Abstract
Introduction
We examined 3-month service use and costs of care for people with mild-to-moderate dementia in Great Britain.
Methods
We analyzed Improving the experience of Dementia and Enhancing Active Life cohort study baseline data on paid care, out-of-pocket expenditure, and unpaid care from participants with dementia (N = 1547) and their carers (N = 1283). In regression analyses, we estimated per-group mean costs of diagnostic and sociodemographic subgroups.
Results
Use of services apart from primary and outpatient hospital care was low. Unpaid care accounted for three-quarters of total costs (mean, £4008 [standard error, £130] per participant). Most participants (87%) received unpaid care equating to 36 hours weekly. Estimated costs for people with Parkinson's dementia were £8609, £4359 for participants with mixed dementia, and £3484 for those with Alzheimer's disease. Total costs were lower for participants with dementia living alone than living with others (£2484 vs. £4360); costs were lower for female than for male participants (£3607 vs. £4272).
Discussion
Costs varied by dementia subtype, carer status, and living arrangement. Policy makers should recognize the high costs of unpaid care for people with dementia, who do not always get the support that they need or would like to receive.
Keywords: Dementia, Costs, Health services, Social care, Unpaid care
1. Introduction
In the United Kingdom, 850,000 people live with dementia; in parallel with global trends, this number looks set to double in the next 20 years [1,2]. The symptoms of dementia can affect individuals' personal and social circumstances, creating challenges to living well [3]. Supporting people with dementia brings its own challenges, and unpaid carers may require support to maintain social roles and resources [4]. Individuals living with the condition may need to make demands on the time and resources of unpaid carers and on services provided by health and social care. Projected growth in the number of people living with dementia will have major cost consequences worldwide [1]. Although costs of dementia care to society are high, the burden of care falls disproportionately on unpaid carers [[5], [6], [7], [8]]. Previous UK person-level studies of care for people with dementia [[9], [10], [11], [12], [13], [14], [15]] have used relatively small samples, covered limited geographical areas, or focused on unconfirmed diagnoses or diagnosis of a single dementia type. This limits the information available to decision makers planning how to meet the needs of people living with dementia and their families [16,17].
This study aims to contribute new evidence on use and associated costs of health, social and unpaid care for people with mild-to-moderate dementia, drawing on baseline data from a large British cohort. We explore associations between diagnostic and sociodemographic characteristics of people with dementia and costs of care.
2. Methods
2.1. Design and sample
We used baseline data from the Improving the experience of Dementia and Enhancing Active Life (IDEAL) program [18,19] (data set version 2.0), following yearly for up to 6 years a cohort of people with mild-to-moderate dementia from baseline (hereafter participants) and, where available, a primary carer (relative/friend providing unpaid support to the participant; hereafter carers) [18]. The first phase of IDEAL, covering the first three time points, was approved by Wales Research Ethics Committee 5 (13/WA/0405) and the Ethics Committee of the School of Psychology, Bangor University (reference 2014-11684) and is registered with the UK Clinical Research Network (16593).
Participants were recruited from National Health Service (NHS) clinics and Join Dementia Research [20] (NHS-funded portal supporting dementia research) in 29 sites across England, Scotland, and Wales. Any community-dwelling person with a clinical dementia diagnosis and Mini-Mental State Examination (MMSE) score >14 was eligible for inclusion [18]. Baseline questionnaires were administered by trained researchers (July 2014–August 2016) using face-to-face interviews with participants. One section, on paid and unpaid care, was administered to both the participant and carer, if the latter was available. Carers self-completed separate questionnaires.
Sample size was powered on planned structural equation model analyses of measures of capability of living well and was large enough to permit subgroup analyses for age, sex, dementia subtype, whether people lived alone, living situation, and relationship with carer [18]. The baseline sample comprised 1547 participants and 1283 carers. Most participants with dementia were recruited from England (90%), with 5% each from Scotland and Wales.
2.2. Measures
Questionnaire measures and costing methods are summarized herewith (details in Supplementary Material 1).
2.2.1. Use of paid and unpaid care
Information on health and social care services, medications, assistive equipment, unpaid care, and costs to carers of missing work was collected using an adapted Client Service Receipt Inventory [21]. Questions on health and social care services and unpaid care were asked of participant and carer, or only the participant where no carer was involved in the study. Carer questionnaires asked about working time given up to provide care (lost working time).
2.3. Sociodemographic characteristics
We examined associations of baseline costs with sociodemographic characteristics and dementia subtype. We do not focus on dementia-related needs here (activities of daily living, cognition, behavior, and comorbidities), as these were measured at baseline and therefore up to 3 months after costs were incurred (explored elsewhere in IDEAL study [[22], [23], [24], [25]]). Participant characteristics examined were age groups, sex, dementia subtype, education, National Statistics Socio-economic Classification 5 variable version [26], quintiles of gross annual income (participant and spouse/partner), household tenure, living alone/with others, and participating carer status (none, spousal [spouse/partner], or nonspousal [friend/other family]). Separate regressions examined associations of lost working time costs with carer characteristics: age groups, sex, carer status, socioeconomic status, and education.
2.4. Costing methods
Community health and social care contacts and assistive equipment were weighted by nationally applicable unit costs [27]. Base year for prices was 2014/15. For hospital costs, we applied NHS Reference Costs figures [28]. Mental health medication costs were taken from NHS prescription costs analysis [29]. Hours of unpaid care provided by relatives/friends during the previous 3 months were valued at opportunity cost, applying the minimum wage (in England) [30,31]. Costs of carers' and other relatives/friends' lost working time during the previous 3 months were calculated using Annual Survey of Hours and Earnings data [32]. Paid and unpaid care and out-of-pocket costs were estimated from participant questionnaires. Individual cost items were summed to give category subtotals (Table 1), in turn summed to give overall paid and unpaid care cost totals. Costs of lost working time were calculated and reported separately.
Table 1.
Mean costs (£) of care during prior 3 months
| Cost categories (£) | Mean | SE |
|---|---|---|
| Health and social care (N = 1547) | ||
| Primary and community health care costs | 142 | 7 |
| Community mental health costs | 67 | 4 |
| Community social care costs∗ | 175 | 15 |
| Day care services costs | 143 | 12 |
| Hospital costs | 404 | 39 |
| Total medication costs† | 62 | 3 |
| Costs of equipment paid for by social services‡ | 10 | 1 |
| Costs of equipment paid for by NHS‡ | 6 | 1 |
| Total services and medication costs§ | 1008 | 48 |
| Out-of-pocket costs to the person and to relatives and friends (N = 1547) | ||
| Costs of equipment purchased by self or family | 41 | 2 |
| Costs of condition-related travel to participant & carers¶ | 10 | 2 |
| Costs of unpaid care and lost working time | ||
| From carer questionnaires (N = 1283) | ||
| Lost working time costs to carers# | 158 | 24 |
| From participant with dementia questionnaires (N = 1547) | ||
| Unpaid care costs∗∗ | 2928 | 114 |
| Lost working time costs to carers†† | 20 | 2 |
| Total costs of paid and unpaid care (N = 1547) | ||
| Total paid and unpaid carer costs‡‡ | 4008 | 130 |
NOTE. Results of multiply imputed data (34 complete data sets).
Abbreviations: SE, standard error; NHS, National Health Service.
Includes costs of respite in residential accommodation.
Costs of dementia and central nervous system medications.
Costs over prior 3 months.
Assumes all community care costs fall to social services.
Costs of travel to appointments related to problems with thinking, memory, and behavior by participant and carer or participant only if no carer was involved.
Production costs to carers—variables for participating carer from carer questionnaire respondents.
Unpaid carer costs include costs of hours of unpaid care by unpaid carer and by other friends and relatives.
Lost working time costs to carers—from participant questionnaire respondents (other friends and relatives' lost working time).
