Abstract
Background
Identification of signaling pathways altered at early stages after cardiac ischemia/reperfusion (I/R) is crucial to develop timely therapies aimed at reducing I/R injury. The expression of G protein-coupled receptor kinase 2 (GRK2), a key signaling hub, is up-regulated in the long-term in patients and in experimental models of heart failure. However, whether GRK2 levels change at early time points following myocardial I/R and its functional impact during this period remain to be established.
Methods
We have investigated the temporal changes of GRK2 expression and their potential relationships with the cardioprotective AKT pathway in isolated rat hearts and porcine preclinical models of I/R.
Findings
Contrary to the maladaptive up-regulation of GRK2 reported at later times after myocardial infarction, successive GRK2 phosphorylation at specific sites during ischemia and early reperfusion elicits GRK2 degradation by the proteasome and calpains, respectively, thus keeping GRK2 levels low during early I/R in rat hearts. Concurrently, I/R promotes decay of the prolyl-isomerase Pin1, a positive regulator of AKT stability, and a marked loss of total AKT protein, resulting in an overall decreased activity of this pro-survival pathway. A similar pattern of concomitant down-modulation of GRK2/AKT/Pin1 protein levels in early I/R was observed in pig hearts. Calpain and proteasome inhibition prevents GRK2/Pin1/AKT degradation, restores bulk AKT pathway activity and attenuates myocardial I/R injury in isolated rat hearts.
Interpretation
Preventing transient degradation of GRK2 and AKT during early I/R might improve the potential of endogenous cardioprotection mechanisms and of conditioning strategies.
Keywords: GRK2, AKT, Ischemia-reperfusion, Cardioprotection, Pin1, Proteasome, Calpains
Abbreviations: GPCR, G-protein coupled receptor; GRK2, G-protein coupled receptor kinase 2; HF, heart failure; I/R, Ischemia/Reperfusion; MI, myocardial infarction; RISK, reperfusion injury salvage kinase pathway
Research in context.
Evidence before this study
Acute myocardial infarction (MI) is a major cause of death and disability. Prompt restoration of blood flow to the ischemic area is key for reducing infarct size and mortality, but can itself trigger additional myocardial damage, termed reperfusion injury. Despite advances in the protocols allowing rapid and effective reperfusion in MI patients, therapies adequately targeting reperfusion injury remain elusive. Therefore, it is crucial to gather information about the status of key cardiac signaling pathways at early stages after I/R to identify potential new targets for intervention. G protein-coupled receptor kinase 2 (GRK2) is a very relevant signaling hub in cardiac physiopathology, reported to be upregulated in the long-term in patients and in experimental models of heart failure. However, whether GRK2 protein levels are altered at early time points following myocardial I/R, the molecular mechanisms involved and the impact of such changes in GRK2-related signaling networks has not been fully investigated.
Added value of this study
We find that, contrary to the up-regulation taking place at later time points after MI, GRK2 levels are transiently reduced during ischemia and at the onset of reperfusion in rat and porcine preclinical models. GRK2 proteolysis is achieved by the combined action of proteasome and calpain protein degradation pathways, by mechanisms involving dynamic changes in the phosphorylation status of GRK2. Moreover, we suggest that such early GRK2 proteolysis would have an impact on the levels of the AKT kinase, a very important component of the cardioprotective pathways triggered in such pathological contexts. GRK2 proteolysis in early I/R would favor concurrent degradation of the functionally related Pin1 prolyl-isomerase and AKT proteins, leading to impaired overall AKT catalytic ability despite hyper-activation of remaining protein, resulting in reduced global capacity of this pathway to counteract cardiac injury. Importantly, preventing early GRK2/Pin1/AKT degradation by the combined administration of proteasome and calpain inhibitors can attenuate I/R-myocardial injury in isolated rat heart models.
Implications
The timely use of calpain and proteasome inhibition may help to reinforce therapeutic strategies aimed at reducing I/R injury.
Alt-text: Unlabelled Box
1. Introduction
Acute myocardial infarction (MI) as a result of coronary artery occlusion is a major cause of death and disability [1,2]. Prompt restoration of blood flow by means of thrombolytic or primary percutaneous coronary intervention (PPCI) is key for reducing infarct size and mortality. However, the process of restoring blood flow can itself trigger additional myocardial damage (termed reperfusion injury), as a result of abrupt changes in pH and calcium homeostasis, alterations in metabolic and inflammatory mediators and sudden burst of reactive oxygen species. These changes can promote ventricular arrhythmiasmicrovascular obstruction, myocardial stunning or myocardial death, leading to increased prevalence of chronic heart failure (HF) in surviving patients [1,[3], [4], [5], [6]].
Whereas the development of systems of care for emergent myocardial reperfusion and advances in PPCI technology are increasingly allowing rapid and effective reperfusion in MI patients, therapies adequately targeting reperfusion injury remain elusive and constitute one of the top unmet needs in cardiology [2,7,8]. Brief cycles of myocardial I/R applied either before (ischemic preconditioning) a prolonged coronary artery occlusion plus reperfusion event or immediately after reflow (ischemic post-conditioning) in experimental models can reduce infarct size by activating different combinations of endogenous cardioprotective pathways [1,2,4,9]. Activation of kinases such as AKT and ERK1/2, important components of the so-called reperfusion injury salvage kinase pathway (RISK) pathway, plays a key cardioprotective role, and modulation of this pathway by endogenous mediators or pharmacological agents has been postulated as a relevant strategy to prevent I/R injury [1,4,10,11]. Given the limited efficacy of these current therapeutic strategies, it is crucial to gather information about the status of key cardiac signaling pathways at early stages after I/R [6] to identify potential new targets for intervention.
G protein-coupled receptor kinase 2 (GRK2) is a central regulator of beta–adrenergic and many other G protein-coupled receptors (GPCRs) involved in cardiovascular physiopathology. In addition, data in different cell types put forward GRK2 as a key regulatory node in non-GPCR pathways via the modulation of insulin and growth factor signaling, the PI3K/AKT route, MAPK cascades, NO bioavailability or mitochondrial function, which are also instrumental in cardiac function and dysfunction (reviewed in [[12], [13], [14], [15], [16], [17]]. In particular, myocardial GRK2 has been reported to participate in apoptotic pathways after I/R by mechanisms involving its mitochondrial targeting [18,19]. Maladaptive increased GRK2 expression has been described in the failing human heart of patients and in experimental models of HF due to chronic hypertensive or ischemic disease, and its genetic ablation or inhibition has been reported to be cardioprotective in the long-term in animal models by reducing adverse post-infarction remodeling, purportedly as a result of the integrated actions of GRK2 in myocardial contractile function and cardiac metabolism (reviewed in [15,[20], [21], [22], [23]]. However, whether GRK2 protein levels are altered at early time points following myocardial I/R, the molecular mechanisms involved and the impact of such changes in GRK2-related signaling networks has not been fully investigated.
We report that, contrary to the up-regulation reported at later time points after MI, GRK2 levels are transiently reduced during ischemia and at the onset of reperfusion in preclinical models, due to the combined action of proteasome and calpain pathways, by mechanisms involving dynamic changes in the phosphorylation status of GRK2. Our data suggest that such early GRK2 proteolysis would favor concurrent degradation of the functionally related Pin1 prolyl-isomerase and AKT proteins, thus impairing overall AKT functionality and cardioprotection, whereas the combined administration of proteasome and calpain inhibitors prevents early GRK2/Pin1/AKT degradation and counteracts I/R myocardial injury.
2. Materials and methods
2.1. Experimental protocol of ischemia/reperfusion in rats
The experimental procedures conformed to the Guide for the Care and Use of Laboratory Animals published by the United States National Institute of Health (NIH Publication Eighth Edition, 2011) and were approved by the Research Commission on Ethics of the Hospital Vall d'Hebron. Male Sprague-Dawley rats (Charles River,Barcelona, Spain) weighing 250 to 300 g were anaesthetized with sodium pentobarbital (100 mg/kg). Hearts were removed, mounted onto a Langendorff apparatus, and perfused with a modified Krebs-Henseleit bicarbonate buffer as previously described. Hearts were perfused normoxically for 60 min (Nx group) or for 20 min and then subjected to 40 min of global ischemia followed by reperfusion of different duration (I/R group). Ischemic preconditioning protocol consisted of two cycles of 5 min of ischemia and 5 min of reperfusion applied before index ischemia (PreCo group). Ischemic postconditioning was achieved with a previously established protocol commenced immediately after ischemia consisting of 6 cycles of 10-second reperfusion-10-second occlusion (PosCo group) [[24], [25], [26]]. In additional groups of hearts, the membrane-permeable calpain inhibitor SNJ-1945 (Senju Pharmaceutical Co,Ltd) at 10 μM [27], the proteosome inhibitor bortezomib (Cell Signaling Technology) at 1 μM and 10 μM, the PI3K inhibitor LY-294002 (10 μM, Sigma-Aldrich), or the indicated combinations of SNJ-1945; LY-294002 and 10 μM bortezomib or their vehicle (0.01% DMSO) were added to the perfusion media during the 10 min prior to 40 min of ischemia and the first 10 min of reperfusion.
The selective contractile inhibitor blebbistatin (10 μM, Merck) was added during the first 5 min of reperfusion in all the experiments involving ischemia/reperfusion, except in those experiments specifically aimed to assess cell death and infarct size. Blebbistatin prevents cell death by reducing the mechanical stress caused by the excessive and irreversible contractile activation occurring at the onset of reperfusion. It was used to discard the possibility that any variation on the measured parameters was a mere consequence of differences in cell death associated with sarcolemmal disruption [26].
2.2. Experimental protocol of ischemia/reperfusion in pigs
Transient myocardial ischemia in pigs was performed as previously described [28]. Farm pigs (25–30 kg) were pre-medicated with tiletamine–zolazepam (4–6 mg/kg, IM) and xylazine (1–2 mg/kg, IM), anaesthetised with propofol-lipuro 1% (1.5–2.5 mg/kg, IV, followed by continuous infusion at 11 mg/kg/h) and fentanyl (5 mg/kg, IV, followed by continuous infusion at 3–6 mg/kg/h), and mechanically ventilated. A mid-sternotomy was performed, and the left anterior descending (LAD) coronary artery was dissected free at its midpoint. Lead II of ECG, left ventricular (LV) pressure and LV dP/dt, coronary bloodflow, and regional myocardial function were continuously recorded. LAD coronary artery was occluded for 40 min followed by 5 min or 120 min of reperfusion. At the end of the experiment, animals were sacrificed by a pentobarbital overdose (100 mg/kg, IV) and myocardial tissue samples from both, the control region and the area at risk were quickly excised and frozen in liquid nitrogen.
2.3. Myocardial tissue processing
Myocardial rat or porcine tissue was minced and homogenized in 4 vol (v:w) 20 mm Tris-HCl (pH 7.5), 5 mm EDTA, 5 mm EGTA, and protease inhibitors. The homogenate was centrifuged (2000 ×g, 5 min, 4 °C) to obtain a clarified post-nuclear supernatant designated as crude cytoplasmic fraction (SB1). The pellet was rinsed in the same buffer (1/5 of the initial volume) supplemented with 0.5% NP40, extensively vortexed and centrifuged (850 ×g, 10 min, 4 °C) after 15 min ice-cold incubation to obtain a NP40-extracted nuclear fraction (SB2). For determination of GRK2 levels in some conditions, crude cytoplasmic fractions were previously enriched in GRK2 protein by incubating these fractions in the presence of 200–250 mmol/L NaCl, followed by centrifugation at 250,000 ×g for 60 min and desalting by using Amicon Ultra-0.5 Centrifugal Filter Devices according to the manufacturer's instructions. For detection of redox states of PKA RIα, myocardial samples were homogenized using a Polytron grinder with a hypotonic buffer as above but supplemented with 100 mM maleimide, and protein lysates electrophoresed with non-reducing SDS sample buffer (100 mM Tris HCl pH 6.8, 4% SDS, 20% glycerol, bromophenol blue with 100 mM maleimide) or reducing SDS sample buffer (i.e. supplemented with 5% β-mercaptoethanol).
