Abstract
Colorectal cancer (CRC) is one of the most frequently diagnosed cancers and leading cause of cancer-related deaths worldwide. In recent years, there has been a growing realisation that lifestyle plays a major role for CRC development and that intestinal microbiota, which are shaped by lifestyle and nutrition habits, may be critically involved in the pathogenesis of CRC. Although the precise mechanisms for how the microbiota contribute to CRC development and progression remain elusive, increasing evidence suggests a direct causative role for the intestinal microbiota in modulating signalling pathways, anti-tumour immune responses and cell proliferation. Recent advances in understanding host-microbe interactions have shed light onto the putative use of intestinal microbiota as a powerful tool in CRC diagnosis and therapy. Here, we will discuss the role of the intestinal microbiota in CRC pathogenesis, their potential utility as diagnostic markers, and consider how microbes could be used in therapeutic approaches for the treatment of CRC.
Keywords: Colorectal cancer (CRC), Intestinal microbiota, Nutrition, Pathogenesis, Diagnosis, Therapy
1. Introduction
Colorectal cancer (CRC) is the third most frequent cancer in the Western world and the second leading cause of cancer-related death worldwide. [1]. In recent years, despite improvements in screening and treatment options and approaches [2], CRC is still curable only in its early stages. This fact is demonstrated by the age standardised 5-year survival rates that vary between 70% in Korea to 60–69% in USA and Europe to less than 50% in Russia and India. In particular, 5-year survival rates in patients with UICC stage IV disease are still below 20% [3].
CRC aetiology has been linked to hereditary genetic syndromes, family history of CRC, inflammatory bowel disease (IBD), and, particularly, environmental and lifestyle risk factors, such as obesity, alcoholism, tobacco, and diet [4]. It is anticipated that the number of patients suffering from CRC will significantly rise within the next twenty years, associated with a rise in Western lifestyle practices and diet, particularly as these Western influences become more common also in Asia, Africa, and South America [5]. Clear associations have been demonstrated between the risk of developing CRC and consumption of certain food types, such as red meat, and nutrients, which act to either promote or reduce the risk of developing CRC [6]. Most of these environmental factors have been associated with an effect on the indigenous intestinal microbiota, causing a detrimental alteration known as dysbiosis [7]. Accordingly, the intestinal microbiota or its derived metabolites may be acting as the direct environmental modifiers for the risk to develop CRC, given the known associations of dietary habits on systemic immune responses, inflammation, intestinal immune responses to bacteria, as well as intestinal microbiota composition and functionality overall. This profound effect of microbiota on determining immune responses is exemplified by observations from the use of antibiotics in early childhood, which have been associated with increased colonic adenoma formation in later life, the precursor lesion to CRC [8,9].
Given recent technological and technical advances, there have been significant strides forward in our understanding of the relevance for intestinal microbiota in health and disease. In particular, through identification of several microbial signatures associated with CRC and related pathologies such as IBD. Despite these advances and the significant potential for modulating intestinal microbiota for the host benefit as part of a future personalised/precision medicine approach, our knowledge about how microbiota may be used as a diagnostic tool or which bacterium or mix of bacteria could treat or prevent CRC is still in its infancy. This highlights the need to move away from spurious, often retrospectively identified associations, greater scrutiny to prove causation and not just correlation, as well as highlighting the importance of functional data to better understand the complex microbiota-host interplay.
2. Intestinal microbiota composition and CRC
The human intestine is a highly populated microbial ecosystem. The bacterial concentration increases from 107–8 cells per gram of faecal content in the small intestine to >1011 in the colon [10]. In healthy subjects, the gut is primarily populated by a core microbiota composed of obligate anaerobes belonging mainly to the phyla, Firmicutes and Bacteroidetes and to a lesser extent to Actinobacteria, Proteobacteria, and Verrucomicrobia [11]. However, specific insults to the gut may lead to disturbances in the composition of intestinal microbiota, a term named dysbiosis. Such intestinal dysbiosis is associated with a number of inflammatory diseases along the gastrointestinal tract, including IBD [12,13], coeliac disease [14,15], or lymphocytic gastritis [16]. However, it is unclear whether dysbiosis occurs as a cause or consequence of inflammation, particularly, whether intestinal dysbiosis represents the result of an inflammatory process or if, factors leading to inflammation (diet, obesity, host genetics) are responsible for the development of an altered microbial composition.
Nevertheless, the onset of CRC has been strongly associated with intestinal dysbiosis and an altered composition of microbiota at the intestinal mucosal surface of the tumour as well as the tumour-adjacent tissue [17], [18], [19]. Two recent meta-analyses of faecal metagenomes revealed that on a functional level, bacterial metagenomes associated with CRC have increased expression of genes involved in protein, mucin and choline metabolism as well as for gluconeogenesis and fermentation pathways, but reduced expression of genes associated with carbohydrate degradation. Given such metagenomes have also been linked with enhanced secondary bile acid production, this may suggest an association between CRC-associated intestinal microbiota pattern and a fat- and meat-rich nutrition [20,21].
3. Intestinal microbiota and CRC pathogenesis
There is increasing evidence to support that a number of bacteria, e.g. Fusobacterium nucleatum or Bacteroides fragilis are associated with the onset of CRC in humans [22]. This effect may be due to the intestinal microbiota themselves, the toxins the bacteria may produce, and/or the metabolites formed as fermentation by-products, all of which can profoundly influence intestinal homeostasis, leading to pro- or anti-inflammatory immune responses and subsequent development of CRC [23]. The inflammatory state that is driven by intestinal microbiota is thought to have an impact on CRC development and progression [23]. Typically, CRC develops in a stepwise process resulting in the accumulation of genetic and epigenetic alterations. During this process there is a transition from normal to hyperproliferative epithelium, known as hyperplasia. When hyperplasia occurs, the intestinal epithelium loses its characteristic architecture and functions, and becomes dysplastic. Dysplasia can result in the development of non-malignant adenomas, known as polyps, which eventually may invade the submucosa and lead to a carcinoma. Microbiota have been demonstrated to play a key role in this adenoma to carcinoma, tumour formation and progression pathway [24,25].
More recently, the microbial formation of biofilms has been proposed as a driving factor in the early stage of CRC development. Biofilm formation allow bacteria to attach together and protect themselves from the action of external agents. Expansion of bacteria within biofilms negatively affects the intestinal epithelium and reduces the levels of E-cadherin in colonic crypts. As a consequence, intestinal permeability increases and the inflammatory response driven by interleukin (IL)-6 and signal transducer and activator of transcription (STAT) 3 is activated [26]. Of note, this biofilm formation is mainly associated with right-sided colon adenomas and cancers, whilst biofilm formation is infrequently demonstrated in left-sided CRC [27]. Examination of biofilms attached to the colonic mucosa of patients with familial adenomatous polyposis, has demonstrated a predominance of E. coli and B. fragilis which may be contributing to the high risk for developing CRC in those patients [28]. The importance of biofilms has been underlined, by findings supporting their oncogenic potential and an ability to induce CRC in mice regardless of whether they are derived from tumour tissue or healthy human colonic tissue [29].
In CRC patients, the impairment of the intestinal epithelium, and resulting intestinal barrier dysfunction, facilitates the translocation of microbes from the lumen to the lamina propria and the induction of cytokines, including IL-17 and IL-23, which act to maintain the inflammatory microenvironment within the tumour [30]. In a study using IL-10 knockout mice, modification of intestinal microbiota driven by intestinal inflammation, promoted the expansion of genotoxic bacteria resulting in tumour formation [31]. Interestingly, obesity, which is characterised by systemic low-level inflammation and intestinal dysbiosis, has also been linked to an increased risk for CRC development. This increased risk in obese patients may be due to observed associations including a decreased proportion of Bacteriodetes in the intestine [32], and the overall bacterial richness being lower compared to lean people [33].