All costs derived from participant questionnaire data: includes all paid service and medications costs, out-of-pocket costs, unpaid carer costs, and lost working time costs to other friends and relatives.
2.5. Missing data and data imputation
Missing data and imputation models are described in Supplementary Material 1. Proportions of cases missing service use data ran at 4% to 5%; 9% of cases were missing data on care provided by carers; 6% to 8% of cases were missing carer questionnaire data on lost earnings. Imputation by chained equations was carried out in Stata 15 (StataCorp LP, College Station, TX) [33,34]. Equations for imputing variables from participant questionnaires included use, costs, and characteristics to be used in regression analyses. Equations for imputing carers' questionnaire variables included carer socioeconomic status, lost earnings, and employment.
2.6. Analyses
Differences in costs for sociodemographic and diagnostic subgroups were examined through multivariate regressions. Generalized linear models [35] were fitted to cost subcategories and total costs, assuming gamma distribution and log-link function to accommodate anticipated skew in cost data distribution. Two-part models were fitted to cost data with substantial numbers of zeros using the user-written Stata command <<twopm>> [36]. In the first part, logit models were applied to a binary indicator for nonzero costs (henceforth, models of receipt); in the second, generalized linear models were applied to positive costs. The same vector of covariates was used in each part. For factor variables with more than two levels, we tested joint significance of all levels by following a previously described procedure [37] implemented in Stata's multiple imputation suite of commands [33] to obtain a P value across regression estimates from multiply imputed data sets. A 5% significance level was applied to tests of model coefficients. We estimated average marginal effects (henceforth, estimated means), for each level of each factor at observed values of each case. Differences in costs between subgroups were judged significant if 95% confidence intervals (95% CIs) of subgroup estimated means did not overlap. Results of analyses conducted on each complete data set generated by imputation were combined using Rubin's rules [38].
3. Results
3.1. Sample
More than half of the participants were aged older than 74 years, whereas 9% were aged younger than 65 years (Table 2). Mean age was 76.4 (standard deviation, 8.6). There were more men (56%) than women. More than half of the participants (55%) had Alzheimer's disease. A fifth lived alone. Two-thirds (67%) had a spousal carer; 17% had no participating carer. Of participants living with others, 10% did not have a participating carer. Participants with no carer had mean baseline MMSE 1.07 points higher than those with carers (24.12; 95% CI, 23.74–24.50 vs. 23.05; 95% CI, 22.83–23.25; t = 4.34; P < .001, N = 1474). Carers were younger than participants (mean age, 69.1 years; standard deviation, 11.1); 69% were females. On National Statistics Socio-economic Classification 5, 43% of carers (and 41% of participants) were in the top category. About 53% of carers aged younger than 65 years were in paid employment, whereas this proportion dropped to 12% in the 65 to 69 age band and less than 3% in the 70+ age bands. Most nonspousal carers (83%) were the adult children of participants.
Table 2.
Demographic and socioeconomic characteristics
| Participants with dementia (N = 1547) | N (%) |
|---|---|
| Age bands (y) | |
| Younger than 65 | 136 (9) |
| 65–69 | 178 (12) |
| 70–74 | 260 (17) |
| 75–79 | 370 (24) |
| 80+ | 603 (39) |
| Sex | |
| Male | 872 (56) |
| Female | 675 (44) |
| Carer status | |
| Spouse/partner | 1039 (67) |
| Family/friend | 244 (16) |
| No carer involved | 264 (17) |
| Dementia subtypes | |
| Alzheimer's disease | 858 (55) |
| Vascular dementia | 171 (11) |
| Mixed (Alzheimer's and vascular) | 326 (21) |
| Frontotemporal dementia | 54 (3) |
| Parkinson's disease dementia | 44 (3) |
| Dementia with Lewy bodies | 53 (3) |
| Unspecified/other | 41 (3) |
| Socioeconomic classification∗ | |
| Managerial, administrative, and professional occupations | 639 (41) |
| Intermediate occupations | 271 (18) |
| Small employers and own account workers | 173 (11) |
| Lower supervisory and technical occupations | 151 (10) |
| Semiroutine and routine occupations | 313 (20) |
| Lives alone (self-reported)∗ | |
| No | 1241 (80) |
| Yes | 306 (20) |
| Income quintiles† | |
| First quintile (lowest) | 431 (28) |
| Second quintile | 277 (18) |
| Third quintile | 257 (17) |
| Fourth quintile | 330 (21) |
| Fifth quintile (highest) | 252 (16) |
| Education | |
| No qualification | 430 (28) |
| School certificate age 16 | 274 (18) |
| School certificate age 18 | 529 (34) |
| College-level | 314 (20) |
| Tenure | |
| Rents and other forms of tenure | 249 (16) |
| Owns | 1298 (84) |
| Carers (N = 1283) | N (%) |
|---|---|
| Age bands (y) | |
| Younger than 65 | 369 (29) |
| 65–69 | 208 (16) |
| 70–74 | 267 (21) |
| 75–79 | 223 (17) |
| 80+ | 216 (17) |
| Sex | |
| Male | 402 (31) |
| Female | 881 (69) |
| Socioeconomic classification∗ | |
| Managerial, administrative, and professional occupations | 549 (43) |
| Intermediate occupations | 335 (26) |
| Small employers and own account workers | 92 (7) |
| Lower supervisory and technical occupations | 92 (7) |
| Semiroutine and routine occupations | 216 (17) |
| Education∗ | |
| No qualification | 275 (21) |
| School certificate age 16 | 285 (22) |
| School certificate age 18 | 390 (30) |
| College-level | 333 (26) |
NOTE. Socioeconomic classification = National Statistics Socio-economic Classification 5 levels.
Proportions and numbers of observations estimated from imputed data sets.
Joint income of the person with dementia and spouse/partner.
3.2. Use and costs of individual resource items
3.2.1. Paid care services, medications, assistive equipment, and adaptations
During the prior 3 months, 65% of participants saw a general practitioner, 48% a practice nurse, and 16% a community mental health nurse or psychiatrist (Table 3). Other health professionals (e.g., specialist nurses, psychologists) were seen by 10% or fewer. Just more than half had hospital outpatient or day-case treatment; 14% visited an accident and emergency department. Only 6% had an inpatient admission, staying a week on average. Seventy-one percent had taken dementia medications; 23% had taken other central nervous system medications.
Table 3.
