Abstract
Background
Cartilaginous endplate (CEP) degeneration is considered as one of the major causes of intervertebral disc degeneration (IVDD) which causes low back pain. Recent studies have proved that epigenetic alteration is involved in a variety of diseases. This work explored the role of histone methyltransferase enhancer of zeste homologue 2 (EZH2) in CEP degeneration, as well as its underlying epigenetic mechanisms, and confirmed the effect of EZH2 knockdown on delaying IVDD development.
Methods
Western blotting, immunofluorescence staining, and ChIP assay were applied to demonstrate the molecular mechanism of EZH2 in CEP tissue. The therapeutic potential of EZH2 was investigated using puncture-induced rat models.
Findings
The EZH2 expression was upregulated in human and rat CEP tissue. It was also found that the overexpression of EZH2 suppressed the expression of Collagen II, aggrecan and Sox-9, and promoted the expression of ADTAMTS5 and MMP13 in rat endplate chondrocytes (EPCs), which could be reversed by EZH2 silencing. The correlation between EZH2 and Sox-9 was further explored, while overexpression of Sox-9 could reverse the effect of EZH2 in rat EPCs. Moreover, inhibition of EZH2 upregulated the level of Sox-9 by demethylating H3K27me3 at Sox-9 promoter sites, revealing the regulatory mechanism of EZH2 on Sox-9. Meanwhile, puncture-induced rat models showed that EZH2 knockdown exerted a protective effect on CEP and disc degeneration.
Interpretation
This study reveals that EZH2 inhibition is a promising strategy for mitigating the symptoms and progression of IVDD.
Funding
: This study was funded by the Natural Science Foundation of Zhejiang Province (Y16H060034). Authors declare that the funders had no involvement in the study design, data analysis and interpretation of the results.
Keywords: EZH2, Sox-9, Epigenetic alteration, Cartilaginous endplate, Intervertebral disc degeneration
Research in context
Evidence before this study
Cartilaginous endplate (CEP) degeneration is considered as one of the major causes of intervertebral disc degeneration (IVDD) which causes low back pain. Sox-9 is essential for cartilage development and is a transcriptional factor necessary for chondrogenesis, which is required in several successive steps of chondrocyte differentiation. Recent studies have proved that epigenetic alteration is involved in a variety of diseases, and histone methyltransferase enhancer of zeste homologue 2 (EZH2) serves as an essential force in the epigenetic regulation responsible for inflammation, autoimmunity and a wide range of malignancies. However, the role of EZH2 in CEP and disc degeneration and its underlying mechanism remain unclear.
Added value of this study
In this study, we identified the regulatory effect of EZH2 on the expression of degeneration-related genes and Sox-9 in rat endplate chondrocytes (EPCs). We found that overexpression of Sox-9 could reverse the effect of EZH2 in rat EPCs, indicating the correlation between EZH2 and Sox-9. Moreover, inhibition of EZH2 upregulated the level of Sox-9 by demethylating H3K27me3 at Sox-9 promoter sites, revealing the regulatory mechanism of EZH2 on Sox-9. Meanwhile, puncture-induced rat models showed that EZH2 knockdown exerted a protective effect on CEP and disc degeneration.
Implications of all the available evidence
This study identifies that inhibition of EZH2 ameliorates cartilage endplate degeneration and attenuates the progression of intervertebral disc degeneration via demethylation of Sox-9. This study may provide a promising strategy for mitigating the symptoms and progression of IVDD.
1. Introduction
Low back pain (LBP) affects nearly 80% of the population in their different stages of lifetime, affecting the quality of people's daily life and even leads to disability, resulting in heavy economic burden worldwide [1]. Among other influential factors, intervertebral disc degeneration (IVDD) has been found to be a major cause of LBP [2].
The intervertebral disc is avascular tissue composed of nucleus pulposus (NP), the annulus fibrosus (AF), and the cartilaginous endplate (CEP) [3]. Being a thin layer of hyaline bridging between the disc and the vertebrae, CEP not only allows passage of nutrients and metabolites through vertebral blood vessels but also provides biomechanical support by dispersing the pressure between discs [4], [5]. The interrelation between CEP and intervertebral disc has been studied for a long time. In a large population study, Williams et al. demonstrated that endplate defect was significantly and independently associated with disc degeneration at every lumbar disc level [6]. On the other hand Lu et al. reported through MRI studies that endplate change consisting of endplate concave angle and Modic change, was positively correlated with the degree of disc degeneration [7]. Thus CEP degeneration could be one of the major pathologic bases directly related to disc degeneration, which provides a potential therapeutic target. However, little is known about the specific molecular mechanism of CEP that mediates it therapeutic effects.
Sox-9 is essential for cartilage development and is a transcriptional factor necessary for chondrogenesis, and is required in several successive steps during chondrocyte differentiation [8]. Sox-9 inhibits the conversion of chondrocytes into hypertrophic chondrocytes, thereby preventing subsequent endochondral ossification [9]. Previous studies demonstrated the correlation between Sox-9 and intervertebral disc. The of Sox-9 was low in the aging and degenerating disc and its deficiency triggered the degeneration of CEP, resulting in IVDD [10]. In contrast, overexpression of Sox-9 significantly delayed the occurrence of disc degeneration, indicating the protective role of Sox-9 in IVDD progression [11]. However the underlying regulatory mechanism of Sox-9 remains unclear.
Recent studies reported that DNA methylation of Sox-9 plays an important role in various diseases [12], [13], [14]. Kim et al. found that epigenetic change of Sox-9 promoter contributed to the development of osteoarthritis (OA) by increasing DNA methylation, increasing methylation of gene-inactivating histone residues, and decreasing histone acetylation [14]. In our view, the epigenetic regulation of Sox-9 may serve as a potential target for CEP degeneration and disc degeneration, but the specific regulatory factors which cause epigenetic changes at the Sox-9 promoter need to be further explored.
Histone methyltransferase enhancer of zeste homologue 2 (EZH2), the catalytic subunit of the polycomb repressor complex 2, exerts its function of genomic silencing through adding three methyl groups to methylation of histone H3 lysine 27 (H3K27) genes [15]. Aberrant activation of EZH2 was found in osteoarthritis and rheumatoid arthritis [16], [17]. Wang et al. revealed that inactivation of EZH2 could attenuate chondrocyte dysfunction and osteoarthritis progression by reducing H3K27me3 enrichment in the miR-128a promoter [18]. Overexpressed EZH2 dramatically decreased the Sox-9 level in chondrocytes as revealed in our previous study [19]. In the present work, it was hypothesized that high expression of EZH2 inhibits the level of Sox-9, causing CEP and disc degeneration. Additionally, we postulated that EZH2 silencing may inhibit the degeneration of cartilaginous endplate and even the whole intervertebral disc through demethylation of Sox-9. Thus, this study explored the protective effect of EZH2 on IVDD.
2. Materials and methods
2.1. Ethics statement
All surgical interventions, treatments and postoperative animal care procedures were strictly performed in accordance with the guidelines for Animal Care and Use outlined by the Committee of Wenzhou Medical University. Human CEP tissue collection and experiments that involved human CEP were approved by the Second Affiliated Hospital and Yuying Children's Hospital of Wenzhou Medical University Ethics Committee and followed the guidelines of the Helsinki Declaration [20].
2.2. Reagents and antibodies
Recombinant rat IL-1β was obtained from Peprotech (Rocky Hill, NJ, USA). EPZ005687 was obtained from MedChemExpress (1396772-26-1). Antibodies against EZH2 and Sox-9 were purchased from Cell Signaling Technology (Beverly, MA, USA). Antibodies against H3K27me3 and normal rabbit IgG were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Antibodies against β-actin, aggrecan, Collagen II, ADAMTS5 and MMP13 were purchased from Abcam (Cambridge, MA, USA).
