Abstract
Introduction
Reminiscence therapy has been shown to improve mental health and quality of life in dementia; however, reminiscence therapy is often delivered by therapists instead of being technology-enabled. This study evaluated the preliminary efficacy of Memory Matters (MM), an iPad reminiscence game on mood, social interaction, quality of life, and behavioral and psychological symptoms of dementia.
Methods
This pilot study used an experimental design where participants were randomized on a 2:2:1 ratio to three arms: individual MM (one-on-one with an interventionist), group MM (2–3 participants per session), or waitlist control. MM was delivered for 30 minutes a session, twice a week for six weeks, followed by six-week self-play. Outcomes were assessed at the baseline, six weeks, and 12 weeks by data collectors blinded to group allocation. Data were analyzed using intention-to-treat analysis and analysis of covariance.
Results
The sample (n = 80) was 82.1 ± 7.8 years in age with 58% female, 15.3 ± 3.3 years of education. Mood did not differ, except for apathetic mood between group MM and control arm at 12 weeks (P = .051). Social interaction improved for individual MM compared with group MM (t = 2.38, P = .017) and control (t = 2.84, P = .005) at six weeks, but not 12 weeks. Other outcomes did not differ.
Discussion
MM improved social interaction and possibly mood. Future studies are needed to evaluate the efficacy of MM with a sufficient sample size.
Keywords: Dementia, Social interaction, Mood, Quality of life, Reminiscence
1. Introduction
Dementia is a global crisis and the 7th leading cause of death [1]. It is estimated that about 50 million people worldwide have dementia currently, and the number will reach 74.7 million by 2030 and 152 million by 2050 with nearly 10 million new cases each year [1]. Dementia is a major cause for disability and dependency and the cost of caring for people with dementia exceeded $1 trillion in 2018 and will reach $2 trillion by 2030 [1]. Dementia negatively impacts mood and quality of life (QoL) [1,2].
Several nonpharmacological treatments have been recommended as frontline treatments to alleviate dementia symptoms, including reminiscence therapy [3,4]. Reminiscence therapy recalls the life and experiences of a person with the aim to help the person maintain or improve mental health [3,4]. Studies have shown that reminiscence therapy improves cognition [5], depression [6], life satisfaction [7], behavioral and psychological symptoms of dementia (BPSD) [8,9], and communication between family caregivers and people with dementia [[10], [11], [12]].
Despite the accumulating evidence, a challenge in translating reminiscence therapy from research to practice is access because a therapist is often needed to deliver reminiscence therapy. This challenge can be overcome using mobile multimedia and gaming platforms, which led to the development of Memory Matters (MM), an iPad reminiscence app [13]. Gaming platforms such as the MM capitalize on the enjoyment of playing games and can help to offset the considerable time commitments necessary to train professional staff to deliver reminiscence therapy [[14], [15]]. Currently, only a few technology-enabled gaming platforms for delivering therapies in dementia have been developed [16], showing positive effects on mood and interaction [[17], [18]].
Another emerging finding from the literature is a potential differential effect of reminiscence delivery format [8,9]. Group reminiscence therapy positively affects QoL in some participants [[19], [20], [21], [22], [23]], whereas personalized approach may be essential for other participants [[17], [24]]. However, no studies have examined the effects of delivery format for gaming-based reminiscence. Hence, the purpose of this experimental study was to evaluate the preliminary efficacy of MM that was delivered one on one versus in a group format on mood, social interaction, QoL, and BPSD in people with dementia.
2. Methods
2.1. Design
This pilot study used an experimental design to randomize participants on a 2:2:1 allocation ratio to individual MM (one-on-one), group MM (2–3 participants), or waitlist control (usual activity and then crossed over to individual MM). MM was delivered by three interventionists for 30 minutes a session, twice a week for 6 weeks, and then participants played MM on their own or with facility staff for another six weeks. Mood, QoL, social interaction, and BPSD were assessed at the baseline, 6 weeks, and 12 weeks by data collectors blinded to group allocations. At 6 weeks, exit interviews were conducted one on one with the participants and caregivers using a semistructured interview guide. People with dementia received $20 after completing the baseline, 6-week, and 12-week data collection ($60 total), and caregivers received $10 ($30 total). This study was approved by the University of Minnesota Institutional Review Board.