Use of paid and unpaid care and costs: means (SE) across the sample and for users of each type of care during the prior 3 months
| Item | All |
Users |
|||||
|---|---|---|---|---|---|---|---|
| Intensity |
Costs (£) |
Observations∗ |
Intensity |
Costs (£) |
|||
| Mean (SE) | Minimum | Maximum | % | Mean (SE) | |||
| Paid care | |||||||
| Primary and community health† | |||||||
| GP––office | 1.38 (0.05) | 68 (2) | 995 | 1012 | 65 | 2.12 (0.06) | 104 (3) |
| GP––home | 0.09 (0.01) | 7 (1) | 79 | 88 | 5 | 1.63 (0.13) | 139 (11) |
| GP––telephone | 0.33 (0.03) | 7 (1) | 268 | 282 | 18 | 1.83 (0.1) | 40 (2) |
| Practice nurse | 0.95 (0.05) | 11 (1) | 725 | 749 | 48 | 1.98 (0.08) | 24 (1) |
| District nurse | 0.6 (0.15) | 22 (5) | 125 | 136 | 8 | 7.15 (1.6) | 264 (59) |
| Physiotherapist/occupational therapist | 0.3 (0.03) | 16 (2) | 169 | 180 | 11 | 2.68 (0.21) | 139 (11) |
| Specialist nurse | 0.15 (0.02) | 10 (1) | 120 | 131 | 8 | 1.86 (0.16) | 130 (11) |
| Community mental health† | |||||||
| Community mental health nurse | 0.3 (0.03) | 10 (1) | 242 | 258 | 16 | 1.86 (0.11) | 63 (4) |
| Psychiatrist | 0.19 (0.01) | 44 (3) | 236 | 249 | 16 | 1.22 (0.04) | 281 (9) |
| Psychologist | 0.1 (0.02) | 13 (3) | 50 | 61 | 4 | 2.75 (0.49) | 379 (67) |
| Social care† | |||||||
| Social work | 0.11 (0.02) | 6 (1) | 70 | 76 | 5 | 2.25 (0.32) | 124 (18) |
| Home care | 7.76 (0.86) | 76 (8) | 168 | 178 | 11 | 69.3 (5.76) | 681 (57) |
| Meals on wheels | 0.73 (0.19) | 4 (1) | 23 | 29 | 2 | 44.4 (7.43) | 260 (43) |
| Cleaner | 2.73 (0.18) | 26 (2) | 354 | 372 | 24 | 11.55 (0.52) | 108 (5) |
| Laundry service | 0.37 (0.07) | 10 (2) | 45 | 52 | 3 | 11.74 (1.35) | 313 (36) |
| Sitting service | 0.3 (0.08) | 13 (4) | 34 | 38 | 2 | 13.11 (2.86) | 575 (126) |
| Carer support | 0.69 (0.2) | 30 (9) | 48 | 56 | 3 | 20.79 (5.12) | 913 (225) |
| Respite days‡ | 0.08 (0.02) | 9 (3) | 16 | 18 | 1 | 7.5 (0.97) | 880 (113) |
| Day center days | 2.25 (0.2) | 133 (12) | 187 | 194 | 12 | 18.23 (1.07) | 1076 (63) |
| Lunch club visits | 1.3 (0.16) | 10 (1) | 135 | 147 | 9 | 14.34 (1.3) | 112 (10) |
| Hospital care | |||||||
| ED visits | 0.14 (0.01) | 27 (2) | 149 | 161 | 10 | 1.41 (0.09) | 275 (13) |
| Admission 1, days | 0.41 (0.1) | 160 (36) | 84 | 92 | 6 | 7.21 (1.6) | 2824 (548) |
| Admission 2, days | 0.02 (0.01) | 11 (5) | 12 | 14 | 1 | 2.92 (0.59) | 1344 (466) |
| Admission 3, days | 0 (0) | 1 (0) | 3 | 4 | 0 | 0.72 (0.33) | 260 (128) |
| Outpatients§ | 1.46 (0.07) | 205 (9) | 789 | 802 | 52 | 2.83 (0.12) | 398 (13) |
| Medications | |||||||
| CNS | 0.28 (0.02) | 10 (2) | 353 | 367 | 23 | 1.2 (0.03) | 38 (6) |
| Dementia | 0.75 (0.01) | 52 (3) | 1096 | 1109 | 71 | 1.05 (0.01) | 71 (4) |
| Unpaid care and travel to appointments | |||||||
| Unpaid carer | |||||||
| Hours helping | 410.6 (16.54)¶ | 2675 (107)¶ | 1226 | 1246 | 87 | 470.03 (18.21)¶ | 3052 (118)# |
| Work weeks lost∗∗ | 0.08 (0.03) | 43 (14) | 12 | 16 | 1 | 7.48 (0.97) | 4146 (602) |
| Hours cut down†† | 11.32 (1.57) | 115 (20) | 76 | 83 | 6 | 184.02 (15.1) | 1878 (256) |
| Other friends/relatives | |||||||
| Hours helping | 31.79 (4.29) | 207 (28) | 374 | 394 | 25 | 128.22 (16.31) | 833 (106) |
| Days lost work | 0.23 (0.03) | 20 (2) | 103 | 113 | 7 | 3.3 (0.24) | 294 (21) |
| Travel to appointments | |||||||
| Number of trips | 1.45 (0.08) | 10 (2) | 752 | 765 | 49 | 2.95 (0.13) | 21 (3) |
NOTE. People with dementia questionnaire N = 1547; carers questionnaire N = 1283 unless otherwise indicated. Results of multiply imputed data (34 complete data sets).
Abbreviations: SE, standard error; GP, general practitioner; ED, emergency department; CNS, central nervous system.
The number of cases with use of each item varied over the 34 complete data sets produced by the multiple imputation process, as indicated by the columns for minimum and maximum observations. Percentage (%) reflects the estimated mean proportion of the sample across the combined 34 data sets.
Items are face-to-face visits unless otherwise stated; items report responses from the participant with dementia questionnaire data set unless otherwise stated.
Respite in residential homes.
Outpatient visits and procedures.
Hour estimates exclude respondents reporting other numbers of hours caring per week, N = 1412.
Costs reported exclude respondents reporting other numbers of hours caring per week, N = 1412. Over the full sample, N = 1547, the imputed cost of unpaid hours helping was £2721 (SE, £107); the cost of unpaid hours helping by those with nonzero costs (minimum N = 1352, maximum N = 1375) was £3087 (SE, £119).
Days lost over the prior 3 months (variable from the carer questionnaire).
All hours cut down are assumed to have occurred over the prior 3 months (variable from the carer questionnaire).
Use of home-based social care was generally low. More participants reported using services of a cleaner (24%) than a home carer (11%). Overall day center attendance was modest (12%), but day center users averaged 18 attendances during 3 months or 2.6 times weekly; day center costs constituted the largest element of social care costs (£133, standard error [SE], £12). Of home care users, 53% reported that they or their families paid all, and 13% paid some, of the costs. All paid the full costs of cleaners. Two-thirds reported using equipment and adaptations (Supplementary Material 2, Table S2.1): most commonly mobility aids but also pendant alarms (13%) and calendar clocks (12%).
3.2.2. Unpaid care and lost working time
Most participants (87%) received weekly help from friends/relatives, averaging 470 (SE, 18.2) hours during 3 months (i.e., 36 hours per week). Thirty percent (N = 456) of friends/relatives assisted participants with personal care; 44% (N = 678) made sure participants were safe; 68% (N = 1048) helped with finances; 70% (N = 1078) with practical matters; and 74% (N = 1140) with escorting to appointments. Only 1% of carers completing carers' questionnaires had given up work (past 3 months), and 6% had cut down work; 7% of other friends/relatives completing participant questionnaires cut down on work.
3.3. Subtotal and total costs
Mean 3-month cost of health and social care was £1004 (SE, £48) (Table 1). Hospital care (accident and emergency department, inpatient, and outpatient) contributed most to this total, followed by community social services (home care and residential respite care). Unpaid care costs were far higher than paid care costs (£2928; SE, £114). Total costs (paid, unpaid, out-of-pocket costs) were £4008 (SE, £130).
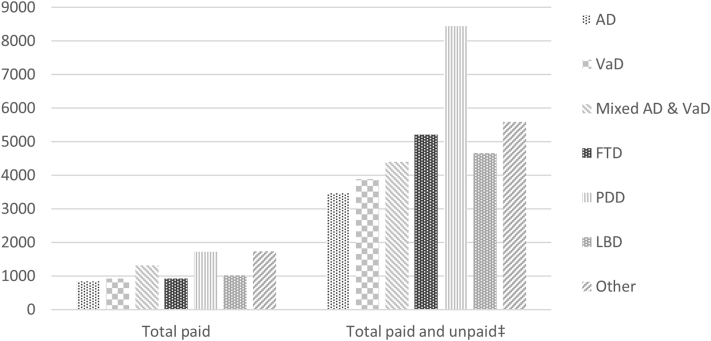
Almost all participants (99%) incurred some costs during 3 months (Supplementary Material 2, Fig. S2.1). A third had some community social care costs. Subtotal and total costs of paid and unpaid care were summarized by sociodemographic and diagnostic subgroups (Supplementary Material 2, Tables S2.2–S2.3). Mean total costs for participants with Parkinson's dementia (Fig. 1) were substantially greater than costs for participants with other dementias. Examining carer data on lost working time (Supplementary Material 2, Table S2.4), costs of carers aged younger than 65 years were more than six times higher than those aged older than 74 years, as might be expected.
