Abstract
Background
SWELL1 was recently demonstrated to be an indispensable part of the volume-regulated anion channel (VRAC). VRAC is reported to participate in cell proliferation, survival, and migration. However, the correlation between SWELL1 and hepatocellular carcinoma (HCC) remains poorly-understood. In this study, we tried to explore the role of SWELL1 in HCC.
Methods
Immunohistochemistry and quantitative real-time-PCR (qRT-PCR) was used to measure SWELL1 expression in HCC samples obtained from patients with HCC. The effects of SWELL1 on HCC cell proliferation, apoptosis, and metastasis were analysed by corresponding cytological experiments including Cell Counting Kit-8 (CCK8), colony-forming, 5-ethynyl-2′-deoxyuridine (EdU), cell cycle analysis, TUNEL, Annexin V and PI staining, wound healing, transwell, and so on. BALB/c nude mice were used for the in vivo assays. qRT-PCR and western blotting was performed for molecular mechanisms.
Findings
SWELL1 was highly expressed in HCC tissues, and related to the poor prognosis. In vitro, the over-expression of SWELL1 significantly induced cell proliferation and migration, and inhibited apoptosis, whereas suppressing SWELL1 had the opposite effects. Moreover, knockdown of SWELL1 suppressed the growth and metastasis of HCC in vivo. Further experiments revealed that SWELL1 induced cell growth by activating the cyclinD1/CDK2 pathway via the connection with PKCa at the signalling level, and regulated cell migration through the JNK pathway in HCC.
Interpretation
SWELL1 acts as a promoter in the growth and metastasis of HCC cells and may be a potential intervention target for HCC.
Fund
This work is supported by the National Natural Science Foundation of China (No. 81572422, 81700515).
Keywords: Hepatocellular carcinoma, Proliferation, Apoptosis, Metastasis, SWELL1
Abbreviations: HCC, hepatocellular carcinoma; VRAC, volume-regulated anion channel; qRT-PCR, quantitative real-time-PCR; IHC, immunohistochemistry; ANOVA, analysis of variance; GAPDH, Glyceraldehyde 3-phosphate dehydrogenase; PVDF, polyvinylidene difluoride; BSA, bovine serum albumin; CCK8, Cell Counting Kit-8; EdU, 5-ethynyl-2′-deoxyuridine; ROS, cellular reactive oxygen species; MMP, mitochondrial membrane potential; EMT, epithelial-to-mesenchymal transition; PKCa, protein kinase C alpha; SPHK1, sphingosine kinase 1; S1P, sphingosine-1-phosphate; DAPI, 4′, 6-diamidino-2-phenylindole; RVD, regulatory volume decrease; PL, phospholipase; DCFH-DA, dichloro-dihydro-fluorescein diacetate; LCK, lymphocyte-specific protein tyrosine kinase; PI3K, phosphoinositide 3-kinase
Research in context.
Evidence before this study
Recently, SWELL1 was confirmed to be an indispensable component of VRAC. Beyond its pivotal role in cell volume regulation, VRAC is involved in cell proliferation, apoptosis, and migration. In fact, most reported studies on SWELL1 have focused on the VRAC, and the role of SWELL1 itself in tumours is poorly understood. Currently, the role of SWELL1 in HCC has not been investigated.
Added value of this study
In this study, we found that the expression of SWELL1 in HCC tissues was much higher than that in pericarcinous tissues and related to a poorer prognosis for patients with HCC. The over-expression of SWELL1 in HCC promoted cell proliferation and migration and suppressed apoptosis. Further experiments revealed that SWELL1 induced cell growth by activating the cyclinD1/CDK2 pathway via connecting with PKCa at the signalling level, and regulated cell migration through the JNK pathway in HCC.
Implications of all the available evidence
Our results suggest that SWELL1 acts as a promoter in the growth and metastasis of HCC cells and may be a potential intervention target for HCC. The results of our study will aid in better understanding the functional capacity of SWELL1 and the progression of HCC.
Alt-text: Unlabelled Box
1. Introduction
Hepatocellular carcinoma (HCC) is a major health concern and one of the leading causes of cancer-associated mortality worldwide [1]. HCC is characterised by rapid metastasis and development, reducing the time for the treatment of patients [2]. Although the treatment level has improved in recent years, the prognosis of HCC remains unsatisfying [2]. Therefore, identifying predictive tumour biomarkers of HCC to ensure an early diagnosis and effective treatments is critical. SWELL1, a member of the four-transmembrane protein family, was originally identified in a woman who lacked B cells in the peripheral blood and was found to have congenital agamma-globulinaemia [3]. Moreover, recent studies have confirmed that SWELL1 is an essential component of volume-regulated anion channel (VRAC), and knockdown of SWELL1 dramatically reduces endogenous VRAC currents in various cell types [4,5]. VRAC is not only an important volume regulator for cell volume homeostasis, but also involved in various cellular functions, including cell proliferation, differentiation, survival, migration, swelling-induced exocytosis, and intercellular communication [[6], [7], [8], [9]]. The SWELL1-mediated functions are so extensive and complex that many concrete mechanisms remain to be elucidated. To date, the role of SWELL1 in HCC has not been investigated. In this study, we found that the expression of SWELL1 in HCC tissues was much higher than that in pericarcinous tissues and related to a poorer prognosis for patients with HCC. In addition, SWELL1 induced cell growth by activating the cyclinD1/CDK2 pathway via connecting with PKCa at the signalling level, and regulate cell migration through the JNK pathway in HCC.
2. Materials and methods
2.1. Patients and samples
All of the samples (liver cancer samples and their adjacent nontumourous samples) were obtained from patients who underwent surgical resection of HCC in our hospital. None of the patients received any preoperative chemotherapy or radiotherapy. For each pair of samples, most of the tissues were stored in an ultra-low temperature freezer, and the rest were fixed in 4% paraformaldehyde for Immunohistochemistry (IHC). Detailed clinicopathological characteristics of the patients are presented in Supplementary Table S1. Informed consent was obtained from all of the patients. The study was performed according to the guidelines of the Ethics Committee of the Tongji Hospital and approved in accord with the ethical standards of World Medical Association Declaration of Helsinki.
2.2. RNA extraction and quantitative real-time-PCR (qRT-PCR)
The RNA extraction was performed using Trizol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. The total RNA was reverse-transcribed into cDNA using a Prime-Script® RT Reagent Kit (TaKaRa, Otsu, Japan). Then the qRT-PCR was performed by SYBR (TaKaRa, Otsu, Japan) using an ABI Step One Real-Time PCR System (Applied Biosystem, Carlsbad, CA, USA). The qRT-PCR conditions for the reactions were as follows: 95 °C for 30 s, 95 °C for 5 s and 60 °C 30 s for 40 cycles. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as the housekeeping gene. The relative mRNA expression levels were calculated using the 2−△△CT method and normalized to the control samples. The y-axis in the results of qRT-PCR represents the “fold change” in gene expression. For the better confirmation of SWELL1 expression levels in the HCC tissues, thirty-four pairs of liver cancer samples and their adjacent nontumourous samples were randomly chosen from the sample cohort for qRT-PCR analysis. Besides GAPDH, β-actin was also used as the housekeeping gene in the qRT-PCR of HCC tissues. The primers were designed using NCBI Primer-BLAST (Supplementary Table S2). For the primer of SWELL1, the amplified products are 145 bp and have been validated by the PCR products sequencing (Supplementary Fig. S1).
2.3. Tissue microarray and immunohistochemistry
All tissue samples were paraformaldehyde-fixed and paraffin-embedded. The tissue microarray was prepared by Zuocheng Biotech (Shanghai, China). The tissue microarray and tissue samples were incubated with primary antibodies overnight at 4 °C, followed by incubation with secondary antibodies for one hour at 25 °C. The specific immunoreaction was visualised using a horseradish peroxidase-diaminobenzidine staining kit. For the IHC of the tissue microarray, the SWELL1 antibody or its isotype control antibody was used as the primary antibody for the validation of specificity. In addition, the immunofluorescence staining with the SWELL1 antibody was also performed in HCC cell lines to validate the results of the IHC (Supplementary Fig. S2a). To distinguish the SWELL1 expression between HCC and non-HCC cells in HCC samples, the antibodies of Ki67, CD31, and MPO were used in the IHC for supporting the malignancy of cancer samples and excluding the infiltrating immune cells and vascular endothelial cells. The results were provided in Supplementary Fig. S2a. In order to separate the differences of SWELL1 expression in the clinical tumour samples, we used a scoring method by multiplying the staining intensity score and the extent of staining score [10]. The staining intensity was scored as follows: zero, no staining; one, weak staining; two, moderate staining, and three, strong staining. The extent of staining score was evaluated according to the positive staining area: one, ≤25%; two, 26%–50%; three, 51%–75%, and four, ≥76%. The samples were defined as low expression if the final score was zero–five, whereas the samples with scores of six–twelve were defined as high expression. The antibodies used are listed in Supplementary Table S3.
2.4. Small interfering RNA, plasmid, and lentiviral shRNA
Transfection with a small interfering RNA (siRNA; Juneng Company, Wuhan, China) and plasmid (Genechem Company, China) was performed using Lipofectamine 3000 (Invitrogen, USA) according to the standardised protocol. The sequences of siRNA for SWELL1 were as follow: siRNA1 5′-GCAGCAACUUCUGGUUCAATT-3′, siRNA2 5′ -CCGUCUACUACGUGCACAATT-3′. The siRNA was used to suppress SWELL1 expression in SMMC-7721 and Huh7 cells, and a recombinant plasmid construct of the gene was used to achieve the over-expression of SWELL1 in Sk-hep-1 and HCCLM3 cells. In order to establish stable knockdown cell lines, lentiviruses (Genechem Company, Shanghai, China) were used as the vector to carry the siRNA2 sequences and to transfect SMMC-7721 cells. The sequence (5′-TTCTCCGAACGTGTCACGT-3′) was used as the negative control for SWELL1 shRNA. The knockdown and over-expression of SWELL1 were verified by qRT-PCR and western blotting (Supplementary Fig. S3a–b). SPHK1 siRNA: 5′-GCAGCUUCCUUGAACCAUUTT-3′. PKCa siRNA: 5′-GCUCCACACUAAAUCCGCATT-3′. The scrambled control siRNA sequence (5′-UUCUCCGAACGUGUCACGUTT-3′) was used as negative control for the siRNA of SWELL1, SPHK1, and PKCa.