2.2. Sample
2.2.1. Recruitment and screening
Participants were recruited using a variety of strategies, including advertisements; study flyer posting and distribution to senior facilities and community centers; referral from community partners; and presentations at community and professional events. Respondents to recruitment were screened over the phone (~5 minutes) and in person (~30 minutes) by a trained graduate research assistant (RA).
In-person interview took place at a location convenient to participants and caregivers who received the consent form in advance. During the interview, the RA explained the consent in detail and answered all questions. Afterwards, the RA assessed the participant's capacity to consent using the UCSD Brief Assessment of Capacity to Consent. If deemed to have the capacity to consent, potential participants signed their own consent forms. Otherwise, they signed assent and family caregivers signed surrogate consent. Consent was also obtained from family and professional caregivers.
2.2.2. Eligibility criteria
The inclusion criteria for people with dementia were English-speaking; diagnosis of dementia confirmed by healthcare providers; stable on dementia medication(s) for at least 1 month (no dosing changes in the past month); having an identified caregiver; and signed informed consent or surrogate consent/assent. The inclusion criteria for caregivers were English-speaking; 21 years of age or older; and self-identified as the family caregiver or direct care provider for a potential participant. Direct care providers are defined as interacting with potential participants at least 3 times per week for at least 3 months and signed informed consent. There were no exclusion criteria.
2.2.3. Sample size and power
A repeated-measures analysis of covariance (ANCOVA) was utilized to capitalize on the experimental design. Sample size was determined using power analysis procedures [25]. To identify a medium statistical effect (standardized regression coefficient = .15) at an alpha level of .05 and statistical power of .80, a sample size of 97 people with dementia was required.
2.3. Setting
All data collection and intervention delivery took place in a private room at the participant's residence. Data collection with family caregivers or direct care providers was conducted immediately after the participant interview either in person or over the phone.
2.4. MM intervention
MM was developed to engage people with dementia in interactive activities designed to tap their long-term memories [20]. These games can be played in “solo mode” or “social/group mode” where people with dementia can play the games solo (individual MM) or with others (group MM). MM is a simple matching game and has hundreds of colorful images of well-known objects (e.g., toys, tools, foods), pets and animals, locations, clothing and fashion, fads, places, and historical events that were well known or part of the common culture from 1930s to 1960s. Images, sounds, and music are intended to help “trigger” autobiographical memories. After each match is made, a “Did you know” screen appears and is designed to promote recall, reflection, and reminiscing. A “Side Show” option makes it possible to click through the images without playing the game and a “My Memories” option makes it possible to create games or slide shows using personal and family photos.
2.5. Study procedures
2.5.1. Staff training
Our staff included one primary and two substitute interventionists and two graduate RAs. All staff were trained by the investigators using the study protocol before implementation. The primary interventionist further trained the substitute interventionists via on-site training which included demonstrations, observations, and return demonstrations until they reached 100% agreement in intervention delivery. Two RAs were hired because the first RA graduated three months before the completion of the study. In addition to investigator training, the second RA was trained by the first RA until they reached 100% agreement in protocol implementation. The RAs recruited and screened participants for eligibility, were blinded to participant's group assignments, and collected outcome data. Continuing training was provided through weekly meetings, semiannual booster training, and whenever there was a protocol change.
2.5.2. Randomization
A random assignment scheme was created before recruitment. Randomization was completed via a priori list generated from http://randomizer.org on a 2:2:1 allocation ratio. Randomization scheme was placed inside 100 opaque envelops that were sequentially numbered and stored in the interventionist's office.
2.5.3. Data collection
Baseline data collection was conducted either after the in-person interview on the same day or scheduled for another time, whichever was convenient to the participants. Each data collection session took 1–2 hours to complete. The RAs confirmed the appointments or reminded the participants of the appointments 1–4 days before a scheduled interview. The RAs reached out to the participants within 24 hours of a missed interview to reschedule. On completing baseline data collection, participants and their family caregivers or facility staff were formally enrolled in the study.