Fig. 1.
Paid care costs and total costs of paid, out-of-pocket, and unpaid care (£) of participants with dementia, by diagnostic subtype. NOTE. Costs derived from participant with dementia questionnaires. Abbreviations: AD, Alzheimer's disease; VaD, vascular dementia; FTD, frontotemporal dementia; PDD, Parkinson's disease dementia; LBD, dementia with Lewy bodies; Other, unspecified/other. ‡Unpaid care and lost working time costs derived from participant with dementia questionnaires.
3.4. Model results
Relationships between paid and unpaid care and socioeconomic and diagnostic factors were explored in two-part models (Supplementary Material 2, Tables S2.5a, S2.5b, S2.6). Estimated mean costs are presented in Tables 4 and 5.
Table 4.
Marginal means (95% CIs) (£) from two-part models of paid care cost categories and GLM of total paid care costs
| Variable | Primary care |
Mental health |
Social care |
Day services |
Hospital |
Medications |
Equipment |
Total paid |
|---|---|---|---|---|---|---|---|---|
| Mean (95% CI) | ||||||||
| Sex | ||||||||
| Male | 146 (129, 163) | 69 (57, 82) | 171 (127, 216) | 160 (121, 199) | 436 (340, 533) | 60 (51, 69) | 15 (11, 19) | 1059 (928, 1189) |
| Female | 137 (119, 155) | 65 (50, 79) | 183 (138, 229) | 125 (87, 164) | 358 (273, 443) | 65 (54, 77) | 18 (13, 22) | 949 (831, 1068) |
| Age bands (y) | ||||||||
| Younger than 65 | 190 (131, 249) | 144 (96, 192) | 132 (50, 213) | 88 (30, 145) | 428 (226, 631) | 64 (40, 89) | 12 (5, 20) | 1046 (765, 1326) |
| 65–69 | 139 (107, 172) | 63 (39, 86) | 111 (43, 179) | 80 (30, 131) | 396 (218, 573) | 56 (38, 74) | 11 (5, 18) | 856 (643, 1069) |
| 70–74 | 133 (107, 158) | 85 (60, 109) | 133 (70, 195) | 153 (81, 226) | 418 (284, 552) | 64 (48, 79) | 13 (7, 18) | 992 (807, 1176) |
| 75–79 | 131 (110, 152) | 46 (33, 60) | 98 (59, 138) | 162 (99, 225) | 461 (326, 596) | 59 (47, 72) | 13 (9, 18) | 942 (790, 1094) |
| 80+ | 144 (125, 163) | 59 (46, 72) | 255 (196, 313) | 163 (121, 205) | 359 (264, 454) | 66 (54, 78) | 21 (15, 27) | 1086 (940, 1232) |
| Diagnosis | ||||||||
| AD | 127 (113, 141) | 61 (50, 71) | 149 (113, 185) | 127 (94, 159) | 312 (249, 374) | 63 (55, 72) | 13 (10, 16) | 852 (760, 944) |
| VaD | 147 (111, 184) | 51 (29, 73) | 177 (97, 257) | 202 (116, 288) | 286 (152, 420) | 20 (9, 31) | 14 (8, 20) | 890 (678, 1102) |
| Mixed | 163 (136, 190) | 90 (64, 116) | 175 (115, 235) | 136 (84, 188) | 621 (415, 826) | 77 (59, 94) | 17 (11, 22) | 1256 (1022, 1490) |
| FTD | 86 (48, 124) | 36 (12, 61) | 298 (48, 548) | 323 (34, 611) | 345 (125, 564) | 24 (4, 45) | 24 (−4, 52) | 1025 (616, 1435) |
| PDD | 328 (190, 466) | 68 (24, 113) | 556 (117, 994) | 205 (28, 383) | 650 (176, 1123) | 105 (39, 171) | 67 (27, 106) | 2001 (1107, 2895) |
| DLB | 173 (100, 247) | 130 (51, 210) | 220 (49, 391) | 118 (−2, 238) | 310 (89, 530) | 86 (39, 133) | 21 (4, 37) | 1026 (607, 1445) |
| Unspecified/other | 128 (64, 192) | 91 (20, 163) | 397 (47, 747) | 134 (−29, 297) | 1028 (16, 2040) | 78 (24, 132) | 43 (9, 77) | 1839 (851, 2828) |
| Carer relationship | ||||||||
| Spouse/partner | 144 (126, 162) | 72 (59, 84) | 117 (81, 153) | 138 (102, 175) | 397 (302, 492) | 67 (57, 78) | 14 (10, 18) | 958 (835, 1081) |
| Family/friend | 162 (120, 204) | 57 (31, 82) | 317 (185, 448) | 221 (118, 325) | 491 (308, 674) | 48 (32, 65) | 21 (13, 29) | 1320 (1023, 1616) |
| No carer involved | 117 (91, 143) | 59 (36, 83) | 208 (123, 293) | 94 (43, 145) | 344 (195, 492) | 58 (41, 75) | 17 (11, 24) | 895 (696, 1095) |
| Level of education | ||||||||
| No qualifications | 144 (119, 170) | 71 (52, 89) | 149 (93, 205) | 153 (99, 208) | 354 (245, 463) | 71 (55, 86) | 18 (13, 23) | 977 (809, 1146) |
| School certificate age 16 | 132 (107, 157) | 68 (46, 90) | 171 (100, 242) | 166 (96, 236) | 346 (230, 463) | 54 (40, 68) | 14 (8, 20) | 955 (773, 1136) |
| School certificate age 18 | 150 (129, 171) | 65 (51, 80) | 190 (134, 246) | 160 (115, 206) | 437 (319, 555) | 65 (53, 76) | 16 (11, 21) | 1081 (922, 1240) |
| College-level | 133 (106, 160) | 66 (45, 88) | 210 (127, 293) | 78 (36, 120) | 458 (288, 629) | 56 (41, 71) | 16 (7, 24) | 977 (779, 1174) |
| Household status | ||||||||
| Lives with others | 134 (120, 148) | 67 (57, 77) | 150 (112, 189) | 147 (114, 180) | 397 (325, 468) | 63 (55, 71) | 15 (11, 19) | 965 (864, 1066) |
| Lives alone | 173 (131, 214) | 68 (38, 98) | 236 (148, 323) | 137 (72, 202) | 428 (225, 632) | 60 (41, 80) | 19 (12, 25) | 1162 (884, 1441) |
| Socioeconomic classification | ||||||||
| Managerial | 138 (118, 158) | 61 (47, 74) | 196 (141, 251) | 158 (105, 211) | 382 (279, 486) | 69 (56, 82) | 15 (10, 21) | 1029 (875, 1183) |
| Intermediate | 153 (122, 184) | 82 (58, 107) | 139 (82, 197) | 125 (69, 181) | 512 (334, 690) | 63 (46, 81) | 15 (8, 21) | 1081 (865, 1296) |
| Small employers | 139 (106, 173) | 71 (42, 99) | 173 (76, 270) | 126 (55, 196) | 321 (168, 475) | 48 (31, 65) | 18 (9, 26) | 886 (669, 1102) |
| Lower supervisory | 123 (92, 154) | 73 (43, 103) | 163 (57, 268) | 171 (81, 262) | 503 (263, 742) | 49 (30, 67) | 23 (11, 35) | 1069 (782, 1355) |
| Semiroutine | 150 (119, 180) | 65 (43, 86) | 185 (105, 265) | 138 (82, 195) | 347 (228, 466) | 64 (46, 82) | 16 (10, 22) | 953 (773, 1133) |
| Tenure | ||||||||
| Rental tenant and other tenure | 147 (116, 177) | 62 (41, 84) | 270 (142, 397) | 159 (90, 228) | 520 (315, 726) | 66 (46, 87) | 43 (28, 59) | 1252 (981, 1524) |
| Owner-occupier | 141 (128, 154) | 68 (59, 78) | 155 (123, 187) | 141 (113, 169) | 381 (313, 450) | 62 (55, 69) | 10 (8, 12) | 959 (867, 1051) |
| Income quintile | ||||||||
| First quintile | 145 (120, 171) | 60 (41, 78) | 161 (101, 220) | 169 (103, 234) | 407 (266, 548) | 68 (51, 85) | 16 (11, 21) | 1016 (822, 1211) |
| Second quintile | 138 (109, 167) | 66 (45, 88) | 180 (95, 265) | 186 (111, 262) | 349 (223, 474) | 64 (46, 82) | 22 (13, 31) | 1031 (829, 1233) |
| Third quintile | 154 (122, 185) | 50 (31, 70) | 155 (80, 231) | 163 (82, 244) | 393 (253, 533) | 56 (41, 71) | 16 (9, 24) | 976 (780, 1173) |
| Fourth quintile | 143 (116, 169) | 71 (47, 94) | 222 (121, 323) | 102 (43, 161) | 442 (273, 612) | 61 (46, 76) | 12 (6, 17) | 1029 (810, 1247) |
| Fifth quintile | 126 (96, 155) | 95 (61, 128) | 176 (78, 275) | 83 (32, 134) | 411 (247, 574) | 61 (42, 79) | 13 (4, 22) | 972 (738, 1207) |
NOTE. Results using multiply imputed data (34 complete data sets).