2.5. Growth and metastasis assays in vivo
All experimental procedures involving animals were performed in accord with the “Guide for the Care and Use of Laboratory Animals” prepared by the National Academy of Sciences and published by the National Institutes of Health (NIH publication 86–23 revised 1985). BALB/c nude mice (four weeks old, male) were purchased from the animal centre of Tongji Medical College, Huazhong University of Science and Technology (Wuhan, China). For the in vivo growth assay, nude mice were randomly divided into two groups (eight per group), and 1 × 107 cells were subcutaneously injected into the mice. We measured the length and width of the tumours with a digital Vernier calliper. Tumour volume = (length×width2)/2. After three weeks later, the mice were sacrificed, and the subcutaneous tumours were resected and fixed with 4% paraformaldehyde. For the in vivo metastasis assay, nude mice were randomly divided into two groups (six per group), and 1 × 106 cells were injected into the tail vein of the mice. After eight weeks, the mice were sacrificed, and the liver and lung tissues were removed and fixed. All of the above tissue samples were stained with H&E.
2.6. Western blotting
For western blotting, the protein samples were separated on 10% polyacrylamide gels, and electrophoretically transferred to polyvinylidene difluoride (PVDF) membranes. Then, according to the instructions of primary antibodies, the membranes were blocked in 5% skim milk or bovine serum albumin (BSA) for one hour, and were incubated with a primary antibody overnight at 4 °C. Next, the membranes were washed three times with TBST, and incubated with an anti-rabbit or anti-mouse IgG secondary antibody for one hour at 25 °C. The protein signals were detected with an ECL assay kit (Amersham, Buckinghamshire, UK). GAPDH was used as a loading control. The blot density of the western blotting was quantified using the ImageJ software (National Institutes of Health, Bethesda, MD). In addition, the corresponding isotype control antibody was used for the validation of the specificity about SWELL1 antibody (Supplementary Fig. S2b). The primary and secondary antibodies used are listed in Supplementary Table S3.
Other materials and methods used in this study are described in Additional file 1 (Supplementary materials and methods).
2.7. Statistical analyses
All data were analysed using SPSS Statistics software version 17.0 (SPSS Inc., USA). All experiments were performed in triplicate unless otherwise specified, and the results are presented as the mean ± SD.
The Kolmogorov-Smirnov test and Shapiro-Wilk test were used to verify the normal distribution. The expression of SWELL1 mRNA in HCC tissues was compared using a Wilcoxon signed-rank test (continuous variables). The chi-squared test or Fisher's extract test was used to analyse the relationship between clinicopathological characteristics and SWELL1 expression (categorical variables). The Kaplan-Meier method was used to construct the survival curve, and the differences between the curves were compared by a log-rank test. The statistical comparisons of continuous variables between two groups were performed using Student's t-test. One-way analysis of variance (ANOVA) followed by a Dunnett t-test or Tukey test was used to compare the differences among groups more than two (continuous variables). All the tests were two-sided, and P < .05 was considered statistically significant.
3. Results
3.1. The expression of SWELL1 is up-regulated in HCC
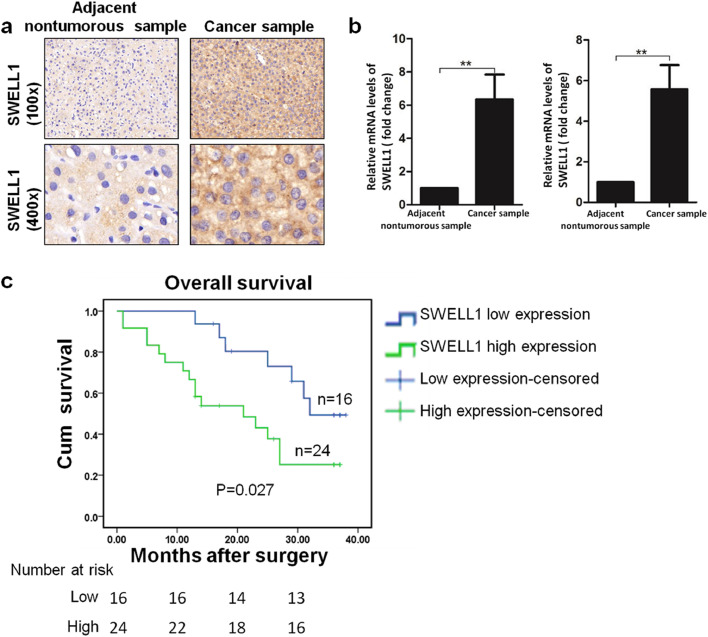
We analysed the IHC results and found that the expression of SWELL1 was significantly up-regulated in HCC samples (87/126, 69%; Fig. 1a). We also randomly selected 34 pairs of samples and assessed SWELL1 expression with qRT-PCR. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and β-actin were both used as the housekeeping gene for the better confirmation. The results were in agreement with the IHC results (Fig. 1b). The correlations between SWELL1 expression and the clinicopathological characteristics of the 126 patients with HCC are shown in Supplementary Table S1. The expression of SWELL1 showed a positive correlation with tumour size, metastasis, and HBsAg(+). No significant correlations were observed with respect to gender, age, tumour differentiation, and AFP. Moreover, according to the overall survival of our follow-up patients over three years, as evaluated in the Kaplan-Meier survival analysis, patients with high expression of SWELL1 had a significantly poorer prognosis than those with low expression of SWELL1 (Fig. 1c). Taken together, SWELL1 expression was significantly up-regulated in HCC samples and related to a poor prognosis.
Fig. 1.
The correlations between SWELL1 expression and HCC samples. (a) Representative images of IHC (n = 126) showing SWELL1 expression in the liver cancer sample and the adjacent nontumourous sample. (b) SWELL1 mRNA expression in 34 pairs of liver cancer samples and their adjacent nontumourous samples was measured by qRT-PCR. GAPDH (left) and β-actin (right) were used as the housekeeping gene. The results were calculated using the 2−△△CT method and normalized to the nontumourous sample. The “fold change” value represents the ratio of the SWELL1 mRNA expression levels in the liver cancer sample and the adjacent nontumourous sample. **P < .01. Data are represented as the mean ± SD of three independent experiments and compared using a Wilcoxon signed-rank test. (c) The Kaplan-Meier survival curves according to the expression of SWELL1 in the follow-up patients. The log-rank test was used to compare the differences between the curves.
3.2. SWELL1 promotes HCC cell proliferation in vitro
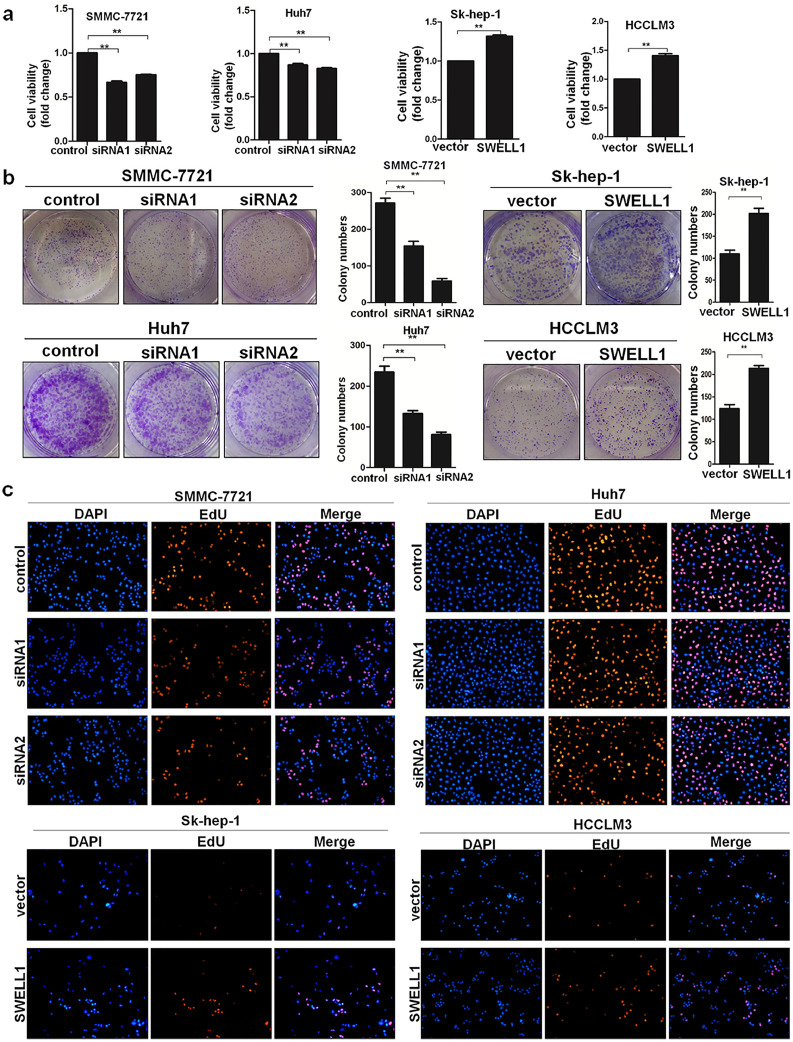
According to the results of the Cell Counting Kit-8 (CCK8) and colony-forming assay (Fig. 2a–b), the over-expression of SWELL1 enhanced cell proliferation and colony-forming ability. Conversely, suppressing SWELL1 had the opposite effects. In addition, we performed the 5-ethynyl-2′-deoxyuridine (EdU) assay to further confirm these findings. Similarly, the results showed that there were more EdU-positive cells among those with high expression of SWELL1, and suppressing SWELL1 clearly reduced the number of cells positive for EdU (Fig. 2c). Thus, these results indicate that SWELL1 promotes HCC cell proliferation in vitro.
Fig. 2.
SWELL1 promotes HCC cell proliferation in vitro. (a) The CCK8 assay results performed in SMMC-7721, Huh7, Sk-hep-1, and HCCLM3 cells. (b) The colony-forming assay results for SMMC-7721, Huh7, Sk-hep-1, and HCCLM3 cells. (c) Representative images of the EdU assay in HCC cells. **P < .01. Data are represented as the mean ± SD of three independent experiments and compared using Student's t-test (Sk-hep-1 and HCCLM3 cells), or one-way ANOVA followed by a Dunnett t-test (SMMC-7721 and Huh7 cells).