Another prominent cause of CRC is colitis-associated cancer (CAC). CAC develops from long-standing colitis in patients with IBD and greater risk for CAC has clearly been associated with greater inflammatory burden over time. Patients suffering from IBD have a reported increase in their risk of CRC development by up to 20% after 30 years [34]. The increased incidence of CRC in IBD patients may depend on several factors, including extent and severity of intestinal inflammation, disease severity, duration of disease, treatment as well as management and surveillance strategies [35]. Similar to CRC, dysbiosis of the intestinal microbiota has been reported to play an essential role in IBD pathogenesis [12]. E. coli NC101 mono-colonization studies have further supported the link between inflammation and CRC onset. In this instance, the intestinal inflammation in IL-10 deficient mice has been demonstrated to promote a specific phenotype of intestinal dysbiosis and the subsequent mono-colonization with this E. coli strain enhanced colon tumourigenesis in vivo [31].
There is also increased evidence for an important role of F. nucleatum in CRC pathogenesis. The presence of the gram-negative anaerobic bacterium F. nucleatum in CRC tissue has been inversely correlated with the number of CD3+ T-cells in CRC tissue suggesting a possible involvement of this bacterium in the regulation of anti-tumour immune responses [36]. On a functional level, F. nucleatum is able to inhibit the activity of natural killer (NK) cells targeting cancer cells via binding of the bacteria-derived protein Fap2 on exclusively human T cell immunoglobulin and ITIM domain (TIGIT) molecule [37], suggesting that F. nucleatum is involved in regulating tumour immune-evasion processes, which are critical for the development and progression of CRC. However, F. nucleatum does not only seem to be involved in CRC pathogenesis, but also in modulating response to CRC therapy. Particularly, F. nucleatum is able to induce the activation of autophagy machinery, in an effect mediated by targeting of specific microRNAs. Additionally, F. nucleatum also interferes with the activation of the TLR4 pathway. The end-point of these mechanisms, results in promotion of chemoresistance of CRC cells in vivo [38]. Accordingly, F. nucleatum has been more frequently detected in cancerous tissue from patients with recurrent tumours and is associated with worse survival rates of CRC patients. As well as in recurrent tumours, F. nucleatum has been found in tissue derived from CRC metastases. The potential of microbial modulation has been demonstrated from the use of antibiotic treatment targeting F. nucleatum, which is efficient to reduce not only bacterial load, but also tumour cell growth and proliferation in human CRC xenograft models [39]. Supplementation of diet with foods rich in fibre has also been associated with lower risk for F. nucleatum –positive CRC, again supporting a potential role of the microbiota as mediator between diet and CRC [40].
Aside from gut bacteria themselves, bacteria-derived virulence factors may also be involved in the promotion and development of CRC. F. nucleatum secretes an anchoring adhesion molecule named FadA. The link between FadA and E-cadherin on intestinal epithelial cells results in an impairment of the paracellular adhesions, allowing the penetration of pathogens to the submucosa. This drives a consequent inflammatory response and activates β-catenin signaling to further enhance the activity of pro-oncogenic pathways [41], [42], [43]. B. fragilis is another gut microbe that has been shown to be enriched when analysing microbial composition from the intestine of patients with CRC. B. fragilis subgroup ETBF produces the toxin fragylisin, which is a metalloproteinase that cleaves E-cadherin on colonocytes. Fragylisin increases mucosal permeability, enhances IL-17 cytokine secretion as well as STAT3 activation, and is increased in patients with advanced CRC [44]. A recent elegant approach used a specific bacterial enzyme expressed by intratumoural bacteria, specifically the long isoform of bacterial cytidine deaminase, to demonstrate a contribution to gemcitabine resistance via degradation of this chemotherapeutic agent in CRC tumours. Of interest, this enzyme is expressed e.g. by Klebsiella pneumonia or E. coli strain K-12 and the authors were able to demonstrate that antibiotic treatment eradicating those bacteria enhanced the efficacy of gemcitabine in mouse CRC models in vivo [45].
Reactive oxygen species (ROS) and reactive nitrogen species (RNS) induce inflammation and DNA damage. Oxidative stress causes DNA mutations, dysplasia, and thus facilitates tumour development [46]. Although ROS are generated by macrophages and neutrophils during inflammatory processes, they can be produced as well by certain microbiota species including Enterococcus faecalis [47]. These findings strongly support the idea that particular microbes, which are enriched in an inflammatory microenvironment, contribute or even cause the onset of CRC. A summary of microorganism that have been linked to CRC development, their associated virulence factors, and proposed pathogenic mechanisms are highlighted in Table 1.
Table 1.
| Microorganism | Phylum | Oxigen requirement | Natural reservoir | Effectors | Mechanism identified in models | Reference |
|---|---|---|---|---|---|---|
| Bacteroides fragilis (ETBF) | Bacteroidetes | Strict anaerobe | GI tract | Fragilysin, Bft toxin | Fragilysin cleavage E-cadherin and increase mucosal permeability | [44,78] |
| Porphyromonas spp | Bacteroidetes | Strict anaerobe | Oral cavity | [79] | ||
| Prevotella spp | Bacteroidetes | Strict anaerobe | Oral cavity | [61,80] | ||
| Odoribacter spp | Bacteroidetes | Strict anaerobe | GI tract | [61] | ||
| Alistipes finegoldii | Bacteroidetes | Strict anaerobe | GI tract | IL-6/STAT3 activation in Il-10-/- mice | [81] | |
| Streptococcus gallolyticus | Firmicutes | Facultative anaerobe | GI tract | Selective adhesion to collagen I, IV, fibronectin, and fibrinogen | [82] | |
| Peptostreptococcus anaerobius | Firmicutes | Strict anaerobe | GI tract | Activates TLR2/4-ROS-cholesterol axis | [83] | |
| Enterococcus faecalis | Firmicutes | Facultative anaerobe | GI tract | Production of extracellular O2− | [47] | |
| Streptococcus bovis | Firmicutes | Facultative anaerobe | GI tract | [44,82] | ||
| Fusobacterium nucleatum | Fusobacteria | Strict anaerobe | Oral cavity | Adhesin FadA, Fap2 | FadA binds E-cadherin on ECs and affects paracelullar adhesions | [41], [42], [43] |
| Leptotrichia spp | Fusobacteria | Strict anaerobe | Oral cavity | [84,85] | ||
| E. coli (B2 strain) | Proteobacteria | Facultative anaerobe | GI tract | Colibactin | DNAse activity | [86] |
| Campilobacter jejuni | Proteobacteria | Microaerophilic | GI tract | Cytolethal distending toxin (CDT) | DNAse activity | [87] |
| Helicobacter hepaticus | Proteobacteria | Microaerophilic | GI tract | [88] |
4. Microbiota as diagnostic markers
As demonstrated, the causative relationship between the intestinal microbiota and CRC development is complex. Identification of candidates in a one microorganism-one disease model is challenging, highlighted by the difficulty in dissociating effect and cause. However, notable progress in the field has been made through identification of microbial signatures associated with and specific to patients with CRC. Flemer et al., found an increased abundance of Bacteroides, Roseburia, Ruminococus, and Oscilobacter in mucosal samples from patients with CRC (n = 70) compared to healthy controls (n = 56). Moreover, the authors observed regional differences between distal and rectal versus proximal cancers from the same mucosal samples [48]. A decrease in Firmicutes and Bacteroidetes, the most prominent phyla in healthy individuals and lactic acid bacteria have also been associated with CRC [25,49]. Even accounting for the differences in microbial composition from different stages of disease and treatment, there is still significant inter-individual heterogeneity, with no single OTU being identified as causative in all individuals with CRC. This is perhaps unsurprising given the increasing evidence of molecular subtypes of CRC and different pathogenic mechanisms driving each individual subtype [50]. Instead, co-abundance groups (CAGs) of organisms determining the community structure are more informative and could be used in the early detection of CRC [48,51].