2.4. Western blot and dot blot analysis
Proteins were separated by SDS-PAGE for Western blot analysis or applied directly on nitrocellulose membranes in a single spot for immunodetection (dot-blot) as previously described [29]. Protein bands were detected by the chemiluminescence method (ECL, Amersham Pharmacia Biotech) or with the Odyssey Imaging Systems (LI-COR Biosciences). Bands were quantified by laser-scanner densitometry with a Biorad GS-700 scanner or by the software included in the Odyssey Infrared Imaging System. The rabbit polyclonal antibodies raised against phosho-Ser/Thr-PKA substrate, phosho-Ser/Thr-AKT substrate, pSer473-AKT and AKT and the mouse monoclonal anti-Acetylated-Lysine antibody were all from Cell Signaling Technologies. Rabbit primary antibodies raised against ERK1/2, Lamin B1 and PKA catalytic subunit were from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA). Mouse anti-PKA RI-subunit was from BD Transduction Laboratories. Anti-Pin1 rabbit polyclonal and GAPDH mouse monoclonal (6C5) antibodies were provided by Upstate Biotechnology and Abcam, respectively. Anti-pSer670-GRK2 and anti-pSer685-GRK2 polyclonal antibodies were from Biosource International and from Immunoway. Anti-acetyl-K40-tubulin and anti-tubulin antibodies were purchased from SIGMA. GRK2 protein was immunodetected with a rabbit polyclonal antibody Ab C-15 (sc-562) (Santa Cruz). Vertical dotted lines in western blots indicate juxtaposed lanes that come from the same gel but were non-adjacent.
2.5. Determination of Pin1 acetylation
Myocardial tissue was lysed in immunoprecipitation buffer (50 mM HEPES pH 7.5, 150 mM NaCl, 1%Triton, 10% Glycerol, 10 mM NaF,1 mM sodium orthovanadate, plus protease inhibitors). Upon centrifugation (15,000 ×g, 10 min), supernatants were incubated with a specific anti-Pin1 polyclonal antibody (Upstate Biotechnology or Millipore). Immune complexes were resolved in 15% SDS/PAGE and transferred to nitrocellulose membranes. After incubation with antibody anti-Acetyl lysine (Clone 4G12, Millipore), blots were stripped and reproved with a polyclonal antibodies directed against the immunoprecipitated Pin1 [30]. The amount of Pin1 acetylation was normalized to the amount of the immunoprecipitated protein, and data were represented as the fold over control normoxic conditions [29,30].
2.6. Cell culture and transfection and protein degradation assays
HEK293 were obtained from the American Type Culture Collection (ATCC) and maintained in DMEM supplemented with 10% (v/v) foetal bovine serum (FBS) at 37 °C in a humidified 5% CO2 atmosphere. Transient transfections with the indicated combinations of cDNA were performed using the Lipofectamine/Plus method, following manufacturer's instructions.
Metabolic labelling and pulse-chase experiments were performed as described [31]. HEK293 cells previously co-transfected with PKA catalytic subunit or empty vector and GRK2wt, GRK2-K220R or GRK2-S685A constructs were labeled by keeping the cells for 2 h in methionine and cysteine-free Dulbecco's modified Eagle's medium (DMEM) and then incubated for 15–30 min in DMEM supplemented with 250 μCi/ml of [35S]methionine and [35S]cysteine labeling mixture (NEN Life Science Products).The plates were washed with phosphate-buffered saline and chased for the indicated times in DMEM plus 10% fetal bovine serum. The proteolysis inhibitors lactacystin (Calbiochem, La Jolla, CA), ALLN (Sigma), or PD150606 (Calbiochem), the PKA activator Forskolin (Sigma) or the PKA inhibitor PKI (Calbiochem) were added 90 min before metabolic labeling and maintained during the chase periods. At different chase times (1−3h), cells were harvested using radioimmune precipitation assay lysis buffer (20 mm Tris-HCl, pH 7.5, 150 mm NaCl, 1% sodium deoxycholate, 0.5% Nonidet P-40, 0.1% SDS, with a mixture of protease inhibitors). GRK2 protein was immunoprecipitated with a specific rabbit polyclonal antibody AbFP1 previously validated [32]. Immunoprecipitates were resolved by SDS/PAGE and transferred to PVDF membranes to be treated with the Enhancer Autoradiography Starter Kit (EABiotech Ltd.) according to the manufacturer's protocol. Band density of 35S-labeled GRK2 was quantified by laser densitometry analysis and data were corrected according to total GRK2 protein detected by immunoblotting [32].
2.7. Quantification of cell death
Lactate dehydrogenase (LDH) activity was spectrophotometrically measured in the coronary effluent throughout the reperfusion period. After 60 min of reperfusion, heart slices were incubated at 37 °C for 10 min in 1% 2,3,5-triphenyltetrazolium chloride and imaged under white light to outline the area of necrosis as previously described [25]. Previous studies demonstrated that in the Langendorff rat model, reperfusion for 60 min is sufficient for acute assessment of infarct size [26,33].
2.8. Statistical analysis
Data analysis was performed using GraphPad Prism for Windows. Means between groups were compared by 2-way or one-way ANOVA with Bonferroni's or Tukey's post hoc test, respectively, or with unpaired Student's t-test as indicated in the Figure legends. All results are expressed as mean ± SEM.
3. Results
3.1. GRK2 is degraded in ischemia and reperfusion conditions by different mechanisms
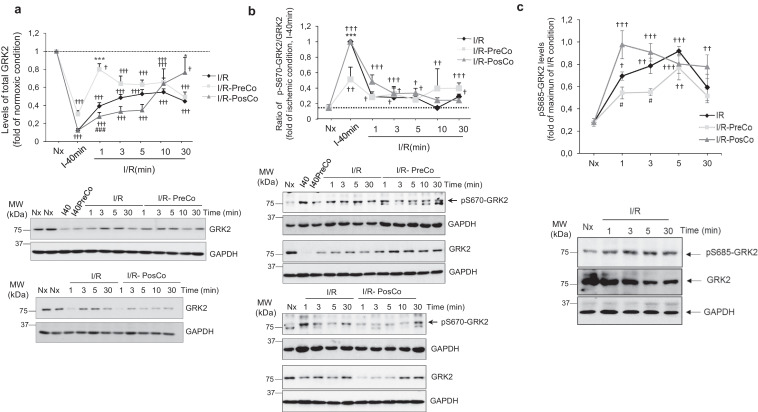
Potential changes in GRK2 levels were analyzed during myocardial ischemia and the early time points following reperfusion, by using a well-established experimental model of cardiac ischemia/reperfusion (IR) in isolated rat hearts [24,25,34]. Notably, 40 min of ischemia promoted a marked decay in cardiac GRK2 protein levels compared to control conditions, and GRK2 down-regulation was maintained after 30 min of reperfusion, when a circa 50% decrease compared to normoxic GRK2 expression was noted (Fig. 1A). Neither pre- nor post-conditioning conditions, performed as described previously [24,25], were able to prevent the ischemia-promoted GRK2 down-regulation. These treatments did not fully restore GRK2 protein levels during the 30 min of reperfusion, although slightly higher kinase levels at early (preconditioning) or later (post-conditioning) reperfusion times were observed. No overt changes in the subcellular distribution of the kinase were observed in either I or in I/R experimental conditions (Fig. S1), suggesting that the rapid down-modulation of GRK2 levels was related to protein degradation.
Fig. 1.
Ischemia, reperfusion and conditioning treatments trigger marked and dynamic changes in cardiac GRK2 protein levels and phosphorylation status. Isolated rat hearts were exposed to 40 min of ischemia alone 40or with preconditioning I40PreCo(I) followed by reperfusion for the times indicated (I/R) in the presence or absence of preconditioning (I/R-PreCo) or post-conditioning (I/R-PosCo) interventions as detailed in Methods. Control group (Nx, normoxia condition) was exposed to60 min of continuous perfusion without ischemia. (A) GRK2 protein levels were analyzed by western-blot with a specific total anti-GRK2 antibody as detailed in Methods. GAPDH expression was used as loading control. (B–C) The extent of GRK2 phosphorylation on different residues (Ser670 or Ser685) was monitored in cardiac lysates by western-blot using phosphosite-specific antibodies. Data were normalized to total GRK2 levels GAPDH expression was used as loading control. In all panels, data are mean ± SEM, n = 3–4 rats per condition. In all panels, data were analyzed by comparing the different experimental situations (I/R, I/R-PreCo and I/R-PosCo) to the normoxic condition [2-way ANOVA followed by Bonferroni's post-hoc test, †p < .05; ††p < .01, †††p < .001]. In addition, we compared I/R pre-Co and I/R post-Co conditions versus I/R alone (*p < .05; ***p < .001) or between conditioning situations (#p < .05; ###p < .001) [1-way ANOVA and Tukey's post hoc test]. Representative blots are shown.
GRK2 can be rapidly degraded by the proteasome, in a process dependent on its previous phosphorylation at the S670 residue [35,36]. Interestingly, a dramatic increase of GRK2 S670-phosphorylation status was noted in ischemic conditions (Fig. 1B), a pattern consistent with the involvement of the S670-GRK2 phosphorylation/proteasome pathway in kinase downmodulation during this period. However, S670 phosphorylation rapidly returned to normoxic levels upon reperfusion in all conditions tested, suggesting that additional mechanisms were involved in maintaining GRK2 protein levels low during the initial phase of reperfusion.
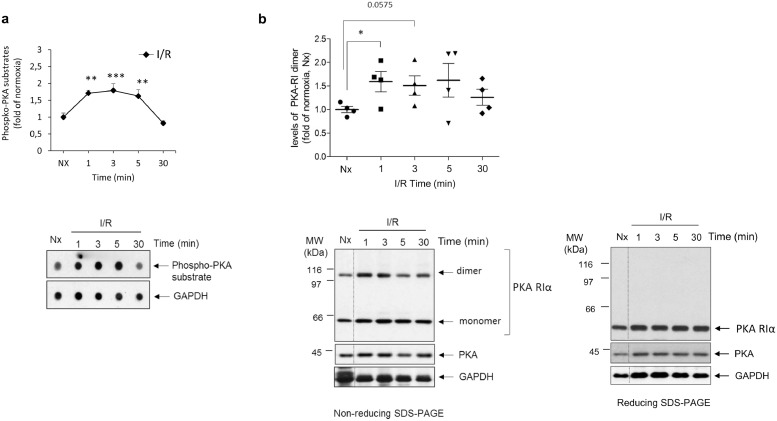
We searched for other regulatory post-translational modifications during this period. GRK2 is phosphorylated by PKA on S685, a residue highly conserved across species, leading to increased binding to Gβγ subunits and subsequent translocation to the plasma membrane [37]. We uncovered a rapid (1 min) and transient (peak at 5 min) increase of GRK2 phosphorylation at S685 upon reperfusion (Fig. 1C). A similar pattern was observed under pre- or post-conditioning conditions, although the extent of S685 phosphorylation was slightly but significantly decreased (pre-conditioning) or enhanced (post-conditioning) at early reperfusion times. The pattern of phospho-S685 GRK2 during early reperfusion trailed the rapid and transient increase in total PKA activity detected in these cardiac samples (Fig. 2A), consistent with previous reports of PKA activation during I/R as a result of GPCR stimulation or oxidative stress [38,39]. Oxidation-dependent formation of a disulfide bond between the RIα subunit of PKA leading to dimerization can promote PKA activation independently of cAMP [39]. Notably, the transient changes in global PKA activity in reperfused isolated rat heart were parallel to those observed in the dimerization status of PKA-RIα (Fig. 2B), suggesting that this mechanism of PKA stimulation might play a relevant role in such early reperfusion contexts.