3.3. SWELL1 promotes G1/S transition in HCC cells
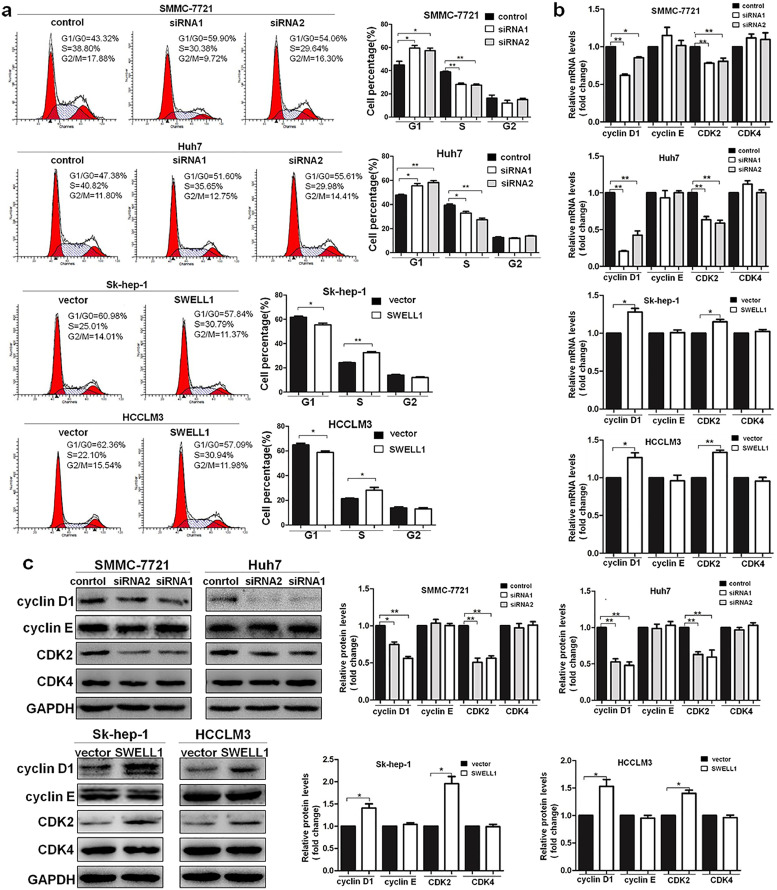
To analyse the effects of SWELL1 on cell cycle progression, we performed the cell cycle analysis. The results indicated that high expression of SWELL1 increased the number of cells in S peak and decreased number of cells in G1/G0 phase. In contrast, inhibition of SWELL1 expression had the opposite effects (Fig. 3a). We also analysed the expression of the cell cycle-related proteins cyclinD1, cyclinE, CDK2, and CDK4 by qRT-PCR (Fig. 3b) and western blotting (Fig. 3c). The results indicated that the expression of the G1 phase regulatory proteins cyclinD1 and CDK2 at both the RNA and protein levels was significantly higher, after increasing SWELL1 expression. Decreasing SWELL1 expression had the opposite effects. By contrast, the expression of cyclinE and CDK4 did not change with SWELL1 expression. Therefore, these results indicate that SWELL1 promotes G1/S transition in HCC cells.
Fig. 3.
SWELL1 promotes the G1/S transition of HCC cells. (a) The results of cell cycle analysis with flow cytometry. (b, c) The expression of the cell cycle-related proteins cyclinD1, cyclinE, CDK2, and CDK4 determined by qRT-PCR (b) and western blotting (c). GAPDH was used as the loading control. The blot density of the western blotting was quantified using the ImageJ software. *P < .05, **P < .01. Data are represented as the mean ± SD of three independent experiments and compared using Student's t-test (Sk-hep-1 and HCCLM3 cells), or one-way ANOVA followed by a Dunnett t-test (SMMC-7721 and Huh7 cells).
3.4. SWELL1 protects HCC cells from apoptosis
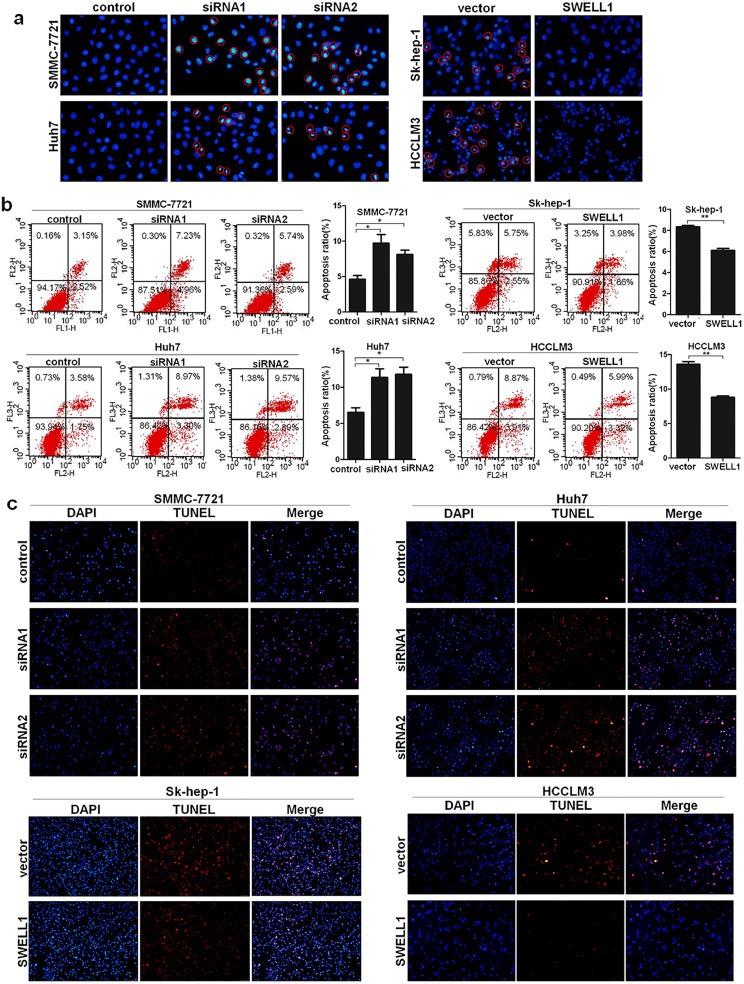
The effects of SWELL1 on apoptosis in HCC cells were preliminary analysed using Hoechst 33258 staining. The results indicated that cells with high SWELL1 expression had much fewer apoptotic cells, while suppressing SWELL1 expression had the opposite effect (Fig. 4a). In addition, the results of Annexin V and PI staining showed that cells with high SWELL1 expression had a clearly lower apoptotic rate, while suppressing SWELL1 expression led to more apoptotic cells (Fig. 4b). The TUNEL assay was used to further confirm these results, revealing that cells with high SWELL1 expression had a lower level of red fluorescence and that decreasing SWELL1 expression clearly increased the red fluorescence (Fig. 4c), indicating that SWELL1 suppressed apoptosis in HCC cells, which agreed with our previous assay results.
Fig. 4.
SWELL1 protects HCC cells from apoptosis. (a) Representative images of Hoechst 33258 staining in SMMC-7721, Huh7, Sk-hep-1, and HCCLM3 cells. (b) Results of Annexin V and PI staining by flow cytometry. (c) Representative images of the TUNEL assay. *P < .05, **P < .01. Data are represented as the mean ± SD of three independent experiments and compared using Student's t-test (Sk-hep-1 and HCCLM3 cells), or one-way ANOVA followed by a Dunnett t-test (SMMC-7721 and Huh7 cells).
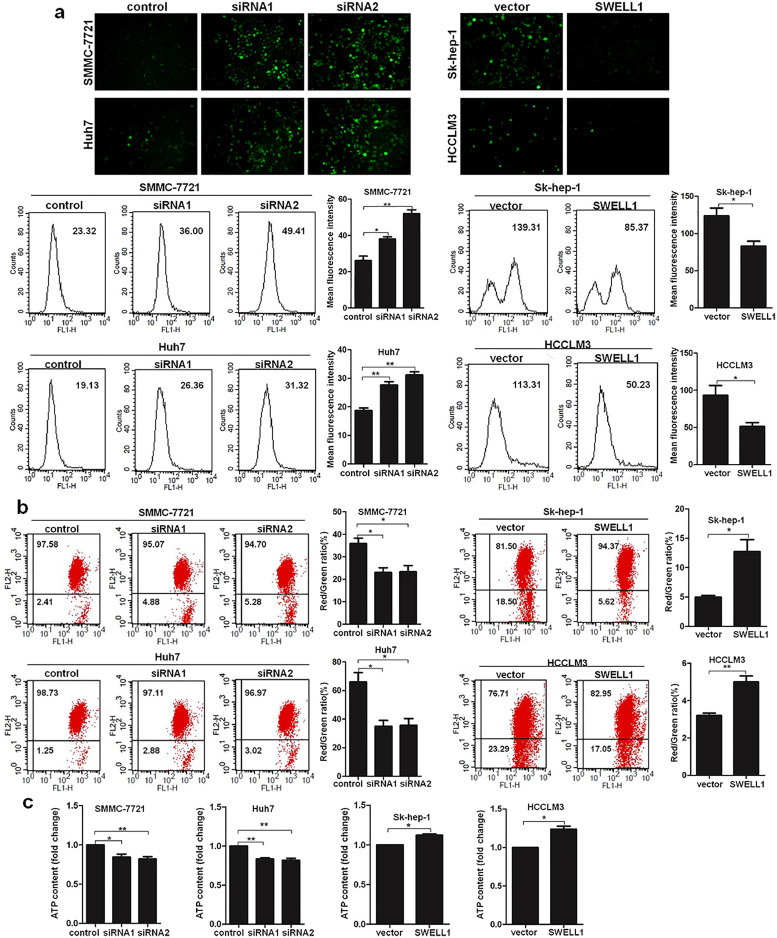
Next, cellular reactive oxygen species (ROS) measurements were performed using the fluorescence microscope and FACScan flow cytometer. The dichloro-dihydro-fluorescein diacetate (DCFH-DA) was used as the fluorescent probe. The results showed that cells with high SWELL1 expression had a significantly lower fluorescence intensity, indicating decreased ROS production. In contrast, suppressing SWELL1 increased ROS production (Fig. 5a). ROS production correlates closely with mitochondrial dysfunction, and mitochondrial dysfunction can be indicated by changes in mitochondrial membrane potential (MMP) [11,12]. As shown in Fig. 5b, there was less MMP loss in cells overexpressing SWELL1 and clearly more MMP loss in cells with decreased SWELL1 expression. In addition, intracellular ATP was also measured. As shown in Fig. 5c, cells overexpressing SWELL1 had elevated levels of ATP, while decreasing SWELL1 expression reduced the intracellular ATP content. These results also verified the stimulatory effect of SWELL1 on HCC cell proliferation. Together, these results indicate that SWELL1 protects HCC cells from apoptosis by preventing mitochondrial dysfunction.