On enrollment of a participant, the primary interventionist opened the envelope to reveal a participant's group assignment to one of three groups: MM Individual, MM Group, or control and recorded the assignment on the randomization scheme. The investigators and RAs were blinded to participants' group assignments. Outcomes were assessed again at 6 and 12 weeks by the RAs.
2.5.4. Delivery of assigned activity
Within two weeks of baseline data collection, the participants started their assigned activity. In the first session, the interventionist introduced MM to the participant and his/her family caregivers or direct care provider through demonstration and discussion, and then delivered the intervention to the participant. Participants in the individual MM arm received an iPad and an interventionist met with them one on one to play the game together for 30 minutes, twice per week for 6 weeks. Participants in the group MM arm played MM with others in a group led by an interventionist for 30 minutes, twice per week for 6 weeks.
In a session, the interventionist asked the participant(s) to select a game such as pets and animals. After a match is made, the interventionist read the “Did you know” screen message before clicking on the Next button. When a participant expressed that he/she was done with the game (such as telling the interventionist or showing no longer interested), then the interventionist asked him/her to pick another game. In group MM, the participants took turns to select the game and agreed to play the selected game. In a typical session, the participant(s) played an average 2–3 games.
After six weeks, an iPad with MM was left with the participants in the individual MM arm who were encouraged to play as many times as possible alone or with others. For participants in the group MM arm, an iPad was given to the activity director who was trained by the interventionist to continue group MM in his/her facility for 6 weeks. Participants in the control arm continued care as usual for 12 weeks and then received individual MM for 6 weeks.
2.6. Outcomes and their measures
The study outcomes included mood (primary outcome), QoL, social interaction, and BPSD (secondary outcomes). Demographics and cognition were measured at the baseline and controlled as covariates when appropriate.
2.6.1. Mood
Mood was assessed using the Alzheimer's Disease and Related Dementia Mood Scale (AMS). The AMS measures five moods (hostile, apathetic, sad, contented, and spirited) in people with dementia. A caregiver was asked to rate 34 measures of moods from 1 (never exhibited) to 5 (always exhibited) to derive a total score for each mood. The scores range from 8 to 40 for hostile mood, from 5 to 25 for apathetic mood, 4 to 20 for sad mood, 5 to 25 for contented mood, and 12 to 60 for spirited mood. Higher scores indicate a higher level of a particular mood. The AMS has an interrater reliability of 0.78–0.85 and an internal consistency of 0.73–0.92. It takes five minutes to administer [26].
2.6.2. Quality of life
Participants rated their perceived QoL on the widely used Cantril QoL ladder (QoL ladder). Although there is no established psychometrics for the QoL ladder, it has been one of the most widely used QoL measures. The QoL ladder is scored from 0 to 100 with higher scores indicating better QoL [27].
2.6.3. Social interaction
Social interaction was measured using the Pleasant Events Schedule–AD (PES-AD short version) which has 20 items of events. The caregivers are asked to first rate how many times an event happened in the last month (frequency) for participants and then how much the participants enjoy the activity. Items are rated according to their frequency during the past month and how much they enjoy the activity. The frequency is rated as 0 (not at all), 1 (1 to 6 times), or 3 (7 or more times). Enjoyment was rated as 0 (not at all), 1 (somewhat), and 2 (a great deal). An overall summary score assessing frequency of engagement in enjoyable activities is generated by multiplying the frequency and enjoyment scores for an item and summed to derive a total score (range from 0 to 80). The PES-AD has demonstrated excellent reliability and validity. This measure takes about five minutes to administer [28].
2.6.4. BPSD severity
BPSD was measured using the Neuropsychiatric Inventory Questionnaire Caregiver (NPI-Q). The NPI-Q asks the caregiver to rate the presence of 12 symptoms. For the symptoms that are present, the caregiver further rates the severity of the symptom from 1 (mild) to 3 (severe). The total scores for severity range from 0 to 36 and for caregiver distress from 0 to 60 with higher scores indicating more severity and distress. The NPI-Q has a Cronbach's α = 0.756 and test-retest reliability of 0.99. It takes about five minutes to administer [29].
