.14201. [DOI] [PubMed] [Google Scholar]
- 51.Hamidi A., Song J., Thakur N., Itoh S., Marcusson A., Bergh A. TGF-beta promotes PI3K-AKT signaling and prostate cancer cell migration through the TRAF6-mediated ubiquitylation of p85alpha. Sci Signal. 2017;10(486) doi: 10.1126/scisignal.aal4186. pii: eaal4186. [DOI] [PubMed] [Google Scholar]
- 52.Yi J.Y., Shin I., Arteaga C.L. Type I transforming growth factor beta receptor binds to and activates phosphatidylinositol 3-kinase. J Biol Chem. 2005;280(11):10870–10876. doi: 10.1074/jbc.M413223200. [DOI] [PubMed] [Google Scholar]
- 53.Chow J.Y., Quach K.T., Cabrera B.L., Cabral J.A., Beck S.E., Carethers J.M. RAS/ERK modulates TGFbeta-regulated PTEN expression in human pancreatic adenocarcinoma cells. Carcinoenesis. 2007;28(11):2321–2327. doi: 10.1093/carcin/bgm159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brunet A., Bonni A., Zigmond M.J., Lin M.Z., Juo P., Hu L.S. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96(6):857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 55.Yang J.Y., Zong C.S., Xia W., Yamaguchi H., Ding Q., Xie X. ERK promotes tumorigenesis by inhibiting FOXO3a via MDM2-mediated degradation. Nat Cell Biol. 2008;10(2):138–148. doi: 10.1038/ncb1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.