In a multi-cohort study including Chinese, Austrian, American, German, and French patients, meta-analysis of shotgun metagenomics on CRC identified a group of bacteria consistently enriched in CRC across multiple populations. In addition to differences in bacterial composition, some bacterial pathways were positively correlated with CRC, such as Lipopolysaccharide (LPS)-related pathway, biosynthetic pathways, or pathways being relevant for metabolism of cofactors and vitamins [52]. In a further metagenome-wide association study involving Chinese and Danish patients with CRC, the authors identified two new species associated with CRC, Parvimonas micra and Solobacterium moorei. The authors also discovered 20 gene markers significantly associated with CRC microbiomes in the Chinese cohort. From those, four were validated in the Danish cohort and later in external French and Austrian cohorts [53]. Given these promising findings, stool microbiota have been further investigated as a putative non-invasive biomarker for CRC detection [17,54].
In a cohort of 170 CRC patients and 200 healthy control subjects the presence of F. nucleatum in faecal samples from cancer patients was identified as a potential biomarker for the onset of CRC. Presence of only F. nucleatum demonstrated high sensitivity and specificity of about 80%. The combination of four bacteria in the faeces, F. nucleatum (Fn), Bacteroides clarus (Bc), Clostridium hathewayi (Ch) and one undefined species (m7), improved these values only to a small extent. However, the combination of those four bacteria with the result of the faecal immunochemical test (FIT) increased the sensitivity to detect CRC up to 92.8% and even up to 100% in stage II CRC. Such an approach offers significant promise for incorporation into routine clinical practice to aid diagnosis. In addition to diagnosis, levels of F. nucleatum within the CRC tumour tissue have also been associated with poorer clinical outcomes and prognosis [55]. Whilst this prognostic element would need further investigation and validation, the clinical utility of such a biomarker would be even further enhanced if both predictive and prognostic capability were to be demonstrated.
In recent years, extracellular bacterial peptides encrypted in the intestinal exoproteome have been proposed to possess bioactivity and immunomodulatory capacity to maintain the mucosal barrier [56], [57], [58]. Beyond these roles, the potential for bacterial peptides to be used as biomarkers of gut homeostasis have also been reported. IgA serum levels elevated in response to certain bacterial peptides have been proposed as a diagnostic biomarker to help discriminate patients with IBD from healthy controls. In the same study, the authors demonstrated that IgA serum levels distinguished between patients with CD and those with UC, in both the active or quiescent disease. Analysis of the bacterial peptides in thus study showed capacity to modulate the intestinal cytokine milieu on resting conditions and in the presence of LPS [59]. Bacterial peptides may offer significant promise as potential biomarkers not only in IBD, but also as a promising tool for the development, diagnosis, and progression of CRC.
Despite the wide-ranging studies which have identified associations and potential for microbiota to be used in diagnosis of CRC, there remains a lack of consensus on many fronts. The discordant findings from these studies may be due to differences in the collection, storage, and processing of faecal samples, DNA extraction, amplification, as well as the bioinformaticworkflow consisting of sequencing, normalization of data, and subsequent bioinformaticanalyses [60]. Despite these limitations, microbial markers are a promising tool in the detection of CRC. Future research should focus on the detection of early stages of CRC, such as polyps, adenomas and other related CRC (pre-)lesions, which have been a limitation of microbial markers to date, as most studies have focused on detection for more advanced stages of the disease [61,62].
5. Microbiota as therapeutic approach in CRC
The intestinal microbiota is characterised by temporal stability and a pronounced capacity for resilience. However, acute perturbations initiated from either the host or the microbial side may require prolonged periods for the microbiota to recover. This perturbation and resulting microbial dysbiosis can elicit permanent changes locally or in other tissues, that may lead to chronic diseases such as IBD [63] The establishment of native microbiota and the interplay with the host immune system is crucial for the development during childhood. It has been recently reported that early-life antibiotic exposure, caesarean section, and formula feeding can lead to an immature microbiota, having adverse consequences later in life, including increased risk of obesity, diabetes, IBD, asthma, and various allergic or hypersensitivity reactions [64]. Therefore, the ultimate and elusive goal in cancer therapy remains the discovery of bacterial species or a combination of them that either prevent the onset of CRC, promote anti-tumour responses or enhance the efficacy of existing medications.
In recent years, diverse studies have shown the possibility of applying dietary strategies to modulate the intestinal microbiota. The aim of such work is to encourage the growth of certain bacterial strains that may convert indigestible dietary components into a large variety of metabolites with beneficial effects for the host [65]. It is now well recognised that “Western” diets rich in animal fats and red meat, while poor in fruit and vegetable intake predispose to inflammatory and metabolic diseases, as well as CRC [66], [67], [68]. Dietary fibre has been identified as an important source of beneficial bacterial metabolites i.e. short-chain fatty acids (SCFAs). Dietary fibre is resistant to digestion and absorption in the small intestine, but specific members of the resident intestinal microbiota can ferment these polysaccharides, including resistant starch, cellulose, pectins, oligosaccharides, and lignins, either partially or completely [69]. Compelling evidence for the impact of diet on risk of developing CRC was provided by a study where a native African cohort who have generally a low CRC rate, and African-American cohort switched their diets for 2 weeks. The dietary changes from traditional high-fibre diet to Western diets and vice versa resulted in rapid and reciprocal changes in the microbiome, affecting the levels of SCFAs, including butyrate, and the mucosal biomarkers of CRC risk [70]. The importance of dietary fibre has also been demonstrated in a meta-analysis where CRC risk could be reduced by 10% per additional 10 g/day of total dietary fibre intake [71]. With rapidly expanding understanding of pre- and probiotics, it is increasingly likely that bioactive food components and certain members of the microbiota could offer hope for therapeutic use for the prevention and treatment of CRC.
In addition to its association with CRC development, intestinal microbiota also have local and systemic effect in modulating the efficacy and toxicity of cancer therapy [72]. The first important findings on response to cancer therapeutics, were observed when intestinal microbiota were found to control the response to cancer chemotherapy by regulating myeloid-derived cell functions within the tumour microenvironment. In this study, the authors showed that LPS is essential to prime myeloid cells via TLR4 [73]. A further study underlying the importance of microbial composition for treatment response, studied the pro-carcinogenic phenotype expressed by some genetically mutated mice when stool transfer to wild-type mice was performed by faecal microbiota transplantation (FMT). In a similar way, FMT from patients responding to cancer therapy to germ-free mice endowed those mice with the ability to respond to the therapy [74]. Whilst the direct and indirect biological mechanisms need to be determined, these data support that intestinal microbiota composition affect the efficacy of anti-cancer therapies.
In efforts to discover the underlying biology of host-microbial interactions, a consortium of 11 bacteria in the stool of healthy individuals were able to consistently induce the number of IFNγ+CD8+ cytotoxic T-cells in the intestine of germ-free mice. Notably, those 11 bacteria induced CD8+ T-cells without additionally enhancing intestinal or systemic inflammation. The effect of those 11 bacteria, appears to be mediated via a CD103+ dendritic cell- and major histocompatibility (MHC) class Ia molecule-dependent pathway. On a functional level, the colonization of mice undergoing a subcutaneous CRC mouse model with those 11-bacteria resulted in enhanced efficacy of checkpoint inhibitor treatment for CRC therapy [75]. Whilst these initial findings offer promising signals, clearly further studies are needed to translate microbiota-based therapies into clinical practice.