Fig. 2.
Cardiac ischemia/reperfusion induces rapid and transient oxidation-dependent activation of PKA. (A) Global PKA activity was assessed in lysates from normoxic or reperfused isolated rat hearts (40 min of sustained ischemia followed by the indicated reperfusion times) by dot-blot, using a pan-specific phospho-substrate antibody that broadly detects phosphorylated proteins by PKA as indicated in Methods. GAPDH expression was used as loading control. Data are mean ± SEM, n = 3–4 rats per condition. **p < .01; ***p < .001 compared to I/R 30 min [1-way ANOVA and Tukey's post hoc test]. A representative dot-blot is shown. (B) I/R promotes oxidation-related dimerization of the regulatory PKA-RIα subunit in parallel to global PKA activation. Lysates as in panel A were probed with a specific PKA-RI antibody that recognizes both monomers and dimers of this regulatory subunit linked by disulfide bond formation. The dimer formation detected in non-reducing SDS-PAGE resolving conditions was fully reversed in the presence of 2-mercaptoethanol (reducing SDS-PAGE). The expression of the PKA catalytic subunit and of GAPDH was used as loading controls. Data are mean ± SEM, n = 3–4 rats per condition. *p < .05 for the indicated comparisons [Student's t-test]. Representative blots are shown.
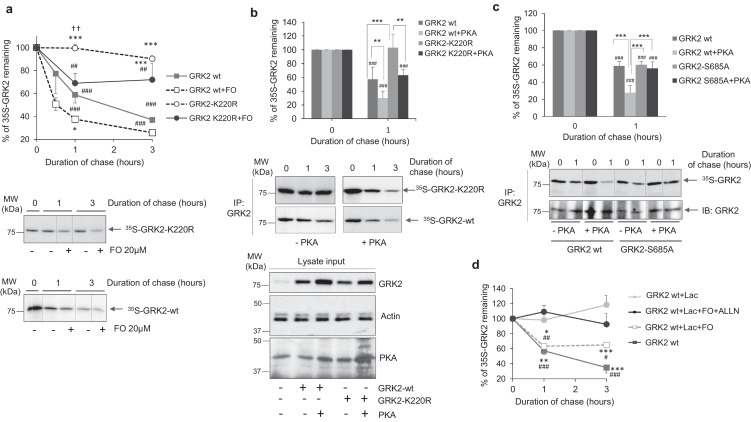
The pattern of total and phospho-S685 GRK2 levels during reperfusion suggested that this phosphorylation might help to keep GRK2 downregulated. Pulse-chase experiments in HEK-293 cells, a well-established model for characterizing GRK2 degradation mechanisms [20,35,40], indicated that forskolin (FO), a direct stimulator of adenylyl cyclase activity, and thus of the PKA pathway, increased the basal turnover of GRK2 (Fig. 3A), as well as triggered phosphorylation of GRK2 on S685 (Fig. S2). Interestingly, FO also enhanced the degradation of the catalytically-inactive GRK2-K220R mutant (Fig. 3A), previously reported to be impaired in GPCR-induced proteasome-mediated degradation [41]. This suggested that the GRK2-destabilizing effect of FO involved alternative proteolytic pathways.
Fig. 3.
PKA-mediated phosphorylation stimulates GRK2 degradation in a calpain-dependent manner. (A) Activation of endogenous PKA increases the turnover of both wild-type GRK2 (GRK2-wt) andof the catalytically inactive GRK2-K220R mutant. HEK-293 cells were transiently transfected with GRK2 or GRK2-K220R constructs and the turnover of these proteins in the presence or absence of forskolin (FO, 20 μM), a stimulator of the PKA pathway, was assessed by metabolic labeling with 35S followed by GRK2 immunoprecipitation with a specific antibody and fluorography detection of 35S-labeled GRK2 (35S-GRK2) as described in Methods. (B) GRK2 degradation is enhanced in the presence of the constitutively active PKA catalytic subunit. HEK-293 cells were co-transfected with the indicated constructs and GRK2 turnover monitored as above. (C) GRK2 phosphorylation on S685 is required for PKA-induced downregulation. HEK-293 cells were transiently transfected with GRK2-wt or the PKA phosphorylation-defective site mutant GRK2-S685A and their stability in the presence or absence of the active catalytic subunit of PKA assessed as in previous panels. Immunoprecipitated GRK2 was assessed by fluorography and by western blot as a control (below). (D) PKA-triggered GRK2 degradation is abrogated upon addition of calpain inhibitors. The stability of GRK2 expressed in HEK-293 cells was investigated in the presence of the indicated combinations of the forskolin (FO, 20 μM), the proteasome inhibitor lactacystin Lac, (5 μM) and the broad-spectrum calpain protease inhibitor N-Acetyl-Leu-Leu-norleucinal (ALLN, 5 μM). In all panels data are the mean ± SEM of at least 3 independent experiments performed in duplicate. Panel A, ***p < .001 compared to untreated GRK2-wt overexpressing cells<, ##p < .01, ### p < .001compared to 0 h, and ††p < .01 comparing GRK2-K220R degradation in the absence or presence of FO. Panels B–C, **p < .01, ***p < .001 for the indicated comparisons and ## p < .01, ### p < .001 compared to 0 h. Panel D,*p < .05; **p < .01, ***p < .001 comparing to GRK2-wt plus lactacystin condition, and # p < .05, ### p < .001 compared to 0 h [One-way ANOVA, Tukey post hoc analysis]. Representative gel fluorographies are shown.
It is worth noting that the fostering effect of FO in GRK2 degradation was fully prevented upon addition of a PKA inhibitor (Fig. S2B), whereas over-expression of the catalytic subunit of PKA enhanced the decay of both wild-type GRK2 and the K220R mutant (Fig. 3B), confirming the involvement of PKA downstream of FO in the modulation of GRK2 stability. Moreover, a GRK2-S685A mutant (unable to be phosphorylated by PKA) displayed a stability similar to WT GRK2 in basal conditions, but lacked the PKA-stimulated component of GRK2 degradation, demonstrating that phosphorylation on S685 was required for PKA activity-induced GRK2 downregulation (Fig. 3C).
Consistent with the involvement of alternative PKA-dependent proteolytic pathways, the proteasome inhibitor lactacystin fully inhibited the basal degradation of wt GRK2, whereas only partially attenuated GRK2 proteolysis in the presence of FO stimulation (Fig. 3D). On the other hand, PKA-triggered GRK2 degradation was completely abrogated upon addition of broad spectrum (ALLN, Fig. 3D) or specific (PD150606, Fig. S2C) inhibitors of calpains, a family of Ca2 + -dependent cysteine proteases reportedly over-activated in myocardial reperfusion, leading to myocardial injury through the proteolysis of different proteins [[42], [43], [44]], suggesting that calpain-mediated proteolyisis of phospho-S685-GRK2 might take place in early I/R conditions.
Overall, our data suggested that dynamic and sequential phosphorylation of GRK2 at S670 and S685 during ischemia and in early reperfusion would trigger GRK2 degradation by the proteasome and via calpains, respectively, thus maintaining low GRK2 protein levels during such initial period after heart damage.
3.2. AKT degradation takes place in early I/R resulting in decreased overall activity of this pathway
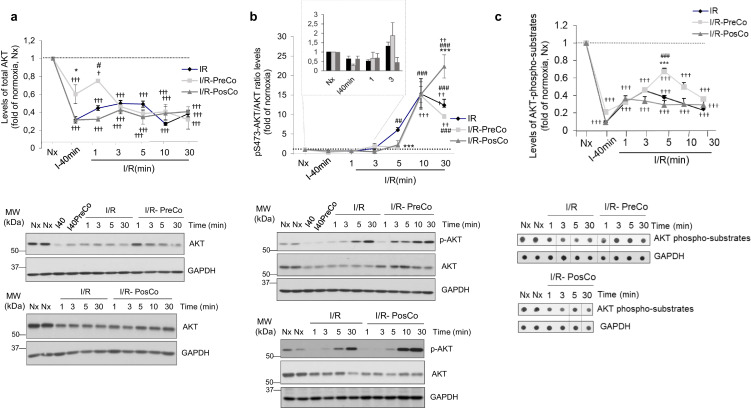
We investigated whether such transient GRK2 downmodulation was related to changes in other key cardiac signaling pathways. Total ERK1/2 protein levels were not significantly altered during I/R or upon pre- or post-conditioning (Fig. S3A), indicating that I/R does not promote a general decay in signaling proteins. Interestingly however, total AKT protein levels were markedly diminished by ischemia and maintained low during the first 30 min of reperfusion (Fig. 4A), with a pattern similar to that detected for GRK2. Moreover, AKT and GRK2 protein levels displayed a similar trend of changes upon pre- conditioning, which led to a less marked AKT decrease after ischemia and at very early reperfusion times (Fig. 4A). As for GRK2, no manifest changes in the nuclear/cytoplasmic distribution of AKT were observed in either I or in I/R experimental conditions (Fig. S3B), suggesting the occurrence of protein degradation processes.
Fig. 4.
Ischemia/reperfusion promotes a marked loss of total AKT protein and an overall decrease in global AKT-mediated substrate phosphorylation, despite the robust stimulation status of the remaining AKT protein. Rat heart lysates as in Fig. 1 were analyzed with specific antibodies for total AKT (A) or for AKT phosphorylated at Ser473 (B) as detailed in Methods. Data were normalized by GAPDH (panel A) or total AKT (panel B) loading. (C) Global activity of AKT towards its substrates was assessed in cardiac lysates from the indicated conditions by dot-blot, using a pan phospho-AKT substrate-specific antibody, and data normalized by GAPDH loading. In all panels, normalized data were represented as fold-change with respect to normoxic situation and are mean ± SEM, n = 3–4 rats per condition. Data were analyzed by comparing the different experimental situations (I/R, I/R-PreCo and I/R-PosCo) to the normoxic condition (2-way ANOVA followed by Bonferroni's post-hoc test, †p < .05; ††p < .01, †††p < .001). In addition, we compared I/R pre-Co and I/R post-Co conditions versus I/R alone (*p < .05; ***p < .001) or between conditioning situations (#p < .05; ##p < .01, ###p < .001) [1-way ANOVA and Tukey's post hoc test]. Representative blots are shown.