Fig. 5.
Effect of SWELL1 on the levels of ROS, MMP, and intracellular ATP. (a) Results of the measurement of ROS in SMMC-7721, Huh7, Sk-hep-1, and HCCLM3 cells by fluorescence microscope and flow cytometry. (b) Results of the MMP measurement by flow cytometry. The ratio of red/green fluorescence intensity was defined as the changes in MMP in HCC cells. (c) Results of intracellular ATP content. *P < .05, **P < .01. Data are represented as the mean ± SD of three independent experiments and compared using Student's t-test (Sk-hep-1 and HCCLM3 cells), or one-way ANOVA followed by a Dunnett t-test (SMMC-7721 and Huh7 cells). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
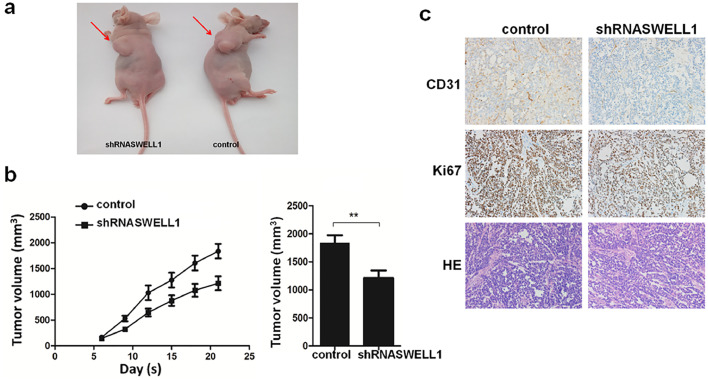
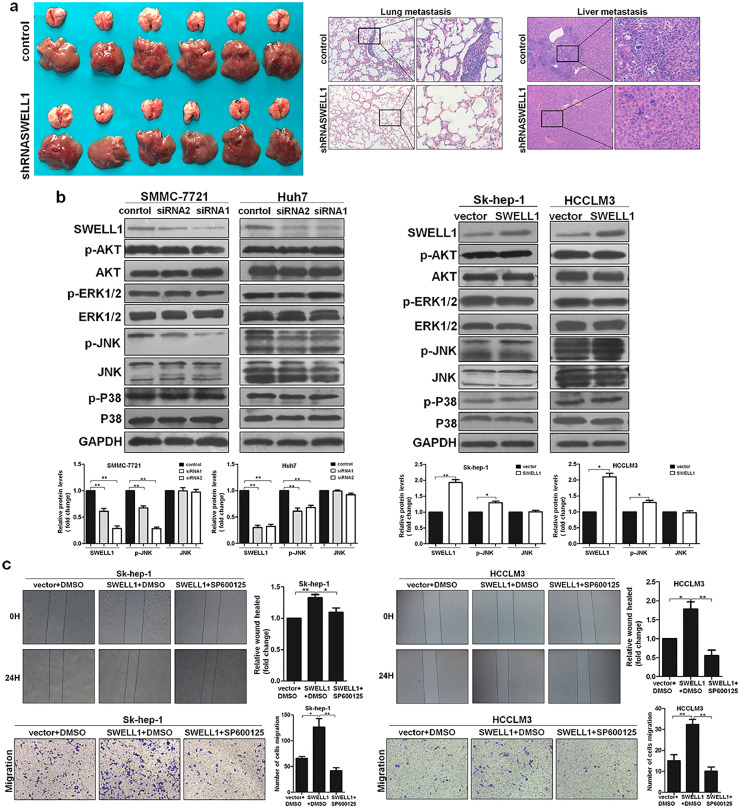
3.5. SWELL1 enhances the cell growth of HCC in vivo
To study the role of SWELL1 in HCC cell growth in vivo, we developed the nude mouse subcutaneous xenograft models. The experiment consisted of two groups of mice: one injected with SMMC-7721-shRNASWELL1 cells and the other with SMMC-7721-control cells. The effect on tumour growth was assessed by measuring the tumour size every three days from the sixth day after injection. Among the subcutaneously injected mice, those injected with SMMC-7721-shRNASWELL1 cells had obviously smaller tumours and lower tumour volume than those in the control group (Fig. 6a–b). Moreover, we also analysed the expression of Ki67 and CD31 using IHC and found that the shRNASWELL1 group had clearly lower expression of Ki67 and CD31 (Fig. 6c). In conclusion, these results confirmed that SWELL1 enhances the growth of HCC cells.
Fig. 6.
SWELL1 promotes cell growth of HCC in vivo. (a) Representative image of the nude mice with tumours in each group (n = 8 per group). (b) The tumour growth curves and mean tumour size of the two groups of nude mice injected with SMMC-7721-shSWELL1 or SMMC-7721-control cells (n = 8 per group). We measured the tumour size for the first time on the 6th day and then measured it every 3 days. The mean tumour size of the two groups of mice was measured on day 21. **P < .01. Data are represented as the mean ± SD and compared using Student's t-test. (c) Representative H&E staining and expression of Ki67 and CD31 determined by IHC in tumours in the two groups.
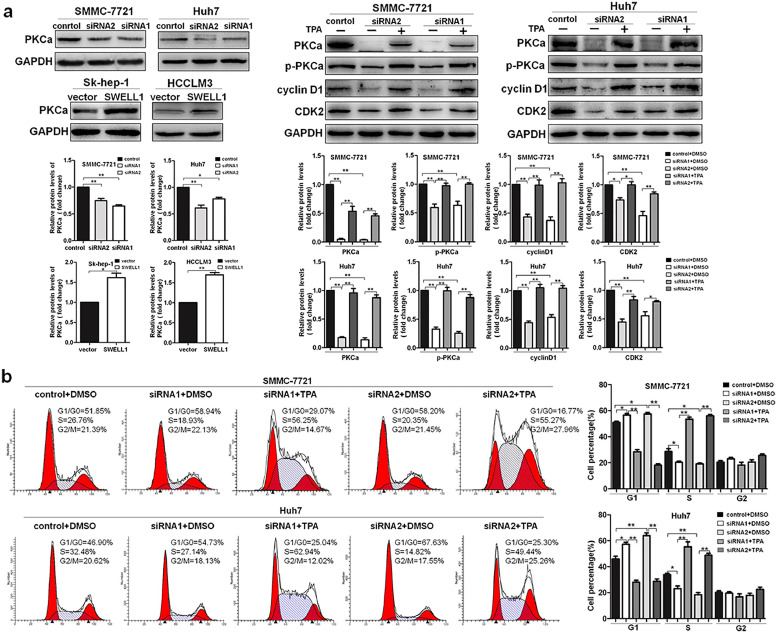
SWELL1 promotes HCC cell growth via connecting with protein kinase C alpha at the signalling level Protein kinase C alpha (PKCa) is involved in cell proliferation and has complex effects on the tumour growth [13,14]. However, role that PKCa plays in the regulation of HCC cell proliferation is unclear. To investigate this role, we analysed the expression of PKCa by western blotting and found that upregulating SWELL1 expression increased PKCa expression, while decreasing SWELL1 expression suppressed PKCa expression (Fig. 7a). To further clarify whether PKCa is involved in SWELL1-medicated HCC growth, we used TPA, an activator of PKCa. The results indicated that the inhibitory effect on the expression of PKCa, p-PKCa, cyclinD1, and CDK2 caused by suppressing SWELL1 was clearly abolished by TPA (Fig. 7a). To further verify this finding, we performed a cell cycle analysis and found that TPA terminated the inhibitory effects caused by decreasing SWELL1 expression on G1/S transition in HCC (Fig. 7b). Taken together, these results indicate that SWELL1 could promote HCC cell growth via the PKCa-cyclinD1/CDK2 pathway. However, PKCα is found to positively regulate the activity of VRAC in various studies [[15], [16], [17]], which indicating a upstream regulatory role of PKCα on SWELL1. Therefore, we also analysed the regulation of PKCα on SWELL1 in HCC for confirmation. The results suggested that decreasing PKCα expression obviously suppressed the expression of SWELL1, and the inhibitory effect on the expression of SWELL1, cyclinD1, CDK2, including PKCa and p-PKCa was terminated by SWELL1 over-expression (Fig. 8a). The cell cycle analysis also agreed with this (Fig. 8b). Therefore, in this part of study, the results indicate that the HCC cell growth can be regulated by the PKCα-SWELL1-cyclinD1/CDK2 pathway. Combining the above results, it seems that SWELL1 have a connection with PKCα at the signalling level which contributes to the HCC cell growth.
Fig. 7.
SWELL1 promotes HCC cell growth via the PKCa-cyclinD1/CDK2 pathway. (a) The expression of PKCa, p-PKCa, cyclinD1, and CDK2 was assessed by western blotting with or without TPA (an activator of PKCa). GAPDH was used as the loading control. The blot density of the western blotting was quantified using the ImageJ software. (b) The results of cell cycle analysis with flow cytometry after cells were pre-treated with TPA. *P < .05, **P < .01. Data are represented as the mean ± SD of three independent experiments and compared using Student's t-test (Sk-hep-1 and HCCLM3 cells), or one-way ANOVA followed by a Tukey test (SMMC-7721 and Huh7 cells).
Fig. 8.