The most well recognised probiotics nowadays are those from the genera Lactobacillus and Bifidobacterium. In a randomised controlled trial, oral supplementation of lyophilised live L. acidophilus and B. bifidum was shown to prevent intestinal toxicity in cancer patients treated with both radiotherapy and cisplatin [76]. These clinical data support the use of probiotic bacteria such as L. acidophilus to promote an anti-tumour effect while preventing the toxicity of the drug. Interestingly, two recent studies have shown the influence of intestinal microbiota on the efficacy of anticancer therapy with immune checkpoint inhibitors. The anti-cytotoxic T-lymphocyte-associated antigen 4 (CTLA4) causes epithelial barrier disruption, allowing the penetration of bacteria into the lamina propria and an alteration in the composition of intestinal microbiota. In a mouse model of subcutaneous melanoma tumours, which poorly respond to anti-CTLA4 therapy, the relative abundance of Bacteroidales and Burkholderiales decreased in favour of Clostridiales. Restoring B. thetaiotaomicron and B. fragilis in microbiota-depleted mice enhanced the efficacy of the anti-CTLA4 therapy, by inducing the maturation of dendritic cells within the tumours and TH1 response in tumour-draining lymph nodes. The authors concluded that anti-CTLA4 therapy promoted not only antitumour, but also anticommensal immunity, which served as adjuvant for the anticancer therapy [74].
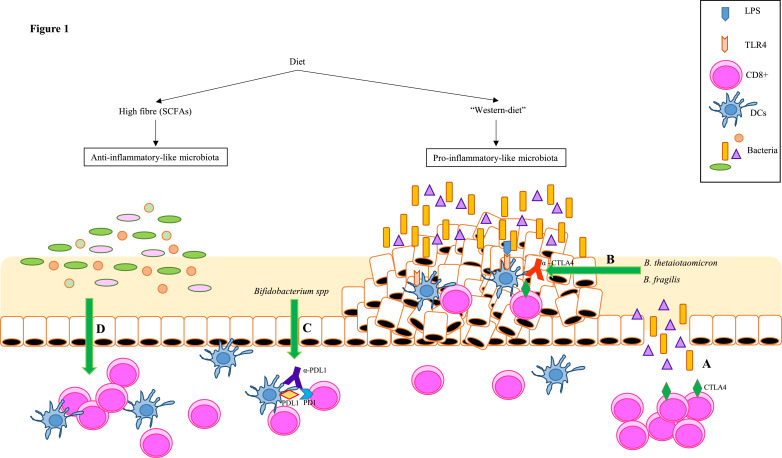
Unlike anti-CTLA4, programmed cell death protein ligand 1 (PD-L1) blockade does not affect the intestinal barrier. However, Sivan and colleagues demonstrated that intestinal microbiota plays again a role in the efficacy of the PD-L1 immune checkpoint inhibitor blockade. The authors showed that mice from different vendors developed subcutaneous tumours at a different rate, which correlated to a higher infiltration of CD8+ T cells in tumour tissue and lower progression of tumours. When analysing the intestinal microbiota they found that response to anti-PD-L1 therapy correlated with higher relative abundance of Bifidobacterium species. This suggests that anti-PD-L1 therapy against PD-L1-PD1 interaction is enhanced by the presence of the certain probiotic, such as Bifidobacterium spp [77]. Although these two studies were conducted in melanoma models, immunotherapy is increasingly being used for the treatment of other cancers, including CRC. The different strategies that could be followed for CRC treatment using intestinal microbiota-based approaches are summarised in Fig. 1.
Fig. 1.
Potential microbiota therapies to treat CRC. Diet plays an important role in establishing the composition of intestinal microbiota. While a fibre-rich diet will lead to an anti-inflammatory-like microbiota, predominant in SCFAs producing bacteria, a western diet rich in animal fats and read meat, will lead to a pro-inflammatory microenvironment that may predispose to CRC. Several studies have demonstrated the role of microbiota in response to cancer therapy: (A) L. acidophilus and B. bifidum are able to prevent intestinal toxicity in CRC patients treated with both radiotherapy and cisplatin [70]. (B) CTLA4 causes epithelial disruption and the consequent penetration of bacteria into the lamina propria. However, this a priori adverse effect allows B. thetaiotaomicron and B. fragilis to enhance the efficacy of anti-CTLA4 therapy by inducing the maturation of dendritic cells within the tumours (C) [68]. (D) Bifidobacterium spp enhaces anti-PDL1 therapy [71]. (E) A defined commensal consortium of 11 bacteria has been shown to induce IFNγ+CD8+ T cells without enhancing intestinal inflammation in a CD103+ dendritic cell and MHC-I - dependent manner [69].
6. Summary
The intestinal microbiome has been demonstrated to be critically involved in the pathogenesis of CRC. A broad number of high quality studies have demonstrated the importance of intestinal microbiota, and accordingly diet, life-style and nutrition as its modifying factors, for the development of the disease. In addition to a large number of studies demonstrating an association between specific bacteria and CRC onset, a wealth of functional data exist that reliably demonstrate the involvement of bacteria in pathways and molecular mechanisms that culminate in the onset of CRC. There have been promising initial findings for the use of intestinal microbiota as predictive and/or prognostic markers for CRC. In terms of translational potential, there is significant optimise for modulation of intestinal microbiota as a potential tool for treating CRC. In order to develop on these promising advances to date, a greater understanding of the complex interplay between the intestinal microbiota and the onset of CRC is critical to utilize the intestinal microbiota for the treatment of CRC.
7. Outstanding questions
The major limiting factor for translation of microbiota to identify therapeutic targets or even to be used as therapeutic agents, is the fact that most of our knowledge about intestinal microbiota and disease pathogenesis relies on associations, usually retrospectively identified, and, often derived from animal models. However, to use the intestinal microbiota or microbiota-based products in complex disease areas such as oncology where there is the additional complexity of multiple therapeutic agents, we need to obtain a greater understanding on the functional role of microbiota, particularly with respect to its immunologic, metabolic and cell physiologic consequences in the human host. Obtaining such a critical understanding of the functional relationship between the intestinal microbiota and host physiology would enable us to design and deliver personalised/precision medicine approaches. The aim of such an approach would be to allow specific modulation of the intestinal microbiome for any one individual and to deliver microbiota-based products, which can be used to directly treat disease for that individual. One could envisage, that such an approach could be used to enhance the efficacy of existing therapies or to reduce the extent of side effects. The ultimate aim of a precision oncology approach should be to promote development of nutritional therapies, composed to foster of specific functional groups of bacteria, which are able to act via the intestine to exert beneficial effects on human health and disease. Whilst such a vision may currently seem futuristic. Given rapid advances in technology and bioinformatic approaches, greater understanding about the functional role of microbiota, as well as large prospectively studied and well-phenotyped cohorts, we anticipate the potential use of microbiota modulation in the near future and look forward to the use of such an approach to help deliver the goal of precision medicine for patients with colorectal cancer.
8. Search strategy and selection criteria
Data for this Review were identified by searches of MEDLINE, Current Contents, PubMed, and references from relevant articles using the search terms “microbiota”, “colorectal carcinoma”, and “colon cancer”. Abstracts and reports from meetings were included only when they related directly to previously published work. Only articles published in English between 1980 and 2019 were included.
CRediT authorship contribution statement
Ana Montalban-Arques: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing - original draft. Michael Scharl: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing - review & editing.
Declaration of Competing Interest
The authors have no financial conflicts of interest to disclose.
Acknowledgements
The authors are grateful to Dr. Nuru Noor for critically reading of the manuscript.
This work has been supported by grants to MS from the Stiftung Experimentelle Biomedizin and the Swiss National Science Foundation (grants No. 314730_166381/1, 314730_166381/2, CRSII3_154488/1, and 320030_184753/1).
