Abstract
Lymphoepithelioma-like carcinoma (LELC) is histologically similar in form to nasopharyngeal carcinoma (NPC) and is an epithelial tumor that is suggested to be involved in infection with Epstein-Barr virus (EBV), but it is rare to occur in the colon. A 35-year-old woman was found to have a rectal wall thickening by follow-up computed tomography (CT) image after sigmoidectomy and left salpingo-oophorectomy. Biopsy under colonoscopy revealed recurrence of ovarian cancer, and she underwent a low anterior resection. Pathological diagnosis was LELC. Although LELC of the stomach has been reported to have a high EBV infection rate as NPC, EBV infection was not detected in our case. Pelvic lymph node dissection was also performed, and metastasis was recognized around the iliac artery. There have been few reports of LELC occurring in the rectum, and there are no reports of distant metastasis only to the pelvic lymph node. We consider it a very valuable case, and report it with literature references.
Keywords: Lymphoepithelioma-like carcinoma (LELC), Rectum, Pelvic lymph node
1. Introduction
Lymphoepithelioma-like carcinoma (LELC) is a tumor accompanied by highly lymphocytic infiltration with follicle formation among poorly differentiated tumor vacuoles, and epithelial tumor which is histologically similar to nasopharyngeal carcinoma (NPC). We reported primary rectal LELC with only common iliac lymph node metastasis.
2. Case report
A 35-year-old woman was found to have a rectal wall thickness by computed tomography (CT) during a periodic medical follow up. Enhanced CT showed slight enhancement (Fig. 1). Fluorodeoxyglucose-positron emission tomography (FDG-PET) showed accumulation in the rectum, right common iliac lymph node, right ovary and uterine mucosa (Fig. 2). Colonoscopy revealed a semicircular Type 1 lesion near the anastomosis of previous operation (Fig. 3). Histopathological findings of biopsy showed poorly differentiated carcinoma. Immunohistochemical staining revealed that PAX-8 was positive and CDX-2 was negative.
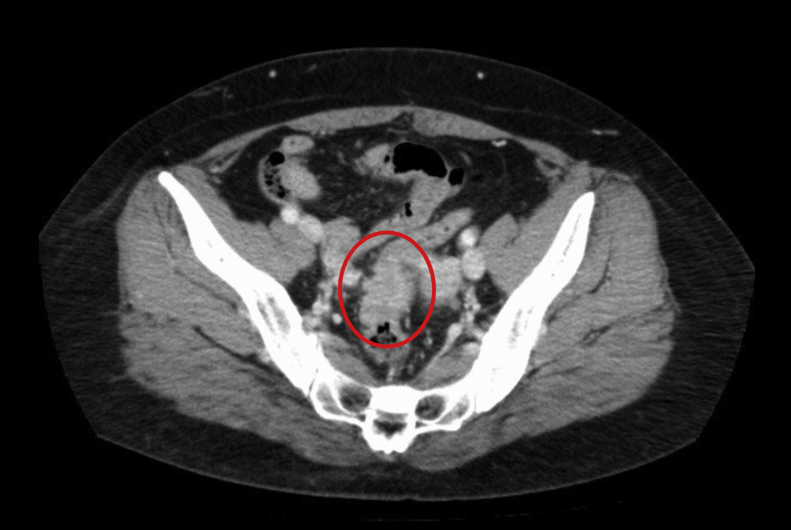
Fig. 1.
Enhanced computed tomography (CT) image.
There is a light high-density area in the rectum (RS), no invasion image around the rectum is observed.
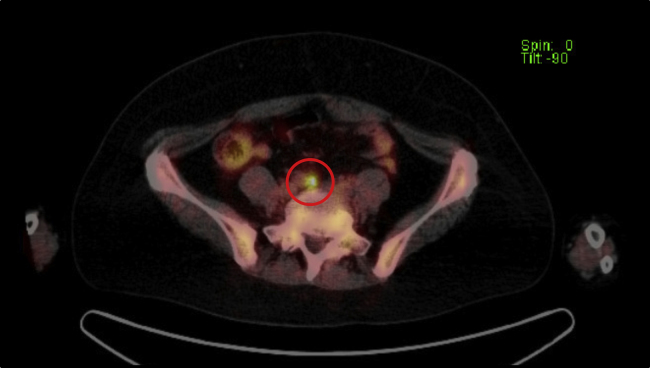
Fig. 2.