2.3. Human cartilaginous endplate and endplate chondrocytes culture
The normal human CEP tissue (Pfirrmann grades I, n = 4) was obtained from patients undergoing internal fixation for the treatment of lumbar fracture. The degenerated CEP tissues from IVDD patients (Pfirrmann grades II, n = 5; Pfirrmann grades III, n = 4; Pfirrmann grades V, n = 5) were collected for further experiments according to the Pfirrmann grading scale. There were no other complications related to IVDD, such as diabetes mellitus, in the patients whose CEP tissues were collected during surgery. CEP tissues were cut into 5-μm sagittal sections and embedded in paraffin for histological analysis. To obtain primary human endplate chondrocytes (EPCs), hyaline cartilage was cut into 1 mm3 and washed three times with phosphate-buffered saline (PBS) before digestion with 0.25% type II collagenase for 4 h at 37 °C. After washing in PBS and resuspension, chondrocytes were cultured in a six-well plate at a seeding density of 2 × 105 cells per ml in DMEM/ F12 supplemented with 10% FBS and 1% antibiotic in 5% CO2 at 37 °C. Chondrocytes no later than first passage were used for the experiments.
2.4. Primary rat endplate chondrocytes culture
Cartilaginous endplate tissues were collected from the tails of 2-week-old pups (Sprague–Dawley rats, either sex) under a dissecting microscope. The tissues were digested in 2 mg/mL 0.1% type II collagenase for 4 h at 37 °C. The digested cartilage tissues were then incubated in DMEM (Gibco, Invitrogen, Grand Island, NY, USA) with 10% fetal bovine serum (FBS; HyClone, Thermo Scientific, Logan, UT, USA) and antibiotics (1% penicillin/ streptomycin) and the mixture was maintained in 5% CO2 at 37 °C. The cells were harvested using 0.25% trypsin EDTA (Gibco, Invitrogen) after they had formed a confluent. EPCs were added into 10-cm culture plates at the appropriate density. The complete medium was changed every day and the first two and three passage EPCs were used for the subsequent experiments. The chondrocytes were cultured in an incubator maintained with 5% CO2 at 37 °C.
2.5. Lentivirus transfection
Lenti-EZH2-control, Lenti-EZH2, Lenti-shEZH2, Lenti-Sox-9-control and Lenti-Sox-9 lentiviral particles were produced by triple transfections of 293T cells (Invitrogen, Carlsbad, USA) with the vectors of pLVX-EZH2-control, pLVX-EZH2, pLVX-shEZH2, pLVX-Sox-9-control or pLVX-Sox-9, respectively. The EPCs were transfected with the lentivirus and siRNA at a confluence of 30–50%; >95% of the cells were viable after 12 h. The medium was changed, the cells incubated for 3 more days, and passaged for further experiments. The efficacy of transfection was measured via western blotting.
2.6. Western blotting
EPCs were lysed in ice-cold RIPA with 1 mM PMSF (phenylmethanesulfonyl fluoride, Beyotime). The protein concentration in the samples was measured using the BCA protein assay kit (Beyotime). The proteins were separated via sodium dodecyl sulfate–polyacrylamide gel electrophoresis and then transferred to a polyvinylidene difluoride membrane (Millipore, USA) followed by blocking with 5% nonfat milk. The bands were subsequently probed with primary antibodies specific to EZH2 (1:1000), Sox-9 (1:1000), H3K27me3 (1:1000), aggrecan (1:1000), Collagen II (1:1000), ADAMTS5 (1:1000), MMP13 (1:1000), and β-actin (1:1000) overnight at 4 °C, prior to incubation with the respective secondary antibodies. Finally, the intensity of the bands was quantified using Image Lab 3.0 software (Bio-Rad).
2.7. Immunofluorescence
EPCs were washed in PBS, fixed in 4% paraformaldehyde and permeated in 0.1% Triton X-100 for 15 min. The cells were then blocked with 5% bovine serum albumin for 1 h at 37 °C, rinsed with PBS and incubated with primary antibodies in a humid chamber overnight at 4 °C. The cells were washed and incubated with secondary antibodies for 1 h at room temperature and labeled with DAPI for 5 min. Images of each slides were obtained randomly with a fluorescence microscope (Olympus Inc., Tokyo, Japan). Images were prepared for presentation using Adobe Photoshop 6.0 (San Jose, CA, USA).
2.8. Quantitative RT-PCR
Total RNA was isolated using TRIzol (Invitrogen) according to the manufacturer's instructions. cDNA was synthesized from 1 μg of RNA via the One Step RT-PCR Kit (TaKaRa). Quantitative real-time PCR was performed using the iQTM SYBR Green Supermix PCR kit with the iCycler apparatus system (Bio-Rad). The primer sequences were as follows: for EZH2, collagen II and aggrecan, GAPDH was used as the invariant housekeeping gene internal control.
2.9. Chromatin immunoprecipitation analysis
Chondrocytes were fixed with 1% formaldehyde and lysed. Chromatin was sheared through sonication and pretreated with normal rabbit serum and protein A beads (Upstate/Millipore, Zug, Switzerland). For ChIP qPCR, 1–2 μg of antibody [anti-H3K27me3 or normal IgG] was added. Chromatin was precipitated with protein A beads, washed, eluted, reverse-crosslinked, digested with proteinase K and analyzed by quantitative real-time PCR.
2.10. Animal model
6-week old adult male Sprague Dawley rats were anesthetized by intraperitoneal injection with 2% (w/v) pentobarbital (40 mg/kg) and randomly divided into 3 groups: SHAM + NS, IVDD + NS, and IVDD + siEZH2. As described in previous studies, the experimental level rat tail disc (Co7/8) was located by digital palpation on the coccygeal vertebrae and confirmed by counting the vertebrae from the sacral region in a trial radiograph [21]. Needles (27 G) were used to puncture the whole layer of annulus fibrosus though the tail skin. To ensure that the needle puncture was not too deep, the length of the needle was decided according to the annulus fibrosus and the nucleus pulposus dimensions, which were measured in the preliminary experiment and found to be about 4 mm. All the needles were kept in the disc for 1 min. The si-EZH2 or saline constructs were injected into the disc. The SHAM group received an injection of saline only. After the surgery, the rats were treated as described until sacrifice.
2.11. Histological assessment
The rats were killed by an intraperitoneal overdose injection of 10% chloral hydrate and the tails were harvested. The human CEP tissues were collected during operation. The specimens were decalcified and fixed in formaldehyde, dehydrated and embedded in paraffin. The tissues were cut into 5-μm sections. Slides of each disc were stained with hematoxylin-eosin (HE staining) and Safranin-Orange (SO staining). Images were captured using a light microscope.
2.12. Immunohistochemical analysis
The sections embedded in paraffin were deparaffinized and rehydrated while endogenous peroxidase was blocked by 3% hydrogen peroxide. The sections were incubated with 0.4% pepsin (Sangon Biotech, Shanghai, China) in 5 mM HCl at 37 °C for 20 min for antigen retrieval. The sections were incubated with 5% bovine serum albumin for 30 min at room temperature, followed by incubation with primary antibody overnight at 4 °C, and finally with HRP conjugated secondary antibody. The rate of positive cells in each section was quantitated by observers who were blinded to the experimental groups. Five mice of each group were used for quantitative analysis.
2.13. Magnetic resonance imaging
Magnetic resonance imaging (MRI) of the coccyx was performed 8 weeks after surgery. All rats were anesthetized throughout the examinations, with their tails straightened. Five rats of each group (total, 15) were subjected to sagittal and horizontal T2-weighted imaging with a 3.0-T clinical magnet (Philips Intera Achieva 3.0MR). T2-weighted sections were set as follows: a fast-spin echo sequence with a time-to-repetition of 5400 ms and a time-to-echo of 920 ms; a 320 (h) × 256 (v) matrix; a field of view of 260°; and four excitations. The section thickness was 2 mm and the gap was 0 mm. All MR images were analyzed in a blinded manner using the IVDD classification by Pfirrmann et al. [22].