Abbreviations: 95% CIs, 95% confidence intervals; GLM, generalized linear model; AD, Alzheimer's disease; VaD, vascular dementia; FTD, frontotemporal dementia; PDD, Parkinson's disease dementia; DLB, dementia with Lewy bodies; Other, unspecified/other; Managerial, managerial, administrative, and professional occupations; Small employers, small employers and own account workers; Lower supervisory, lower supervisory and technical; Semiroutine, semiroutine and routine.
Table 5.
Marginal means (95% CIs) (£) from two-part models of out-of-pocket, unpaid care time, and lost work time costs and GLM of total costs of paid and unpaid care
| Variable | Out-of-pocket |
Unpaid care time |
Lost work time |
Total paid and unpaid |
|---|---|---|---|---|
| Mean (95% CI) | ||||
| Sex | ||||
| Male | 53 (47, 60) | 3107 (2759, 3455) | 15 (9, 22) | 4272 (3883, 4662) |
| Female | 50 (43, 57) | 2639 (2273, 3006) | 25 (17, 34) | 3607 (3219, 3995) |
| Age bands (y) | ||||
| Younger than 65 | 36 (25, 47) | 3546 (2508, 4583) | 53 (17, 89) | 4748 (3661, 5835) |
| 65–69 | 39 (29, 50) | 2357 (1798, 2915) | 28 (10, 46) | 3346 (2735, 3957) |
| 70–74 | 46 (35, 56) | 2699 (2168, 3231) | 8 (1, 15) | 3774 (3188, 4360) |
| 75–79 | 50 (41, 58) | 2902 (2427, 3378) | 18 (8, 27) | 3876 (3378, 4374) |
| 80+ | 63 (55, 71) | 3084 (2631, 3537) | 19 (12, 27) | 4215 (3743, 4686) |
| Dementia subtype | ||||
| AD | 46 (41, 52) | 2591 (2291, 2890) | 18 (12, 24) | 3498 (3189, 3807) |
| VaD | 47 (34, 60) | 2855 (2140, 3570) | 25 (9, 42) | 3773 (3046, 4500) |
| Mixed AD & VaD | 53 (43, 63) | 2973 (2445, 3502) | 19 (8, 30) | 4337 (3715, 4958) |
| FTD | 68 (37, 100) | 3838 (2228, 5448) | 22 (−2, 47) | 4783 (3189, 6378) |
| PDD | 117 (72, 163) | 6258 (3441, 9075) | 17 (−11, 46) | 8572 (5380, 11,763) |
| DLB | 72 (43, 101) | 3368 (1988, 4749) | 63 (2, 124) | 4618 (3065, 6172) |
| Other | 52 (28, 75) | 3761 (1932, 5591) | 21 (−33, 74) | 5684 (3480, 7888) |
| Carer status | ||||
| Spouse/partner | 51 (45, 57) | 3052 (2745, 3359) | 9 (5, 13) | 4120 (3771, 4469) |
| Family/friend | 64 (49, 80) | 3645 (2654, 4637) | 95 (50, 139) | 5037 (3988, 6086) |
| No carer involved | 41 (31, 52) | 1461 (1050, 1871) | 17 (3, 31) | 2467 (2003, 2931) |
| Level of education | ||||
| No qualifications | 49 (40, 57) | 3140 (2583, 3697) | 22 (11, 33) | 4266 (3663, 4870) |
| School certificate age 16 | 51 (40, 61) | 2435 (1953, 2918) | 11 (3, 20) | 3411 (2881, 3941) |
| School certificate age 18 | 53 (45, 60) | 3005 (2579, 3430) | 23 (13, 33) | 4163 (3697, 4630) |
| College-level | 55 (43, 67) | 2925 (2298, 3553) | 22 (9, 36) | 3846 (3210, 4481) |
| Household status | ||||
| Lives with others | 51 (46, 57) | 3333 (3003, 3662) | 29 (19, 38) | 4360 (4007, 4713) |
| Lives alone | 52 (39, 66) | 1033 (724, 1342) | 12 (6, 17) | 2484 (1980, 2989) |
| Socioeconomic classification | ||||
| Managerial | 50 (43, 57) | 2685 (2270, 3101) | 19 (10, 27) | 3857 (3383, 4331) |
| Intermediate | 57 (46, 69) | 3242 (2516, 3967) | 21 (10, 33) | 4336 (3590, 5083) |
| Small employers and own account workers | 46 (33, 59) | 2709 (2001, 3417) | 25 (5, 46) | 3549 (2830, 4268) |
| Lower supervisory and technical | 50 (36, 64) | 2898 (2138, 3657) | 14 (−2, 30) | 4028 (3184, 4872) |
| Semiroutine | 56 (43, 68) | 3284 (2605, 3963) | 22 (10, 35) | 4250 (3563, 4938) |
| Tenure | ||||
| Rental tenant and other tenure | 41 (32, 51) | 3112 (2440, 3783) | 29 (14, 43) | 4503 (3746, 5260) |
| Owner-occupier | 54 (49, 59) | 2884 (2618, 3150) | 18 (13, 24) | 3899 (3615, 4183) |
| Income quintile | ||||
| First quintile | 48 (38, 58) | 3375 (2754, 3996) | 16 (8, 23) | 4414 (3769, 5060) |
| Second quintile | 59 (45, 72) | 3025 (2413, 3637) | 22 (9, 35) | 4063 (3407, 4719) |
| Third quintile | 51 (40, 63) | 3185 (2497, 3873) | 25 (7, 42) | 4261 (3541, 4982) |
| Fourth quintile | 56 (45, 67) | 2632 (2109, 3155) | 26 (7, 46) | 3750 (3147, 4354) |
| Fifth quintile | 45 (33, 58) | 2294 (1728, 2859) | 25 (2, 48) | 3325 (2659, 3990) |
NOTE. Results using multiply imputed data (34 complete data sets).
Abbreviations: 95% CIs, 95% confidence intervals; GLM, generalized linear model; AD, Alzheimer's disease; VaD, vascular dementia; FTD, frontotemporal dementia; PDD, Parkinson's disease dementia; DLB, dementia with Lewy bodies; Other, unspecified/other; Managerial, managerial, administrative, and professional occupations; Semiroutine, semiroutine and routine.
