Introduction
Hidradenitis suppurativa (HS) is a chronic and inflammatory skin follicular disease in the apocrine gland–bearing areas of the body. It is characterized by recurrent painful nodules, cysts, and abscesses that can rupture and lead to the formation of sinus tracts and scarring. The most common HS locations are the axillary, inguinal, and anogenital regions. Squamous cell carcinoma (SCC) is an uncommon complication of this disease. Fewer than 100 cases are reported in the literature.1,2 We describe the case of a young woman presenting a vulvar SCC complicating an HS.
Case report
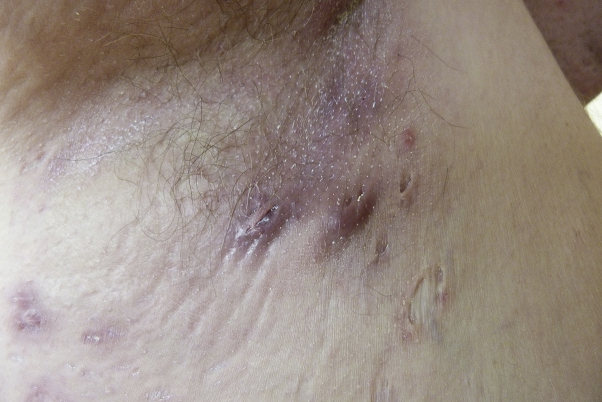
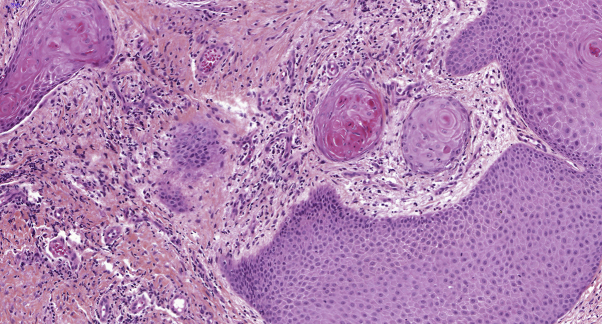
A 31-year-old woman was seen in our dermatology department for recurrent abscesses in the axillary, inguinal, and genital areas that evolved since the age of 13 years. She had no medical history apart from active smoking, reported at 10 cigarettes per day for 10 years, and overweight (body mass index, 25 kg/m2). She had 1 daughter who was born 1 year before. She had no history of human papilloma virus (HPV), and the last Pap smear was performed 2 years before the consultation. Examination found typical scars and abscesses in the axillary, inguinal, and genital regions (Fig 1) that led to the diagnosis of HS. There was no sign of other skin diseases, such as lichen sclerosus. She also presented with a 1.5-cm lesion involving the left labia majora, which was infiltrated and rough (Fig 2). According to the patient, this lesion appeared 6 months after an abscess. The skin biopsy on the vulvar lesion found an infiltrating well-differentiated SCC, and the tumor cells presented dyskeratosis (Fig 3). The bilateral inguinal ultrasound scan found several bilateral lymph nodes. Two fine-needle aspirations were performed in the lymph nodes, and cytologic examination did not show any tumoral cells. Positron emission tomography did not identify distant metastases. A treatment with doxycycline, 100 mg/d, was introduced for the HS. For the SCC, a left anterolateral radical vulvectomy with a bilateral inguinal sentinel lymph node biopsy was performed, and a gluteal lotus petal flap was used for vulvar reconstruction. The pathology report confirmed an infiltrating well-differentiated SCC; the deep and lateral margins were 9 mm and 10 mm, respectively, but no lymph nodes were involved. The International Federation of Gynecology and Obstetrics' staging was IB, and pTNM was pT1b, N0, R0. The histologic signs did not suggest an HPV-induced SCC because it was a well-differentiated SCC. The lesion was negative for P16 by immunohistochemistry, and there were no vulvar intraepithelial neoplasia lesions around the tumor. The Pap smear was normal. No adjuvant treatment was performed.
Fig 1.
Lesions of HS in the axillary region.
Fig 2.
Large tumor involving the left labia majora.
Fig 3.
Infiltrating well-differentiated squamous cell carcinoma shows dyskeratosis. (Hematoxylin-eosin and saffron stain; original magnification: ×20.)
Discussion
SCC is a rare and severe complication of HS. Lavogiez et al2 reported 10 SCCs in their cohort of 217 patients with HS and reported an incidence of 4.6%. Jourabchi et al1 described 80 cases of SCC arising from HS. The average age of the reported cases was 52.4 years, with an average of 25.5 years of HS duration before SCC diagnosis. The SCC locations were mainly perianal/perineal and gluteal, and 86.5% of patients were male.1 Seven additional cases were published more recently,3, 4, 5 with 1 axillary case in a 56-year-old man.4 The youngest case was described by Williams et al6 in a 27-year-old patient.
In the literature, we identified 7 cases of vulvar SCC in patients with HS (Table I).7, 8, 9, 10 Among these cases, the mean duration of HS before the SCC was 25 years. In our patient, symptoms were present for 18 years, but the diagnosis of HS was not established. The mean age at diagnosis of SCC is 55 years, whereas our patient was only 31 years old; she was the youngest case of a vulvar SCC in HS described. Three cases of the 7 had a metastatic evolution. There is frequently a delay in the diagnosis of SCC in HS because of the difficulty of differentiating a new lesion of chronic disease from a malignant lesion. This delay can explain why metastatic evolution is frequent: 55.1% of patients in Jourabchi's study.1
Table I.