2.14. Statistical analysis
Statistical analysis was performed using the Stata 20.0 software (IBM, Armonk, NY, USA). Data are expressed as the mean ± standard deviation (SD). Data were analyzed by one-way analysis of variance (ANOVA) followed by Tukey's test for comparison between the two groups. Differences were considered significant when P < 0.05.
3. Results
3.1. The up-regulation of EZH2 in human degenerated CEP
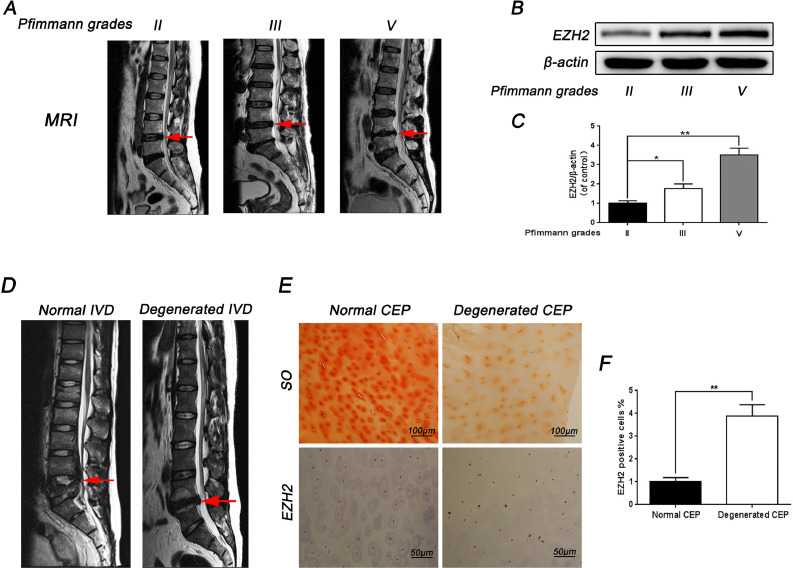
To ascertain whether EZH2 expression was correlated with CEP degeneration, immunohistochemical analysis of human CEP tissue was used to compare the level of EZH2 in normal and degenerated human CEP. It was found that the expression of EZH2 increased in degenerated human CEP (Fig. 1D and 1E). Results of immunohistochemical staining further confirmed the effect of EZH2 (Fig. 1E). In addition, human CEP tissue obtained from patients with different degenerative degrees were subjected to western blotting to measure the protein level of EZH2 (Fig. 1A). As shown in Fig. 1B and 1C, it was found that the expression level of EZH2 in CEP samples increased with the degree of disc degeneration. Thus, it was concluded that EZH2 could be associated with CEP degeneration.
Fig. 1.
The expression of EZH2 increased in human degenerated CEP. (A) Representative MR images of three different degrees of IVDD patients (Pfirrmann grades: II, III, V). (B) The expression of EZH2 from EPCs of different degrees of IVDD patients was analyzed by western blot. (C) Quantification of EZH2 immunoblots of EPCs of different degrees of IVDD patients. (D) Representative MR images of normal CEP of lumbar fracture patients (Pfirrmann grades I) and degenerated CEP of IVDD patients (Pfirrmann grades V). (E) SO staining of normal and degenerated CEP, and IHC staining for EZH2 of human normal and degenerated CEP tissue. (F) Percentages of EZH2-positive cells detected in human normal and degenerated CEP tissue. All experiments were performed three times, and data are reported as the mean ± SD. *P < 0.05, **P < 0.01.
3.2. The expression of EZH2 is increased in IL-1β-induced rat EPCs and rat IVDD model
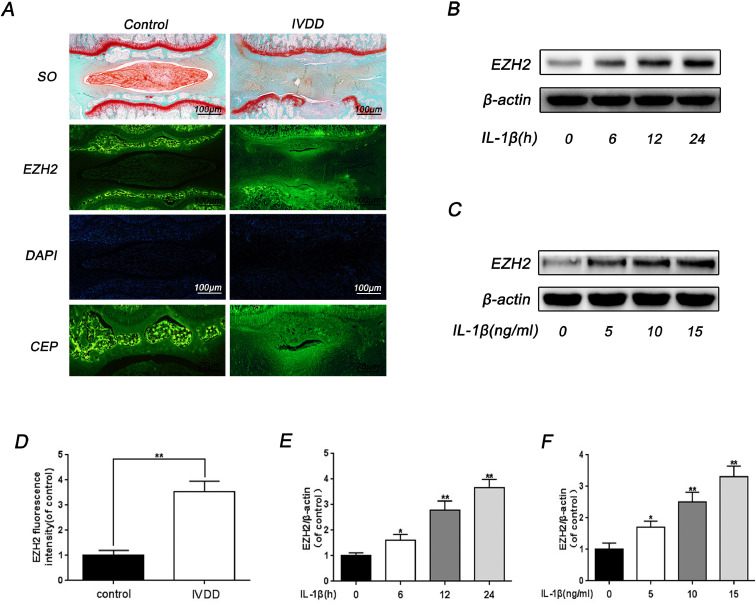
The pro-inflammatory cytokine IL-1β was closely related with degenerated CEP and it was found that IL-1β treatment upregulated the protein level of EZH2 both in a dose- and time- dependent manner in rat EPCs (Fig. 2B, 2C, 2D and 2E). Moreover, the role of EZH2 in CEP was explored using immunofluorescence on rat IVDD model. Interestingly, it was found that a percentage of EZH2 positive EPCs increased in CEP from IVDD tissue (Fig. 2A and 2D). These results are basically consistent with studies in human CEP, indicating that EZH2 is activated and up-regulated in degenerated CEP.
Fig. 2.
The expression of EZH2 increased in IL-1β-induced rat EPCs and rat IVDD model. (A) Representative SO staining and immunofluorescence staining of EZH2 in rat CEP tissue (Scale bar: 100 μm in SO staining, Scale bar: 100 μm or 75 μm in immunofluorescence staining). (B) The effect of different durations of treatment with IL-1β on rat EPCs. The EPCs were incubated with 10 ng/ml IL-1β for 0, 6, 12, 24 h. (C) The effect of different concentrations of IL-1β on rat EPCs. The EPCs were incubated with 0, 5, 10, 15 ng/ml IL-1β for 24 h. (D) Quantitation of immunofluorescence staining of EZH2 in the CEP region was detected by image J. (E, F) Quantification of EZH2 immunoblots of different concentrations and different durations of treatment with IL-1β on rat EPCs. All experiments were performed three times, and data are reported as the mean ± SD. *P < 0.05, **P < 0.01.
3.3. Effect of EZH2 on the expression of degeneration-related genes and Sox-9 in rat EPCs
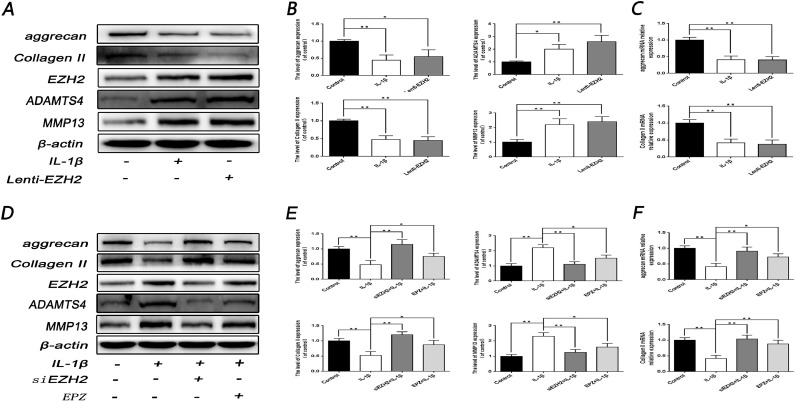
Since chondrocytes degeneration was correlated with anabolic genes, such as collagen II and aggrecan, and catabolic genes, such as ADAMTS5 and MMP13, the relationship between EZH2 and the degeneration-related genes was examined through Western blotting. It was firstly revealed that the overexpression of EZH2 significantly induced the decrease of collagen II and aggrecan, and an increase of ADTAMTS5 as well as MMP13 in protein and mRNA level, which was consistent with the result of IL-1β treatment (P < 0.05) (Fig. 3A, 3B and C3). Conversely, up-regulation of ADTAMTS5 and MMP13, as well as down-regulation of collagen II and aggrecan, induced by IL-1β stimulation, were reversed by EZH2 inhibitor EPZ005687 (5.6 μmol/L) and EZH2 knockdown (P < 0.05) (Fig. 3D, 3E and 3F).