Such pattern of decreased AKT protein levels during I/R was puzzling, since the cardio-protective and pro-survival AKT pathway is considered to be robustly enhanced in these conditions to counterbalance I/R-promoted injury, as inferred from enhanced AKT phosphorylation at activating residues [1,4,11]. In our experimental setting, we also detected such marked increase of stimulated (phosphor-S473) AKT during reperfusion and upon pre- or post-conditioning conditions (Fig. 4B). However, the concurrent alteration in total AKT protein levels appears to have been disregarded or not addressed in detail in other studies. Our data point to a more complex scenario in which I/R triggers both AKT protein downmodulation and activation of the remaining kinase. Notably, when the “global” or bulk AKT activity was tested in cardiac extracts using a pan-AKT substrate antibody, we observed a marked decrease throughout the I/R period considered (Fig. 4C), with a similar pattern upon post- or pre-conditioning, although the later condition allowed slightly higher substrate phosphorylation at 5 min after reperfusion. Our data indicated that hyper-activation of the remaining AKT during reperfusion might notbe able to fully compensate for the decay in protein levels, leading to a clear overall downmodulation of the “catalytic potency” of this key cardioprotective pathway in early I/R contexts, what would counteract the beneficial effects of treatments specifically aimed at enhancing the AKT activation status.
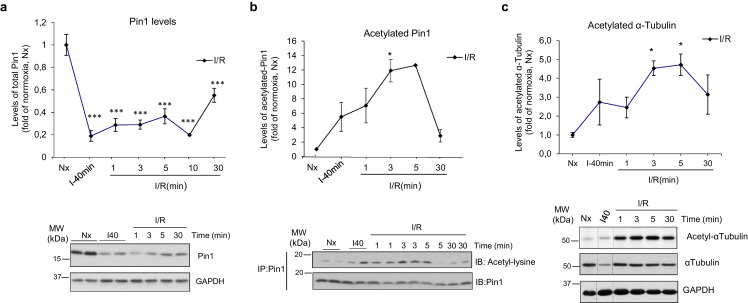
In search of molecular mechanisms underlying AKT protein decay and potentially connecting transient GRK2 and AKT downmodulation, we focused on the prolyl-isomerase Pin1, a central player in the control of folding, activity, and stability of proteins [45,46]. Pin1 is a critical positive factor in the regulation of AKT stability in cancer cells by binding to activated AKT and preserving the protein from degradation by the proteasome [46,47]. Pin1 has also been described as a relevant factor in cardiac hypertrophy, directly binding to AKT and MEK and fostering hypertrophic signaling [48]. Interestingly, we found a clear reduction of Pin1 levels after ischemia and during early reperfusion times (Fig. 5A), with a pattern resembling that of AKT protein, suggesting a link between Pin1 loss and decreased AKT stability. A similar pattern of Pin1 changes was evident in nuclear and cytoplasmic cellular fractions, consistent with Pin1 degradation events (Fig. S3C).
Fig. 5.
Ischemia/reperfusion alters the protein levels and the acetylation status of the prolyl-isomerase Pin1. (A) Total content of Pin1 protein was analyzed with a specific anti-Pin1 antibody in the indicated conditions. GAPDH expression was used as loading control (B). The acetylation status of Pin1 in the same experimental conditions was determined with a pan anti-Acetyl lysine antibody after immunoprecipitation of total Pin1 from myocardial lysates as detailed in Methods. The amount of Pin1 acetylation was normalized to the amount of the immunoprecipitated protein. (C) Ischemia-reperfusion causes strong hyper-acetylation of myocardial α-tubulin. The levels of acetylated and total α-tubulin in the indicated conditions were analyzed with site-specific anti-acetyl-K40-tubulin and total anti-tubulin antibodies. Total α-tubulin and GAPDH served as loading controls. In all panels, normalized data were represented as fold-change with respect to the normoxic situation, and are the mean ± SEM, n = 3–4 rats per condition. *p < .05, ***p < .001 compared to normoxia [1-way ANOVA and Tukey's post hoc test]. Representative blots are shown.
Interestingly, GRK2 is a regulator of Pin1 stability and functionality in other cell types [30]. GRK2 activates HDAC6, a cytoplasmic lysine deacetylase targeting tubulin and many other proteins, thus triggering de-acetylation of Pin1 at a specific residue, therefore favoring Pin1 protein stabilization and stimulation of prolyl-isomerase activity [30,49]. We thus assessed whether Pin1 acetylation might be triggered upon GRK2 downregulation during I/R. This treatment induced a marked increase in Pin1 protein acetylation, which peaked at 5 min of reperfusion (Fig. 5B). A similar pattern was detected for the acetylation levels of the canonical HDAC6 substrate tubulin (Fig. 5C), strongly suggesting an impaired HDAC6 activation by GRK2 in I/R conditions.
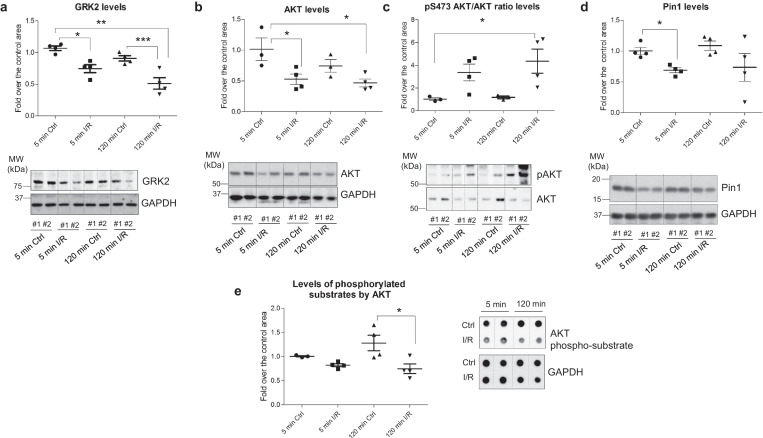
Importantly, a comparable pattern of concurrent down-modulation of GRK2, AKT, and Pin1 protein levels in early stages of myocardial reperfusion was also detected in porcine hearts subjected to in situ transient ischemia. The levels of these proteins in the area at risk were significantly decreased at 5 min of reperfusion with respect to control area of the same animal (Fig. 6A–D). After 120 min of reperfusion, total AKT and Pin1 protein expression values were still slightly lower than those of the matched control area, whereas a marked GRK2 down-modulation remained. In line with the mechanistic role of PKA in promoting GRK2 down-modulation observed in the rat model, increased levels of PKA-phosphorylated substrates were detected at 120 min of reperfusion (Fig. S4). Of note, although an enhanced AKT phosphorylation status was apparent at these I/R stages (Fig. 6C) as previously reported in such conditions [50,51], the global or bulk capacity of active AKT to phosphorylate its targets was markedly reduced (Fig. 6E), akin to the observed decline in total AKT protein levels and in line with our data in the isolated rat heart model (see Fig. 4). Although whether such early and dynamic changes in GRK2/AKT/Pin1 levels take place in patients during cardiac I/R cannot be directly investigated, the data obtained in this relevant translational large animal model supports the potential clinical relevance of these alterations.
Fig. 6.
Concurrent down-modulation of GRK2, AKT, and Pin1 protein levels in early stages of reperfusion in porcine hearts. Samples from non-ischemic (controls) or from the ischemic area at risk of lysates obtained from porcine hearts after 40 min of ischemia and 5 or 120 min of reperfusion as detailed in Methods were analyzed with specific antibodies for GRK2 (A), total AKT (B) AKT phosphorylated at Ser473 (C) or total Pin1 levels (D). Data were normalized by GAPDH (panels A, B, D) or total AKT (panel C) loading. (E) Global activity of AKT towards its substrates was assessed in pig cardiac lysates from the indicated conditions by dot-blot, using a pan phospho-AKT substrate-specific antibody, and data normalized by GAPDH loading. In all panels, normalized data were represented as fold-change with respect to control situation (mean value of myocardial control area at 5 min of I/R) and are mean ± SEM, n = 3–5 pigs per condition. *p < .05; **p < .01, ***p < .001 compared with the conditions indicated with the lines [1-way ANOVA and Tukey's post hoc test]. Representative blots are shown.
3.3. Combined use of proteasome and calpain inhibitors prevents GRK2/Pin1/AKT degradation and attenuates I/R myocardial injury
Overall, our data suggested a complex interconnection among GRK2, Pin1 and AKT in early cardiac I/R. Changes in the phosphorylation status of GRK2 would trigger transient reduction of kinase levels by the combined action of proteasome and calpain pathways. GRK2 degradation would favor Pin1 proteolysis by altering its acetylation status, which in turn would facilitate AKT degradation, leading to impaired overall AKT catalytic ability despite hyper-activation of remaining protein, resulting in reduced global capacity to counteract cardiac injury.
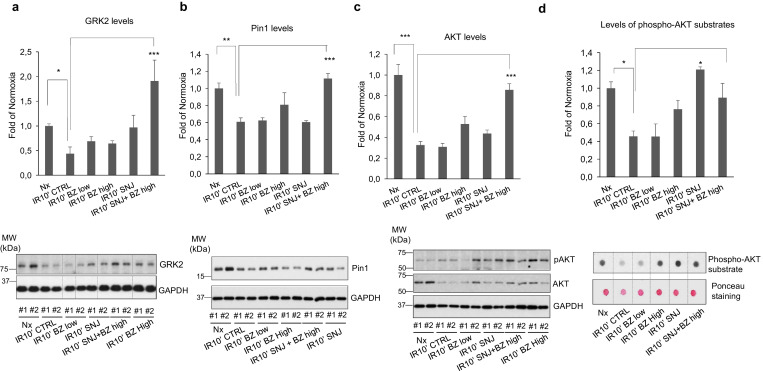
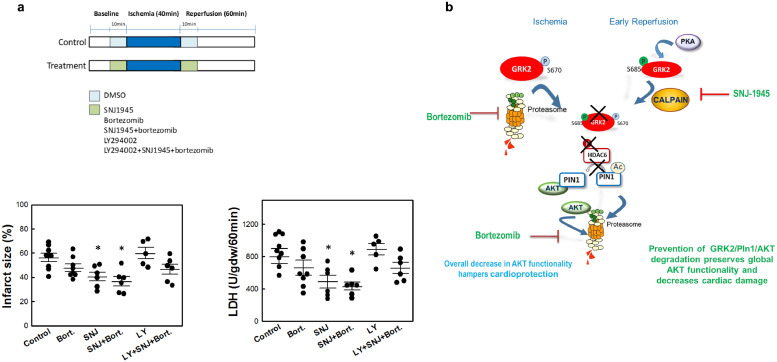
We therefore tested the effect of the administration of the clinically used proteasome inhibitor Bortezomib (Bz) and/or the calpain inhibitor SNJ-1945 (SNJ) on cardiac GRK2, Pin1 and AKT levels in isolated heartsrats subjected to 40 min of ischemia and reperfusion for 10 min. A slight significant prevention of AKT degradation was observed in the presence of BZ, and a tendency was detected for GRK2 and AKT protection upon administration of SNJ, but only the combined treatment with BZ and SNJ fully prevented the downregulation of GRK2, Pin1 and AKT in such conditions (Fig. 7A-C), allowing the maintenance of levels close to those detected in normoxic conditions. Importantly, the administration of proteasome or calpain inhibitors or their combined delivery significantly rescued bulk AKT functionality, as assessed by the overall phosphorylation of AKT substrates during reperfusion (Fig. 7D). This is consistent with the fact that these treatments tend to protect AKT protein from degradation while preserving kinase activation triggered by reperfusion, as indicated by AKT phospho-S473 status (see representative blot in Fig. 7C), thus allowing a normalization of pAKT/AKT ratios. Moreover, the administration of the SNJ calpain inhibitor or the combined delivery of SNJ and the proteasome inhibitor BZ prior to a protocol of 40 min of ischemia followed by 60 min of reperfusion significantly decreased infarct size and LDH release. Of note, this effect was not observed in the presence of a PI3K inhibitor, indicating that calpain and proteasome inhibitors exert its protective action by mechanism involving the PI3K/Akt pathway (Fig. 8A). Overall, our results postulate that preventing GRK2/Pin1/AKT degradation at early times in I/R may help to counteract myocardial injury.