2.6.5. Covariates
Age, gender, and education were assessed at the baseline. Cognition was measured with the Mini–Mental State Examination (MMSE) at baseline.
2.7. Data analysis
Intention-to-treat analysis was applied. After data cleaning to confirm data entry accuracy, the extent of missing data was first evaluated. Overall missing data were <1%. Descriptive statistics were performed with means and standard deviations for continuous variables and frequency for discrete variables and ensure the statistical assumptions for the tests were met. ANCOVAs were used to test for group differences at the baseline. ANCOVAs controlling for covariates were calculated using Proc GENMOD and Proc MIANALYZE in SAS were used to test for between group differences. Mean differences in outcomes at six and 12 weeks were tested after adjusting for their baseline values and covariates.
3. Results
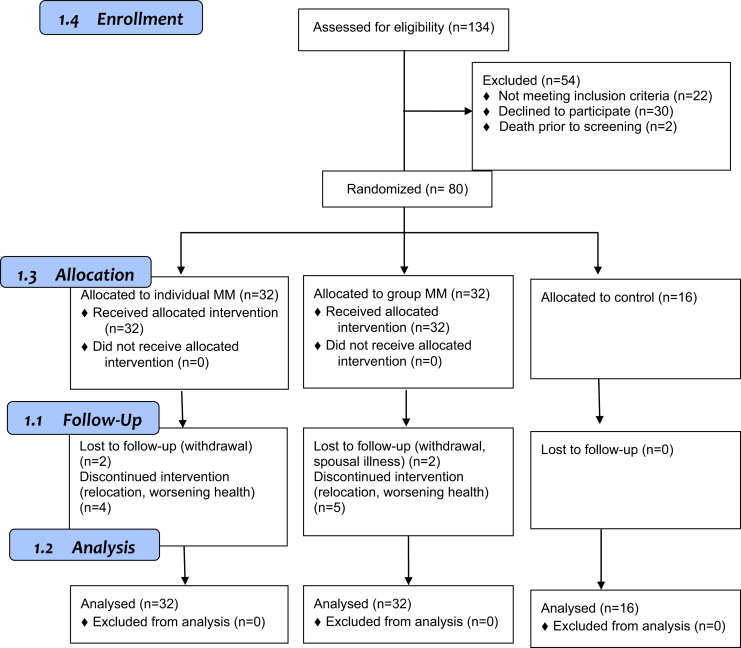
Among 134 potential participants, 54 did not meet the eligibility criteria and 80 were enrolled in the study (Fig. 1). Thirty-two participants were randomized to the group MM arm, 32 to the individual MM arm, and 16 to the control arm. The average age of the participants (n = 80) was 82.1 ± 7.8 years with 58% female and 15.3 ± 3.3 years of education. Their average baseline MMSE score was 17.5 ± 6.7. There were no significant differences in demographics and cognition among the three groups (Table 1).
Fig. 1.
Enrollment flow diagram.
Table 1.
Characteristics of the study sample
| All (N = 80) | Group MM (n = 32) | Individual MM (n = 32) | Control (n = 16) | P value | |
|---|---|---|---|---|---|
| Sex | .794 | ||||
| Male | 34 (43%) | 15 (47%) | 13 (41%) | 6 (38%) | |
| Female | 46 (58%) | 17 (53%) | 19 (59%) | 10 (63%) | |
| Age (years) | 82.1 ± 7.8 (62–98) | 81.7 ± 7.7 (67–94) | 80.7 ± 7.0 (67–91) | 85.6 ± 10.0 (62–98) | .112 |
| Education (years) | 15.3 ± 3.3 (8–24) | 15.9 ± 3.8 (8–24) | 15.2 ± 2.97 (10–22) | 14.3 ± 2.7 (12–20) | .291 |
| Baseline MMSE | 17.5 ± 6.7 (6–29) | 16.1 ± 7.3 (6–29) | 18.6 ± 5.8 (6–28) | 18.3 ± 7.0 (6–29) | .280 |
NOTE. Number and frequency for categorical variables; mean ± SD (range) for continuous variable. Percentages may not sum to 100% due to rounding.