The PKCα-SWELL1-cyclinD1/CDK2 pathway regulates HCC cell growth, and the upstream role of SPHK1 in the SWELL1-PKCa pathway. (a) The expression of SWELL1, PKCa, p-PKCa, cyclinD1, and CDK2 was analysed by western blotting after PKCa expression was suppressed with or without SWELL1 over-expression. (b) The results of cell cycle analysis with flow cytometry after suppressing PKCa with or without SWELL1 over-expression. (c) The expression of SWELL1, PKCa, and p-PKCa was analysed by western blotting after SPHK1 expression was suppressed with or without high SWELL1 expression in HCC cells. GAPDH was used as the loading control. The blot density of the western blotting in (a) and (c) was quantified using the ImageJ software. *P < .05, **P < .01. Data are represented as the mean ± SD of three independent experiments and compared using Student's t-test (when two groups are compared), or one-way ANOVA followed by a Tukey test (when three groups are compared).
3.6. Sphingosine kinase 1 promotes HCC cell proliferation via the SWELL1-PKCa pathway
Sphingosine kinase 1 (SPHK1), the most important synthetase for sphingosine-1-phosphate (S1P), is involved in tumour progression in various cancer cells [[18], [19], [20], [21]]. Studies have shown that VRAC can be activated by S1P and that high expression of SPHK1/S1P promotes the proliferation of HCC [21,22]. Because SWELL1 is an essential component of VRAC, we speculated about the possible relationship between SPHK1 and SWELL1 in the proliferation of HCC. The results suggested that inhibiting SPHK1 expression with siRNA significantly suppressed SWELL1 expression, and this suppressive effect was terminated when combined with SWELL1 over-expression (Fig. 8c). In addition, the changes in PKCa and p-PKCa were consistent with the changes in the expression of SPHK1 and SWELL1 (Fig. 8c). Taken together, these findings indicate that SPHK1 may promote HCC cell proliferation via the SWELL1-PKCa pathway.
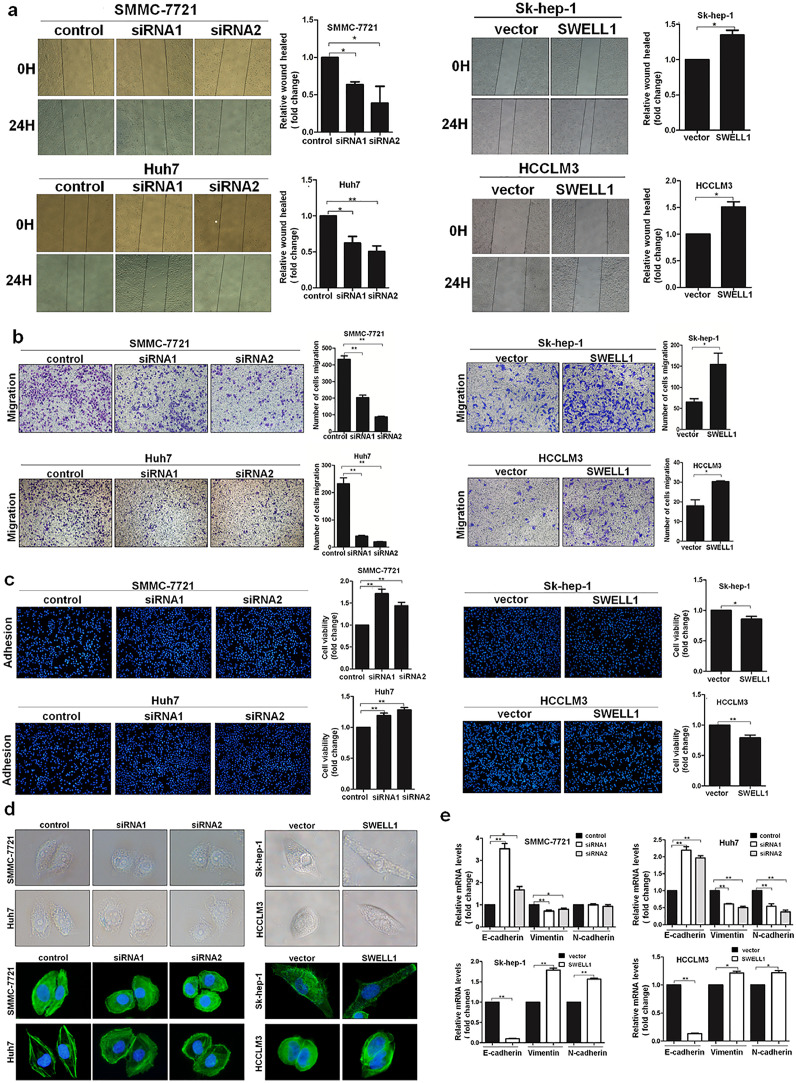
3.7. SWELL1 promotes HCC cell migration in vitro
In the wound healing assay, up-regulation of SWELL1 dramatically enhanced the migration distance of cells, whereas the migration ability was reduced in cells suppressing SWELL1 (Fig. 9a). In addition, we also used the transwell migration assay to evaluate this effect. In accord with the above results, the number of migratory cells was clearly higher with SWELL1 over-expression and decreased when SWELL1 expression was suppressed (Fig. 9b). Cell adhesion is closely linked to tumour metastasis [23]. Thus, cell adhesion experiments using 4′, 6-diamidino-2-phenylindole (DAPI) staining and CCK8 assays were adopted. As shown in Fig. 9c, the results from both indicated that the up-regulated expression of SWELL1 suppressed cell adhesion, whereas the down-regulated expression of SWELL1 enhanced the adhesion ability. Thus, these results indicate that SWELL1 suppresses cell adhesion in HCC, which agrees with its role in promoting cell migration.
Fig. 9.
SWELL1 promotes HCC cell migration and EMT in vitro. (a) Representative images of the wound healing assay performed with SMMC-7721, Huh7, Sk-hep-1, and HCCLM3 cells. The migration of cells was quantified as the percentage of the wound-healed area. (b) Transwell migration assays for SMMC-7721, Huh7, Sk-hep-1, and HCCLM3 cells. The number of migratory cells was quantified. (c) Effects of SWELL1 on the adhesion ability of HCC cells. The cell adhesion ability was assessed using two methods: DAPI staining and the CCK8 method. (d) Morphological changes observed by optical microscopy and cellular F-actin staining observed by fluorescence microscopy. (e) The expression of the EMT markers E-cadherin, N-cadherin, and Vimentin examined by qRT-PCR. *P < .05, **P < .01. Data are represented as the mean ± SD of three independent experiments and compared using Student's t-test (Sk-hep-1 and HCCLM3 cells), or one-way ANOVA followed by a Dunnett t-test (SMMC-7721 and Huh7 cells).
3.8. SWELL1 promotes epithelial-mesenchymal transition in HCC cells
Epithelial-mesenchymal transition (EMT) is closely related to tumour metastasis [24]. Our results presented above indicated that SWELL1 promoted HCC cell migration, so we wanted to determine the role of SWELL1 in EMT. Morphological changes were assessed using an optical microscope and cellular F-actin staining. Both results showed that increasing SWELL1 expression aided cells in obtaining the fibroblastic mesenchymal morphology, while decreasing SWELL1 expression enhanced the epithelial morphological characteristics of HCC cells (Fig. 9d). The expression of the EMT markers E-cadherin, N-cadherin and Vimentin was examined by qRT-PCR (Fig. 9e). In accordance with the morphological changes, the expression of E-cadherin (epithelial marker) was down-regulated and the expression of N-cadherin and Vimentin (mesenchymal markers) was up-regulated when SWELL1 expression was increased. Conversely, decreasing SWELL1 expression increased E-cadherin expression and decreased the expression of N-cadherin and Vimentin (except for the expression of N-cadherin in SMMC-7721 cells). Collectively, these results indicated that SWELL1 promotes EMT in HCC cells.
3.9. SWELL1 promotes the metastasis of HCC in vivo
To determine the role of SWELL1 in HCC metastasis in vivo, we developed the nude mouse models through tail vein injections. The two groups of this experiment were the same as the in vivo growth assay. The role of SWELL1 in tumour metastasis was evaluated using gross observations and pathological examinations of the livers and lungs collected from the mice. As shown in Fig. 10a, mice injected with SMMC-7721-shRNASWELL1 cells had less metastasis in the livers and lungs, which was in accord with the lower CD31 expression. In conclusion, these results confirmed that SWELL1 promotes the metastasis of HCC.
Fig. 10.
SWELL1 promotes metastasis of HCC in vivo and the study on molecular mechanism. (a) Images of the livers and lungs of nude mice showing the metastasis (n = 6 per group) and the representative H&E staining of liver and lung tissues. (b) Protein levels of SWELL1, ERK1/2, P38, AKT, and JNK were analysed by western blotting. GAPDH was used as the loading control. The blot density of the western blotting was quantified using the ImageJ software. *P < .05, **P < .01. Data are represented as the mean ± SD of three independent experiments and compared using Student's t-test (Sk-hep-1 and HCCLM3 cells), or one-way ANOVA followed by a Dunnett t-test (SMMC-7721 and Huh7 cells). (c) Wound healing and transwell migration assays were performed in Sk-hep-1 and HCCLM3 cells with or without SP600125. *P < .05, **P < .01. Data are represented as the mean ± SD of three independent experiments and compared using one-way ANOVA followed by a Tukey test.
3.10. SWELL1 regulates HCC cell migration through the JNK signalling pathway
To investigate the underlying molecular mechanism of SWELL1-mediated cell migration, we explored the MAPK signalling pathways, which has been reported to play an important role in the metastasis of HCC [25]. As shown in Fig. 10b, the expression of p-JNK increased in the case of SWELL1 over-expression, whereas inhibiting SWELL1 significantly decreased the level of p-JNK. In addition, we used a JNK inhibitor (SP600125) to confirm this result. Based on the results of the wound healing and transwell assays, the stimulatory effect of SWELL1 on cell migration was significantly suppressed by SP600125 (Fig. 10c). Taken together, these results indicate that SWELL1 promotes the migration of HCC cells via the JNK pathway.