Fig. 7.
The combined inhibition of proteasome and calpain activities preserves GRK2, AKT and Pin1 levels and recovers AKT functionality in post-ischemic reperfused hearts. (A–C) Isolated rat hearts were treated with the calpain inhibitor SNJ-1945 (SNJ, 10 μM) or the proteasome inhibitor Bortezomib (BZ) at high (10 μM) or low dose (1 μM), alone or in combination as indicated, during the 10 min prior to ischemia (40 min) and maintained during reperfusion (10 min). Myocardial lysates from the different groups were analyzed for determination of total protein expression levels of GRK2 (A), Pin 1 (B) and AKT (C). GAPDH or non-specific band proteins were used as loading controls for normalization. The AKT activation status assessed in the same samples by using a specific anti-phospho-Ser473 AKT antibody (C). (D) Global activity of AKT towards its substrates is preserved in post-ischemic reperfused hearts upon joint inhibition of proteasome and calpains. Bulk AKT activity was assessed in cardiac lysates from the indicated conditions as in Fig. 4C. Data were normalized with Ponceau protein staining. In all panels, normalized data were represented as fold-change with respect to normoxic situation and are mean ± SEM, n = 4 rats per condition. *p < .05; **p < .01; ***p < .001 compared with the conditions indicated with the lines [1-way ANOVA and Tukey's post hoc test]. Representative blots are shown.
Fig. 8.
Cardiac ischemia-reperfusion injury is attenuated by combined-inhibition of proteasome and calpain activities. (A) Isolated rat hearts were treated during the 10 min prior to ischemia (40 min) and the first 10 min of a total period of 60 min reperfusion with the indicated combinations of the calpain inhibitor SNJ-1945 (SNJ, 10 μM), the proteasome inhibitor Bortezomib (BZ) (10 μM) and the PI3K inhibitor LY-294002 (10 μM).After the reperfusion period, total LDH released during reperfusion (expressed as units of activity released per gram of dry weight during the first 60 min of reperfusion, U/gdw/60 min) and infarct size (expressed as the percentage of ventricular mass in the different experimental groups) were determined as detailed in Methods. Data are the mean ± SEM, n = 6–9 rats per condition. *p < .05 vs untreated I/R control group [1-way ANOVA and Tukey's post-hoc test]. (B) Scheme of the molecular mechanisms underlying GRK2/Pin1/AKT degradation during myocardial ischemia and early reperfusion. See text for details.
4. Discussion
Our data reveal several unforeseen alterations taking place in relevant cardiac signaling pathways during the early stages of I/R in rat and porcine experimental models that might provide a rationale for therapeutic strategies aimed at fostering cardioprotection mechanisms. First, we uncover that, contrary to the reported up-regulation of cardiac GRK2 taking place at later times after MI [[21], [22], [23]], successive GRK2 phosphorylation at specific sites during ischemia and in early reperfusion would sequentially elicit GRK2 degradation by the proteasome and calpains, respectively, thus keeping GRK2 protein levels low during early I/R. Second, we unveil a marked and previously uncharacterized decrease of AKT protein levels during this period, likely related to concurrent changes in the Prolyl isomerase Pin1 (a known AKT stabilizing factor), which results in an overall down-modulation of this key cardioprotective pathway in early I/R contexts, despite the high activation status of the remaining AKT upon reperfusion. Third, we show that hindering GRK2/Pin1/AKT degradation by the combined administration of proteasome and calpain inhibitors can attenuate I/R-myocardial injury in isolated ratheart experimental models (scheme in Fig. 8B).
The prevailing view to date is that GRK2 levels are increased in the heart of chronic HF patients with dilated or ischemic cardiomyopathy, as a result of sympathetic nervous system hyperactivity, leading to enhanced GRK2 mRNA expression. Such augmented GRK2 levels may initially help the myocardium to counterbalance the beta-adrenergic overdrive and reduce the risk of tachyarrhythmia, but persisting high GRK2 activity is maladaptive, resulting in GPCR desensitization and downregulation, defective contractility, insulin resistance, mitochondrial dysfunction and apoptosis, paving the way to HF [13,21,23].
Strikingly, we find that following myocardial I/R, GRK2 protein levels markedly decrease during the ischemic phase and are kept low during the early phase of reperfusion. Our data suggest that enhanced Ser670-GRK2 phosphorylation during ischemia would trigger its degradation by the proteasome, whereas phosphorylation at Ser685 taking place in early reperfusion would favor calpain-mediated GRK2 proteolysis. Of note, these phosphorylation sites are highly conserved across species, (murine, dog, pig and humans), consistent with preserved modulatory mechanisms.
To our knowledge, the pattern of GRK2 protein levels at such initial times of I/R has not been previously described. In murine models, cardiac GRK2 protein upregulation has been reported as early as 3 and 7 days after acute myocardial injury [13,52]. However, in line with our results, in a canine model of MI GRK2 levels were decreased in the subepicardial border and the infarct zone at 6 and 24 h after ligation of the coronary artery, without apparent changes in mRNA levels, and such GRK2 decrease in ischemic cardiac tissue was blocked by treatment with proteasome inhibitors, resulting in a significant cardioprotection against malignant ventricular tachyarrhythmias [[53], [54], [55]]. In the brain, oxygen deprivation triggers a marked decrease in GRK2 content along enhanced GRK2 phosphorylation at Ser670 in the absence of changes in mRNA levels, in a process blocked by proteasome inhibitors [56,57].
Upon phosphorylation at S670 GRK2 can be rapidly degraded by the proteasome [31,35], and GPCR stimulation facilitates β-arrestin-dependent ERK1/2 activation and subsequent phosphorylation of GRK2 at this residue [35]. Thus, the observed burst in phospho-Ser670 GRK2 after ischemia points to the occurrence of a very active proteasome-dependent degradation of the kinase in this condition. Although an ischemia-triggered inhibition of GRK2 transcription and/or translation cannot be ruled out, a previous report showed that during stop-flow and low flow ischemia in the isolated perfused rat heart GRK2 mRNA even increased after 20 min of ischemia and then returned to baseline after 40 min of ischemia onset [58], further supporting a predominant role for GRK2 proteolysis in this context.
GRK2 is phosphorylated at S670 by several kinases in a context-and stimulus-dependent way, including ERK1/2 [49,59,60], p38MAPK [61] or Cdk2 [31]. CDK2 is highly activated during myocardial ischemia [10,62] and CDK2-mediated GRK2 S670 phosphorylation can trigger proteasome-dependent GRK2 degradation in a GPCR-independent way [31], suggesting that this regulatory axis would be active during the ischemic phase.
The fact that GRK2 protein levels remained low during early reperfusion in the absence of enhanced S670 phosphorylation suggested the involvement of an alternative degradation pathway. We find that GRK2 is rapidly and transiently phosphorylated by PKA at S685 upon reperfusion, and that PKA stimulation can trigger calpain-dependent GRK2 proteolysis in a cellular model. Consistent with sequential proteasome and calpain GRK2 degradation during I/R, the combined inhibition of these proteolytic pathways completely prevents GRK2 protein decay in vivo.
Calpains are ubiquitous Ca2 + -dependent cysteine proteases activated in pathological conditions associated with Ca2+ overload. Calpains are thus strongly activated at the onset of myocardial reperfusion, contributing to myocardial injury through the proteolysis of key structural membrane, cytoskeletal components and regulatory enzymes [42,44,63]. Notably, PKA-phosphorylated GRK2 would only be targeted by calpains during the reperfusion phase upon intracellular pH normalization, since during ischemia ongoing acidosis inhibits these proteases [42].
However, the functional relationships among PKA stimulation, calpain activation status and GRK2 degradation are not straightforward and may involve additional regulatory layers. While phosphorylation of GRK2 by PKA switches on degradation by calpain, PKA has been reported to inhibit calpains upon βAR-triggered cAMP stimulation, and such mechanism appears to participate in the molecular mechanisms of cardioprotection upon ischemic preconditioning [64]. This apparent paradox may be reconciled by the existence of compartmentalized pools of these proteins and of distinct localization and activation mechanisms of PKA isoforms via regulatory anchoring proteins termed AKAPs [65]. In the vicinity of the plasma membrane, βAR-dependent production of cyclic-AMP reportedly promotes activation of AKAP79-bound type II PKA II leading to phosphorylation of GRK2 [37] and potentially also calpains, which subsequent inhibition would partially protect substrates such as fodrin and receptor-bound GRK2 from proteolysis in such locations. On the other hand, we find that reperfusion, in parallel to increased global PKA activity and S685-GRK2 phosphorylation, enhances the oxidation-dependent dimerization status of PKA-RΙα, an event leading to PKA activation independently of cAMP and driving its localization at the myofilament compartment [39,66], where a significant pool of GRK2 protein is present via association with sarcomeric a-actinin [67]. We postulate that PKA-mediated GRK2 phosphorylation at such location would particularly favor its subsequent degradation by nearby calpains, less likely to be targeted there by the reported βAR-PKA inhibitory phosphorylation mechanism. Interestingly, at early reperfusion times after preconditioning we observed comparatively higher GRK2 levels and decreased S685 phosphorylation status, consistent with diminished oxidation-dependent PKA activation in such conditions due to decreased ROS production [68], despite enhanced βAR-PKA stimulation also taking place in such conditions [64].
The cardio-protective and pro-survival AKT pathway is considered to be up-regulated during reperfusion to compensate I/R-promoted injury, and enhanced stimulatory AKT phosphorylation is found in such conditions. Activation of the Akt cascade is central to several conditioning strategies and plays a relevant cardio-protective role within the reperfusion injury salvage kinase pathway (RISK) pathway, whereas inhibitors of the upstream kinase PI3K block conditioning and cardioprotection [1,4,10,11]. However, we uncover that a concurrent and marked reduction in total AKT protein levels is triggered by ischemia and maintained during the first 30 min of reperfusion, resulting in an overall decrease of global AKT activity in cardiac extracts. The fact that such changes in total Akt levels at early times of I/R has not been investigated in detail before may be explained by the circumstance that in this type of studies total AKT levels are usually employed as loading controls to normalize the pAkt phosphorylation status, without direct comparison to other loading controls that would allow assessing possible changes in Akt protein levels (for instance see [11,51,[69], [70], [71]] However, a few reports do show an apparent decrease in total Akt levels compared to normoxia in I/R-related conditions [26,72,73] although this fact or the mechanisms involved were not discussed in detail.
Our results indicate that hyper-stimulation of the remaining AKT enzyme during reperfusion might not be able to fully compensate for the decay in protein levels taking place during early I/R. Such Akt protein down modulation would thus likely counteract the beneficial effects of therapeutic approaches specifically aimed at fostering Akt stimulation, suggesting that preventing GRK2/Pin1/Akt degradation would safeguard global Akt catalytic and foster the efficacy of cardioprotective drugs or conditioning strategies (see section below).