Abbreviations: MM, Memory Matters; MMSE, Mini–Mental State Examination.
At baseline, the participants reported often having contented mood (17.7 ± 4.7) and feeling spirited (37.1 ± 9.7), occasionally to sometimes having apathetic (11.7 ± 2.9) moods, and occasionally feeling sad (6.6 ± 2.8) and hostile (14.0 ± 4.8). Their baseline QoL was high (83.4 ± 18.4) and social interaction was moderate (37.2 ± 14.6). The participants exhibited an average 3.7 ± 2.6 BPSD at a severity of 5.6 ± 4.9 at the baseline. There were no significant differences in these outcomes at baseline among the three groups (Table 2).
Table 2.
Comparison of baseline descriptive statistics on outcomes among groups
| All (N = 80) | Group MM (n = 32) | Individual MM (n = 32) | Control (n = 16) | |
|---|---|---|---|---|
| Mood | ||||
| Apathetic | 11.7 ± 2.9 | 12.1 ± 3.2 | 11.5 ± 3.0 | 11.3 ± 1.9 |
| Content | 17.7 ± 4.7 | 16.7 ± 4.9 | 18.7 ± 4.1 | 17.7 ± 4.6 |
| Hostile | 14.0 ± 4.8 | 14.6 ± 4.9 | 13.2 ± 3.9 | 14.7 ± 5.8 |
| Sad | 6.6 ± 2.8 | 7.0 ± 3.3 | 6.0 ± 1.9 | 6.9 ± 3.0 |
| Spirited | 37.1 ± 9.7 | 36.8 ± 10.5 | 37.8 ± 9.7 | 36.2 ± 8.0 |
| QoL | 83.4 ± 18.4 | 80.3 ± 19.7 | 85.5 ± 18.2 | 85.5 ± 14.8 |
| Social interaction | 37.2 ± 14.6 | 34.9 ± 13.2 | 39.8 ± 14.8 | 36.3 ± 15.8 |
| BPSD | ||||
| Number | 3.7 ± 2.6 | 4.1 ± 2.5 | 3.1 ± 2.4 | 3.9 ± 2.9 |
| Severity | 5.6 ± 4.9 | 7.7 ± 6.2 | 5.5 ± 6.6 | 6.3 ± 5.7 |
| Caregiver distress | 6.5 ± 6.3 | 6.6 ± 4.6 | 4.5 ± 4.4 | 5.9 ± 5.9 |
NOTE. The numbers represent mean ± SD.
Abbreviations: MM, Memory Matters; BPSD: Behavioral and psychological symptoms of dementia; QoL, quality of life.
There were no significant differences in mood among the three groups at six and 12 weeks (Table 3). There was a trend of reduced apathetic mood among participants in the group MM arm at 12 weeks in comparison with the control group (P = .051).
Table 3.