Fluorodeoxyglucose-positron emission tomography (FDG-PET).
The right common iliac lymph node is clarified in the delayed image [SUV max 5.7].
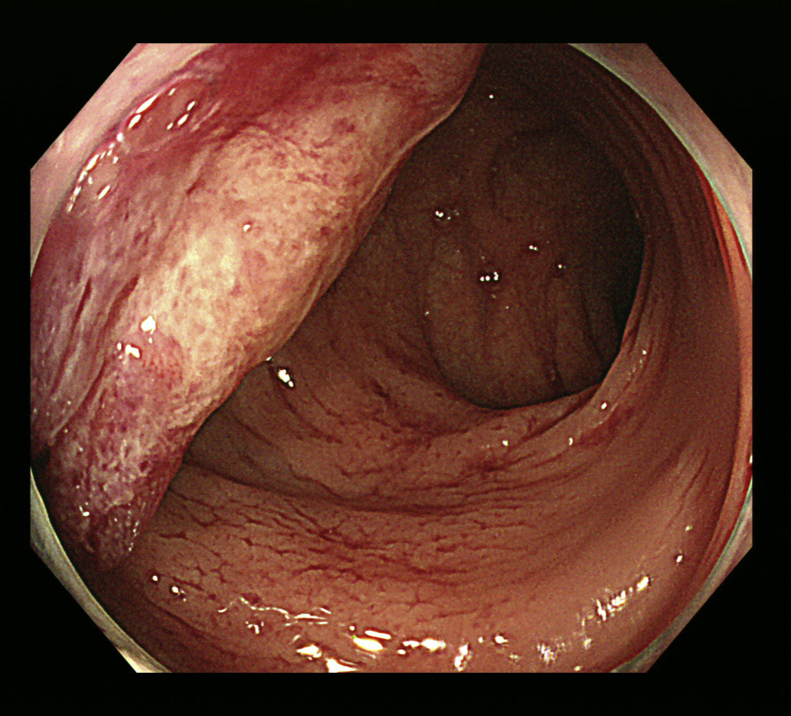
Fig. 3.
Colonoscopy examination.
A semicircular Type 1 lesion is observed on the insertion length 17 cm.
History: At the age of 31, the patient underwent sigmoidectomy with lymph nodes dissection, left salpingo-oophorectomy, and right ovarian cystectomy. During the operation, the cystic part of ovarian cancer ruptured. Histopathological diagnosis of sigmoid colon was moderately differentiated adenocarcinoma with stage 1 according to UICC and the stage of ovarian cancer was 1b according to FIGO.
Preoperative diagnosis: We considered two patterns of tumor origin.
First pattern is that peritoneal dissemination of ovarian cancer sprinkled at the previous operation directly invaded into the rectum. The other pattern is that colon and lymph node metastasis originated from right ovarian cancer grew up.
In any case, it is essential for definitive diagnosis to perform right salpingo-oophorectomy. Therefore, she underwent a right salpingo-oophorectomy, total hysterectomy and pelvic lymph node dissection performed by gynecologists, following an anterior resection by gastrointestinal surgeons.
Intraoperative findings: First, she underwent a right salpingo-oophorectomy and total hysterectomy performed by gynecologists. Because pathological diagnosis during the operation of the right common iliac lymph node was positive, she underwent an additional pelvic and paraaortic lymph node dissection. Subsequently, she underwent an anterior resection with a lymph node dissection that simultaneously removed previous anastomosis.