We postulate that GRK2-related changes in the Prolyl isomerase Pin1 may provide functionally link altered GRK2 and AKT protein levels during I/R. AKT undergoes proteasomal degradation in different cell types, and phosphorylation by mTORC2 at S473 can decrease AKT stability in addition to allowing full kinase activation [47,74]. Of note, Pin1, a known modulator of protein folding, activity and stability [45] and a relevant player in cardiac hypertrophy [48] and cardiac calcium handling [75], reportedly acts as a key positive factor in the regulation of AKT stability in cancer cells by binding to Ser473-activated AKT and protecting it from degradation by the proteasome, so decreased Pin1 expression facilitates AKT degradation [46,47]. Pin1 can also be degraded by the proteasome [76,77], and decreased expression takes place in liver I/R by unknown mechanisms [78]). GRK2 controls Pin1 stability and functionality in cancer cells by modulating its acetylation status via HDAC6, so decreased GRK2 expression fosters Pin1 acetylation and favors its degradation [30]. Consistently, we find that cardiac GRK2 downregulation during I/R correlates with a marked reduction in total Pin1 levels, along with its enhanced acetylation. Such decrease in this AKT stabilizing factor would in turn facilitate the observed concurrent AKT degradation.
Supporting a potential clinical relevance of our findings, a pattern of concomitant down-modulation of GRK2/AKT/Pin1 protein levels in early stages of cardiac I/R was also observed in pigs, a very relevant large animal model used in translational studies [28,51,79]. One limitation of our study is the relatively low number of animals per condition, arising from the logistic complexity of this type of experiments, including comparison of expression levels during myocardial ischemia and different early time points following reperfusion, as well as pre- or post-conditioning conditions in the rat model, However, the fact that the key changes reported are observed at different time points, in independent experiments in other rat cohorts using calpain and proteasome inhibitors and in a large animal pig model support the main conclusions of this work.
Consistent with this notion of related proteolysis events of GRK2 (via both proteasome and calpains), Pin1 and AKT (mostly via proteasome) during I/R, the combined inhibition of these degradation pathways prior to I/R fully prevents the degradation of GRK2, Pin1 and AKT, rescues global AKT functionality in rat cardiac extracts, and significantly decreases infarct size and LDH release. Both calpain [[42], [43], [44]] and the proteasome [80,81] have been reported to target a variety of cardiac substrates, including components of several signaling cascades. The susceptibility of specific substrates for degradation may vary depending on their subcellular localization, whereas different proteasome inhibitors inhibit cardiac proteasome subtypes to a different extent [82]. Thus, it is likely that the combined effect of SNJ-1945 and bortezomib may alter the levels of other cardiac signaling pathways in addition to the GRK2/Pin1/AKT axis. However, the fact that the protective effect of these inhibitors in I/R-induced cardiac damage is lost in the presence of an inhibitor of the PI3K/Akt pathway strongly suggests that preventing GRK2/Pin1/AKT degradation in cardiac cell types at early times in I/R and the parallel rescue of global AKT functionality play a relevant role in helping reduce myocardial injury.
Our observation that AKT protein levels fall during early I/R, resulting in an overall decrease of the functionality of this key cardioprotective pathway despite the stimulated status of the remaining AKT upon reperfusion puts forward a strong impediment for treatment approaches aimed only to enhance AKT activation status, as is the case for a variety of conditioning and pharmacological strategies [1,4,[9], [10], [11]], and suggest that treatments using a combination of calpain and proteasome inhibitors at the time of flow restoration in order to inhibit GRK2 and AKT degradation would foster the efficacy of endogenous cardioprotection, cardioprotective drugs or conditioning strategies.
In addition to helping preserve global AKT functionality, shielding GRK2 from degradation during early reperfusion might also protect against malignant ventricular tachyarrhythmias [[53], [54], [55]] and adrenergic-mediated myocardial injury [38]. However, given the reported increase in GRK2 expression at later time points after MI and their overall maladaptive role in cardiac remodeling and progression to HF [12,13,15,16,23], the therapeutic window for strategies aimed at decreasing GRK2 degradation should be carefully established. In mouse models, genetic GRK2 ablation 10 days after MI [83] or treatment with the GRK2 catalytic inhibitor paroxetine initiated 2 weeks after MI [22] improved cardiac function and reduced the adverse remodeling of ischemic nature. In this regard, permanent down-regulation of GRK2 in cardiac-specific knock-out animals protects [18], whereas increased GRK2 protein levels in cardiac transgenic GRK2 mice at the onset of MI aggravates [84] the injury caused by I/R, reportedly related to pro-apoptotic signaling triggered by enhanced targeting of GRK2 to the mitochondrial compartment, likely initiated during the ischemic phase of MI injury, as GRK2 mitochondrial shuttling depends on S670 phosphorylation, which is induced by ischemia. Although such data are consistent with a maladaptive role of high GRK2 levels in the long term, these models do not recapitulate the dynamic changes in GRK2 levels occurring at different phases of ischemia/reperfusion/remodeling and do not rule out a protective role for timely prevention of GRK2 degradation at early points of reperfusion. Interestingly, adenoviral delivery or cardiac transgenic expression of the C-terminal region of GRK2 (βARKct construct, aa 495–689) has a clear cardio-protective effect in both HF and acute MI experimental settings in murine or porcine preclinical models, by mechanisms involving prevention of the interaction and activation of GRK2 by Gβγ subunits released upon GPCR activation and interference with GRK2 phosphorylation at Ser670 and maladaptive targeting to mitochondria (reviewed in [13,21,23]). In other cell types, expression of βARKct also inhibits phosphorylation of endogenous GRK2 at Ser-670 by CDK2 and Pin1/GRK2 association, thus preventing down-regulation of endogenous GRK2 during cell cycle progression [31]. It is thus tempting to suggest that in the cardiac context an additional therapeutic effect of the ectopic expression of βARKct (which bears both the Ser670 and Ser685-related GRK2 phosphodegrons, highly conserved across species) would be to compete with endogenous GRK2 in the phosphorylation by the respective kinases and the action of the degradation machinery during I/R. Therefore, βARKct would contribute to the stabilization of the full-length endogenous GRK2 protein and thus of its cardioprotective Pin1 and AKT partners in the early phase after acute myocardial injury, while still acting as a modulator of GRK2 subcellular distribution and as a potent inhibitor of GRK2 functionality once the maladaptive increase in GRK2 takes place at later stages.
In sum, our data uncover dynamic changes in the central GRK2 and AKT cardiac signaling nodes at early stages after I/R, and suggest that the timely use of calpain and proteasome inhibition in these contexts may be an amenable way to reinforce therapeutic strategies aimed at reducing I/R injury.
Funding
ISCIII, CIBERCV, MINECO Spain, Fundación Areces.
Author contributions
PP and JI designed and performed experiments, prepared figures, analyzed data, wrote and revised manuscript, ARS provided cardiac pig samples and analyzed data, PR performed experiments, analyzed data and prepared figures, DG-D and FM Jr., provided study concept and design, interpreted experiments, analyzed data, supervised the study, wrote and revised manuscript.
Acknowledgements and funding sources
Our laboratories are supported by Instituto de Salud Carlos III, Spain (grant PI-16/00232; RETICS-RIC-RD12/0042/0021 to DGD, co-funded with European Regional Development Fund-FEDER contribution, and grants PI14-00435 and PI17-00576 to PP), by Ministerio de Economía; Industria y Competitividad (MINECO) of Spain (grant SAF2017-84125-R to F.M.); by CIBERCV-Instituto de Salud Carlos III, Spain (grant CB16/11/00479 to DGD and CB16/11/00278 to F.M, co-funded with European Regional Development Fund-FEDER contribution), and Programa de Actividades en Biomedicina de la Comunidad de Madrid-B2017/BMD-3671-INFLAMUNE to F.M. We also acknowledge institutional support to the CBMSO from Fundación Ramón Areces. This work is dedicated to the memory of our colleague and friend Dr. David García-Dorado, who sadly passed away during the final revision stage of this manuscript.
Declaration of Competing Interest
The authors have nothing to disclose.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2019.09.019.
Appendix A. Supplementary data
Supplementary material
References
- 1.Hausenloy D.J., Yellon D.M. Myocardial ischemia-reperfusion injury: a neglected therapeutic target. J Clin Invest. 2013;123(1):92–100. doi: 10.1172/JCI62874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ibanez B., Heusch G., Ovize M., Van de Werf F. Evolving therapies for myocardial ischemia/reperfusion injury. J Am Coll Cardiol. 2015;65(14):1454–1471. doi: 10.1016/j.jacc.2015.02.032. [DOI] [PubMed] [Google Scholar]
- 3.Piper H.M., Garcia-Dorado D., Ovize M. A fresh look at reperfusion injury. Cardiovasc Res. 1998;38(2):291–300. doi: 10.1016/s0008-6363(98)00033-9. [DOI] [PubMed] [Google Scholar]
- 4.Heusch G., Libby P., Gersh B., Yellon D., Bohm M., Lopaschuk G. Cardiovascular remodelling in coronary artery disease and heart failure. Lancet. 2014;383(9932):1933–1943. doi: 10.1016/S0140-6736(14)60107-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garcia-Dorado D., Rodriguez-Sinovas A., Ruiz-Meana M., Inserte J. Protection against myocardial ischemia-reperfusion injury in clinical practice. Rev Esp Cardiol. 2014;67(5):394–404. doi: 10.1016/j.rec.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 6.Binek A., Fernandez-Jimenez R., Jorge I., Camafeita E., Lopez J.A., Bagwan N. Proteomic footprint of myocardial ischemia/reperfusion injury: longitudinal study of the at-risk and remote regions in the pig model. Sci Rep. 2017;7(1) doi: 10.1038/s41598-017-11985-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fuster V. Top 10 cardiovascular therapies and interventions for the next decade. Nat Rev Cardiol. 2014;11(11):671–683. doi: 10.1038/nrcardio.2014.137. [DOI] [PubMed] [Google Scholar]