Group differences in outcome at 6 and 12 weeks
| 6 weeks |
12 weeks |
|||
|---|---|---|---|---|
| Mean ± SD | t (P value) | Mean ± SD | t (P value) | |
| Mood | ||||
| Apathetic | ||||
| Individual versus Group | −0.78 ± 0.65 | −1.19 (.23) | 0.26 ± 0.66 | 0.39 (.69) |
| Individual versus Control | −0.48 ± 0.74 | −0.65 (.52) | −1.32 ± 0.78 | −1.69 (.09) |
| Group versus Control | 0.30 ± 0.80 | 0.37 (.71) | −1.58 ± 0.81 | −1.95 (.051)∗ |
| Contented | ||||
| Individual versus Group | 0.78 ± 0.88 | 0.89 (.37) | 0.65 ± 0.85 | 0.77 (.44) |
| Individual versus Control | 1.29 ± 1.06 | 1.22 (.22) | 1.50 ± 0.98 | 1.53 (.13) |
| Group versus Control | 0.50 ± 1.08 | 0.47 (.64) | 0.85 ± 1.04 | 0.82 (.41) |
| Hostile | ||||
| Individual versus Group | −0.78 ± 0.80 | −0.98 (.33) | −0.02 ± 0.84 | −0.02 (.98) |
| Individual versus Control | −0.70 ± 0.97 | −0.73 (.47) | −0.61 ± 1.02 | −0.60 (.55) |
| Group versus Control | 0.08 ± 0.98 | 0.08 (.94) | −0.59 ± 1.03 | −0.57 (.57) |
| Sad | ||||
| Individual versus Group | −0.73 ± 0.61 | −1.20 (.23) | −0.09 ± 0.60 | −0.16 (.87) |
| Individual versus Control | −1.03 ± 0.71 | −1.45 (.15) | −0.80 ± 0.69 | −1.16 (.25) |
| Group versus Control | −0.31 ± 0.73 | 0.42 (.67) | −0.71 ± 0.74 | −0.96 (.34) |
| Spirited | ||||
| Individual versus Group | 1.62 ± 1.54 | 1.05 (.29) | 0.97 ± 1.47 | 0.66 (.51) |
| Individual versus Control | 1.64 ± 1.86 | 0.88 (.38) | 2.32 ± 1.80 | 1.29 (.20) |
| Group versus Control | 0.02 ± 1.89 | 0.01 (.99) | 1.35 ± 1.84 | 0.74 (.46) |
| QoL | ||||
| Individual versus Group | −2.71 ± 5.04 | −0.54 (.59) | −3.57 ± 4.09 | −0.87 (.38) |
| Individual versus Control | −4.59 ± 5.61 | −0.82 (.41) | −1.12 ± 4.93 | −0.23 (.82) |
| Group versus Control | −1.88 ± 5.92 | 0.32 (.75) | 2.45 ± 5.03 | 0.49 (.63) |
| Social interaction | ||||
| Individual versus Group | 5.41 ± 2.27 | 2.38 (.017)∗ | 1.43 ± 2.70 | 0.53 (.60) |
| Individual versus Control | 7.78 ± 2.75 | 2.84 (.005)† | 1.20 ± 3.30 | 0.36 (.72) |
| Group versus Control | 2.37 ± 2.83 | 0.84 (.40) | −0.23 ± 3.35 | −0.07 (.95) |
| BPSD | ||||
| Number of BPSD | ||||
| Individual versus Group | 0.32 ± 0.47 | 0.67 (.50) | 0.18 ± 0.58 | 0.32 (.75) |
| Individual versus Control | 0.32 ± 0.57 | 0.56 (.58) | 0.74 ± 0.73 | 1.02 (.31) |
| Group versus Control | 0.00 ± 0.58 | 0.00 (.99) | 0.56 ± 0.73 | 0.76 (.45) |
| BPSD Severity | ||||
| Individual versus Group | 0.50 ± 0.85 | 0.59 (.56) | −0.06 ± 0.90 | −0.06 (.95) |
| Individual versus Control | 0.51 ± 0.99 | −0.52 (.60) | 1.00 ± 1.11 | 0.90 (.37) |
| Group versus Control | 0.01 ± 1.04 | 0.01 (.99) | 1.06 ± 1.14 | 0.93 (.35) |
| Caregiver distress | ||||
| Individual versus Group | 0.20 ± 1.23 | 0.16 (.87) | 0.26 ± 1.37 | 0.19 (.85) |
| Individual versus Control | −0.16 ± 1.46 | −0.11 (.91) | 0.93 ± 1.68 | 0.56 (.58) |
| Group versus Control | −0.36 ± 1.48 | −0.24 (.81) | 0.67 ± 1.66 | 0.40 (.69) |
NOTE. The analyses were adjusted by baseline values, age, education, gender, and cognition at the baseline.
Abbreviations: Individual, Intervention was delivered by the interventionist to the participants one-on-one; Group, Intervention was delivered by the interventionist in a group of participants; Control, Control group; BPSD, Behavioral and psychological symptoms of dementia; QoL, quality of life.
.05.
.005.