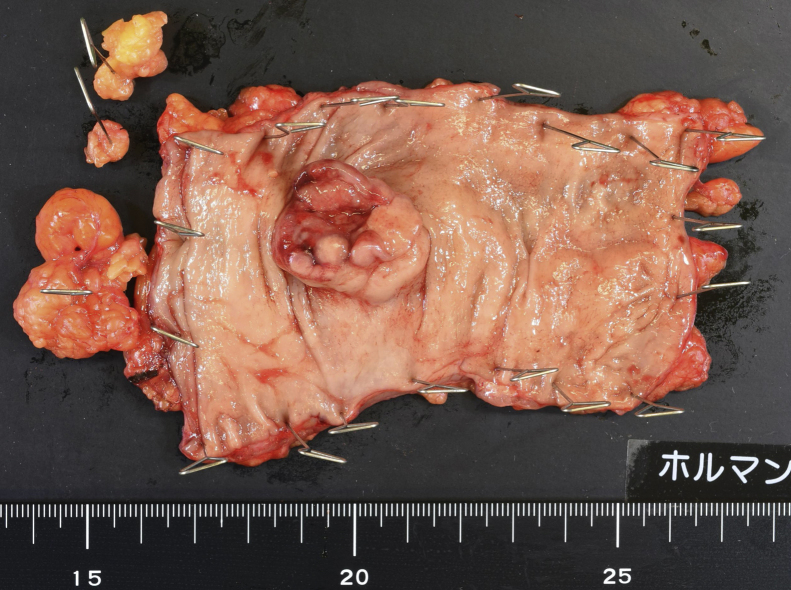
The tumor was macroscopically observed as an elevated lesion, measured as 28 × 23 mm with a central ulcer (Fig. 4). No tumors were observed in gynecologically resected specimens.
Fig. 4.
Excised specimen.
A tumor is 28 × 23 mm in the rectum. With the central part accompanied by ulcers, the limbs were raised like a ulcer mound.
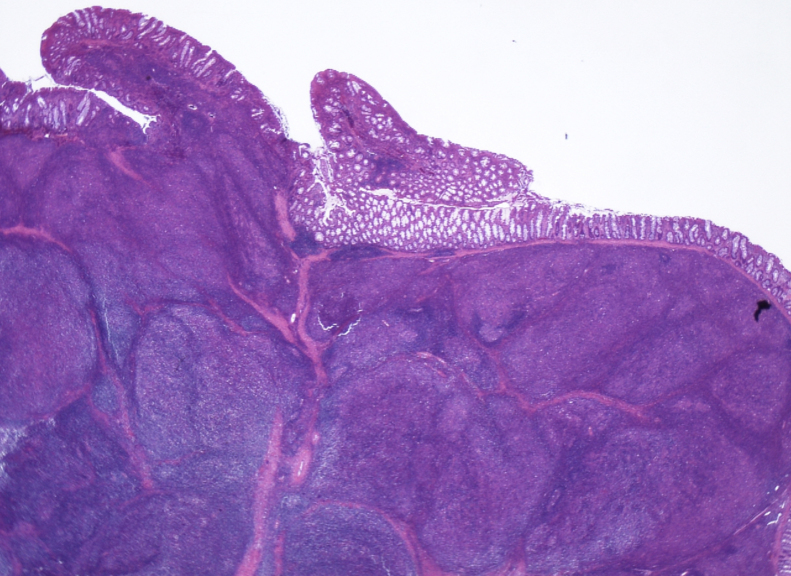
Microscopically, tumor cells associated with nuclear enlargement invasively proliferated in individual cellular, vesicular, or small cord-like sequence, and no glandular structure was observed (Fig. 5). Significant lymphocyte infiltration with lymph follicles was observed, and tumor cells and lymphocytes were unclearly mixed. The tumor proliferated primarily in the submucosal layer and was exposed on the mucosal surface at the central part of the lesion. In the depth, it infiltrated the subserosal layer beyond the muscularis propria. No vascular and neural invasion was observed. The resection margin was negative.
Fig. 5.
HE stains, ×4.
There are many lymphocytes in the background, and follicular-like nodule formation is observed.