Fig. 3.
Effect of EZH2 on the expression of degeneration-related genes in rat EPCs. (A, B) Western blot and its quantification showed the level of aggrecan and Collagen II, MMP13 and ADAMTS4 after treatment with IL-1β or Lenti-EZH2. (C) The mRNA expression of aggrecan and Collagen II, MMP13 and ADAMTS4 were measured by real-time PCR after treatment with IL-1β or Lenti-EZH2. (D, E) Western blot and its quantification showed the level of aggrecan, Collagen II, MMP13 and ADAMTS4 after treatment with IL-1β or siEZH2 or EPZ or combination. (F) The mRNA expression of aggrecan and Collagen II, MMP13 and ADAMTS4 were measured by real-time PCR after treatment with IL-1β or siEZH2 or EPZ or combination. All experiments were performed three times, and data are reported as the mean ± SD. *P < 0.05, **P < 0.01.
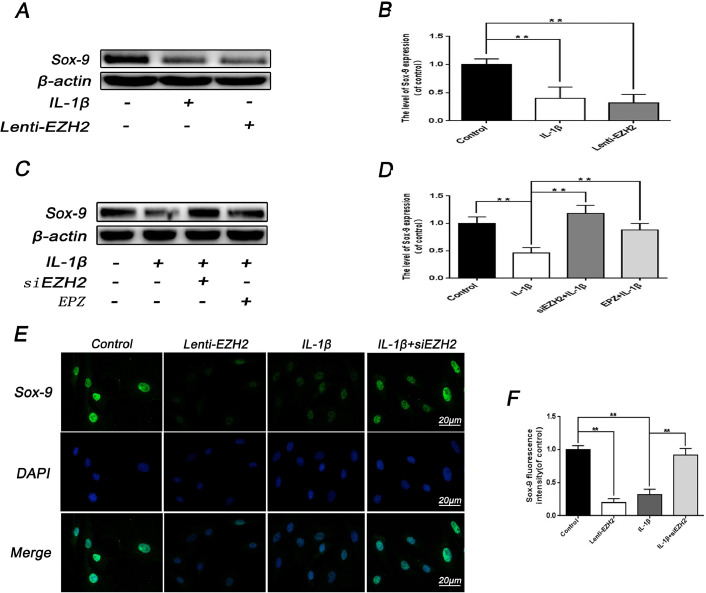
It is generally accepted that Sox-9 plays a crucial role in the extracellular matrix of cartilage, which is potentially related to the upregulation of collagen II and aggrecan [23]. To investigate the relationship between EZH2 and Sox-9, western blotting analysis was performed. Results revealed that Lenti-EZH2 treatment exerted lower Sox-9 lever compared with IL-1β-stimulation (Fig. 4A and 4B), and the treatment of siEZH2 and EPZ005687 reversed the effect of IL-1β on Sox-9 expression (Fig. 4C and 4D). Immunofluorescence staining of Sox-9 further confirmed the effects of EZH2 on Sox-9 (Fig. 4E and 4F).
Fig. 4.
Effect of EZH2 on the expression of Sox-9 in rat EPCs. (A, B) Western blot and its quantification showed the level of Sox-9 after treatment with IL-1β or Lenti-EZH2. (C, D) Western blot and its quantification showed the level of Sox-9 after treatment with IL-1β or siEZH2 or EPZ or combination. (E) Representative image of immunofluorescence staining of Sox-9 in rat EPCs after treatment with IL-1β or siEZH2 or Lenti-EZH2 or combination. Scale bar: 20 μM. (F) Quantitative analysis of immunofluorescence staining of Sox-9 was detected by image J. All experiments were performed three times, and data are reported as the mean ± SD. **P < 0.01.
These findings indicate that EZH2 is strongly linked to CEP degeneration, and its internal mechanism may contribute to Sox-9 regulation.
3.4. Overexpression of Sox-9 abolishes the effect of EZH2
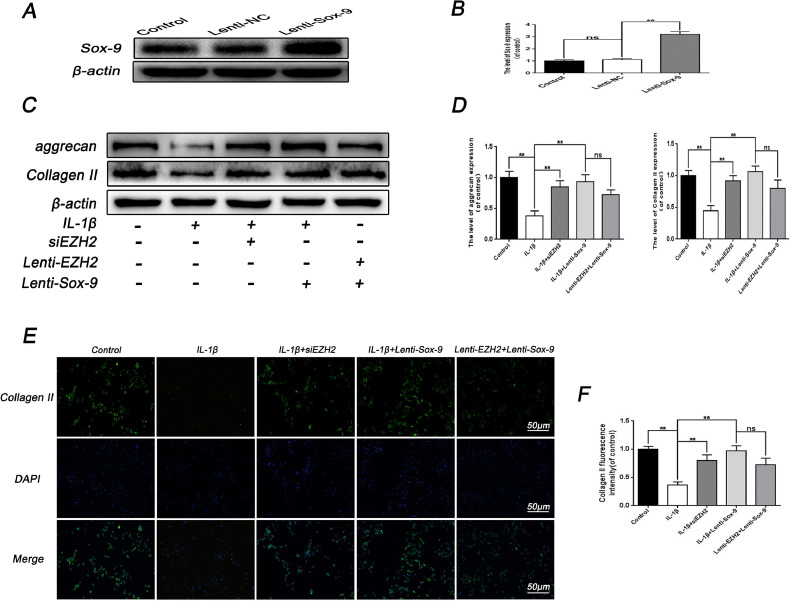
To confirm the mechanism of interaction between EZH2 and Sox-9, Lenti-Sox-9 was used to overexpress Sox-9 protein in EPCs (Fig. 5A and 5B). The expression of Sox-9 was dramatically increased after Lenti-Sox-9 transfection in EPCs. It was found that Lenti-Sox-9 significantly prevented the IL-1β-mediated decrease in aggrecan and collagen II expression, which was consistent with the effect of siEZH2 (Fig. 5C and 5D). Subsequently, western blotting analysis revealed that co-incubation of Lenti-Sox-9 and Lenti-EZH2 increased collagen II and aggrecan expression compared with the IL-1β stimulation. Notably, immunofluorescence staining of collagen II further confirmed the effect of Lenti-Sox-9. In summary, these results imply that the transfection of Lenti-Sox-9 could reverse the effect of EZH2 in EPCs, and ascertain mutual effect of Sox-9 and EZH2.
Fig. 5.
Sox-9 overexpression regulates the effect of EZH2 in EPCs. (A, B) Successful transfection of Lenti-Sox-9 in rat EPCs was analyzed by western blot and its quantification. (C) The expression of aggrecan and Collagen II were analyzed by western blot after treatment with IL-1β or siEZH2 or Lenti-EZH2 or Lenti-Sox-9 or combination. (D) Quantification of aggrecan and Collagen II immunoblots after treatment with IL-1β or siEZH2 or Lenti-EZH2 or Lenti-Sox-9 or combination. (E) Immunofluorescence of Collagen II was observed by fluorescence microscope (OLYMPUS). Scale bar: 50 μM. (F) Quantitation of immunofluorescence staining of Collagen II was detected by image J. All experiments were performed three times, and data are reported as the mean ± SD. **P < 0.01.