Reports of vulvar squamous cell carcinoma in patients with HS
| Study | Age (y) | History of HS (y) | Location | Metastasis Yes/No |
Treatment | Outcome |
|---|---|---|---|---|---|---|
| Makris et al, 20177 | 43 | 2 | Vulva | N | Surgery | Alive 1 year after surgery |
| Rekawek et al, 20168 | 61 | — | Left labia | N | Surgery | Not reported |
| Peña et al, 20159 | 64 | 44 | Right labia | Y | Radiotherapy and chemotherapy | Alive after 19 mo with signs of progressive disease |
| Maclean and Coleman, 200710 | 61 | 40 | Right vulva | Y | Palliative radiotherapy | Died 2 mo after diagnosis |
| Crain, 2005 (cited in Peña et al9) | 44 | 20 | Right labia | Y | Palliative radiotherapy | Died 6 mo after diagnosis |
| Short, 2005 (cited in Peña et al9) | 57 | 15 | Right labia minora | N | Surgery | Not reported |
| Manolitsas, 1999 (cited in Peña et al9) | 52 | 30 | Right vulva | N | Surgery | Not reported |
The development of SCC is multifactorial. Smoking is one of the predisposing factors. In HS, there is an independent loss-of-function mutation in genes encoding essential components of the ϒ-secretase multiprotein complex, which is involved in the regulation of the canonical Notch signaling pathway that is required for hair follicle terminal differentiation and for postnatal hair cycle homeostasis. Notch also acts as a tumor suppressor in nonmelanoma skin cancers, including SCC. The effects of smoking may augment preexisting impairment of Notch signaling in patients with HS, which may increase the risk of SCC development.1 Furthermore, chronic inflammation produces an environment that favors oncogenesis through the dysregulation of tumor suppressor genes and through self-sufficient growth.3
HS is more prevalent in women, but SCC transformation is more frequent in men.1,2 SCC in HS is frequently located in the perineal, perianal, and gluteal areas. These areas are exposed to bacterial, fungal, or viral infections and could promote chronic inflammation and thus the occurrence of SCC. HPV was present in 8 genital or anal tumors; among them, HPV-16 (high risk) was present in 7 cases.2 HPV may be an important contributory factor to SCC arising in HS. In our case, the histologic signs were not in favor of HPV. In the context of HS, the HPV vaccination could be discussed because it has proven efficacious with an acceptable benefit-risk profile in the prevention of cervical cancer and anogenital intraepithelial neoplasia. Immunosuppressive therapies used to treat HS may constitute another risk factor. For the treatment of SCC in chronic HS, a surgical excision with a minimum of 2-cm margins and sentinel lymph node evaluation for metastasis is recommended.2
To our knowledge, fewer than 10 cases of vulvar SCCs complicating HS are described, and we present the youngest case. Because of the difficulty in diagnosing this complication, it is important to perform a skin biopsy of any atypical HS lesion.
Footnotes
Funding sources: None.
Conflicts of interest: None disclosed.
References
- 1.Jourabchi N., Fischer A.H., Cimino-Mathews A. Squamous cell carcinoma complicating a chronic lesion of hidradenitis suppurativa: a case report and review of the literature. Int Wound J. 2017;14(2):435–438. doi: 10.1111/iwj.12671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lavogiez C., Delaporte E., Darras-Vercambre S. Clinicopathological study of 13 cases of squamous cell carcinoma complicating hidradenitis suppurativa. Dermatology. 2010;220(2):147–153. doi: 10.1159/000269836. [DOI] [PubMed] [Google Scholar]
- 3.Chapman S., Delgadillo D., Barber C. Cutaneous squamous cell carcinoma complicating hidradenitis suppurativa: a review of the prevalence, pathogenesis, and treatment of this dreaded complication. Acta Dermatovenerol Alp Pannonica Adriat. 2018;27(1):25–28. [PubMed] [Google Scholar]
- 4.Dessinioti C., Plaka M., Zisimou C. Advanced squamous cell carcinoma of the axillae mimicking hidradenitis suppurativa. J Eur Acad Dermatol Venereol. 2017;31(9):e421–e423. doi: 10.1111/jdv.14235. [DOI] [PubMed] [Google Scholar]
- 5.Juviler P.G., Patel A.P., Qi Y. Infiltrative squamous cell carcinoma in hidradenitis suppurativa: a case report for early surgical intervention. Int J Surg Case Rep. 2019;55:50–53. doi: 10.1016/j.ijscr.2019.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams S.T., Busby R.C., DeMuth R.J. Perineal hidradenitis suppurativa: presentation of two unusual complications and a review. Ann Plast Surg. 1991;26(5):456–462. [PubMed] [Google Scholar]
- 7.Makris G.-M., Poulakaki N., Papanota A.-M. Vulvar, Perianal and perineal cancer after hidradenitis suppurativa: a systematic review and pooled analysis. Dermatol Surg. 2017;43(1):107–115. doi: 10.1097/DSS.0000000000000944. [DOI] [PubMed] [Google Scholar]
- 8.Rekawek P., Mehta S., Andikyan V. Squamous cell carcinoma of the vulva arising in the setting of chronic hidradenitis suppurativa: a case report. Gynecol Oncol Rep. 2016;16:28–30. doi: 10.1016/j.gore.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peña Z.G., Sivamani R.K., Konia T.H. Squamous cell carcinoma in the setting of chronic hidradenitis suppurativa; report of a patient and update of the literature. Dermatol Online J. 2015;21(4) [PubMed] [Google Scholar]
- 10.Maclean G.M., Coleman D.J. Three fatal cases of squamous cell carcinoma arising in chronic perineal hidradenitis suppurativa. Ann R Coll Surg Engl. 2007;89(7):709–712. doi: 10.1308/003588407X209392. [DOI] [PMC free article] [PubMed] [Google Scholar]