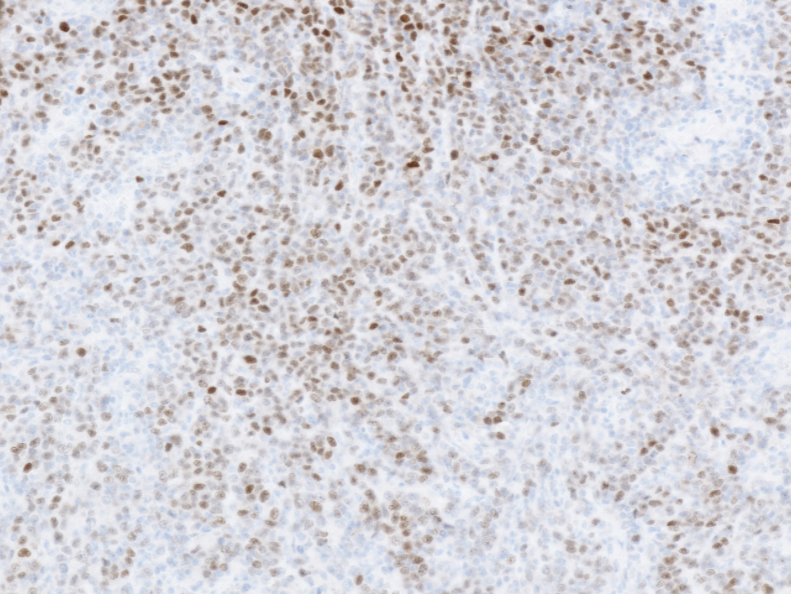
Immunohistochemically, the tumor cells showed AE1/AE3 positive, CAM5.2 positive, CDX-2 negative, PAX-8 positive (also partially positive for infiltrating lymphocytes) (Fig. 6), CD56 negative, Synaptophysin negative, ChromigraninA negative. In the infiltrating lymphocytes, CD3-positive cells were slightly superior to CD20-positive cells. EBV-encoded small RNA in situ hybridization (EBER-ISH) was negative. Dissection lymph nodes showed poorly differentiated cancer metastasis.
Fig. 6.
PAX-8, ×4.
PAX8 is weak to moderate positive in the nucleus of cancer cells.
There were no neoplastic lesions in gynecologically resected specimens and no pelvic lymph nodes metastasis (0/33).
Postoperative course was uneventful, and she was discharged on the 12th postoperative day.
3. Discussion
LELC is a tumor with similar histologic features to NPC, first reported by Schmincke in 1921.1 It is a poorly differentiated adenocarcinoma characterized by diffuse lymphocyte infiltration. In our case, a lymphocyte severely infiltrated throughout the poorly differentiated adenocarcinoma. If the patient was diagnosed with colon cancer, it would be atypical that PAX-8, which was usually positive for Murhalian tumor (most of gynecologic cancers) was positive. However, the area occupied by the carcinoma cells showed that it was diagnosed with primary colon cancer. Moreover, it was diagnosed with LELC because it involved a marked lymphocyte infiltration.
According to previous reports, it is considered that LELC develop in the stomach, salivary gland and so on, but development in the colon is rare.2, 3 When searching from 1950 to 2016 by PubMed with "Lymphoepithelial-like carcinoma or Lymphoepithelioma-like carcinoma" and "colon" as a keyword, only 11 cases, including our could be found, as shown in Table 1.2, 3, 4, 5, 6, 7, 8, 9, 10, 11
Table 1.
LELC cases occurred in the colon. S/C: sigmoid colon, T/C: transverse colon, A/C: ascending colon, N/A: not available, EBER-ISH: EBV-encoded small RNA in situ hybridization, UC: ulcerative colitis, HNPCC: hereditary non-polyposis colorectal cancer.