3.5. Inhibition of EZH2 ameliorates the H3K27me3 occupation within Sox-9 promoter
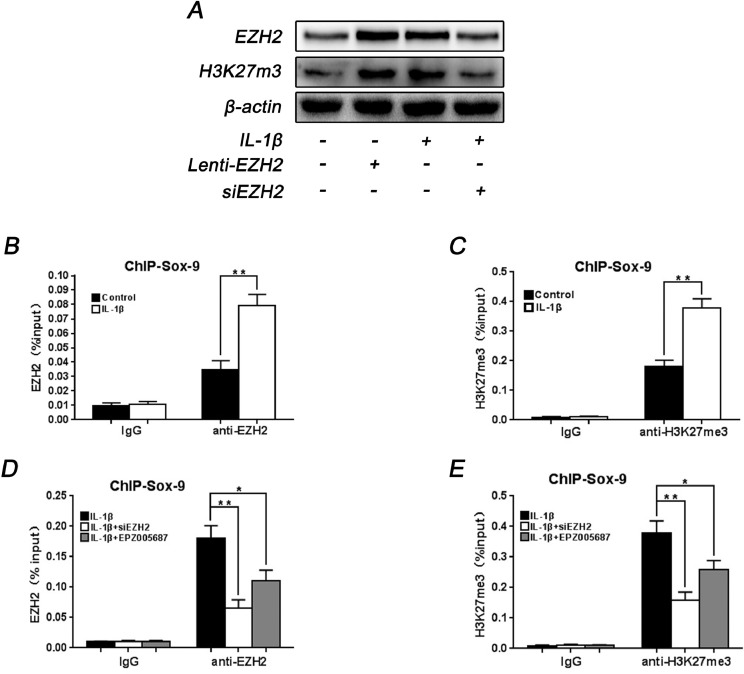
To evaluate epigenetic changes, the variation of histone methylation was investigated. Several studies demonstrated that EZH2 can induce methylation of H3K27, a silenced chromatin marker. According to Western blot analysis, H3K27 methylation increased following the treatment of Lenti-EZH2 in accordance with the effect of IL-1β, while siEZH2 transfection decreased the level of H3K27me3 induced by IL-1β (Fig. 6A), indicating the effect of EZH2 on H3K27 methylation in rat EPCs. To further examine the binding of EZH2 to Sox-9 promoters in normal and IL-1β-stimulated rat EPCs, ChIP analysis was carried out to measure the binding affinity. When promoters of Sox-9 were ChIP-ed with anti-EZH2 antibody, there was higher affinity of EZH2 within Sox-9 promoter in IL-1β-stimulated rat EPCs (Fig. 6B). Subsequently, ChIP qPCR was used to verify the presence of H3K27me3 on the Sox-9 promoter. Compared with normal rat EPCs, repressive H3K27me3 was markedly elevated at the Sox-9 promoter areas in IL-1β-stimulated EPCs (Fig. 6C), indicating the vital role of repressive H3K27me3 in Sox-9 promoter in degenerated rat EPCs.
Fig. 6.
Effect of EZH2 on H3K27me3 occupation within Sox-9 promoter in rat EPCs. (A) Western blot showed the expression of EZH2 and H3K27me3 after treatment with IL-1β or siEZH2 or Lenti-EZH2 or combination. (B) The promoter of Sox-9 was ChIP-ed with anti-EZH2 antibody or IgG control. IL-1β-induced EPCs showed a higher level of EZH2 occupation at the promoter of Sox-9. (C) The promoter of Sox-9 was ChIP-ed with anti-H3K27me3 antibody or IgG control. The repressive trimethylation of H3K27 at the Sox-9 promoter was higher in IL-1β-induced EPCs compared with normal EPCs. (D) Inhibition of EZH2 reduced EZH2 binding to the promoters of Sox-9 in IL-1β-stimulated EPCs. (E) Inhibition of EZH2 decreased H3K27me3 levels at the promoters of Sox-9 in IL-1β-stimulated EPCs. All experiments were performed three times, and data are reported as the mean ± SD. *P < 0.05, **P < 0.01.
Afterwards, the effect of EZH2 on Sox-9 activity in degenerated rat EPCs was explored via ChIP analysis. The results showed that EZH2 binding to the promoter of Sox-9 decreased dramatically when treated with siEZH2 or EPZ005687 in IL-1β-stimulated rat EPCs. Interestingly, it was also found that inhibition of EZH2 in IL-1β-stimulated rat EPCs decreased the level of repressive H3K27me3 in the Sox-9 promoter. Collectively, these results support the hypothesis that depletion of EZH2 can reverse the degeneration of CEP via Sox-9 regulation, and increase Sox-9 expression by decreasing the level of H3K27me3 in Sox-9 promoter.
3.6. EZH2 knockdown prevents CEP and disc degeneration in vivo
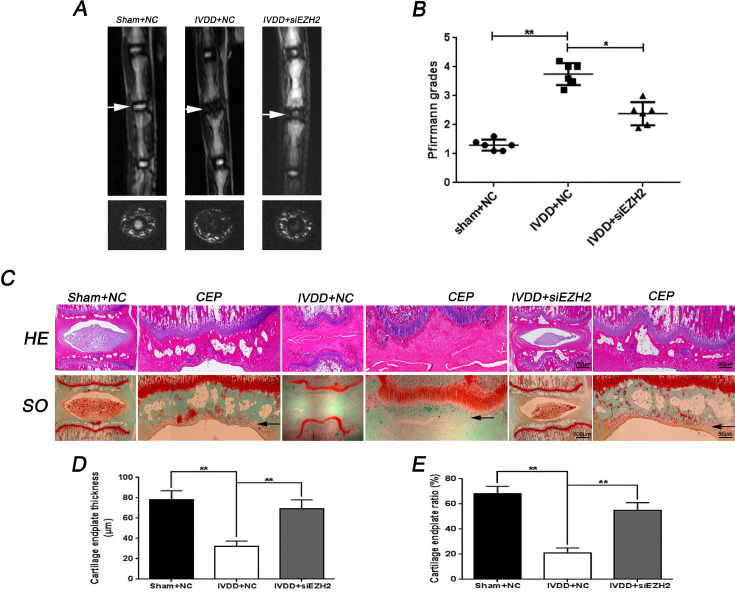
Finally, the potential therapeutic effect of EZH2 knockdown on IVDD progression in vivo was evaluate. A rat IVDD model was established through disc puncture surgery. MRI was performed at 8 weeks to assess the level of disc degeneration in rats. MR image revealed that the intensity of T2-weighted signal intensities after puncture induction were higher in the siEZH2 treated group than in the control group (Fig. 7A). In addition, the Pfirrmann MRI grade scores, which indicate the degree of disc degeneration, were significantly lower in rats transfected with siEZH2 than in the controls (Fig. 7B). Histological analysis of IVDD was performed by HE staining and SO staining. HE staining results showed that the structure of CEP and nucleus pulposus disappeared, and the fibrous ring was markedly irregular in IVDD + NC group, while in IVDD + siEZH2 group CEP as well as nucleus pulposus tissues were better preserved and regular fibrous ring were still found within the discs (Fig. 7C). The SO staining demonstrated that loss of CEP and nucleus pulposus tissue was alleviated by siEZH2 transfection compared to the IVDD + NC group (Fig. 7C). Furthermore, the CEP thickness and the proportion of EPCs in the CEP areas were significantly higher after siEZH2 treatment compared with the IVDD + NC group (Fig. 7D and 7E).
Fig. 7.