3.4.1. Receipt and costs of paid care
In first-part models (Tables S2.5a, S2.5b), diagnostic subtype was associated with receipt of most service categories except mental health services. Age was associated with receipt of mental health and social care services. Relationship to carer, living alone, socio-economic classification, and income were also related to receiving social care, people living alone being nearly twice as likely as those living with others to use social care. Second-part models indicated that diagnostic subtype was associated with primary and community care, mental health care, hospital care, and medication costs.
Examining marginal effects of diagnostic subtypes (Table 3), costs of primary and community health care, social care, and medication were highest for those with Parkinson's dementia compared with other dementias. Mental health costs were higher in younger than 65 years age group than in other age groups. Social care costs of participants with nonspousal carers (£317) were 2.7 times higher than of those with spousal carers (£117). Social care costs of participants aged 80+ were higher than those of participants in other age bands. Total paid care costs were highest in those with Parkinson's dementia (£2001): 2.3 times those of participants with Alzheimer's disease (£852) and 2.2 times those of participants with vascular dementia (£890). Costs of participants with nonspousal carers (£1320) were 27% higher than costs of those with spousal carers (£958); and 47% higher than costs of participants with no carer (£895).
3.4.2. Unpaid care
First-part models (Table S2.6) indicated that people with nonspousal carers were three times more likely to have unpaid care than people with spousal carers. People without a participating carer were half as likely as people with spousal carers to have unpaid care. People living alone were 77% less likely to have unpaid care than those living with others. In the second-part unpaid care models, no characteristics showed significant associations with costs. Estimated mean costs of unpaid care (Table 4) for participants with no participating carer were £1461: 60% less than costs of participants with nonspousal carers (£3645) and half the costs of participants with spousal carers (£3052). The estimated mean cost of unpaid care for participants living alone was less than a third of the cost of care for participants living with others.
In a model of carers' lost working time (Table S2.7), the likelihood of carers aged 65+ having lost working time was significantly lower than that of carers younger than 65 years (Table S2.7), unsurprisingly given carers' age and employment profiles. The estimated cost (Table S2.8) of lost working time was £387 (95% CI, 205–569) for carers younger than 65 years, far higher than for older carers.
3.4.3. Total costs
Participant sex and diagnosis and carer status were significantly associated with total paid, unpaid, and out-of-pocket costs (Table S2.6). Costs of female participants were 16% lower than those of males. The costs of Parkinson's dementia were nearly 2.5 times the costs of Alzheimer's disease and one quarter more than those of participants with mixed dementia. Relative to costs of people with spousal carers, costs for participants without carers were 40% lower and costs for participants with nonspousal carers were 22% higher. Costs of participants living alone were 44% lower than of those living with others.
Marginal effects estimates of total costs (Table 5) for women were lower than for men (£3607 vs. £4272). Estimated costs for people with Parkinson's dementia were £8609, £4359 for participants with mixed dementia, and £3484 for those with Alzheimer's disease. Estimates for participants without participating carers were £2467, less than half of those of participants with nonspousal carers (£5037) and 60% less than those for participants with spousal carers (£4120). Estimated costs for participants with dementia living alone (£2484) were £1876 less than for participants living with others (£4360).
4. Discussion
In this large-scale British cohort of people with dementia and their carers, we examined receipt and costs of health and social care services and unpaid care. Most participants visited a general practitioner (65%), and half attended outpatient appointments; use of other individual health and care services was low. Dementia subtype was associated with receipt and costs across sectors. In particular, Parkinson's disease dementia was associated with higher probability of paid care receipt and higher paid care costs. Living alone was positively associated with receipt of social care and negatively associated with receipt of unpaid carer time, receipt and costs of friends/relatives' lost working time, and total costs. Carer status was associated with receipt of several categories of paid and unpaid care, but direction of association varied among spousal, nonspousal, and no-carer groups. Total costs for women, adjusting for diagnosis, socioeconomic characteristics, and carer status, were lower than for men. This trend was also seen in unpaid care costs but not total paid care costs. Similarly, Del Bono et al. [39] found that older women supply more care hours than older men, suggesting that gender-related differences in providing care might act as a driver. The proportion of women was much larger in the sample of carers than in the sample of people with dementia. Given that more men than women participants were recruited and that there were many spousal carers, it is unsurprising that more than two-thirds of carers were females, and this preponderance may have boosted overall unpaid care cost estimates. Numerous studies have reported similar proportions of female carers of people with dementia [8], [40].
Relatively little individual-level information has been collected previously on care use and costs of people with dementia in Britain. Jones et al. [9] estimated 3-month costs of health and social care of people with dementia as £1159 (2014/15 prices). Comparisons are not straightforward as that study's sample (N = 249) was smaller than ours and recruited people with lower MMSE scores (3–26), who could be in residential care. In a study comparing service use in two small dementia samples in 2001 (N = 122) and 2010 (N = 84) [11], 53% saw a practice nurse, comparable to that found here. However, reported proportions in contact with other services were much higher than for the IDEAL cohort: 26% saw a district nurse, 31% a home care worker, and 54% a social worker. Gustavsson et al. [10] examined costs of people with Alzheimer's dementia, finding total monthly costs of care in the UK sample (2014/15 prices, uprated from 2007) to be £951 (mild dementia; N = 86) and £1361 (moderate dementia; N = 81). These figures appear comparable to those for the baseline IDEAL sample (mild-to-moderate dementia).
In line with other evidence [1,2,6,41], we found that unpaid care costs of dementia were much higher than paid service costs, accounting for three-quarters of the total. Recent cost-of-illness calculations [42] estimated that 42% of total costs of all individuals with dementia in England fell to unpaid care; another 25% were social care costs borne by individuals themselves. A recent systematic review [6] reported the share of total costs of dementia attributable to unpaid care as between 60% and 70%. Our estimate is for community-dwelling individuals and is higher than in some studies: in the study by Gustavsson et al. [10], the share of total costs attributable to unpaid care was 64% (mild dementia) and 57% (moderate dementia). Differences between these and our estimates may reflect different methods for calculating unpaid care costs, different sample bases, and shrinkage of paid care available between 2007 and 2014, leaving unpaid carers to fill the gap [14]. Although we valued unpaid care time at minimum wage, the proportion of total costs accounted for by unpaid care would have been even higher had we used a valuation such as national average wage or (taking a replacement cost approach) costs to social care providers of paying home carers for the time. We did not find that unpaid care costs were associated with socioeconomic status, contrary to previous findings [43]. In terms of dementia subtypes and variations in cost, and contrary to the study by Costa et al. [44], we found unpaid care costs higher for Parkinson's-type dementia than for Alzheimer's disease participants.
Our study benefited from a large sample, drawn from across Great Britain, with sufficient numbers of people with less common dementia subtypes to allow comparisons. Limitations include reliance on self-report data with attendant difficulties of reporting biases such as forward and backward telescoping [[45], [46], [47]], particularly in a sample with cognitive impairment (albeit mild to moderate) and for participants with no participating carer. Self-reported carer costs were estimated from bands of carer time; there are more detailed methods of tracking carer time (e.g., time diaries), but they impose heavier respondent burdens. To avoid additional carer effort, information was not collected on their own use of health or social care services. Data were limited to snapshots of retrospective service use during 3 months to minimize inaccuracy of recall [45,47]; analyses based on linked health records over longer retrospective periods are planned.
Compared to having a spousal carer, having a nonspousal carer was associated with a greater likelihood of someone with dementia receiving unpaid care; and the absence of a carer was associated with lower likelihood of receiving unpaid care. These differences could be due in part to differences in cognitive functioning. Although the baseline MMSE scores we observed were not contemporaneous with the period over which costs were reported, nonetheless it is possible that having no carer was associated with higher levels of cognitive functioning and consequently less need for care. Likewise the high prevalence of comorbidity (three-quarters had at least one comorbid condition [22]) may be associated with higher use of services and unpaid care. This could not be investigated with only baseline data because of potential simultaneity of comorbidity incidence and costs but will be examined with data from multiple cohort sweeps. Most nonspousal carers were adult children, who might be more likely to report providing care than spousal carers. Lack of clarity about the person to whom participants with no participating carer were referring when answering unpaid care questions may have resulted in lower reporting of care by this group (Supplementary Material 1). We subsequently revised the unpaid care questions in later data collections to clarify roles played by participating and other unpaid carers.