Bibliography
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(Nov (6)):394–424. doi: 10.3322/caac.21492. PubMed PMID: 30207593. Epub 2018/09/13. [DOI] [PubMed] [Google Scholar]
- 2.Jeon J., Du M., Schoen R.E., Hoffmeister M., Newcomb P.A., Berndt S.I. Determining risk of colorectal cancer and starting age of screening based on lifestyle, environmental, and genetic factors. Gastroenterology. 2018;154(Jun (8)):2152–2164. doi: 10.1053/j.gastro.2018.02.021. e19. PubMed PMID: 29458155. PMCID: PMC5985207. Epub 2018/02/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allemani C., Matsuda T., Di Carlo V., Harewood R., Matz M., Niksic M. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391(Mar (10125)):1023–1075. doi: 10.1016/S0140-6736(17)33326-3. 17PubMed PMID: 29395269. PMCID: PMC5879496. Epub 2018/02/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Imperiale T.F., Ransohoff D.F. Risk for colorectal cancer in persons with a family history of adenomatous polyps: a systematic review. Ann Intern Med. 2012;156(10):703–709. doi: 10.7326/0003-4819-156-10-201205150-00006. May 15PubMed PMID: 22586009. Epub 2012/05/16. [DOI] [PubMed] [Google Scholar]
- 5.Arnold M., Sierra M.S., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66(4):683–691. doi: 10.1136/gutjnl-2015-310912. AprPubMed PMID: 26818619. Epub 2016/01/29. [DOI] [PubMed] [Google Scholar]
- 6.Song M., Garrett W.S., Chan A.T. Nutrients, foods, and colorectal cancer prevention. Gastroenterology. 2015;148(May (6)):1244–1260. doi: 10.1053/j.gastro.2014.12.035. e16. PubMed PMID: 25575572. PMCID: PMC4409470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shanahan F., van Sinderen D., O'Toole P.W., Stanton C. Feeding the microbiota: transducer of nutrient signals for the host. Gut. 2017;66(Sep (9)):1709–1717. doi: 10.1136/gutjnl-2017-313872. PubMed PMID: 28663354. Epub 2017/07/01. [DOI] [PubMed] [Google Scholar]
- 8.Cao Y., Wu K., Mehta R., Drew D.A., Song M., Lochhead P. Long-term use of antibiotics and risk of colorectal adenoma. Gut. 2018;67(Apr (4)):672–678. doi: 10.1136/gutjnl-2016-313413. PubMed PMID: 28377387. PMCID: PMC5628103. Epub 2017/04/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gensollen T., Iyer S.S., Kasper D.L., Blumberg R.S. How colonization by microbiota in early life shapes the immune system. Science. 2016;352(6285):539–544. doi: 10.1126/science.aad9378. Apr 29PubMed PMID: 27126036. PMCID: PMC5050524. Epub 2016/04/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riviere A., Selak M., Lantin D., Leroy F., De Vuyst L. Bifidobacteria and butyrate-producing colon bacteria: importance and strategies for their stimulation in the human gut. Front Microbiol. 2016;7:979. doi: 10.3389/fmicb.2016.00979. PubMed PMID: 27446020. PMCID: PMC4923077. Epub 2016/07/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eckburg P.B., Bik E.M., Bernstein C.N., Purdom E., Dethlefsen L., Sargent M. Diversity of the human intestinal microbial flora. Science. 2005;308(Jun (5728)):1635–1638. doi: 10.1126/science.1110591. 10PubMed PMID: 15831718. PMCID: PMC1395357. Epub 2005/04/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ni J., Wu G.D., Albenberg L., Tomov V.T. Gut microbiota and IBD: causation or correlation? Nat Rev Gastroenterol Hepatol. 2017;14(Oct (10)):573–584. doi: 10.1038/nrgastro.2017.88. PubMed PMID: 28743984. PMCID: PMC5880536. Epub 2017/07/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henson M.A., Phalak P. Microbiota dysbiosis in inflammatory bowel diseases: in silico investigation of the oxygen hypothesis. BMC Syst Biol. 2017;11(Dec (1)):145. doi: 10.1186/s12918-017-0522-1. PubMed PMID: 29282051. PMCID: PMC5745886. Epub 2017/12/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Girbovan A., Sur G., Samasca G., Lupan I. Dysbiosis a risk factor for celiac disease. Med Microbiol Immunol. 2017;206(Apr (2)):83–91. doi: 10.1007/s00430-017-0496-z. PubMed PMID: 28204873. Epub 2017/02/17. [DOI] [PubMed] [Google Scholar]
- 15.Marasco G., Di Biase A.R., Schiumerini R., Eusebi L.H., Iughetti L., Ravaioli F. Gut microbiota and celiac disease. Dig Dis Sci. 2016;61(Jun (6)):1461–1472. doi: 10.1007/s10620-015-4020-2. PubMed PMID: 26725064. Epub 2016/01/05. [DOI] [PubMed] [Google Scholar]
- 16.Montalban-Arques A., Wurm P., Trajanoski S., Schauer S., Kienesberger S., Halwachs B. Propionibacterium acnes overabundance and natural killer group 2 member D system activation in corpus-dominant lymphocytic gastritis. J Pathol. 2016;240(Dec (4)):425–436. doi: 10.1002/path.4782. PubMed PMID: 27538697. PMCID: PMC5111592. Epub 2016/10/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liang Q., Chiu J., Chen Y., Huang Y., Higashimori A., Fang J. Fecal bacteria act as novel biomarkers for noninvasive diagnosis of colorectal cancer. Clin Cancer Res. 2017;23(Apr (8)):2061–2070. doi: 10.1158/1078-0432.CCR-16-1599. PubMed PMID: 27697996. Epub 2016/10/05. [DOI] [PubMed] [Google Scholar]
- 18.Feng Q., Liang S., Jia H., Stadlmayr A., Tang L., Lan Z. Gut microbiome development along the colorectal adenoma-carcinoma sequence. Nat Commun. 2015;6(Mar):6528. doi: 10.1038/ncomms7528. PubMed PMID: 25758642. Epub 2015/03/12. [DOI] [PubMed] [Google Scholar]
- 19.Nakatsu G., Li X., Zhou H., Sheng J., Wong S.H., Wu W.K. Gut mucosal microbiome across stages of colorectal carcinogenesis. Nat Commun. 2015;6(Oct):8727. doi: 10.1038/ncomms9727. PubMed PMID: 26515465. PMCID: PMC4640069. Epub 2015/10/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomas A.M., Manghi P., Asnicar F., Pasolli E., Armanini F., Zolfo M. Metagenomic analysis of colorectal cancer datasets identifies cross-cohort microbial diagnostic signatures and a link with choline degradation. Nat Med. 2019;25(Apr (4)):667–678. doi: 10.1038/s41591-019-0405-7. PubMed PMID: 30936548. Epub 2019/04/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wirbel J., Pyl P.T., Kartal E., Zych K., Kashani A., Milanese A. Meta-analysis of fecal metagenomes reveals global microbial signatures that are specific for colorectal cancer. Nat Med. 2019;25(Apr (4)):679–689. doi: 10.1038/s41591-019-0406-6. PubMed PMID: 30936547. Epub 2019/04/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tilg H., Adolph T.E., Gerner R.R., Moschen A.R. The intestinal microbiota in colorectal cancer. Cancer Cell. 2018;33(Jun (6)):954–964. doi: 10.1016/j.ccell.2018.03.004. PubMed PMID: 29657127. Epub 2018/04/17. [DOI] [PubMed] [Google Scholar]
- 23.Louis P., Hold G.L., Flint H.J. The gut microbiota, bacterial metabolites and colorectal cancer. Nat Rev Microbiol. 2014;12(Oct (10)):661–672. doi: 10.1038/nrmicro3344. PubMed PMID: 25198138. [DOI] [PubMed] [Google Scholar]