| Case | Author | Publish yr | Age | Gender | Region | Size (㎜) | Tissue | Depth | LN metastasis | LLN metastasis | EBER-ISH | Background disease |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ① | Our case | 35 | Female | RS | 28 × 23 | por2 | SS | N1(2/17) | #273R(1/1) | Negative | ||
| ② | Sazuka | 2015 | 60 | Female | Rec | 32 × 30 | N/A | MP | N0 | Nothing | *1 | |
| ③ | Mori | 2013 | 70 | Female | S/C | 2 × 5 × 3 | tub2 | SM | N0 | Nothing | *1 | |
| ④ | Delaney | 2012 | 85 | Female | S/C | 42 × 30 × 15 | por | MP | N0 | Nothing | *1 | |
| ⑤ | Taniguchi | 2011 | 88 | Female | T/C | 40 × 35 | por1 | SS | N0 | Nothing | Negative | |
| ⑥ | Kojima | 2007 | 25 | Male | S/C | 7 × 7 | por | SM | N0 | Nothing | Positive | UC |
| ⑦ | Kon | 2001 | 72 | Male | Rec | 20 × 25 × 7 | por | MP | N1(2/22) | Nothing | Positive | |
| ⑧ | De Petris | 1999 | 44?46? | Male | A/C | 9 | tub1 | N/A | N/A | N/A | *1 | HNPCC |
| ⑨ | Samaha | 1998 | 62 | Male | Ce | 28 × 27 × 5 | tub2 | MP | N1(1/22) | Nothing | N/A | HNPCC susp |
| ⑩ | Palazzo | 1995 | 29 | Male | Rec | N/A | N/A | N/A | N/A | N/A | Negative | |
| ⑪ | Vilor | 1995 | 77 | Female | T/C | 120 | por | MP | N/A | N/A | *1 |
*1: negative (surrounding lymph nodes were positive).
Age ranged from 29 to 88 years, 5 males and 6 females. There was a tendency towards the left side colon. There were 6 poorly differentiated cases. Two cases, including ours, were diagnosed as LELC with lymph node metastasis and only our case was confirmed lateral lymph node metastasis. There were only 2 cases in which tumor cells showed positive EBER-ISH, and 6 cases in which surrounding lymph nodes were positive (Table 1).
In our case, the location of the tumor was rectal sigmoid (RS) after sigmoidectomy. According to Kato’s report, among 253 patients undergoing operation for rectal cancer located in RS or Ra, 8 patients (3%) had lateral lymph node metastasis, and only 2 cases had common iliac lymph nodes metastasis.12 Among 776 patients undergoing operation for rectal cancer located in Rb, 97 patients (13%) had lateral lymph node metastasis, and 3 cases had only common iliac lymph nodes metastasis without regional lymph nodes metastasis. In our case, the pelvic lymph node was not metastasized, except the right common iliac lymph node, it was atypical metastatic pathway according to the lymphatic flow. The reasons were considered to be as follows: 1) In this case, the lymphatic flow along the lateral and median sacral artery may have developed because the lymph duct from the pelvis to the inferior mesenteric artery was divided at the previous operation. These lymphatic flows act as an accessory lateral lymph flow, appearing posteriorly from the rectal wall and reach the upper surface of the internal iliac artery near the root of the lateral sacral artery along the sacral base. The lymphatic flow along the median sacral artery reaches the paraaortic lymph node via the lymph nodes present under the promontory.13 The tumor might metastasize to the area considered as distant metastasis because the lymphatic pathway was divided and reconstructed after previous operation. 2) Because LELC is characterized by lymphocytic infiltration in the main tumor, affinity to lymphoid tissue is different from common colorectal cancer, so it is possible that the tumor cells passed through the regional lymph nodes without fixation.
Now those who have advanced rectal cancer usually undergo total mesorectal resection after preoperative radiochemotherapy.14 But it is necessary for us to choose the best therapy from many options individually because LELC is a unique histology. Although there are a few reported complete clinical responses to chemotherapy for LELC of the stomach,15 it is unknown whether chemotherapy or radiotherapy is more effective for LELC. It is desirable to accumulate cases for establishing the concept of diseases including the mechanism of development. And then those data contribute to decide the treatment strategies including perioperative chemotherapy.
4. Conclusion
We reported a case of rectal LELC which had lateral lymph node metastasis.
Financial disclosure
We have no financial information to disclose.
Conflicts of interest
We have no conflict of interest.
Contributor Information
Haruka Oi, Email: haruka.oi62818@gmail.com.
Satoshi Yamamoto, Email: satoshi.hbps@gmail.com.
Yoshiharu Kono, Email: y.kono2.4@gmail.com.
Yukiyoshi Masaki, Email: masaki-y@mghp.ome.tokyo.jp.