5. Conclusions
Estimates of paid and unpaid care costs of IDEAL participants varied by dementia subtype, carer status, and living arrangement. Hospital services accounted for the largest part of paid care costs; unpaid care accounted for three-quarters of total costs. Dementia can increase use of paid and unpaid care for older people with other health conditions [14], and carers do not always get the support they need or would like [48]: the condition requires particular attention from policy makers in funding and planning support for people with dementia, families, and friends. Unpaid carers shoulder most of the costs of supporting people with mild-to-moderate dementia: policy makers should give further consideration to improving financial and instrumental support for carers.
Research in context.
-
1.
Systematic review: We sought person-level studies examining use and costs of paid and unpaid care for people with dementia living in countries of the United Kingdom. Identified studies had one or more limitations: limited geographical coverage, small samples, samples with a single dementia subtype, or unconfirmed dementia diagnoses.
-
2.
Interpretation: In our cross-sectional study, unpaid care accounted for three-quarters of participants' 3-month total care costs. Parkinson's-type dementia was associated with higher costs than other dementias. Costs for participants with no carer were lower than costs of those with a spousal carer. Unpaid care costs were lower in female than male participants with dementia. The role of unpaid carers is a key driver of care costs; carer gender may influence the supply and costs of care.
-
3.
Future directions: We will test associations between costs and dementia subtype, sex, and carer type alongside indicators of need in planned longitudinal analyses.
Acknowledgments
The first phase of the IDEAL program was funded jointly by the Economic and Social Research Council (ESRC, United Kingdom) and the National Institute for Health Research (NIHR, United Kingdom) through grant ES/L001853/2. “Improving the experience of dementia and enhancing active life: living well with dementia” (Investigators: L. Clare, I.R. Jones, C.R. Victor, J.V. Hindle, R.W. Jones, M. Knapp, M.D. Kopelman, R. Litherland, A. Martyr, F.E. Matthews, R.G. Morris, S.M. Nelis, J.A. Pickett, C. Quinn, J.M. Rusted, and J.M. Thom). ESRC is part of UK Research and Innovation. The views expressed are those of the author(s) and not necessarily those of ESRC, UK Research and Innovation, NHS, the NIHR, or the Department of Health and Social Care. The support of the ESRC and NIHR is gratefully acknowledged. The funders had no role in the study design, data collection, data analysis and interpretation, writing of the report, or the decision to submit the article for publication.
Author contributions: M.K., S.M.N., C.Q., A.M., I.R.J., C.R.V., J.A.P., J.V.H., R.W.J., M.D.K., R.G.M., J.R., J.M.T., and L.C. were involved in the original conception and design of the study. C.H. and M.K. were responsible for design of the economic data analysis and interpretation of results. C.H. conducted the analyses. C.H. drafted the article; all authors contributed to the critical revision of the article and approved the final version to be published.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.trci.2019.09.012.
Contributor Information
Catherine Henderson, Email: c.henderson@lse.ac.uk.
IDEAL Programme Team:
L. Clare, I.R. Jones, C.R. Victor, J.V. Hindle, R.W. Jones, M. Knapp, M.D. Kopelman, R. Litherland, A. Martyr, F.E. Matthews, R.G. Morris, S.M. Nelis, J.A. Pickett, C. Quinn, J.M. Rusted, and J.M. Thom
Supplementary Data
References
- 1.Prince M., Wimo A., Guerchet M., Ali G.-C., Wu D.Y.-T., Prina M. Alzheimer's Disease International (ADI); London: 2015. World Alzheimer Report 2015. The global impact of dementia: an analysis of prevalence, incidence, cost and trends. [Google Scholar]
- 2.Prince M., Knapp M., Guerchet M., McCrone P., Prina M., Comas-Herrera A. 2nd ed. Alzheimer's Society; London: 2014. Dementia UK: Update. [Google Scholar]
- 3.Clare L., Wu Y.-T., Jones I.R., Victor C.R., Nelis S.M., Martyr A. A comprehensive model of factors associated with subjective perceptions of “living well” with dementia: findings from the IDEAL study. Alzheimer Dis Associated Disord. 2019;33:36–41. doi: 10.1097/WAD.0000000000000286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clare L., Wu Y.-T., Quinn C., Jones I.R., Victor C.R., Nelis S.M. A comprehensive model of factors associated with capability to “live well” for family caregivers of people living with mild-to-moderate dementia: findings from the IDEAL study. Alzheimer Dis Associated Disord. 2019;33:29–35. doi: 10.1097/WAD.0000000000000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wimo A., Reed C.C., Dodel R., Belger M., Jones R.W., Happich M. The GERAS Study: a prospective observational study of costs and resource use in community dwellers with Alzheimer's disease in three European countries––study design and baseline findings. J Alzheimer Dis. 2013;36:385–399. doi: 10.3233/JAD-122392. [DOI] [PubMed] [Google Scholar]
- 6.Schaller S., Mauskopf J., Kriza C., Wahlster P., Kolominsky-Rabas P.L. The main cost drivers in dementia: a systematic review. Int J Geriatr Psychiatry. 2015;30:111–129. doi: 10.1002/gps.4198. [DOI] [PubMed] [Google Scholar]
- 7.Livingston G., Sommerlad A., Orgeta V., Costafreda S.G., Huntley J., Ames D. Dementia prevention, intervention, and care. Lancet. 2017;390:2673–2734. doi: 10.1016/S0140-6736(17)31363-6. [DOI] [PubMed] [Google Scholar]
- 8.Alzheimer's Association 2018 Alzheimer's disease facts and figures. Alzheimers Dement. 2018;14:367–429. [Google Scholar]
- 9.Jones R.W., Romeo R., Trigg R., Knapp M., Sato A., King D. Dependence in Alzheimer's disease and service use costs, quality of life, and caregiver burden: The DADE study. Alzheimers Dement. 2015;11:280–290. doi: 10.1016/j.jalz.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 10.Gustavsson A., Brinck P., Bergvall N., Kolasa K., Wimo A., Winblad B. Predictors of costs of care in Alzheimer's disease: a multinational sample of 1222 patients. Alzheimers Dement. 2011;7:318–327. doi: 10.1016/j.jalz.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 11.Gilbert C., Wilcock J., Thune-Boyle I., Iliffe S. A comparison of service use by people with dementia in two samples a decade apart. Dementia. 2015;16:96–107. doi: 10.1177/1471301215581504. [DOI] [PubMed] [Google Scholar]
- 12.Dodel R., Belger M., Reed C., Wimo A., Jones R.W., Happich M. Determinants of societal costs in Alzheimer's disease: GERAS study baseline results. Alzheimers Dement. 2015;11:933–945. doi: 10.1016/j.jalz.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 13.Gage H., Cheynel J., Williams P., Mitchell K., Stinton C., Katz J. Service utilisation and family support of people with dementia: a cohort study in England. Int J Geriatr Psychiatry. 2014;30:166–177. doi: 10.1002/gps.4118. [DOI] [PubMed] [Google Scholar]
- 14.Bennett H.Q., Norton S., Bunn F., Robinson L., Rait G., Goodman C. The impact of dementia on service use by individuals with a comorbid health condition: a comparison of two cross-sectional analyses conducted approximately 10 years apart. BMC Med. 2018;16:114. doi: 10.1186/s12916-018-1105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knapp M., Chua K.C., Broadbent M., Chang C.K., Fernandez J.L., Milea D. Predictors of care home and hospital admissions and their costs for older people with Alzheimer's disease: findings from a large London case register. BMJ Open. 2016;6:e013591. doi: 10.1136/bmjopen-2016-013591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Institute for Health and Care Excellence . National Institute for Health and Care Excellence; London: 2017. Dementia: Assessment, Management and Support for People Living with Dementia and their Carers. NICE Guideline 97. Methods, Evidence and Recommendations. p. 418. [PubMed] [Google Scholar]
- 17.Department of Health, London. Prime Minister's challenge on dementia: delivering major improvements in dementia care and research by 2015. 2012. Available at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/215101/dh_133176.pdf. Accessed December 5, 2018
- 18.Clare L., Nelis S.M., Quinn C., Martyr A., Henderson C., Hindle J.V. Improving the experience of dementia and enhancing active life—living well with dementia: study protocol for the IDEAL study. Health Qual Life Outcomes. 2014;12:164. doi: 10.1186/s12955-014-0164-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silarova B., Nelis S.M., Ashworth R.M., Ballard C., Bienkiewicz M., Henderson C. Protocol for the IDEAL-2 longitudinal study: following the experiences of people with dementia and their primary carers to understand what contributes to living well with dementia and enhances active life. BMC Public Health. 2018;18:1214. doi: 10.1186/s12889-018-6129-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Institute for Health Research. 2019. https://www.joindementiaresearch.nihr.ac.uk/content/researchers Available at: Accessed March 2, 2018.