Inhibition of EZH2 ameliorates CEP and disc degeneration in rat IVDD model in vivo. (A) T2-weighted MRI of a rat tail with a needle-punctured disc (the white arrow) at 8 weeks postoperatively in each group (Sham + NC, IVDD + NC, IVDD + siEZH2). (B) The Pfirrmann MRI grade scores were acquired from three groups at week 8. (C) Representative HE and SO staining of disc and CEP in different group (Scale bar: 100 μM in general disc, Scale bar: 50 μM in CEP). (D, E) The quantitation of CEP thickness and chondrocyte/CEP area in different group. All experiments were performed three times, and data are reported as the mean ± SD. *P < 0.05, **P < 0.01.
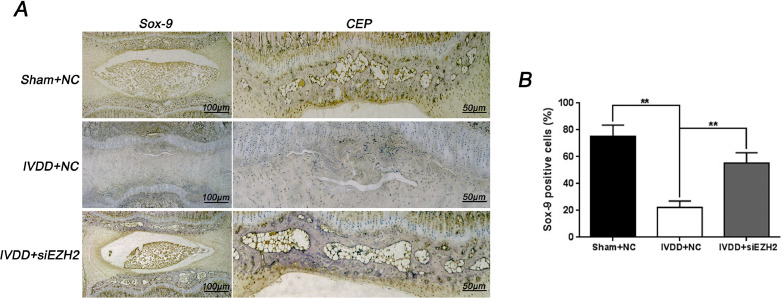
Based on the vitro study results, the activation of Sox-9 by depletion of EZH2 in vivo was further verify. Consistent with in vitro findings, immunohistochemical staining and its corresponding quantification showed that knockdown of EZH2 promoted the Sox-9 expression in CEP and disc tissue (8A and 8B). In summary, these results provide a strong evidence regarding the protective effect of EZH2 inhibition on CEP and disc degeneration in puncture-induced rat model.
4. Discussion
CEP degeneration has been proved to be a predominant pathogenic process leading to IVDD. CEP degeneration may impede nutrient delivery and affects mechanical transduction, and reduced nutrient supply is universally acknowledged to play a vital role in disc degeneration [24]. Jason et al. demonstrated that CEP composition dramatically affected NP cell function and survival, while its deficit resulted in poor diffusion that blocked solute passage and impeded the normal nutrient supply which could impede successful biologic therapies during the development of IVDD [25]. Similarly, Adams suggested that insufficient nutrition would impair the disc's ability to respond to increased loading or injury and make the disc vulnerable to degeneration [26]. Luoma et al. reported that patients with Modic type I change in CEP were more vulnerable to IVDD during 1-year follow-up, indicating that CEP degeneration could be a potential sign of disc degeneration [27]. The molecular mechanism was also explored, and the results showed that CEP degeneration may upregulate inflammatory cytokines and gene expression of matrix degrading enzymes via secretion of inflammatory cytokines and thereby induce disc degeneration [28]. Furthermore, IVDD models were also established by inducing ischemic sub-endplates by blocking the main blood supply gateway [29]. Hence inhibition of CEP degeneration may serve as an effective treatment target for IVDD.
Epigenetic mechanisms play an essential role in embryonic development through gene regulation [30]. Over the past few decades, a large number of researches have demonstrated that aberrant epigenetic modification is always associated with many diseases, including cancer, Alzheimer's disease and many other age-related diseases, providing mechanistic insights into the occurrence and treatment of diseases [31], [32]. It is well known that, histone modifications are deemed as good epigenetic indicators, reflecting gene activation or repression, which can be medicated by specific methyltransferases and demethylases [33]. Methylation of histones H3K27 has been functionally linked to gene repression [34]. In particular, EZH2, a major histone methyltransferase for H3K27, serves as an essential force in the epigenetic regulation responsible for inflammation, autoimmunity and a wide range of malignancies [35], [36]. Inhibition of EZH2 could promote human embryonic stem cell differentiation into mesoderm and more mesenchymal stem cells by reducing H3K27me3 [37]. Recently, EZH2 has been reported to specifically regulate chondrocyte proliferation and hypertrophy [38]. In addition, our previous studies demonstrated that the level of EZH2 was hyperactivated in chondrocytes of OA patients compared to normal humans [19]. The present study investigated the role of EZH2 in CEP degeneration. More importantly, it was found that EZH2 expression increased in human and rat degenerated CEP tissue, and the elevation of the level of EZH2 was induced by IL-1β in a time- and dose-dependent manner in EPCs. Additionally, IL-1β could enhance the level of ADTAMTS4 and MMP13 and lower the level of collagen II and aggrecan, in accordance with the transfection of Lenti-EZH2 in EPCs, and its effect was reversed by siEZH2, indicating the pivotal role of EZH2 during pathogenesis of CEP degeneration. Nevertheless, the underlying molecular mechanism of EZH2 on CEP degeneration requires further clarification.
Fig. 8.
EZH2 inhibition promotes Sox-9 activation during disc and CEP in vivo. (A) Immunohistochemical staining of Sox-9 expression in the disc and CEP samples of each group (Sham + NC, IVDD + NC, IVDD + siEZH2). (Scale bar: 100 μM in general disc, Scale bar: 50 μM in CEP). (B) Relative positive cells of Sox-9 in CEP sample was quantified by image pro plus. Each group consisted of 6 discs sample. All experiments were performed three times, and data are reported as the mean ± SD. **P < 0.01.
Sox-9 is a cartilage master regulator transcription factor responsible for chondrogenesis and chondrocyte differentiation [8]. Sox-9 increases the expression of aggrecan and collagen II, which are the components of cartilage [23]. Previous studies revealed that the mRNA levels of Sox-9 were significantly increased when treated with EZH2 inhibitor [17]. Interestingly, our results revealed that overexpression of EZH2 inhibited the expression of Sox-9 in EPCs, which was in agreement with our previous observation in osteoarthritis chondrocytes [19]. Subsequently, the results revealed that overexpression of Sox-9 reversed the effect of Lenti-EZH2 on expression aggrecan and collagen II. Therefore, EZH2 indeed targeted Sox-9 gene during the pathogenetic process of CEP degeneration.
Methylation of H3K27 in Sox-9 promoter has already been recognized to a crucial role in osteoarthritis chondrocytes [14]. Kumar et al. demonstrated that the expression of Sox-9 in limb bud mesenchymal cells cultured in chondrogenic medium was high due to decreased levels of H3K27me3 in Sox-9 promoter [13]. Wand et al. found that KDM6A can promote chondrogenic differentiation of periodontal ligament stem cell by demethylation of H3K27me3 in Sox-9 promoter, while EZH2 inhibitor can rescue impaired chondrogenic potential by decreasing H3K27me3 after KDM6A depletion [39]. However, whether EZH2 regulates the expression of Sox-9 through targeting H3K27 in Sox-9 promoter during CEP degeneration is unknown and needs further exploration. Based on these results, methylation of H3K27 during Sox-9 promoter maintained a high expression status in degenerated human EPCs compared with normal human EPCs. A higher affinity of EZH2 within Sox-9 promoter was observed in degenerated human EPCs. Silencing of EZH2 may trigger H3K27me3 demethylation at the Sox-9 promoter areas, leading to the high expression of Sox-9. However, overexpression of EZH2 deactivated Sox-9 by catalyzing H3K27me3 methylation in the Sox-9 promoter areas. Herein, we show that inhibition of EZH2 may exert its protective role by removing H3K27me3 from Sox-9 promoter.
Both NF-κB and Wnt/β-catenin signaling have been recognized as master regulators of inflammation and catabolism in several musculoskeletal disorders, such as osteoarthritis and disc degeneration [40], [41], [42], [43]. The interaction between EZH2 and NF-κB or Wnt/β-catenin signaling have been extensively studied. Lee et al. demonstrated that inhibition of EZH2 could attenuate the progression of breast cancers through regulating NF-κB-dependent transcription [44]. Iannetti et al. verified that EZH2 was a direct NF-κB target gene, which was involved in cell senescence and tumorigenesis [45]. In addition, Cheng et al. found that EZH2-mediated epigenetic silencing could lead to the Wnt/β-catenin signaling dysregulation and consequential proliferation of hepatocellular carcinoma cells, which was considered to be a novel therapeutic target [46]. It was previously revealed that EZH2 depletion could reduce the expression of catabolic genes and prevent osteoarthritis development through the Wnt/β-catenin signaling pathway. [19] The present work shows that overexpression of EZH2 upregulates the level of MMP13 and ADTAMTS4 while depletion of EZH2 decreases the IL-1β-induced expression of MMP13 and ADTAMTS4 in endplate chondrocytes. This may be attributed to the regulation of NF-κB or Wnt/β-catenin signaling.