References
- 1.Schmincke A. Uber lymphoepitheliale Geschwiilste. Beitr Path Anat. 1921;68:161–169. [Google Scholar]
- 2.Kon S., Kasai K., Tsuzuki N., Nishibe M., Kitagawa T., Nishibe T. Lymphoepithelioma-like carcinoma of rectum: possible relation with EBV. Pathol Res Pract. 2001;197:577–582. doi: 10.1078/0344-0338-00130. [DOI] [PubMed] [Google Scholar]
- 3.Kojima Y., Mogaki M., Takagawa R., Ota I., Sugita M., Natori S. A case of lymphoepithelioma like carcinoma of the colon with ulcerative colitis. Gastroenterology. 2007;42:181–185. doi: 10.1007/s00535-006-1981-0. [DOI] [PubMed] [Google Scholar]
- 4.Vilor M., Tsutsumi Y. Localization of Epstein-Barr virus genome in lymphoid cells in poorly differentiated adenocarcinoma with lymphoid stroma of the colon. Pathol Int. 1995;45:695–697. doi: 10.1111/j.1440-1827.1995.tb03524.x. [DOI] [PubMed] [Google Scholar]
- 5.Palazzo J.P., Mittal K.R. Lymphoepithelioma-like carcinoma of the rectum in a patient with ulcerative colitis. Am J Gastroenterol. 1996;91:398–399. [PubMed] [Google Scholar]
- 6.Samaha S., Tawfik O., Horvat R., Bhatia P. Lymphoepithelioma-like carcinoma of the colon: report of a case with histologic, immunohistochemical, and molecular studies for Epstein-Barr virus. Dis Colon Rectum. 1998;41:925–928. doi: 10.1007/BF02235379. [DOI] [PubMed] [Google Scholar]
- 7.De Petris G., Lev R., Quirk D.M., Ferbend P.R., Butmarc J.R., Elenitoba-Johnson K. Lymphoepithelioma-like carcinoma of the colon in a patient with hereditary non-polyposis colorectal cancer. Arch Pathol Lab Med. 1999;123:720–724. doi: 10.5858/1999-123-0720-LLCOTC. [DOI] [PubMed] [Google Scholar]
- 8.Tetsutaro S., Yuji S., Toru F., Kentaro T., Ota Takumi. A case of lymphoepithelioma-like carcinoma of the rectum. Chiba Medical J. 2015;91:107–112. [Google Scholar]
- 9.Yasuharu M., Kazunari A., Masaaki Y., Hiroshi S. Lymphoepithelioma-like carcinoma of the colon. Case Rep Gastroenterol. 2013;7:127–133. doi: 10.1159/000348765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.David D., Runjan C. Lymphoepithelioma-like carcinoma of the colon. Int J Clin Exp Pathol. 2012;5:105–109. [PMC free article] [PubMed] [Google Scholar]
- 11.Ryuta T., Yasuhiro N., Daisuke N., Yumi M. Lymphoepithelial-like carcinoma of the colon. Jpn Soc Gastroenterol Surg. 2011;44:591–595. [Google Scholar]
- 12.Takehito K., Takashi T., Hirotoshi O., Akiteru K. A study on lateral lymph node metastasis of rectal carcinoma-with reference to location and the pathway of metastasis. Jpn J Gastroenterol Surg. 1986;19:963–968. [Google Scholar]
- 13.Sauer I., Bacon H.E. Influence of lateral spread of cancer of the rectum on radicability of operation and prognosis. Am J Surg. 1951;81:111–120. doi: 10.1016/0002-9610(51)90196-1. [DOI] [PubMed] [Google Scholar]
- 14.Laura C., Juan P.C., Leire A., Olga L. Current treatment of rectal cancer adapted to the individual patient. Rep Pract Oncol Radiother. 2013;18:353–362. doi: 10.1016/j.rpor.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jun T., Kazuhiko K., Kaoru T., Nobuo B. A case of gastric medullary carcinoma showing a complete histological response after S-1/CDDP neoadjuvant chemotherapy. J Jpn Surg Assoc. 2009;70:1071–1076. [Google Scholar]