- 8.Davidson S.M., Ferdinandy P., Andreadou I., Botker H.E., Heusch G., Ibanez B. Multitarget strategies to reduce myocardial ischemia/reperfusion injury: JACC review topic of the week. J Am Coll Cardiol. 2019;73(1):89–99. doi: 10.1016/j.jacc.2018.09.086. [DOI] [PubMed] [Google Scholar]
- 9.Hausenloy D.J., Yellon D.M. Ischaemic conditioning and reperfusion injury. Nat Rev Cardiol. 2016;13(4):193–209. doi: 10.1038/nrcardio.2016.5. [DOI] [PubMed] [Google Scholar]
- 10.Heusch G. Molecular basis of cardioprotection: signal transduction in ischemic pre-, post-, and remote conditioning. Circ Res. 2015;116(4):674–699. doi: 10.1161/CIRCRESAHA.116.305348. [DOI] [PubMed] [Google Scholar]
- 11.Rossello X., Riquelme J.A., Davidson S.M., Yellon D.M. Role of PI3K in myocardial ischaemic preconditioning: mapping pro-survival cascades at the trigger phase and at reperfusion. J Cell Mol Med. 2018;22(2):926–935. doi: 10.1111/jcmm.13394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Woodall M.C., Ciccarelli M., Woodall B.P., Koch W.J. G protein-coupled receptor kinase 2: a link between myocardial contractile function and cardiac metabolism. Circ Res. 2014;114(10):1661–1670. doi: 10.1161/CIRCRESAHA.114.300513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sato P.Y., Chuprun J.K., Schwartz M., Koch W.J. The evolving impact of g protein-coupled receptor kinases in cardiac health and disease. Physiol Rev. 2015;95(2):377–404. doi: 10.1152/physrev.00015.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cannavo A., Koch W.J. GRK2 as negative modulator of NO bioavailability: implications for cardiovascular disease. Cell Signal. 2018;41:33–40. doi: 10.1016/j.cellsig.2017.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mayor F., Jr., Cruces-Sande M., Arcones A.C., Vila-Bedmar R., Briones A.M., Salaices M. G protein-coupled receptor kinase 2 (GRK2) as an integrative signalling node in the regulation of cardiovascular function and metabolic homeostasis. Cell Signal. 2018;41:25–32. doi: 10.1016/j.cellsig.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 16.Murga C., Arcones A.C., Cruces-Sande M., Briones A.M., Salaices M., Mayor F., Jr. G protein-coupled receptor kinase 2 (GRK2) as a potential therapeutic target in cardiovascular and metabolic diseases. Front Pharmacol. 2019;10:112. doi: 10.3389/fphar.2019.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nogues L., Palacios-Garcia J., Reglero C., Rivas V., Neves M., Ribas C. G protein-coupled receptor kinases (GRKs) in tumorigenesis and cancer progression: GPCR regulators and signaling hubs. Semin Cancer Biol. 2018;48:78–90. doi: 10.1016/j.semcancer.2017.04.013. [DOI] [PubMed] [Google Scholar]
- 18.Fan Q., Chen M., Zuo L., Shang X., Huang M.Z., Ciccarelli M. Myocardial ablation of G protein-coupled receptor kinase 2 (GRK2) decreases ischemia/reperfusion injury through an anti-intrinsic apoptotic pathway. PLoS One. 2013;8(6) doi: 10.1371/journal.pone.0066234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sato P.Y., Chuprun J.K., Grisanti L.A., Woodall M.C., Brown B.R., Roy R. Restricting mitochondrial GRK2 post-ischemia confers cardioprotection by reducing myocyte death and maintaining glucose oxidation. Sci Signal. 2018;11(560) doi: 10.1126/scisignal.aau0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Penela P., Murga C., Ribas C., Tutor A.S., Peregrin S., Mayor F., Jr. Mechanisms of regulation of G protein-coupled receptor kinases (GRKs) and cardiovascular disease. Cardiovasc Res. 2006;69(1):46–56. doi: 10.1016/j.cardiores.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 21.de Lucia C., Eguchi A., Koch W.J. New insights in cardiac beta-adrenergic signaling during heart failure and aging. Front Pharmacol. 2018;9:904. doi: 10.3389/fphar.2018.00904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schumacher S.M., Gao E., Zhu W., Chen X., Chuprun J.K., Feldman A.M. Paroxetine-mediated GRK2 inhibition reverses cardiac dysfunction and remodeling after myocardial infarction. Sci Transl Med. 2015;7(277):277ra31. doi: 10.1126/scitranslmed.aaa0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cannavo A., Komici K., Bencivenga L., D'Amico M.L., Gambino G., Liccardo D. GRK2 as a therapeutic target for heart failure. Expert Opin Ther Targets. 2018;22(1):75–83. doi: 10.1080/14728222.2018.1406925. [DOI] [PubMed] [Google Scholar]
- 24.Inserte J., Garcia-Dorado D., Hernando V., Barba I., Soler-Soler J. Ischemic preconditioning prevents calpain-mediated impairment of Na+/K+-ATPase activity during early reperfusion. Cardiovasc Res. 2006;70(2):364–373. doi: 10.1016/j.cardiores.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 25.Inserte J., Barba I., Hernando V., Garcia-Dorado D. Delayed recovery of intracellular acidosis during reperfusion prevents calpain activation and determines protection in postconditioned myocardium. Cardiovasc Res. 2009;81(1):116–122. doi: 10.1093/cvr/cvn260. [DOI] [PubMed] [Google Scholar]
- 26.Inserte J., Hernando V., Vilardosa U., Abad E., Poncelas-Nozal M., Garcia-Dorado D. Activation of cGMP/protein kinase G pathway in postconditioned myocardium depends on reduced oxidative stress and preserved endothelial nitric oxide synthase coupling. J Am Heart Assoc. 2013;2(1) doi: 10.1161/JAHA.112.005975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poncelas M., Inserte J., Aluja D., Hernando V., Vilardosa U., Garcia-Dorado D. Delayed, oral pharmacological inhibition of calpains attenuates adverse post-infarction remodelling. Cardiovasc Res. 2017;113(8):950–961. doi: 10.1093/cvr/cvx073. [DOI] [PubMed] [Google Scholar]
- 28.Alburquerque-Bejar J.J., Barba I., Inserte J., Miro-Casas E., Ruiz-Meana M., Poncelas M. Combination therapy with remote ischaemic conditioning and insulin or exenatide enhances infarct size limitation in pigs. Cardiovasc Res. 2015;107(2):246–254. doi: 10.1093/cvr/cvv171. [DOI] [PubMed] [Google Scholar]
- 29.Penela P., Ruiz-Gomez A., Castano J.G., Mayor F., Jr. Degradation of the G protein-coupled receptor kinase 2 by the proteasome pathway. J Biol Chem. 1998;273(52):35238–35244. doi: 10.1074/jbc.273.52.35238. [DOI] [PubMed] [Google Scholar]
- 30.Nogues L., Reglero C., Rivas V., Salcedo A., Lafarga V., Neves M. G protein-coupled receptor kinase 2 (GRK2) promotes breast tumorigenesis through a HDAC6-Pin1 axis. EBioMedicine. 2016;13:132–145. doi: 10.1016/j.ebiom.2016.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Penela P., Rivas V., Salcedo A., Mayor F., Jr. G protein-coupled receptor kinase 2 (GRK2) modulation and cell cycle progression. Proc Natl Acad Sci U S A. 2010;107(3):1118–1123. doi: 10.1073/pnas.0905778107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murga C., Penela P., Zafra F., Mayor F., Jr. The subcellular and cellular distribution of G protein-coupled receptor kinase 2 in rat brain. Neuroscience. 1998;87(3):631–637. doi: 10.1016/s0306-4522(98)00145-6. [DOI] [PubMed] [Google Scholar]
- 33.Ferrera R., Benhabbouche S., Bopassa J.C., Li B., Ovize M. One hour reperfusion is enough to assess function and infarct size with TTC staining in Langendorff rat model. Cardiovasc Drugs Ther. 2009;23(4):327–331. doi: 10.1007/s10557-009-6176-5. [DOI] [PubMed] [Google Scholar]
- 34.Inserte J., Hernando V., Ruiz-Meana M., Poncelas-Nozal M., Fernandez C., Agullo L. Delayed phospholamban phosphorylation in post-conditioned heart favours Ca2+ normalization and contributes to protection. Cardiovasc Res. 2014;103(4):542–553. doi: 10.1093/cvr/cvu163. [DOI] [PubMed] [Google Scholar]
- 35.Elorza A., Penela P., Sarnago S., Mayor F., Jr. MAPK-dependent degradation of G protein-coupled receptor kinase 2. J Biol Chem. 2003;278(31):29164–29173. doi: 10.1074/jbc.M304314200. [DOI] [PubMed] [Google Scholar]
- 36.Penela P., Murga C., Ribas C., Lafarga V., Mayor F., Jr. The complex G protein-coupled receptor kinase 2 (GRK2) interactome unveils new physiopathological targets. Br J Pharmacol. 2010;160(4):821–832. doi: 10.1111/j.1476-5381.2010.00727.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cong M., Perry S.J., Lin F.T., Fraser I.D., Hu L.A., Chen W. Regulation of membrane targeting of the G protein-coupled receptor kinase 2 by protein kinase A and its anchoring protein AKAP79. J Biol Chem. 2001;276(18):15192–15199. doi: 10.1074/jbc.M009130200. [DOI] [PubMed] [Google Scholar]
- 38.Schreckenberg R., Bencsik P., Weber M., Abdallah Y., Csonka C., Gomori K. Adverse effects on beta-adrenergic receptor coupling: ischemic postconditioning failed to preserve long-term cardiac function. J Am Heart Assoc. 2017;6(12) doi: 10.1161/JAHA.117.006809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnston A.S., Lehnart S.E., Burgoyne J.R. Ca(2+) signaling in the myocardium by (redox) regulation of PKA/CaMKII. Front Pharmacol. 2015;6:166. doi: 10.3389/fphar.2015.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nogues L., Salcedo A., Mayor F., Jr., Penela P. Multiple scaffolding functions of {beta}-arrestins in the degradation of G protein-coupled receptor kinase 2. J Biol Chem. 2011;286(2):1165–1173. doi: 10.1074/jbc.M110.203406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Penela P., Elorza A., Sarnago S., Mayor F., Jr. Beta-arrestin- and c-Src-dependent degradation of G-protein-coupled receptor kinase 2. EMBO J. 2001;20(18):5129–5138. doi: 10.1093/emboj/20.18.5129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hernando V., Inserte J., Sartorio C.L., Parra V.M., Poncelas-Nozal M., Garcia-Dorado D. Calpain translocation and activation as pharmacological targets during myocardial ischemia/reperfusion. J Mol Cell Cardiol. 2010;49(2):271–279. doi: 10.1016/j.yjmcc.2010.02.024. [DOI] [PubMed] [Google Scholar]
- 43.Aluja D., Inserte J., Penela P., Ramos P., Ribas C., Iniguez M.A. Calpains mediate isoproterenol-induced hypertrophy through modulation of GRK2. Basic Res Cardiol. 2019;114(3):21. doi: 10.1007/s00395-019-0730-5. [DOI] [PubMed] [Google Scholar]
- 44.Inserte J. Calpains in the cardiovascular system. Cardiovasc Res. 2012;96(1):9–10. doi: 10.1093/cvr/cvs245. [DOI] [PubMed] [Google Scholar]
- 45.Zhou X.Z., Lu K.P. The isomerase PIN1 controls numerous cancer-driving pathways and is a unique drug target. Nat Rev Cancer. 2016;16(7):463–478. doi: 10.1038/nrc.2016.49. [DOI] [PubMed] [Google Scholar]
- 46.Liao Y., Wei Y., Zhou X., Yang J.Y., Dai C., Chen Y.J. Peptidyl-prolyl cis/trans isomerase Pin1 is critical for the regulation of PKB/Akt stability and activation phosphorylation. Oncogene. 2009;28(26):2436–2445. doi: 10.1038/onc.2009.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liao Y., Hung M.C. Physiological regulation of Akt activity and stability. Am J Transl Res. 2010;2(1):19–42. [PMC free article] [PubMed] [Google Scholar]