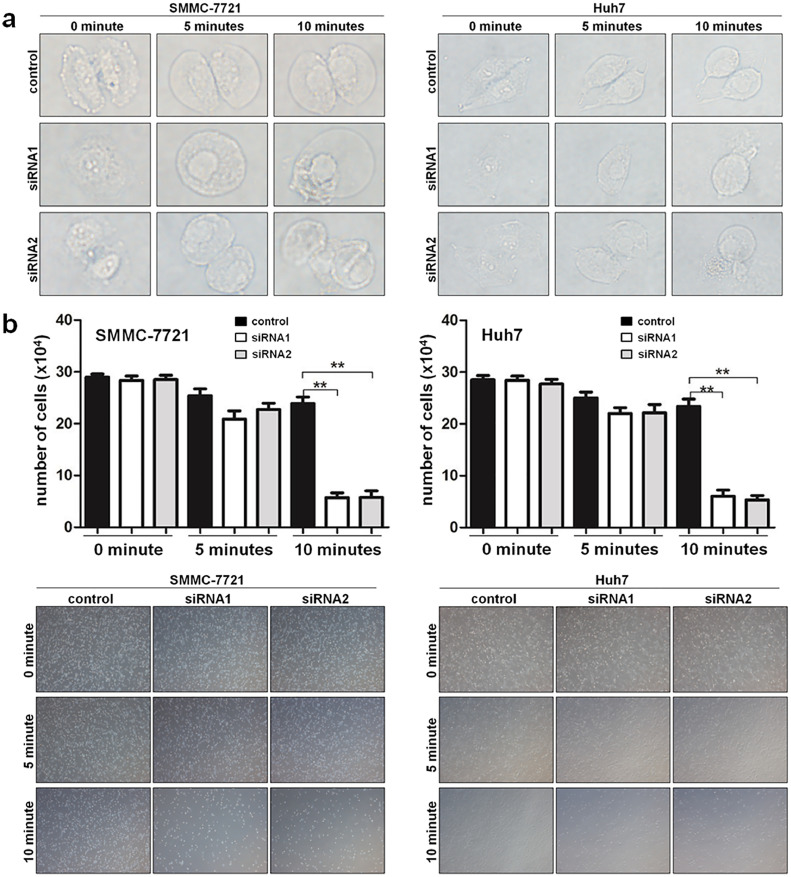
3.11. SWELL1 is required for the function of VRAC in HCC cells
As an important swelling-activated Cl− channel (ICl,swell), the VRAC current is critical for regulatory volume decrease (RVD) [9,26,27]. Knockdown of SWELL1 will severely reduce VRAC currents and damage the function of RVD in various cell types [4,5]. To evaluate whether SWELL1 was required for VRAC in HCC cells, the effect of SWELL1 knockdown on hypotonicity induced RVD was analysed. We preliminary performed the observation of morphological changes in HCC cells after exposure to the hypotonic solution. The results showed that the HCC cells began to swell in response to the hypotonic stress and cells with decreased SWELL1 expression swelled and ruptured faster (Fig. 11a), revealing the worse tolerant ability to the hypotonic stress. Besides, re-incubation of HCC cells after exposure to hypotonic solutions was used to confirm the effect of SWELL1 on RVD. The results showed that decreasing SWELL1 expression obviously reduced the number of the surviving cells after the exposure to hypotonic solutions for 10 min (Fig. 11b), which were consistent with the previous results. Together, suppressing SWELL1 expression could lead to the impaired function of RVD in HCC cells. Therefore, these results indicated that SWELL1 contributes to RVD and is required for the function of VRAC in HCC cells.
Fig. 11.
SWELL1 is required for the function of VRAC in HCC cells. (a) Morphological changes observed in SMMC-7721 and Huh7 cells after exposure to the hypotonic solution. (b) Results of the re-incubation of HCC cells after exposure to hypotonic solutions for 0, 5, or 10 min. Results were assessed by two methods: the number of the surviving cells after the exposure was counted with trypan blue staining and the representative images captured under a microscope. **P < .01. Data are represented as the mean ± SD of three independent experiments and compared using one-way ANOVA followed by a Dunnett t-test. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
SWELL1 is a member of the LRRC8 family and contains 17 leucine-rich repeats and was previously known as LRRC8A [28]. Since SWELL1 was first reported, it has attracted an increasing amount of research attention. Recently, SWELL1 was found to be an indispensable component of VRAC [4,6]. This marked a significant breakthrough, revealing more possibilities for SWELL1. Beyond its pivotal role in cell volume regulation, VRAC is also involved in cell proliferation, apoptosis, and migration [6,8,9]. However, currently, the role of SWELL1 itself in tumours is poorly understood. In fact, most reported studies on SWELL1 have focused on the VRAC. In only a few studies, SWELL1 was suggested to be related to the progression of colorectal cancer, alveolar epithelial carcinoma, and glioblastoma, although evidence for concrete molecular mechanisms was lacking [[29], [30], [31]].
HCC is a very common and severe malignancy [2]. Therefore, we hypothesised that SWELL1 might play a role in the development of HCC and represent a potential intervention target for HCC. To date, there has been no report on the relationship between SWELL1 and HCC. In this study, the results showed that SWELL1 expression was significantly up-regulated in HCC samples and positively associated with metastasis status. Moreover, high expression of SWELL1 was related to a poor prognosis for patients with HCC.
Next, we analysed the effect of SWELL1 on HCC cell growth in vitro and in vivo. For the in vitro analyses, all of the results indicated that SWELL1 promoted cell proliferation by inducting G1/S transition in HCC. The results of the in vivo study also verified this finding. PKCα is closely related to cell proliferation, including cell cycle progression [13]. Additionally, PKCα has also been found to be involved in VRAC regulation [[15], [16], [17]]. Thus, we determined whether PKCα was involved in SWELL1-mediated cell proliferation in HCC. In fact, in terms of proliferation, the effect of PKCα is contradictory [14]. It can act completely different in different cell types [14]. The results in the present study clearly showed that PKCα played a promoting role in the proliferation of HCC. Especially, it is worth noting that the results in this study indicated a connection between SWELL1 and PKCα at the signalling level in HCC cell growth. In the present study, PKCα could regulate the expression of SWELL1, which also had the regulating effects on PKCα expression in turn. Nevertheless, various of the previous studies only indicate the upstream role of PKCα in VRAC regulation, and our results in this study are somewhat inconsistent with them. Currently, the explicit explanation about the upstream regulatory role of SWELL1 on PKCα is lacking. We suspect that other factors or signalling pathways might exist in the regulation of SWELL1 on PKCα, which finally contribute to the current results. Besides, whether the two kinds of regulatory relationships between SWELL1 and PKCα have the same effect on HCC cell growth, or which one is dominant, has not been explored. So, obviously, more specific studies are needed to fully comprehend this issue.
As mentioned above, SPHK1 can enhance HCC cell proliferation [21]. Moreover, S1P has been shown to affect the activity of VRAC [22]. As the most important synthetase of S1P, SPHK1 might be relevant to SWELL1-mediated proliferation in HCC. The results showed that the expression of SWELL1 was decreased by the SPHK1 knockdown, indicating that SPHK1 regulates the SWELL1 expression in HCC. However, it is necessary to mention that the SWELL1 expression is not keeping consistent with the VRAC activity. Besides S1P, VRAC can also be activated by cell swelling, intracellular ionic strength, GTP-γ-S, ATP, phospholipase (PL)A2, intracellular Ca2+, and so on [[32], [33], [34]]. In fact, the activity of VRAC could be reduced when increasing SWELL1 expression [4,5,30]. As reported, although the over-expression of SWELL1 can be detected on the plasma membrane, it will resulted in decreased VRAC current instead of the enhancement [4,5]. The precise mechanism about this remains unclear, and it may be explained as follows. Firstly, as a subunit of VRAC, increasing SWELL1 itself may be not enough for the enhancement of VRAC activity, and other members of the LRRC8 family may be involved [4]. Second, upregulating SWELL1 expression might cause an inappropriate shift in the stoichiometry among SWELL1 with other compositions of VRAC, which is adverse to the VRAC current [5]. Third, the various factors and signalling pathways related to the modulation of VRAC activity may be the limiting factors [4,30]. Besides, the posttranslational modulation of SWELL1 could also be involved [30]. In conclusion, the VRAC activity is affected by various factors, not only depends on the expression of SWELL1. Therefore, although SPHK1 do regulate the SWELL1 expression in the present study, we should not take it for granted. The activation of S1P on VRAC is not a guarantee for the regulation of SPHK1 on SWELL1 expression, which should not be confused. Next, to confirm the effect of SPHK1 on cell proliferation, we also analysed the expression of PKCα. The results indicated that SPHK1 was an upstream factor of SWELL1 and that SPHK1 promoted cell proliferation via the SWELL1-PKCa pathway in HCC. Moreover, given the connection between SWELL1 and PKCα at the signalling level in the above results, the possibly direct regulating effect of SPHK1 on PKCa might exist. Additional work is required for the definitive relationship among SPHK1, SWELL1, and PKCa.
Apoptosis is programmed cell death which is essential for regulating the survival of tumour cells [35]. After demonstrating the proliferating effects of SWELL1, we aimed to determine the role of SWELL1 in apoptosis in HCC cells. The results showed that SWELL1 protected HCC cells from apoptosis. Apoptotic cells generate excess ROS and lose MMP, which in turn reduce the production of ATP [12,36]. In this study, the results of the ROS measurements also indicated the anti-apoptotic role of SWELL1 in HCC. However, it should be noted that DCFH-DA was used as the fluorescent probe for the measurement of cellular ROS in this study. To be precise, this method is an indication of general oxidative stress rather than a very specific proof of cellular ROS [37]. DCFH-DA may be influenced by other reactive intermediates, such as intracellular oxidants, reactive nitrogen species [37,38]. Although it is sensitive and widely used, the limitation should be noted. For further confirmation, MMP and intracellular ATP measurements were analysed, and the results were in agreement with ROS measurements. Moreover, S1P has been found to induce ATP secretion by activating the VRAC [22], and ATP is a known growth factor for tumour cells [39,40]. Thus, to some degree, the enhancement effect of SWELL1 on ATP content also verified the proliferative effect of the SPHK1-SWELL1-PKCa pathway on HCC.
Regarding apoptosis, SWELL1 has been reported in some previous studies to be associated with the regulation of chemotherapy resistance by permitting cisplatin-induced apoptosis [41,42], which seems to be incompatible with its proliferating effects. Conversely, SWELL1 was also found to be an anti-apoptotic factor in a recent study on glioblastoma [31]. Similarly, according to the results of our study, SWELL1 plays an anti-apoptotic role in HCC. The reason why SWELL1 can play completely opposite roles in different tumours is not fully understood. It is possible that the effect of SWELL1 on apoptosis depends on the specific cell type or complex factors in the cellular environment. It has been reported that SPHK1/S1P also protects cells from apoptosis, although it does have a proliferative effect [21,43]. Because SWELL1 expression can be regulated by SPHK1 in HCC, we suspect that the anti-apoptotic effect of SWELL1 may be associated with SPHK1. Further studies are required to completely understand this relationship.