There were significant group differences in social interaction at six weeks. The individual MM arm reported better social interaction than both the group MM arm (P = .017) and the control arm (P = .005) at six weeks (Table 3). This difference was no longer significant at 12 weeks. There was no difference in social interaction between the group MM and control arms at 6 or 12 weeks. There were no significant group differences on QoL and BPSD among the three arms at 6 and 12 weeks (Table 3).
4. Discussions
This pilot study showed that MM did not significantly improve mood except for a trend towards significance on contented and apathetic moods at 12 weeks. The lack of statistical significance is attributable to our small sample size because our power analysis indicated that a sample size of 97 participants is required to identify a medium statistical effect (standardized regression coefficient = 0.15) at an alpha level of .05 and 80% power [25]. However, we were not able to enroll more participants because of the lack of financial resources.
Both the participants and caregivers reported that MM was fun, easy, and enjoyable, and caregivers further reported improved mood in the participants. The quantitative and qualitative findings together suggest that reminiscence therapy likely positively influences mood, especially apathetic moods, which is consistent with the literature [14]. The dose of delivered MM appears to play a role as well. We did not find an effect on mood at six weeks but a trend towards significance at 12 weeks, indicating that there might be a “minimum” dose for reminiscence therapy—a direction for future research.
Our study showed that one-on-one MM significantly increased social interaction over the 6-week guided period, but not at 12 weeks. This finding may be explained by the presence of the interventionists to play MM together. During the follow-up self-play period, MM was rarely played by the participants themselves. This finding suggests that the presence of another person such as a family member is essential for engaging people with dementia in reminiscence. Technology-enabled reminiscence such as MM may reduce the need for professionally trained reminiscence therapist, but is not sufficient by itself to motivate people with dementia to self-initiate its use. In addition, the existing studies rarely used social interaction as a primary outcome [14]. Future research should consider using social interaction as the primary outcome of reminiscence therapy when reminiscence therapy is to be delivered one on one.
Another interesting finding from our study was a potential differential effect of MM delivery formats, showing an effect of group MM on apathetic mood. Group MM may affect apathetic mood by increasing engagement. While previous studies have not examined the effects of delivery format on mood, group reminiscence therapy was found to positively affect QoL [[19], [20], [21], [22], [23]].
Although a recent systematic review found that reminiscence therapy reduced depression and BPSD as well as improved QoL [30], our study showed that MM did not significantly affect QoL and BPSD because of small sample size. In our study, the planned sample size was 97. However, once enrollment began, it quickly became evident that reaching the target recruitment goal was not feasible. Both the number and actual enrollment of eligible participants were considerably lower than expected, so we expanded our recruitments to a 45-mile radius from the university. While such an effort helped to boost enrollment, the cost for delivering MM substantially increased due to increased travel and coordination efforts for the interventionists. Although we reallocated resources to recruitment and intervention delivery solely, we ran out of funding and were only able to meet 82.5% of our enrollment goal. Furthermore, the lack of effect on QoL may be explained by a ceiling effect because our participants rated their QoL high at baseline.
Our study has several strengths. The MM is an innovative iPad-based app reminiscence therapy that can be implemented individually or in a group without requiring training in reminiscence therapy. Using dementia as an inclusion criterion resembles real life and increases the generalizability of our findings. On the other hand, dementia is a clinical syndrome with many different etiologies, which limits the rigor of our study. Our findings are further limited by its small sample size, lack of adjustment for multiple comparisons, and use of usual care as control due to lack of resources. Hence, the results need to be interpreted with caution.
5. Conclusion
This study provided a model of gaming-based reminiscence which can be implemented by lay-person and may improve mood and social interaction in people with dementia. Gaming-based reminiscence increases the potential translation of reminiscence therapy from research to practice. Future studies are needed to test MM's effects with a sufficient sample size.
Research in context.
-
1.
Systematic review: The authors reviewed the literature using traditional sources such as PubMed, meeting abstracts, and presentations. Reminiscence therapy has been shown to improve mental health and quality of life (QoL) in dementia; however, it is often delivered by therapists. Emerging studies showed that technology-enabled reminiscence improved QoL in dementia. However, only a few products have been developed. We have developed an iPad app called Memory Matters to deliver reminiscence to people with dementia.