- 24.Lucas C., Barnich N., Nguyen H.T.T. Microbiota, inflammation and colorectal cancer. Int J Mol Sci. 2017;18(Jun (6)) doi: 10.3390/ijms18061310. PubMed PMID: 28632155. PMCID: PMC5486131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raskov H., Burcharth J., Pommergaard H.C. Linking gut microbiota to colorectal cancer. J Cancer. 2017;8(17):3378–3395. doi: 10.7150/jca.20497. PubMed PMID: 29151921. PMCID: PMC5687151. Epub 2017/11/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li S., Konstantinov S.R., Smits R., Peppelenbosch M.P. Bacterial biofilms in colorectal cancer initiation and progression. Trends Mol Med. 2017;23(Jan (1)):18–30. doi: 10.1016/j.molmed.2016.11.004. PubMed PMID: 27986421. Epub 2016/12/18. [DOI] [PubMed] [Google Scholar]
- 27.Dejea C.M., Wick E.C., Hechenbleikner E.M., White J.R., Mark Welch J.L., Rossetti B.J. Microbiota organization is a distinct feature of proximal colorectal cancers. Proc Natl Acad Sci U S A. 2014;111(Dec (51)):18321–18326. doi: 10.1073/pnas.1406199111. PubMed PMID: 25489084. PMCID: PMC4280621. Epub 2014/12/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dejea C.M., Fathi P., Craig J.M., Boleij A., Taddese R., Geis A.L. Patients with familial adenomatous polyposis harbor colonic biofilms containing tumorigenic bacteria. Science. 2018;359(Feb (6375)):592–597. doi: 10.1126/science.aah3648. PubMed PMID: 29420293. PMCID: PMC5881113. Epub 2018/02/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tomkovich S., Dejea C.M., Winglee K., Drewes J.L., Chung L., Housseau F. Human colon mucosal biofilms from healthy or colon cancer hosts are carcinogenic. J Clin Invest. 2019;130(Mar):1699–1712. doi: 10.1172/JCI124196. PubMed PMID: 30855275. PMCID: PMC6436866. Epub 2019/03/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grivennikov S.I., Wang K., Mucida D., Stewart C.A., Schnabl B., Jauch D. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature. 2012;491(Nov (7423)):254–258. doi: 10.1038/nature11465. PubMed PMID: 23034650. PMCID: PMC3601659. Epub 2012/10/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arthur J.C., Perez-Chanona E., Muhlbauer M., Tomkovich S., Uronis J.M., Fan T.J. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science. 2012;338(Oct (6103)):120–123. doi: 10.1126/science.1224820. PubMed PMID: 22903521. PMCID: PMC3645302. Epub 2012/08/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ley R.E., Turnbaugh P.J., Klein S., Gordon J.I. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444(Dec (7122)):1022–1023. doi: 10.1038/4441022a. PubMed PMID: 17183309. Epub 2006/12/22. [DOI] [PubMed] [Google Scholar]
- 33.Le Chatelier E., Nielsen T., Qin J., Prifti E., Hildebrand F., Falony G. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500(Aug (7464)):541–546. doi: 10.1038/nature12506. PubMed PMID: 23985870. Epub 2013/08/30. [DOI] [PubMed] [Google Scholar]
- 34.Munkholm P. Review article: the incidence and prevalence of colorectal cancer in inflammatory bowel disease. Aliment Pharmacol Ther. 2003;18(Sep (Suppl 2)):1–5. doi: 10.1046/j.1365-2036.18.s2.2.x. PubMed PMID: 12950413. Epub 2003/09/03. [DOI] [PubMed] [Google Scholar]
- 35.Kang M., Martin A. Microbiome and colorectal cancer: unraveling host-microbiota interactions in colitis-associated colorectal cancer development. Semin Immunol. 2017;32(Aug):3–13. doi: 10.1016/j.smim.2017.04.003. PubMed PMID: 28465070. Epub 2017/05/04. [DOI] [PubMed] [Google Scholar]
- 36.Mima K., Sukawa Y., Nishihara R., Qian Z.R., Yamauchi M., Inamura K. Fusobacterium nucleatum and T cells in colorectal carcinoma. JAMA Oncol. 2015;1(Aug (5)):653–661. doi: 10.1001/jamaoncol.2015.1377. PubMed PMID: 26181352. PMCID: PMC4537376. Epub 2015/07/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gur C., Ibrahim Y., Isaacson B., Yamin R., Abed J., Gamliel M. Binding of the Fap2 protein of Fusobacterium nucleatum to human inhibitory receptor tigit protects tumors from immune cell attack. Immunity. 2015;42(Feb (2)):344–355. doi: 10.1016/j.immuni.2015.01.010. PubMed PMID: 25680274. PMCID: PMC4361732. Epub 2015/02/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu T., Guo F., Yu Y., Sun T., Ma D., Han J. Fusobacterium nucleatum promotes chemoresistance to colorectal cancer by modulating autophagy. Cell. 2017;170(Jul (3)):548–563. doi: 10.1016/j.cell.2017.07.008. e16. PubMed PMID: 28753429. PMCID: PMC5767127. Epub 2017/07/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bullman S., Pedamallu C.S., Sicinska E., Clancy T.E., Zhang X., Cai D. Analysis of Fusobacterium persistence and antibiotic response in colorectal cancer. Science. 2017;358(Dec (6369)):1443–1448. doi: 10.1126/science.aal5240. PubMed PMID: 29170280. PMCID: PMC5823247. Epub 2017/11/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mehta R.S., Nishihara R., Cao Y., Song M., Mima K., Qian Z.R. Association of dietary patterns with risk of colorectal cancer subtypes classified by fusobacterium nucleatum in tumor tissue. JAMA Oncol. 2017;3(Jul (7)):921–927. doi: 10.1001/jamaoncol.2016.6374. PubMed PMID: 28125762. PMCID: PMC5502000. Epub 2017/01/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kostic A.D., Chun E., Robertson L., Glickman J.N., Gallini C.A., Michaud M. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe. 2013;14(Aug (2)):207–215. doi: 10.1016/j.chom.2013.07.007. PubMed PMID: 23954159. PMCID: PMC3772512. Epub 2013/08/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rubinstein M.R., Wang X., Liu W., Hao Y., Cai G., Han Y.W. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/beta-catenin signaling via its FadA adhesin. Cell Host Microbe. 2013;14(Aug (2)):195–206. doi: 10.1016/j.chom.2013.07.012. PubMed PMID: 23954158. PMCID: PMC3770529. Epub 2013/08/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McCoy A.N., Araujo-Perez F., Azcarate-Peril A., Yeh J.J., Sandler R.S., Keku T.O. Fusobacterium is associated with colorectal adenomas. PLoS ONE. 2013;8(1):e53653. doi: 10.1371/journal.pone.0053653. PubMed PMID: 23335968. PMCID: PMC3546075. Epub 2013/01/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boleij A., Hechenbleikner E.M., Goodwin A.C., Badani R., Stein E.M., Lazarev M.G. The Bacteroides fragilis toxin gene is prevalent in the colon mucosa of colorectal cancer patients. Clin Infect Dis. 2015;60(Jan (2)):208–215. doi: 10.1093/cid/ciu787. PubMed PMID: 25305284. PMCID: PMC4351371. Epub 2014/10/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Geller L.T., Barzily-Rokni M., Danino T., Jonas O.H., Shental N., Nejman D. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science. 2017;357(Sep (6356)):1156–1160. doi: 10.1126/science.aah5043. PubMed PMID: 28912244. PMCID: PMC5727343. Epub 2017/09/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reuter S., Gupta S.C., Chaturvedi M.M., Aggarwal B.B. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med. 2010;49(Dec (11)):1603–1616. doi: 10.1016/j.freeradbiomed.2010.09.006. PubMed PMID: 20840865. PMCID: PMC2990475. Epub 2010/09/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huycke M.M., Moore D.R. In vivo production of hydroxyl radical by Enterococcus faecalis colonizing the intestinal tract using aromatic hydroxylation. Free Radic Biol Med. 2002;33(Sep (6)):818–826. doi: 10.1016/s0891-5849(02)00977-2. PubMed PMID: 12208369. Epub 2002/09/05. [DOI] [PubMed] [Google Scholar]