- 48.Toko H., Konstandin M.H., Doroudgar S., Ormachea L., Joyo E., Joyo A.Y. Regulation of cardiac hypertrophic signaling by prolyl isomerase Pin1. Circ Res. 2013;112(9):1244–1252. doi: 10.1161/CIRCRESAHA.113.301084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lafarga V., Aymerich I., Tapia O., Mayor F., Jr., Penela P. A novel GRK2/HDAC6 interaction modulates cell spreading and motility. EMBO J. 2012;31(4):856–869. doi: 10.1038/emboj.2011.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hausenloy D.J., Iliodromitis E.K., Andreadou I., Papalois A., Gritsopoulos G., Anastasiou-Nana M. Investigating the signal transduction pathways underlying remote ischemic conditioning in the porcine heart. Cardiovasc Drugs Ther. 2012;26(2):87–93. doi: 10.1007/s10557-011-6364-y. [DOI] [PubMed] [Google Scholar]
- 51.Gent S., Skyschally A., Kleinbongard P., Heusch G. Lschemic preconditioning in pigs: a causal role for signal transducer and activator of transcription 3. Am J Physiol Heart Circ Physiol. 2017;312(3) doi: 10.1152/ajpheart.00749.2016. (H478-H84) [DOI] [PubMed] [Google Scholar]
- 52.White D.C., Hata J.A., Shah A.S., Glower D.D., Lefkowitz R.J., Koch W.J. Preservation of myocardial beta-adrenergic receptor signaling delays the development of heart failure after myocardial infarction. Proc Natl Acad Sci U S A. 2000;97(10):5428–5433. doi: 10.1073/pnas.090091197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yu X., Zhang M., Kyker K., Patterson E., Benovic J.L., Kem D.C. Ischemic inactivation of G protein-coupled receptor kinase and altered desensitization of canine cardiac beta-adrenergic receptors. Circulation. 2000;102(20):2535–2540. doi: 10.1161/01.cir.102.20.2535. [DOI] [PubMed] [Google Scholar]
- 54.Yu X., Huang S., Patterson E., Garrett M.W., Kaufman K.M., Metcalf J.P. Proteasome degradation of GRK2 during ischemia and ventricular tachyarrhythmias in a canine model of myocardial infarction. Am J Physiol Heart Circ Physiol. 2005;289(5):H1960–H1967. doi: 10.1152/ajpheart.00328.2005. [DOI] [PubMed] [Google Scholar]
- 55.Huang S., Patterson E., Yu X., Garrett M.W., De Aos I., Kem D.C. Proteasome inhibition 1 h following ischemia protects GRK2 and prevents malignant ventricular tachyarrhythmias and SCD in a model of myocardial infarction. Am J Physiol Heart Circ Physiol. 2008;294(3):H1298–H1303. doi: 10.1152/ajpheart.00765.2007. [DOI] [PubMed] [Google Scholar]
- 56.Lombardi M.S., van den Tweel E., Kavelaars A., Groenendaal F., van Bel F., Heijnen C.J. Hypoxia/ischemia modulates G protein-coupled receptor kinase 2 and beta-arrestin-1 levels in the neonatal rat brain. Stroke. 2004;35(4):981–986. doi: 10.1161/01.STR.0000121644.82596.7e. [DOI] [PubMed] [Google Scholar]
- 57.Lombardi M.S., Vroon A., Sodaar P., van Muiswinkel F.L., Heijnen C.J., Kavelaars A. Down-regulation of GRK2 after oxygen and glucose deprivation in rat hippocampal slices: role of the PI3-kinase pathway. J Neurochem. 2007;102(3):731–740. doi: 10.1111/j.1471-4159.2007.04576.x. [DOI] [PubMed] [Google Scholar]
- 58.Ungerer M., Kessebohm K., Kronsbein K., Lohse M.J., Richardt G. Activation of beta-adrenergic receptor kinase during myocardial ischemia. Circ Res. 1996;79(3):455–460. doi: 10.1161/01.res.79.3.455. [DOI] [PubMed] [Google Scholar]
- 59.Elorza A., Sarnago S., Mayor F., Jr. Agonist-dependent modulation of G protein-coupled receptor kinase 2 by mitogen-activated protein kinases. Mol Pharmacol. 2000;57(4):778–783. doi: 10.1124/mol.57.4.778. [DOI] [PubMed] [Google Scholar]
- 60.Penela P., Murga C., Ribas C., Salcedo A., Jurado-Pueyo M., Rivas V. G protein-coupled receptor kinase 2 (GRK2) in migration and inflammation. Arch Physiol Biochem. 2008;114(3):195–200. doi: 10.1080/13813450802181039. [DOI] [PubMed] [Google Scholar]
- 61.Liu X., Ma B., Malik A.B., Tang H., Yang T., Sun B. Bidirectional regulation of neutrophil migration by mitogen-activated protein kinases. Nat Immunol. 2012;13(5):457–464. doi: 10.1038/ni.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liem D.A., Zhao P., Angelis E., Chan S.S., Zhang J., Wang G. Cyclin-dependent kinase 2 signaling regulates myocardial ischemia/reperfusion injury. J Mol Cell Cardiol. 2008;45(5):610–616. doi: 10.1016/j.yjmcc.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Neuhof C., Neuhof H. Calpain system and its involvement in myocardial ischemia and reperfusion injury. World J Cardiol. 2014;6(7):638–652. doi: 10.4330/wjc.v6.i7.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Inserte J., Garcia-Dorado D., Ruiz-Meana M., Agullo L., Pina P., Soler-Soler J. Ischemic preconditioning attenuates calpain-mediated degradation of structural proteins through a protein kinase A-dependent mechanism. Cardiovasc Res. 2004;64(1):105–114. doi: 10.1016/j.cardiores.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 65.Diviani D., Reggi E., Arambasic M., Caso S., Maric D. Emerging roles of A-kinase anchoring proteins in cardiovascular pathophysiology. Biochim Biophys Acta. 2016;1863(7):1926–1936. doi: 10.1016/j.bbamcr.2015.11.024. Pt B. [DOI] [PubMed] [Google Scholar]
- 66.Brennan J.P., Bardswell S.C., Burgoyne J.R., Fuller W., Schroder E., Wait R. Oxidant-induced activation of type I protein kinase A is mediated by RI subunit interprotein disulfide bond formation. J Biol Chem. 2006;281(31):21827–21836. doi: 10.1074/jbc.M603952200. [DOI] [PubMed] [Google Scholar]
- 67.Yi X.P., Gerdes A.M., Li F. Myocyte redistribution of GRK2 and GRK5 in hypertensive, heart-failure-prone rats. Hypertension. 2002;39(6):1058–1063. doi: 10.1161/01.hyp.0000019130.09167.3b. [DOI] [PubMed] [Google Scholar]
- 68.Cadenas S. ROS and redox signaling in myocardial ischemia-reperfusion injury and cardioprotection. Free Radic Biol Med. 2018;117:76–89. doi: 10.1016/j.freeradbiomed.2018.01.024. [DOI] [PubMed] [Google Scholar]
- 69.Skyschally A., van Caster P., Boengler K., Gres P., Musiolik J., Schilawa D. Ischemic postconditioning in pigs: no causal role for RISK activation. Circ Res. 2009;104(1):15–18. doi: 10.1161/CIRCRESAHA.108.186429. [DOI] [PubMed] [Google Scholar]
- 70.Skyschally A., Gent S., Amanakis G., Schulte C., Kleinbongard P., Heusch G. Across-species transfer of protection by remote ischemic preconditioning with species-specific myocardial signal transduction by reperfusion injury salvage kinase and survival activating factor enhancement pathways. Circ Res. 2015;117(3):279–288. doi: 10.1161/CIRCRESAHA.117.306878. [DOI] [PubMed] [Google Scholar]
- 71.Yin Z., Gao H., Wang H., Li L., Di C., Luan R. Ischaemic post-conditioning protects both adult and aged Sprague-Dawley rat heart from ischaemia-reperfusion injury through the phosphatidylinositol 3-kinase-AKT and glycogen synthase kinase-3beta pathways. Clin Exp Pharmacol Physiol. 2009;36(8):756–763. doi: 10.1111/j.1440-1681.2009.05148.x. [DOI] [PubMed] [Google Scholar]
- 72.Moreira J.B., Wohlwend M., Alves M.N., Wisloff U., Bye A. A small molecule activator of AKT does not reduce ischemic injury of the rat heart. J Transl Med. 2015;13:76. doi: 10.1186/s12967-015-0444-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kleinbongard P., Skyschally A., Gent S., Pesch M., Heusch G. STAT3 as a common signal of ischemic conditioning: a lesson on "rigor and reproducibility" in preclinical studies on cardioprotection. Basic Res Cardiol. 2018;113(1):3. doi: 10.1007/s00395-017-0660-z. [DOI] [PubMed] [Google Scholar]
- 74.Wu Y.T., Ouyang W., Lazorchak A.S., Liu D., Shen H.M., Su B. mTOR complex 2 targets Akt for proteasomal degradation via phosphorylation at the hydrophobic motif. J Biol Chem. 2011;286(16):14190–14198. doi: 10.1074/jbc.M111.219923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sacchi V., Wang B.J., Kubli D., Martinez A.S., Jin J.K., Alvarez R., Jr. Peptidyl-prolyl isomerase 1 regulates Ca(2+) handling by modulating Sarco(Endo)Plasmic reticulum calcium ATPase and Na(2+)/Ca(2+) exchanger 1 protein levels and function. J Am Heart Assoc. 2017;6(10) doi: 10.1161/JAHA.117.006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Basu A., Das M., Qanungo S., Fan X.J., DuBois G., Haldar S. Proteasomal degradation of human peptidyl prolyl isomerase pin1-pointing phospho Bcl2 toward dephosphorylation. Neoplasia. 2002;4(3):218–227. doi: 10.1038/sj.neo.7900233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Eckerdt F., Yuan J., Saxena K., Martin B., Kappel S., Lindenau C. Polo-like kinase 1-mediated phosphorylation stabilizes Pin1 by inhibiting its ubiquitination in human cells. J Biol Chem. 2005;280(44):36575–36583. doi: 10.1074/jbc.M504548200. [DOI] [PubMed] [Google Scholar]
- 78.Kuboki S., Okaya T., Schuster R., Blanchard J., Denenberg A., Wong H.R. Hepatocyte NF-kappaB activation is hepatoprotective during ischemia-reperfusion injury and is augmented by ischemic hypothermia. Am J Physiol Gastrointest Liver Physiol. 2007;292(1):G201–G207. doi: 10.1152/ajpgi.00186.2006. [DOI] [PubMed] [Google Scholar]
- 79.Raake P.W., Schlegel P., Ksienzyk J., Reinkober J., Barthelmes J., Schinkel S. AAV6.betaARKct cardiac gene therapy ameliorates cardiac function and normalizes the catecholaminergic axis in a clinically relevant large animal heart failure model. Eur Heart J. 2013;34(19):1437–1447. doi: 10.1093/eurheartj/ehr447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pagan J., Seto T., Pagano M., Cittadini A. Role of the ubiquitin proteasome system in the heart. Circ Res. 2013;112(7):1046–1058. doi: 10.1161/CIRCRESAHA.112.300521. [DOI] [PubMed] [Google Scholar]
- 81.Glembotski C.C. Clarifying the cardiac proteasome paradox: protein quality control. Circ Res. 2012;111(5):509–512. doi: 10.1161/CIRCRESAHA.112.275917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kloss A., Meiners S., Ludwig A., Dahlmann B. Multiple cardiac proteasome subtypes differ in their susceptibility to proteasome inhibitors. Cardiovasc Res. 2010;85(2):367–375. doi: 10.1093/cvr/cvp217. [DOI] [PubMed] [Google Scholar]
- 83.Raake P.W., Vinge L.E., Gao E., Boucher M., Rengo G., Chen X. G protein-coupled receptor kinase 2 ablation in cardiac myocytes before or after myocardial infarction prevents heart failure. Circ Res. 2008;103(4):413–422. doi: 10.1161/CIRCRESAHA.107.168336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brinks H., Boucher M., Gao E., Chuprun J.K., Pesant S., Raake P.W. Level of G protein-coupled receptor kinase-2 determines myocardial ischemia/reperfusion injury via pro- and anti-apoptotic mechanisms. Circ Res. 2010;107(9):1140–1149. doi: 10.1161/CIRCRESAHA.110.221010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material