In addition to the in vitro study, rat annulus needle puncture models of IVDD were used to evaluate the effect of siEZH2 transfection in vivo. As a result, EZH2 silencing probably mitigated CEP degeneration. Interestingly, reduced nucleus pulposus loss and decreased irregularity of the fibrous ring were revealed after siEZH2 treatment, suggesting that silencing of EZH2 alleviated the disc degeneration by targeting nucleus pulposus. Collectively, the in vivo results showed that EZH2 depletion may contribute to IVDD therapeutics through targeting CEP and regulating nucleus pulposus.
In conclusion, the current study demonstrates EZH2 is highly expressed in both human and rat degenerated CEP tissue. Depletion of EZH2 rescues CEP degeneration by activating the expression of Sox-9 and further reducing H3K27me3 during its promoter in EPCs. Meanwhile, EZH2 silencing can ameliorate CEP degeneration and nucleus pulposus loss in rats annulus needle puncture models. In summary, inhibition of EZH2 could be a potential therapeutic target for the treatment of IVDD.
Author contributions
Chao Jiang designed the research methods, performed the experiments, analyzed the data, and drafted the manuscript. Qiang Guo, Yu Jin, Jia-Jing Xu, Ze-Ming Sun, Ding-Chao Zhu, Jia-Hao Lin and Nai-Feng Tian participated in the experiments. Liao-Jun Sun, Xiao-Lei Zhang and Yao-Sen Wu designed the research and revised the manuscript. All authors have read and approved the final manuscript.
Declaration of Competing Interest
The authors declare that they have no competing interests.
Acknowledgments
This study was funded by the Natural Science Foundation of Zhejiang Province (Y16H060034). The funder had no involvement in the study design, data analysis and interpretation of the results. We thank all the colleagues for their invaluable assistance during the execution of this study.
Contributor Information
Liao-Jun Sun, Email: sunliaojun@wmu.edu.cn.
Xiao-Lei Zhang, Email: zhangxiaolei@wmu.edu.cn.
Yao-Sen Wu, Email: wuyaosen@wmu.edu.cn.
References
- 1.Deyo R.A., Mirza S.K., CLINICAL PRACTICE Herniated lumbar intervertebral disk. N Engl J Med. 2016;374:1763–1772. doi: 10.1056/NEJMcp1512658. [DOI] [PubMed] [Google Scholar]
- 2.Vergroesen P.P., Kingma I., Emanuel K.S., Hoogendoorn R.J., Welting T.J. Mechanics and biology in intervertebral disc degeneration: a vicious circle. Osteoarthritis Cartilage. 2015;23:1057–1070. doi: 10.1016/j.joca.2015.03.028. [DOI] [PubMed] [Google Scholar]
- 3.Sowa G., Vadalà G., Studer R., Kompel J., Iucu C. Characterization of intervertebral disc aging: longitudinal analysis of a rabbit model by magnetic resonance imaging, histology, and gene expression. Spine (Phila Pa 1976) 2008;33:1821–1828. doi: 10.1097/BRS.0b013e31817e2ce3. [DOI] [PubMed] [Google Scholar]
- 4.Zhu Q., Gao X., Levene H.B., Brown M.D., Gu W. Influences of nutrition supply and pathways on the degenerative patterns in human intervertebral disc. Spine (Phila Pa 1976) 2016;41:568–576. doi: 10.1097/BRS.0000000000001292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grunhagen T., Shirazi-Adl A., Fairbank J.C., Urban J.P. Intervertebral disk nutrition: a review of factors influencing concentrations of nutrients and metabolites. Orthop Clin North Am. 2011;42:465–477. doi: 10.1016/j.ocl.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 6.Rade M., Määttä J.H., Freidin M.B., Airaksinen O., Karppinen J. Vertebral endplate defect as initiating factor in intervertebral disc degeneration: strong association between endplate defect and disc degeneration in the general population. Spine (Phila Pa 1976) 2018;43:412–419. doi: 10.1097/BRS.0000000000002352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xiao L., Ni C., Shi J., Wang Z., Wang S. Analysis of correlation between vertebral endplate change and lumbar disc degeneration. Med Sci Monit. 2017;23:4932–4938. doi: 10.12659/MSM.904315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsuda M., Takahashi S., Takahashi Y., Asahara H. Transcriptional co-activators CREB-binding protein and p300 regulate chondrocyte-specific gene expression via association with Sox9. J Biol Chem. 2003;278:27224–27229. doi: 10.1074/jbc.M303471200. [DOI] [PubMed] [Google Scholar]
- 9.Cha B.H., Kim J.H., Kang S.W., Do H.J., Jang J.W. Cartilage tissue formation from dedifferentiated chondrocytes by codelivery of BMP-2 and SOX-9 genes encoding bicistronic vector. Cell Transplant. 2013;22:1519–1528. doi: 10.3727/096368912X647261. [DOI] [PubMed] [Google Scholar]
- 10.Gruber H.E., Norton H.J., Ingram J.A., Hanley E.N., Jr The SOX9 transcription factor in the human disc: decreased immunolocalization with age and disc degeneration. Spine (Phila Pa 1976) 2005;30:625–630. doi: 10.1097/01.brs.0000155420.01444.c6. [DOI] [PubMed] [Google Scholar]
- 11.Paul R., Haydon R.C., Cheng H., Ishikawa A., Nenadovich N. Potential use of Sox9 gene therapy for intervertebral degenerative disc disease. Spine (Phila Pa 1976) 2003;28:755–763. [PMC free article] [PubMed] [Google Scholar]
- 12.Jazirehi A.R., Arle D., Wenn P.B. Role of epigenetic modifications of Sox 9 in gastric carcinoma. Epigenomics. 2012;4:253. [PubMed] [Google Scholar]
- 13.Kumar D., Lassar A.B. Fibroblast growth factor maintains chondrogenic potential of limb bud mesenchymal cells by modulating DNMT3A recruitment. Cell Rep. 2014;8:1419–1431. doi: 10.1016/j.celrep.2014.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim K.I., Park Y.S., Im G.I. Changes in the epigenetic status of the SOX-9 promoter in human osteoarthritic cartilage. J Bone Miner Res. 2013;28:1050–1060. doi: 10.1002/jbmr.1843. [DOI] [PubMed] [Google Scholar]
- 15.Cao R., Wang L., Wang H., Xia L., Erdjument-Bromage H. Role of histone H3 lysine 27 methylation in polycomb-group silencing. Science. 2002;298:1039–1043. doi: 10.1126/science.1076997. [DOI] [PubMed] [Google Scholar]
- 16.Trenkmann M., Brock M., Gay R.E., Kolling C., Speich R. Expression and function of EZH2 in synovial fibroblasts: epigenetic repression of the WNT inhibitor SFRP1 in rheumatoid arthritis. Ann Rheum Dis. 2011;70:1482–1488. doi: 10.1136/ard.2010.143040. [DOI] [PubMed] [Google Scholar]
- 17.Aury-Landas J., Bazille C., Allas L., Bouhout S., Chesneau C. Anti-inflammatory and chondroprotective effects of the S-adenosylhomocysteine hydrolase inhibitor 3-Deazaneplanocin A, in human articular chondrocytes. Sci Rep. 2017;7:6483. doi: 10.1038/s41598-017-06913-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lian W.S., Ko J.Y., Wu R.W., Sun Y.C., Chen Y.S. MicroRNA-128a represses chondrocyte autophagy and exacerbates knee osteoarthritis by disrupting Atg12. Cell Death Dis. 2018;9:919. doi: 10.1038/s41419-018-0994-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen L., Wu Y., Wu Y., Wang Y., Sun L. The inhibition of EZH2 ameliorates osteoarthritis development through the WNT/β-catenin pathway. Sci Rep. 2016;6:29176. doi: 10.1038/srep29176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Medical. Association World Medical Association declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 21.Issy A.C., Castania V., Castania M., Salmon C.E., Nogueira-Barbosa M.H. Experimental model of intervertebral disc degeneration by needle puncture in Wistar rats. Braz J Med Biol Res. 2013;46:235–244. doi: 10.1590/1414-431X20122429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pfirrmann C.W., Metzdorf A., Zanetti M., Hodler J., Boos N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine (Phila Pa 1976) 2001;26:1873–1878. doi: 10.1097/00007632-200109010-00011. [DOI] [PubMed] [Google Scholar]