Regarding cell migration, our results indicated that SWELL1 promotes the migration of HCC cells. EMT is a crucial step in tumour metastasis [24]. Based on the results in this study, we concluded that SWELL1 induced EMT in HCC cells, which contributed to cell migration. The in vivo metastasis assay further confirmed these results. MAPKs are Ser/Thr protein kinases, which include ERK1/2, JNK, P38, and AKT [44]. The de-regulation of MAPKs has been demonstrated to be associated with various tumour metastasis factors, including in HCC [25,45]. Besides, SWELL1 is reported to have an essential role in the development and function of T lymphocyte by activating AKT via the lymphocyte-specific protein tyrosine kinase (LCK)–ZAP-70–GAB2–phosphoinositide 3-kinase (PI3K) pathway [46]. Furthermore, PI3K/AKT is known as an important pathway for glucose uptake and lipogenesis in adipocytes [47,48]. In the case of adipocyte hypertrophy, SWELL1 can be activated and positively regulate lipogenesis, insulin sensitivity, and glucose import by the SWELL1/LRRD-GRB2-Cav1-IRS1-PI3K-AKT2 signalling pathway including its downstreams AS160, GLUT4, and GSK3β [49]. In the present study, we tried to determine whether MAPKs were related to SWELL1-mediated HCC metastasis. Our results indicated that SWELL1 could regulate HCC cell migration through the JNK pathway. Because of the universality of SWELL1 expression and JNK pathway, the SWELL1-JNK pathway might be also involved in many other cell types and cytological functions. Besides, accelerated glucose metabolism is closely related to various malignant tumours including oral squamous cell carcinoma, nasopharyngeal carcinoma, colon cancer, breast cancer, HCC, and so on [[50], [51], [52], [53], [54]]. Although the PI3K/AKT pathway seems show no effect on SWELL1-mediated HCC metastasis in this study, whether the effect of SWELL1 on glucose import plays a role in HCC may be still worth exploring in the future.
As mentioned before, VRAC is a pivotal ICl,swell for the cell volume homeostasis [9,26,27]. When the hypotonic stress triggers cell swelling, ion channels and transporters are activated for the effluxes of K+, Cl−, and H2O, which in turn contributes to the shrinkage of the cell volume [55,56]. VRAC is reported to have a critical role in this process named RVD [26,27]. Moreover, as an essential component of VRAC, knockdown of SWELL1 will badly damage the function of RVD in various cell types [4,5]. To investigate the role of SWELL1 in VRAC in HCC cells, we analysed the effect of SWELL1 on VRAC-mediated RVD. The results suggested that suppressing SWELL1 expression caused the impaired function of RVD, indicating that SWELL1 is required for the function of VRAC in HCC cells. The results in this present study might help to reveal more possibilities for VRAC in HCC. However, our study about this aspect is very basic and a in-depth and comprehensive understanding needs further experimental research.
Nevertheless, there are still some limitations of this study which necessitate deeper investigations. Firstly, the mechanism of the connection between SWELL1 and PKCα at the signalling level is unclear. Second, inhibiting the VRAC currents by inhibitors will suppress cell proliferation and migration in kinds of cell types [[6], [7], [8]], indicating a potential role of VRAC in tumour intervention. In the present study, although we discussed the effects of SWELL1 in HCC and discovered that SWELL1 is required for VRAC in HCC cells, the direct role of VRAC remains unexplored. Besides, decreasing SWELL1 expression by siRNA will dramatically reduces the VRAC currents, which is reported to be as effective as the VRAC inhibitor [4,5]. So, it is fair to ask whether the VRAC inhibitor has the similar or even more important effects on the cytological function and corresponding mechanism in HCC. Third, whether it exists a interaction between SWELL1 and VRAC in the tumour biology of HCC deserves further consideration.
In conclusion, our results in this study suggested that SWELL1 is up-regulated in HCC tissues and unfavourable for the prognosis of patients with HCC. SWELL1 promotes the growth and metastasis of HCC cells. Thus, SWELL1 may be a potential intervention target for HCC. The results of our study might aid in better understanding the functional capacity of SWELL1. However, further work is needed to fully comprehend the underlying mechanisms regarding the role of SWELL1.
Funding sources
This work is supported by the National Natural Science Foundation of China (No. 81572422, 81700515). Funding sources had no involvements in study design, data collection, data analysis, interpretation, writing of the report.
Author contributions
Panpan Lu completed most of the cytologic experiments and experiments about mechanisms; Qiang Ding completed the in vivo assays and helped to draft the manuscript; Xin Li and Xiaoyu Ji collected the tissue samples; Lili Li collected and analysed the detailed clinicopathological characteristics of the patients; Yuhui Fan finished the IHC and qRT-PCR tests of the tissue samples; Yujia Xia finished the follow-up studies about the patients with HCC; Dean Tian analysed the experimental data; Mei Liu guided the thoughts of the study and amended the manuscript. All authors read and approved the final manuscript.
Declaration of Competing Interest
None potential conflicts of interest were disclosed.
Acknowledgements
Not applicable.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2019.09.007.
Appendix A. Supplementary data
Supplementary material
References
- 1.Yang J.D., Roberts L.R. Hepatocellular carcinoma: A global view. Nat Rev Gastroenterol Hepatol. 2010;7:448–458. doi: 10.1038/nrgastro.2010.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Villanueva A., Hernandez-Gea V., Llovet J.M. Medical therapies for hepatocellular carcinoma: a critical view of the evidence. Nat Rev Gastroenterol Hepatol. 2013;10:34–42. doi: 10.1038/nrgastro.2012.199. [DOI] [PubMed] [Google Scholar]
- 3.Sawada A., Takihara Y., Kim J.Y. A congenital mutation of the novel gene LRRC8 causes agammaglobulinemia in humans. J Clin Invest. 2003;112:1707–1713. doi: 10.1172/JCI18937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qiu Z., Dubin A.E., Mathur J. SWELL1, a plasma membrane protein, is an essential component of volume-regulated anion channel. Cell. 2014;157:447–458. doi: 10.1016/j.cell.2014.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Voss F.K., Ullrich F., Munch J. Identification of LRRC8 heteromers as an essential component of the volume-regulated anion channel VRAC. Science. 2014;344:634–638. doi: 10.1126/science.1252826. [DOI] [PubMed] [Google Scholar]
- 6.Eggermont J., Trouet D., Carton I., Nilius B. Cellular function and control of volume-regulated anion channels. Cell Biochem Biophys. 2001;35:263–274. doi: 10.1385/CBB:35:3:263. [DOI] [PubMed] [Google Scholar]
- 7.Hyzinski-Garcia M.C., Rudkouskaya A., Mongin A.A. LRRC8A protein is indispensable for swelling-activated and ATP-induced release of excitatory amino acids in rat astrocytes. J Physiol. 2014;592:4855–4862. doi: 10.1113/jphysiol.2014.278887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shen M.R., Droogmans G., Eggermont J., Voets T., Ellory J.C., Nilius B. Differential expression of volume-regulated anion channels during cell cycle progression of human cervical cancer cells. J Physiol. 2000;529(Pt 2):385–394. doi: 10.1111/j.1469-7793.2000.00385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jentsch T.J. VRACs and other ion channels and transporters in the regulation of cell volume and beyond. Nat Rev Mol Cell Biol. 2016;17:293–307. doi: 10.1038/nrm.2016.29. [DOI] [PubMed] [Google Scholar]
- 10.Guo H., Lv Y., Tian T. Downregulation of p57 accelerates the growth and invasion of hepatocellular carcinoma. Carcinogenesis. 2011;32:1897–1904. doi: 10.1093/carcin/bgr220. [DOI] [PubMed] [Google Scholar]
- 11.Wu Y.J., Wong B.S., Yea S.H., Lu C.I., Weng S.H. Sinularin induces apoptosis through mitochondria dysfunction and inactivation of the pI3K/Akt/mTOR pathway in gastric carcinoma cells. Mar Drugs. 2016;14 doi: 10.3390/md14080142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yao H., Yuan Z., Wei G. Thevetiaflavone from Wikstroemia indica ameliorates PC12 cells injury induced by OGD/R via improving ROSmediated mitochondrial dysfunction. Mol Med Rep. 2017;16:9197–9202. doi: 10.3892/mmr.2017.7712. [DOI] [PubMed] [Google Scholar]
- 13.Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature. 1988;334:661–665. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- 14.Garg R., Benedetti L.G., Abera M.B., Wang H., Abba M., Kazanietz M.G. Protein kinase C and cancer: what we know and what we do not. Oncogene. 2014;33:5225–5237. doi: 10.1038/onc.2013.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hermoso M., Olivero P., Torres R., Riveros A., Quest A.F., Stutzin A. Cell volume regulation in response to hypotonicity is impaired in HeLa cells expressing a protein kinase Calpha mutant lacking kinase activity. J Biol Chem. 2004;279:17681–17689. doi: 10.1074/jbc.M304506200. [DOI] [PubMed] [Google Scholar]
- 16.Rudkouskaya A., Chernoguz A., Haskew-Layton R.E., Mongin A.A. Two conventional protein kinase C isoforms, alpha and beta I, are involved in the ATP-induced activation of volume-regulated anion channel and glutamate release in cultured astrocytes. J Neurochem. 2008;105:2260–2270. doi: 10.1111/j.1471-4159.2008.05312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roman R.M., Bodily K.O., Wang Y., Raymond J.R., Fitz J.G. Activation of protein kinase Calpha couples cell volume to membrane Cl- permeability in HTC hepatoma and Mz-ChA-1 cholangiocarcinoma cells. Hepatology. 1998;28:1073–1080. doi: 10.1002/hep.510280423. [DOI] [PubMed] [Google Scholar]