- 48.Flemer B., Lynch D.B., Brown J.M., Jeffery I.B., Ryan F.J., Claesson M.J. Tumour-associated and non-tumour-associated microbiota in colorectal cancer. Gut. 2017;66(Apr (4)):633–643. doi: 10.1136/gutjnl-2015-309595. PubMed PMID: 26992426. PMCID: PMC5529966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Backhed F., Ley R.E., Sonnenburg J.L., Peterson D.A., Gordon J.I. Host-bacterial mutualism in the human intestine. Science. 2005;307(Mar (5717)):1915–1920. doi: 10.1126/science.1104816. PubMed PMID: 15790844. Epub 2005/03/26. [DOI] [PubMed] [Google Scholar]
- 50.Guinney J., Dienstmann R., Wang X., de Reynies A., Schlicker A., Soneson C. The consensus molecular subtypes of colorectal cancer. Nat Med. 2015;21(Nov (11)):1350–1356. doi: 10.1038/nm.3967. PubMed PMID: 26457759. PMCID: PMC4636487. Epub 2015/10/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Claesson M.J., Jeffery I.B., Conde S., Power S.E., O'Connor E.M., Cusack S. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012;488(Aug (7410)):178–184. doi: 10.1038/nature11319. PubMed PMID: 22797518. Epub 2012/07/17. [DOI] [PubMed] [Google Scholar]
- 52.Dai Z., Coker O.O., Nakatsu G., Wu W.K.K., Zhao L., Chen Z. Multi-cohort analysis of colorectal cancer metagenome identified altered bacteria across populations and universal bacterial markers. Microbiome. 2018;6(Apr (1)):70. doi: 10.1186/s40168-018-0451-2. PubMed PMID: 29642940. PMCID: PMC5896039. Epub 2018/04/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yu J., Feng Q., Wong S.H., Zhang D., Liang Q.Y., Qin Y. Metagenomic analysis of faecal microbiome as a tool towards targeted non-invasive biomarkers for colorectal cancer. Gut. 2017;66(Jan (1)):70–78. doi: 10.1136/gutjnl-2015-309800. PubMed PMID: 26408641. Epub 2015/09/27. [DOI] [PubMed] [Google Scholar]
- 54.Eklof V., Lofgren-Burstrom A., Zingmark C., Edin S., Larsson P., Karling P. Cancer-associated fecal microbial markers in colorectal cancer detection. Int J Cancer. 2017;141(Dec (12)):2528–2536. doi: 10.1002/ijc.31011. PubMed PMID: 28833079. PMCID: PMC5697688. Epub 2017/08/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mima K., Nishihara R., Qian Z.R., Cao Y., Sukawa Y., Nowak J.A. Fusobacterium nucleatum in colorectal carcinoma tissue and patient prognosis. Gut. 2016;65(Dec (12)):1973–1980. doi: 10.1136/gutjnl-2015-310101. PubMed PMID: 26311717. PMCID: PMC4769120. Epub 2015/08/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ruiz L., Hidalgo C., Blanco-Miguez A., Lourenco A., Sanchez B., Margolles A. Tackling probiotic and gut microbiota functionality through proteomics. J Proteomics. 2016;147(Sep) doi: 10.1016/j.jprot.2016.03.023. 28-39PubMed PMID: 27003613. Epub 2016/03/24. [DOI] [PubMed] [Google Scholar]
- 57.Sanchez B., Bressollier P., Urdaci M.C. Exported proteins in probiotic bacteria: adhesion to intestinal surfaces, host immunomodulation and molecular cross-talking with the host. FEMS Immunol Med Microbiol. 2008;54(Oct (1)):1–17. doi: 10.1111/j.1574-695X.2008.00454.x. PubMed PMID: 18631181. Epub 2008/07/18. [DOI] [PubMed] [Google Scholar]
- 58.Sanchez B., Urdaci M.C., Margolles A. Extracellular proteins secreted by probiotic bacteria as mediators of effects that promote mucosa-bacteria interactions. Microbiology. 2010;156(Nov (Pt 11)):3232–3242. doi: 10.1099/mic.0.044057-0. PubMed PMID: 20864471. Epub 2010/09/25. [DOI] [PubMed] [Google Scholar]
- 59.Fernández-Tomé S., Montalban-Arques A., Díaz-Guerra A., Galvan-Roman J.M., Marin A.C., Mora-Gutiérrez I. Peptides encrypted in the human intestinal microbial-exoproteome as novel biomarkers and immunomodulatory compounds in the gastrointestinal tract. J Funct Foods. 2019;52:459–468. 2019/01/01/ [Google Scholar]
- 60.DeSantis T.Z., Shah M.S., Cope J.L., Hollister E.B. Microbial markers in the diagnosis of colorectal cancer: the promise, reality and challenge. Future Microbiol. 2017;12(Nov):1341–1344. doi: 10.2217/fmb-2017-0185. PubMed PMID: 28972391. Epub 2017/10/04. [DOI] [PubMed] [Google Scholar]
- 61.Zackular J.P., Rogers M.A., MTt Ruffin, Schloss P.D. The human gut microbiome as a screening tool for colorectal cancer. Cancer Prev Res (Phila) 2014;7(Nov (11)):1112–1121. doi: 10.1158/1940-6207.CAPR-14-0129. PubMed PMID: 25104642. PMCID: PMC4221363. Epub 2014/08/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Baxter N.T., MTt Ruffin, Rogers M.A., Schloss P.D. Microbiota-based model improves the sensitivity of fecal immunochemical test for detecting colonic lesions. Genome Med. 2016;8(Apr (1)):37. doi: 10.1186/s13073-016-0290-3. PubMed PMID: 27056827. PMCID: PMC4823848. Epub 2016/04/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Integrative HMPRNC The integrative human microbiome project. Nature. 2019;569(May (7758)):641–648. doi: 10.1038/s41586-019-1238-8. PubMed PMID: 31142853. Epub 2019/05/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bokulich N.A., Chung J., Battaglia T., Henderson N., Jay M., Li H. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci Transl Med. 2016;8(Jun (343)):343ra82. doi: 10.1126/scitranslmed.aad7121. PubMed PMID: 27306664. PMCID: PMC5308924. Epub 2016/06/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Montalban-Arques A., De Schryver P., Bossier P., Gorkiewicz G., Mulero V., Gatlin D.M. Selective manipulation of the gut microbiota improves immune status in vertebrates. Front Immunol. 2015;6:512. doi: 10.3389/fimmu.2015.00512. PubMed PMID: 26500650. PMCID: PMC4598590. Epub 2015/10/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Watson A.J., Collins P.D. Colon cancer: a civilization disorder. Dig Dis. 2011;29(2):222–228. doi: 10.1159/000323926. PubMed PMID: 21734388. Epub 2011/07/08. [DOI] [PubMed] [Google Scholar]