- 23.Kolettas E., Muir H.I., Barrett J.C., Hardingham T.E. Chondrocyte phenotype and cell survival are regulated by culture conditions and by specific cytokines through the expression of Sox-9 transcription factor. Rheumatology (Oxford) 2001;40:1146–1156. doi: 10.1093/rheumatology/40.10.1146. [DOI] [PubMed] [Google Scholar]
- 24.Zhu Q., Gao X., Levene H.B., Brown M.D., Gu W. Influences of nutrition supply and pathways on the degenerative patterns in human intervertebral disc. Spine (Phila Pa 1976) 2016;41:568–576. doi: 10.1097/BRS.0000000000001292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wong J., Sampson S.L., Bell-Briones H., Ouyang A., Lazar A.A. Nutrient supply and nucleus pulposus cell function: effects of the transport properties of the cartilage endplate and potential implications for intradiscal biologic therapy. Osteoarthritis Cartilage. 2019;27:956–964. doi: 10.1016/j.joca.2019.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zehra U., Flower L., Robson-Brown K., Adams M.A., Dolan P. Defects of the vertebral end plate: implications for disc degeneration depend on size. Spine J. 2017;17:727–737. doi: 10.1016/j.spinee.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 27.Kerttula L., Luoma K., Vehmas T., Grönblad M., Kääpä E. Modic type I change may predict rapid progressive, deforming disc degeneration: a prospective 1-year follow-up study. Eur Spine J. 2012;21:1135–1142. doi: 10.1007/s00586-012-2147-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neidlinger-Wilke C., Boldt A., Brochhausen C., Galbusera F., Carstens C. Molecular interactions between human cartilaginous endplates and nucleus pulposus cells: a preliminary investigation. Spine (Phila Pa 1976) 2014;39:1355–1364. doi: 10.1097/BRS.0000000000000372. [DOI] [PubMed] [Google Scholar]
- 29.Yuan W., Che W., Jiang Y.Q., Yuan F.L., Wang H.R. Establishment of intervertebral disc degeneration model induced by ischemic sub-endplate in rat tail. Spine J. 2015;15:1050–1059. doi: 10.1016/j.spinee.2015.01.026. [DOI] [PubMed] [Google Scholar]
- 30.Kim K.C., Friso S., Choi S.W. DNA methylation, an epigenetic mechanism connecting folate to healthy embryonic development and aging. J Nutr Biochem. 2009;20:917–926. doi: 10.1016/j.jnutbio.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karagiannis T.C., Maulik N. Factors influencing epigenetic mechanisms and related diseases. Antioxid Redox Signal. 2012;17:192–194. doi: 10.1089/ars.2012.4562. [DOI] [PubMed] [Google Scholar]
- 32.Kwa F.A., Thrimawithana T.R. Epigenetic modifications as potential therapeutic targets in age-related macular degeneration and diabetic retinopathy. Drug Discov Today. 2014;19:1387–1393. doi: 10.1016/j.drudis.2014.03.026. [DOI] [PubMed] [Google Scholar]
- 33.Kimura H. Histone modifications for human epigenome analysis. J Hum Genet. 2013;58:439–445. doi: 10.1038/jhg.2013.66. [DOI] [PubMed] [Google Scholar]
- 34.Pan M.R., Hsu M.C., Chen L.T., Hung W.C. Orchestration of H3K27 methylation: mechanisms and therapeutic implication. Cell Mol Life Sci. 2018;75:209–223. doi: 10.1007/s00018-017-2596-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu Y., Peng J., Sun T., Li N., Zhang L. Epithelial EZH2 serves as an epigenetic determinant in experimental colitis by inhibiting TNFα-mediated inflammation and apoptosis. Proc Natl Acad Sci USA. 2017;114:E3796–E3805. doi: 10.1073/pnas.1700909114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zingg D., Debbache J., Schaefer S.M., Tuncer E., Frommel S.C. The epigenetic modifier EZH2 controls melanoma growth and metastasis through silencing of distinct tumour suppressors. Nat Commun. 2015;6:6051. doi: 10.1038/ncomms7051. [DOI] [PubMed] [Google Scholar]
- 37.Yu Y., Deng P., Yu B., Szymanski J.M., Aghaloo T. Inhibition of EZH2 promotes human embryonic stem cell differentiation into mesoderm by reducing H3K27me3. Stem Cell Reports. 2017;9:752–761. doi: 10.1016/j.stemcr.2017.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lui J.C., Garrison P., Nguyen Q., Ad M., Keembiyehetty C. EZH1 and EZH2 promote skeletal growth by repressing inhibitors of chondrocyte proliferation and hypertrophy. Nat Commun. 2016;7:13685. doi: 10.1038/ncomms13685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang P., Li Y., Meng T., Zhang J., Wei Y. KDM6A promotes chondrogenic differentiation of periodontal ligament stem cells by demethylation of SOX9. Cell Prolif. 2018;51:e12413. doi: 10.1111/cpr.12413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin T.H., Pajarinen J., Lu L., Nabeshima A., Cordova L.A. NF-κB as a therapeutic target in inflammatory-associated bone diseases. Adv Protein Chem Struct Biol. 2017;107:117–154. doi: 10.1016/bs.apcsb.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ahmed A.S., Berg S., Alkass K., Druid H., Hart D.A. NF-κB-Associated pain-related neuropeptide expression in patients with degenerative disc disease. Int J Mol Sci. 2019;20:E658. doi: 10.3390/ijms20030658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou Y., Wang T., Hamilton J.L., Chen D. Wnt/β-catenin signaling in osteoarthritis and in other forms of arthritis. Curr Rheumatol Rep. 2017;19:53. doi: 10.1007/s11926-017-0679-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hiyama A., Sakai D., Risbud M.V., Tanaka M., Arai F. Enhancement of intervertebral disc cell senescence by WNT/β-catenin signaling-induced matrix metalloproteinase expression. Arthritis Rheum. 2010;62:3036–3047. doi: 10.1002/art.27599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee S.T., Li Z., Wu Z., Aau M., Guan P. Context-specific regulation of Nf-κb target gene expression by EZH2 in breast cancers. Mol Cell. 2011;43:798–810. doi: 10.1016/j.molcel.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 45.Iannetti A., Ledoux A.C., Tudhope S.J., Sellier H., Zhao B. Regulation of p53 and Rb links the alternative Nf-κb pathway to EZH2 expression and cell senescence. PLoS Genet. 2014;10 doi: 10.1371/journal.pgen.1004642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cheng A.S., Lau S.S., Chen Y., Kondo Y., Li M.S. EZH2-mediated concordant repression of WNT antagonists promotes β-catenin-dependent hepatocarcinogenesis. Cancer Res. 2011;71:4028–4039. doi: 10.1158/0008-5472.CAN-10-3342. [DOI] [PubMed] [Google Scholar]