- 18.Li W., Yu C.P., Xia J.T. Sphingosine kinase 1 is associated with gastric cancer progression and poor survival of patients. Clin Cancer Res. 2009;15:1393–1399. doi: 10.1158/1078-0432.CCR-08-1158. [DOI] [PubMed] [Google Scholar]
- 19.Pan J., Tao Y.F., Zhou Z. An novel role of sphingosine kinase-1 (SPHK1) in the invasion and metastasis of esophageal carcinoma. J Transl Med. 2011;9:157. doi: 10.1186/1479-5876-9-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Furuya H., Shimizu Y., Tamashiro P.M. Sphingosine kinase 1 expression enhances colon tumor growth. J Transl Med. 2017;15:120. doi: 10.1186/s12967-017-1220-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeng Y, Liu X, Yan Z, Xie L. Sphingosine 1-phosphate regulates proliferation, cell cycle and apoptosis of hepatocellular carcinoma cells via syndecan-1. Prog Biophys Mol Biol, In Press, Corrected proofs. [DOI] [PubMed]
- 22.Burow P., Markwardt F. When S1P meets ATP. Channels (Austin) 2014;8:385–386. doi: 10.4161/19336950.2014.959408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Friedl P., Mayor R. Tuning collective cell migration by cell-cell junction regulation. Cold Spring Harb Perspect Biol. 2017;9 doi: 10.1101/cshperspect.a029199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nieto M.A., Huang R.Y., Jackson R.A., Thiery J.P. Emt: 2016. Cell. 2016;166:21–45. doi: 10.1016/j.cell.2016.06.028. [DOI] [PubMed] [Google Scholar]
- 25.Ito Y., Sasaki Y., Horimoto M. Activation of mitogen-activated protein kinases/extracellular signal-regulated kinases in human hepatocellular carcinoma. Hepatology. 1998;27:951–958. doi: 10.1002/hep.510270409. [DOI] [PubMed] [Google Scholar]
- 26.Bond T.D., Ambikapathy S., Mohammad S., Valverde M.A. Osmosensitive C1- currents and their relevance to regulatory volume decrease in human intestinal T84 cells: outwardly vs. inwardly rectifying currents. J Physiol. 1998;511(Pt 1):45–54. doi: 10.1111/j.1469-7793.1998.045bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nilius B., Sehrer J., de Smet P., van Driessche W., Droogmans G. Volume regulation in a toad epithelial cell line: role of coactivation of K+ and Cl- channels. J Physiol. 1995;487(Pt 2):367–378. doi: 10.1113/jphysiol.1995.sp020886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jentsch T.J., Lutter D., Planells-Cases R., Ullrich F., Voss F.K. VRAC: molecular identification as LRRC8 heteromers with differential functions. Pflugers Archiv. 2016;468:385–393. doi: 10.1007/s00424-015-1766-5. [DOI] [PubMed] [Google Scholar]
- 29.Zhang H., Deng Z., Zhang D. High expression of leucinerich repeatcontaining 8A is indicative of a worse outcome of colon cancer patients by enhancing cancer cell growth and metastasis. Oncol Rep. 2018;40:1275–1286. doi: 10.3892/or.2018.6556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bach M.D., Sorensen B.H., Lambert I.H. Stress-induced modulation of volume-regulated anions channels in human alveolar carcinoma cells. Physiol Rep. 2018;6 doi: 10.14814/phy2.13869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rubino S., Bach M.D., Schober A.L., Lambert I.H., Mongin A.A. Downregulation of leucine-rich repeat-containing 8A limits proliferation and increases sensitivity of glioblastoma to temozolomide and carmustine. Front Oncol. 2018;8:142. doi: 10.3389/fonc.2018.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kunzelmann K. TMEM16, LRRC8A, bestrophin: chloride channels controlled by Ca(2+) and cell volume. Trends Biochem Sci. 2015;40:535–543. doi: 10.1016/j.tibs.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 33.Best L., Brown P.D. Studies of the mechanism of activation of the volume-regulated anion channel in rat pancreatic beta-cells. J Membr Biol. 2009;230:83–91. doi: 10.1007/s00232-009-9189-x. [DOI] [PubMed] [Google Scholar]
- 34.Estevez A.Y., Bond T., Strange K. Regulation of I(Cl,swell) in neuroblastoma cells by G protein signaling pathways. Am J Physiol Cell Physiol. 2001;281:C89–C98. doi: 10.1152/ajpcell.2001.281.1.C89. [DOI] [PubMed] [Google Scholar]
- 35.Lockshin R.A., Williams C.M. Programmed cell death—I. cytology of degeneration in the intersegmental muscles of the Pernyi Silkmoth. J Insect Physiol. 1965;11:123–133. doi: 10.1016/0022-1910(65)90099-5. [DOI] [PubMed] [Google Scholar]
- 36.Lee Y.J., Oh J.E., Lee S.H. Arctigenin shows preferential cytotoxicity to acidity-tolerant prostate carcinoma PC-3cells through ROS-mediated mitochondrial damage and the inhibition of PI3K/Akt/mTOR pathway. Biochem Biophys Res Commun. 2018;505:1244–1250. doi: 10.1016/j.bbrc.2018.10.045. [DOI] [PubMed] [Google Scholar]
- 37.LeBel C.P., Ischiropoulos H., Bondy S.C. Evaluation of the probe 2′,7′-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem Res Toxicol. 1992;5:227–231. doi: 10.1021/tx00026a012. [DOI] [PubMed] [Google Scholar]
- 38.Bejma J., Ji L.L. Aging and acute exercise enhance free radical generation in rat skeletal muscle. J Appl Physiol (1985) 1999;87:465–470. doi: 10.1152/jappl.1999.87.1.465. [DOI] [PubMed] [Google Scholar]
- 39.Gilbert S.M., Oliphant C.J., Hassan S. ATP in the tumour microenvironment drives expression of nfP2X7, a key mediator of cancer cell survival. Oncogene. 2019;38:194–208. doi: 10.1038/s41388-018-0426-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lemoli R.M., Ferrari D., Fogli M. Extracellular nucleotides are potent stimulators of human hematopoietic stem cells in vitro and in vivo. Blood. 2004;104:1662–1670. doi: 10.1182/blood-2004-03-0834. [DOI] [PubMed] [Google Scholar]
- 41.Sorensen B.H., Dam C.S., Sturup S., Lambert I.H. Dual role of LRRC8A-containing transporters on cisplatin resistance in human ovarian cancer cells. J Inorg Biochem. 2016;160:287–295. doi: 10.1016/j.jinorgbio.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 42.Planells-Cases R., Lutter D., Guyader C. Subunit composition of VRAC channels determines substrate specificity and cellular resistance to Pt-based anti-cancer drugs. EMBO J. 2015;34:2993–3008. doi: 10.15252/embj.201592409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Olivera A., Spiegel S. Sphingosine kinase: a mediator of vital cellular functions. Prostaglandins Other Lipid Mediat. 2001;64:123–134. doi: 10.1016/s0090-6980(01)00108-3. [DOI] [PubMed] [Google Scholar]
- 44.Liebmann C. Regulation of MAP kinase activity by peptide receptor signalling pathway: paradigms of multiplicity. Cell Signal. 2001;13:777–785. doi: 10.1016/s0898-6568(01)00192-9. [DOI] [PubMed] [Google Scholar]
- 45.Wagner E.F., Nebreda A.R. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat Rev Cancer. 2009;9:537–549. doi: 10.1038/nrc2694. [DOI] [PubMed] [Google Scholar]
- 46.Kumar L., Chou J., Yee C.S. Leucine-rich repeat containing 8A (LRRC8A) is essential for T lymphocyte development and function. J Exp Med. 2014;211:929–942. doi: 10.1084/jem.20131379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Garofalo R.S., Orena S.J., Rafidi K. Severe diabetes, age-dependent loss of adipose tissue, and mild growth deficiency in mice lacking Akt2/PKB beta. J Clin Invest. 2003;112:197–208. doi: 10.1172/JCI16885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gonzalez E., McGraw T.E. Insulin-modulated Akt subcellular localization determines Akt isoform-specific signaling. Proc Natl Acad Sci U S A. 2009;106:7004–7009. doi: 10.1073/pnas.0901933106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang Y., Xie L., Gunasekar S.K. SWELL1 is a regulator of adipocyte size, insulin signalling and glucose homeostasis. Nat Cell Biol. 2017;19:504–517. doi: 10.1038/ncb3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen Y.H., Song Y., Yu Y.L., Cheng W., Tong X. miRNA-10a promotes cancer cell proliferation in oral squamous cell carcinoma by upregulating GLUT1 and promoting glucose metabolism. Oncol Lett. 2019;17:5441–5446. doi: 10.3892/ol.2019.10257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang J., Jia L., Liu T. mTORC2-mediated PDHE1alpha nuclear translocation links EBV-LMP1 reprogrammed glucose metabolism to cancer metastasis in nasopharyngeal carcinoma. Oncogene. 2019;38:4669–4684. doi: 10.1038/s41388-019-0749-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhao J., Chen Y., Liu F., Yin M. Overexpression of miRNA-143 inhibits colon cancer cell proliferation by inhibiting glucose uptake. Arch Med Res. 2018;49:497–503. doi: 10.1016/j.arcmed.2018.12.009. [DOI] [PubMed] [Google Scholar]
- 53.Hung M.H., Chen Y.L., Chen L.J. Canagliflozin inhibits growth of hepatocellular carcinoma via blocking glucose-influx-induced beta-catenin activation. Cell Death Dis. 2019;10:420. doi: 10.1038/s41419-019-1646-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sun S., Sun Y., Rong X., Bai L. High glucose promotes breast cancer proliferation and metastasis by impairing angiotensinogen expression. Biosci Rep. 2019;39 doi: 10.1042/BSR20190436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Caplanusi A., Kim K.J., Lariviere E., Van Driessche W., Jans D. Swelling-activated K+ efflux and regulatory volume decrease efficiency in human bronchial epithelial cells. J Membr Biol. 2006;214:33–41. doi: 10.1007/s00232-006-0048-8. [DOI] [PubMed] [Google Scholar]
- 56.Miyazaki H., Shiozaki A., Niisato N., Marunaka Y. Physiological significance of hypotonicity-induced regulatory volume decrease: reduction in intracellular Cl- concentration acting as an intracellular signaling. Am J Physiol Renal Physiol. 2007;292:F1411–F1417. doi: 10.1152/ajprenal.00244.2006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material