- 67.Wu G.D., Chen J., Hoffmann C., Bittinger K., Chen Y.Y., Keilbaugh S.A. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334(Oct (6052)):105–108. doi: 10.1126/science.1208344. PubMed PMID: 21885731. PMCID: PMC3368382. Epub 2011/09/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.De Filippo C., Cavalieri D., Di Paola M., Ramazzotti M., Poullet J.B., Massart S. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci U S A. 2010;107(Aug (33)):14691–14696. doi: 10.1073/pnas.1005963107. PubMed PMID: 20679230. PMCID: PMC2930426. Epub 2010/08/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lim C.C., Ferguson L.R., Tannock G.W. Dietary fibres as "prebiotics": implications for colorectal cancer. Mol Nutr Food Res. 2005;49(Jun (6)):609–619. doi: 10.1002/mnfr.200500015. PubMed PMID: 15864790. Epub 2005/05/03. [DOI] [PubMed] [Google Scholar]
- 70.O'Keefe S.J., Li J.V., Lahti L., Ou J., Carbonero F., Mohammed K. Fat, fibre and cancer risk in African Americans and rural Africans. Nat Commun. 2015;6(Apr):6342. doi: 10.1038/ncomms7342. PubMed PMID: 25919227. PMCID: PMC4415091. Epub 2015/04/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ben Q., Sun Y., Chai R., Qian A., Xu B., Yuan Y. Dietary fiber intake reduces risk for colorectal adenoma: a meta-analysis. Gastroenterology. 2014;146(Mar (3)):689–699. doi: 10.1053/j.gastro.2013.11.003. e6. PubMed PMID: 24216326. Epub 2013/11/13. [DOI] [PubMed] [Google Scholar]
- 72.Roy S., Trinchieri G. Microbiota: a key orchestrator of cancer therapy. Nat Rev Cancer. 2017;17(May (5)):271–285. doi: 10.1038/nrc.2017.13. PubMed PMID: 28303904. Epub 2017/03/18. [DOI] [PubMed] [Google Scholar]
- 73.Guiducci C., Vicari A.P., Sangaletti S., Trinchieri G., Colombo M.P. Redirecting in vivo elicited tumor infiltrating macrophages and dendritic cells towards tumor rejection. Cancer Res. 2005;65(Apr (8)):3437–3446. doi: 10.1158/0008-5472.CAN-04-4262. PubMed PMID: 15833879. Epub 2005/04/19. [DOI] [PubMed] [Google Scholar]
- 74.Vetizou M., Pitt J.M., Daillere R., Lepage P., Waldschmitt N., Flament C. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350(Nov (6264)):1079–1084. doi: 10.1126/science.aad1329. PubMed PMID: 26541610. PMCID: PMC4721659. Epub 2015/11/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tanoue T., Morita S., Plichta D.R., Skelly A.N., Suda W., Sugiura Y. A defined commensal consortium elicits CD8 T cells and anti-cancer immunity. Nature. 2019;565(Jan (7741)):600–605. doi: 10.1038/s41586-019-0878-z. PubMed PMID: 30675064. Epub 2019/01/25. [DOI] [PubMed] [Google Scholar]
- 76.Chitapanarux I., Tungkasamit T., Petsuksiri J., Kannarunimit D., Katanyoo K., Chakkabat C. Randomized control trial of benzydamine HCl versus sodium bicarbonate for prophylaxis of concurrent chemoradiation-induced oral mucositis. Support Care Cancer. 2018;26(Mar (3)):879–886. doi: 10.1007/s00520-017-3904-4. PubMed PMID: 28942587. Epub 2017/09/25. [DOI] [PubMed] [Google Scholar]
- 77.Sivan A., Corrales L., Hubert N., Williams J.B., Aquino-Michaels K., Earley Z.M. Commensal bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015;350(Nov (6264)):1084–1089. doi: 10.1126/science.aac4255. PubMed PMID: 26541606. PMCID: PMC4873287. Epub 2015/11/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sears C.L., Islam S., Saha A., Arjumand M., Alam N.H., Faruque A.S. Association of enterotoxigenic bacteroides fragilis infection with inflammatory diarrhea. Clin Infect Dis. 2008;47(Sep (6)):797–803. doi: 10.1086/591130. PubMed PMID: 18680416. PMCID: PMC3045827. Epub 2008/08/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ahn J., Sinha R., Pei Z., Dominianni C., Wu J., Shi J. Human gut microbiome and risk for colorectal cancer. J Natl Cancer Inst. 2013;105(Dec (24)):1907–1911. doi: 10.1093/jnci/djt300. PubMed PMID: 24316595. PMCID: PMC3866154. Epub 2013/12/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gao R., Kong C., Huang L., Li H., Qu X., Liu Z. Mucosa-associated microbiota signature in colorectal cancer. Eur J Clin Microbiol Infect Dis. 2017;36(Nov (11)):2073–2083. doi: 10.1007/s10096-017-3026-4. PubMed PMID: 28600626. [DOI] [PubMed] [Google Scholar]
- 81.Moschen A.R., Gerner R.R., Wang J., Klepsch V., Adolph T.E., Reider S.J. Lipocalin 2 protects from inflammation and tumorigenesis associated with gut microbiota alterations. Cell Host Microbe. 2016;19(Apr (4)):455–469. doi: 10.1016/j.chom.2016.03.007. PubMed PMID: 27078067. Epub 2016/04/15. [DOI] [PubMed] [Google Scholar]
- 82.Abdulamir A.S., Hafidh R.R., Abu Bakar F. The association of streptococcus bovis/gallolyticus with colorectal tumors: the nature and the underlying mechanisms of its etiological role. J Exp Clin Cancer Res. 2011;30(Jan):11. doi: 10.1186/1756-9966-30-11. PubMed PMID: 21247505. PMCID: PMC3032743. Epub 2011/01/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tsoi H., Chu E.S.H., Zhang X., Sheng J., Nakatsu G., Ng S.C. Peptostreptococcus anaerobius induces intracellular cholesterol biosynthesis in colon cells to induce proliferation and causes dysplasia in mice. Gastroenterology. 2017;152(May (6)):1419–1433. doi: 10.1053/j.gastro.2017.01.009. e5. PubMed PMID: 28126350. Epub 2017/01/28. [DOI] [PubMed] [Google Scholar]
- 84.Warren R.L., Freeman D.J., Pleasance S., Watson P., Moore R.A., Cochrane K. Co-occurrence of anaerobic bacteria in colorectal carcinomas. Microbiome. 2013;1(May (1)):16. doi: 10.1186/2049-2618-1-16. PubMed PMID: 24450771. PMCID: PMC3971631. Epub 2014/01/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gao R., Gao Z., Huang L., Qin H. Gut microbiota and colorectal cancer. Eur J Clin Microbiol Infect Dis. 2017;36(May (5)):757–769. doi: 10.1007/s10096-016-2881-8. PubMed PMID: 28063002. PMCID: PMC5395603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Veziant J., Gagniere J., Jouberton E., Bonnin V., Sauvanet P., Pezet D. Association of colorectal cancer with pathogenic Escherichia coli: focus on mechanisms using optical imaging. World J Clin Oncol. 2016;7(Jun (3)):293–301. doi: 10.5306/wjco.v7.i3.293. PubMed PMID: 27298769. PMCID: PMC4896897. Epub 2016/06/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.He Z., Gharaibeh R.Z., Newsome R.C., Pope J.L., Dougherty M.W., Tomkovich S. Campylobacter jejuni promotes colorectal tumorigenesis through the action of cytolethal distending toxin. Gut. 2019;68(Feb (2)):289–300. doi: 10.1136/gutjnl-2018-317200. PubMed PMID: 30377189. PMCID: PMC6352414. Epub 2018/11/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fox J.G., Ge Z., Whary M.T., Erdman S.E., Horwitz B.H. Helicobacter hepaticus infection in mice: models for understanding lower bowel inflammation and cancer. Mucosal Immunol. 2011;4(Jan (1)):22–30. doi: 10.1038/mi.2010.61. PubMed PMID: 20944559. PMCID: PMC3939708. Epub 2010/10/15. [DOI] [PMC free article] [PubMed] [Google Scholar]