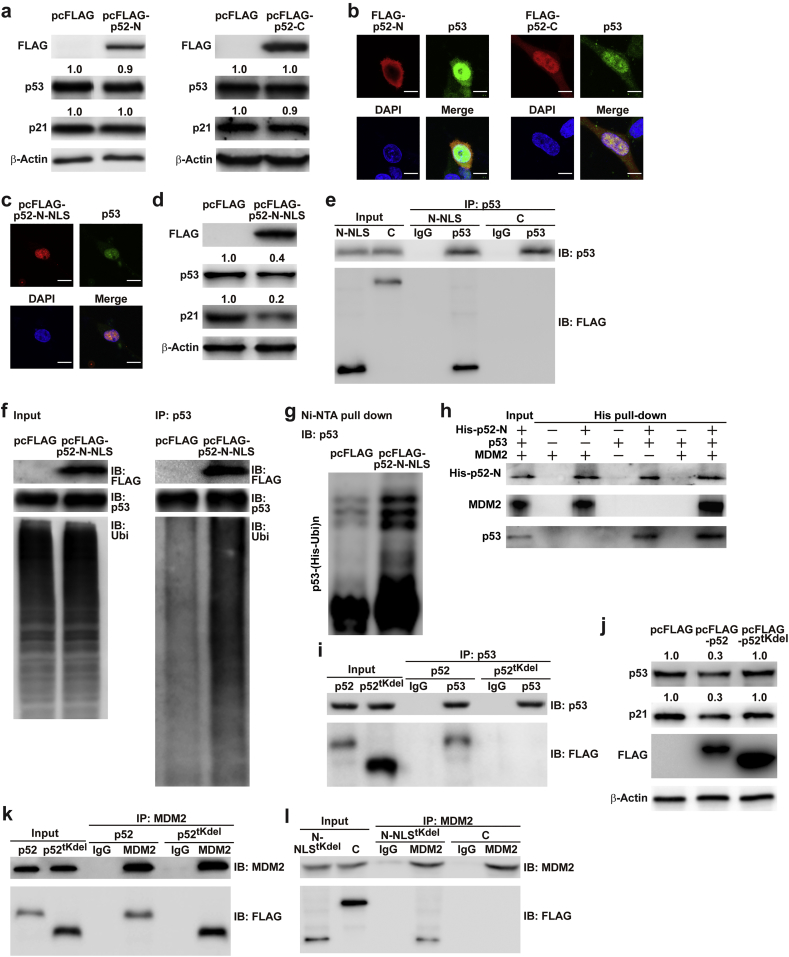
Fig. 6.
p52-ZER6 N-terminus binds and regulates MDM2/p53 complex integrity. (a) p53 protein expression level in HCT116 cells overexpressing N-terminus (left) or C-terminus (right) of p52-ZER6. (b–c) Co-localisation of p52-ZER6 N- or C-terminal fragments (FLAG-p52-N and FLAG-p52-C, respectively; b); or NLS-fused p52-ZER6 N-terminal fragment (FLAG-p52-N-NLS; c) and p53 in HCT116 cells, as determined by immunofluorescence staining. Scale bars, 10 μm. (d) p53 and p21 protein expression levels in HCT116 cells overexpressing FLAG-p52-N-NLS, as analysed using western blotting. (e) Physical interaction between p52-ZER6 fragments and p53 in HCT116 cells transfected with FLAG-p52-N-NLS or FLAG-p52-C overexpression vectors and pcp53, as determined by immunoprecipitation. Cell lysates were immunoprecipitated against IgG or anti-p53 antibody. The presence of p52-ZER6 fragments were detected by immunoblotting with anti-FLAG antibody. (f–g) p53 ubiquitination levels in HCT116 cells overexpressing FLAG-p52-N-NLS were analysed using anti-ubiquitin immunoblotting of cell lysates immunoprecipitated with anti-p53 antibody (f), or using in vivo ubiquitination assay conducted using Ni-NTA pull-down under denaturing condition followed by immunoblotting (g). Cells were treated with MG132 to inhibit proteasomal degradation. (h) Physical interactions between the N-terminus of p52-ZER6 (His-p52-N), MDM2 and p53, as determined by an in vitro His pull-down assay. (i) Physical interaction between p53 and p52-ZER6 without tKRAB domain (FLAG-p52tKdel) in HCT116 cells overexpressing p53 and FLAG-p52tKdel. Cell lysates were immunoprecipitated against anti-p53 antibody. The presence of p52-ZER6 fragments were detected by immunoblotting with anti-FLAG antibody. (j) p53 and p21 protein expression levels in HCT116 cells overexpressing FLAG-p52tKdel, as examined using western blotting. (k) Physical interaction between MDM2 and FLAG-p52tKdel in HCT116 cells. Cell lysates were immunoprecipitated against anti-MDM2 antibody. The presence of p52-ZER6 fragments were detected by immunoblotting with anti-FLAG antibody. (l) Physical interactions between MDM2 and FLAG-p52-C or NLS-fused N-terminal of p52-ZER6 lacking tKRAB domain (FLAG-N-p52-NLStKdel) in HCT116 cells. Cell lysates were immunoprecipitated against anti-MDM2 antibody. The presence of p52-ZER6 fragments was detected by immunoblotting with anti-FLAG antibody. For inputs of immunoprecipitation assay, 40 μg of corresponding samples were loaded. For input of His pull-down assay, 40 ng of corresponding sample was loaded. Ubi, ubiquitin; IP: immunoprecipitation; IB: immunoblotting. Cells transfected with pcFLAG were used as controls. β-Actin was used as western blotting loading control.