- 21.Beecham J.K., Knapp M.R.J. Costing psychiatric interventions. In: Thornicroft G., Brewin C., Wing J.K., editors. Measuring Mental Health Needs. 2nd ed. Gaskell; London: 2001. pp. 220–224. [Google Scholar]
- 22.Nelis S.M., Wu Y.T., Matthews F.E., Martyr A., Quinn C., Rippon I. The impact of comorbidity on the quality of life of people with dementia: findings from the IDEAL study. Age Ageing. 2019;48:361–367. doi: 10.1093/ageing/afy155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu Y.T., Clare L., Hindle J.V., Nelis S.M., Martyr A., Matthews F.E. Dementia subtype and living well: results from the Improving the experience of Dementia and Enhancing Active Life (IDEAL) study. BMC Med. 2018;16:140. doi: 10.1186/s12916-018-1135-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu Y.T., Clare L., Jones I.R., Martyr A., Nelis S.M., Quinn C. Inequalities in living well with dementia—the impact of deprivation on well-being, quality of life and life satisfaction: results from the improving the experience of dementia and enhancing active life study. Int J Geriatr Psychiatry. 2018;33:1736–1742. doi: 10.1002/gps.4998. [DOI] [PubMed] [Google Scholar]
- 25.Martyr A., Nelis S.M., Quinn C., Rusted J.M., Morris R.G., Clare L. The relationship between perceived functional difficulties and the ability to live well with mild-to-moderate dementia: findings from the IDEAL programme. Int J Geriatr Psychiatry. 2019;34:1251–1261. doi: 10.1002/gps.5128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Office for National Statistics . Vol. 3. Palgrave Macmillan; Basingstoke: 2010. (Standard Occupational Classification 2010). The National Statistics Socio-economic Classification: (Rebased on the SOC2010) User Manual. [Google Scholar]
- 27.Curtis L., Burns A. Personal Social Services Research Unit; Canterbury: 2015. Unit Costs of Health and Social Care 2015. [Google Scholar]
- 28.Department of Health . Department of Health; London: 2015. National Schedule of Reference Costs 2014-15. [Google Scholar]
- 29.Health and Social Care Information Centre . Health and Social Care Information Centre; 2015. Prescription Cost Analysis England 2014. [Google Scholar]
- 30.Curtis L. Personal Social Services Research Unit; Canterbury: 2012. Unit Costs of Health and Social Care 2012. [Google Scholar]
- 31.Koopmanschap M.A., van Exel J.N., van den Berg B., Brouwer W.B. An overview of methods and applications to value informal care in economic evaluations of healthcare. Pharmacoeconomics. 2008;26:269–280. doi: 10.2165/00019053-200826040-00001. [DOI] [PubMed] [Google Scholar]
- 32.Office for National Statistics . Office for National Statistics; London: 2014. Annual Survey of Hours and Earnings: 2014 Provisional Results. Statistical Bulletin. [Google Scholar]
- 33.StataCorp . StataCorp LP; College Station: 2017. STATA Multiple-Imputation Reference Manual: Release 15. [Google Scholar]
- 34.StataCorp . StataCorp LP; College Station: 2017. Stata: Release 15. Statistical Software. [Google Scholar]
- 35.McCullagh P., Nelder J.A. Chapman and Hall; London: 1983. Generalized Linear Models. [Google Scholar]
- 36.Belotti F., Deb P., Manning W.G., Norton E.C. twopm: Two-part models. Stata J. 2015;15:3–20. [Google Scholar]
- 37.Li K.H., Meng X.L., Raghunathan T.E., Rubin D.B. Significance levels from repeated P-values with multiply-imputed data. Stat Sinica. 1991;1:65–92. [Google Scholar]
- 38.Rubin D.B. Wiley; Chichester: 1987. Multiple Imputation for Nonresponse in Surveys. [Google Scholar]
- 39.Del Bono E., Sala E., Hancock R. Older carers in the UK: are there really gender differences? New analysis of the individual sample of anonymised records from the 2001 UK Census. Health Soc Care Community. 2009;17:267–273. doi: 10.1111/j.1365-2524.2008.00826.x. [DOI] [PubMed] [Google Scholar]
- 40.Lenox-Smith A., Reed C., Lebrec J., Belger M., Jones R.W. Resource utilisation, costs and clinical outcomes in non-institutionalised patients with Alzheimer's disease: 18-month UK results from the GERAS observational study. BMC Geriatr. 2016;16:195. doi: 10.1186/s12877-016-0371-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bakker C., de Vugt M.E., van Vliet D., Verhey F.R., Pijnenburg Y.A., Vernooij-Dassen M.J. The use of formal and informal care in early onset dementia: results from the NeedYD study. Am J Geriatr Psychiatry. 2013;21:37–45. doi: 10.1016/j.jagp.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 42.Wittenberg R., Knapp M., Hu B., Comas-Herrera A., King D., Rehill A. The costs of dementia in England. Int J Geriatr Psychiatry. 2019;34:1095–1103. doi: 10.1002/gps.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hojman D.A., Duarte F., Ruiz-Tagle J., Budnich M., Delgado C., Slachevsky A. The cost of dementia in an unequal country: the case of Chile. PLoS One. 2017;12:e0172204. doi: 10.1371/journal.pone.0172204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Costa N., Ferlicoq L., Derumeaux-Burel H., Rapp T., Garnault V., Gillette-Guyonnet S. Comparison of informal care time and costs in different age-related dementias: a review. Biomed Res Int. 2013;2013:852368. doi: 10.1155/2013/852368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bhandari A., Wagner T. Self-reported utilization of health care services: improving measurement and accuracy. Med Care Res Rev. 2006;63:217–235. doi: 10.1177/1077558705285298. [DOI] [PubMed] [Google Scholar]
- 46.Evans C.J., Crawford B. Data collection methods in prospective economic evaluations: how accurate are the results? Value Health. 2000;3:277–286. doi: 10.1046/j.1524-4733.2000.34005.x. [DOI] [PubMed] [Google Scholar]
- 47.Thorn J.C., Coast J., Cohen D., Hollingworth W., Knapp M., Noble S.M. Resource-use measurement based on patient recall: issues and challenges for economic evaluation. Appl Health Econ Health Pol. 2013;11:155–161. doi: 10.1007/s40258-013-0022-4. [DOI] [PubMed] [Google Scholar]
- 48.Carter D., Rigby A. Alzheimer's Society; London: 2017. Turning Up the Volume: Unheard Voices of People with Dementia. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.