-
2.
Interpretation: Our findings show that Memory Matters could improve mood and social interaction in people with dementia. Technology-enabled reminiscence such as Memory Matters increases the translation of reminiscence therapy from research to practice.
-
3.
Future directions: This study provided a model of gaming-based reminiscence which can be implemented by lay-person. Future studies are needed to test the effects of technology-enabled reminiscence at sufficient sample size and identify means to better engage people with dementia.
Acknowledgments
The research study reported in this publication was supported by the Small Business Innovation Research grant from the National Institute on Aging of the National Institutes of Health that was awarded to Moai Technologies, LLC (Award #: 9R44AG053923-02). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Authors' contributions: F.Y. designed and implemented the study, analyzed data, and drafted the manuscript. M.A.M. cleaned and analyzed data and drafted the manuscript. J.E.G. designed the study and drafted the manuscript. K.J. implemented the study, collected data, and drafted the manuscript. D.K. designed the study, analyzed data, and drafted the manuscript.
Footnotes
The authors have declared that no conflict of interest exists.
References
- 1.World Health Organization Dementia. 2017. https://www.who.int/news-room/fact-sheets/detail/dementia
- 2.Alzheimer's Association 2019 Alzheimer's disease facts and figures. 2019. https://www.alz.org/media/Documents/alzheimers-facts-and-figures-2019-r.pdf
- 3.Spector A, Orrell M, Davies S, Woods RT. Reminiscence therapy for dementia. Cochrane Database Syst Rev. 2000:CD001120. doi: 10.1002/14651858.CD001120. [DOI] [PubMed] [Google Scholar]
- 4.Woods B, Spector A, Jones C, Orrell M, Davies S. Reminiscence therapy for dementia. Cochrane Database Syst Rev. 2005:CD001120. doi: 10.1002/14651858.CD001120.pub2. [DOI] [PubMed] [Google Scholar]
- 5.Huang H.C., Chen Y.T., Chen P.Y., Huey-Lan Hu S., Liu F., Kuo Y.L. Reminiscence therapy improves cognitive functions and reduces depressive symptoms in elderly people with dementia: a meta-analysis of randomized controlled trials. J Am Med Dir Assoc. 2015;16:1087–1094. doi: 10.1016/j.jamda.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 6.Van Bogaert P., Tolson D., Eerlingen R., Carvers D., Wouters K., Paque K. SolCos model-based individual reminiscence for older adults with mild to moderate dementia in nursing homes: a randomized controlled intervention study. J Psychiatr Ment Health Nurs. 2016;23:568–575. doi: 10.1111/jpm.12336. [DOI] [PubMed] [Google Scholar]
- 7.Wu L.F., Koo M. Randomized controlled trial of a six-week spiritual reminiscence intervention on hope, life satisfaction, and spiritual well-being in elderly with mild and moderate dementia. Int J Geriatr Psychiatry. 2016;31:120–127. doi: 10.1002/gps.4300. [DOI] [PubMed] [Google Scholar]
- 8.Cotelli M., Manenti R., Zanetti O. Reminiscence therapy in dementia: a review. Maturitas. 2012;72:203–205. doi: 10.1016/j.maturitas.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 9.Woods B., O'Philbin L., Farrell E.M., Spector A.E., Orrell M. Reminiscence therapy for dementia. Cochrane Database Syst Rev. 2018;3:CD001120. doi: 10.1002/14651858.CD001120.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lai C.K., Chi I., Kayser-Jones J. A randomized controlled trial of a specific reminiscence approach to promote the well-being of nursing home residents with dementia. Int Psychogeriatr. 2004;16:33–49. doi: 10.1017/s1041610204000055. [DOI] [PubMed] [Google Scholar]
- 11.Yasuda K., Kuwabara K., Kuwahara N., Abe S., Tetsutani N. Effectiveness of personalised reminiscence photo videos for individuals with dementia. Neuropsychol Rehabil. 2009;19:603–619. doi: 10.1080/09602010802586216. [DOI] [PubMed] [Google Scholar]
- 12.O'Shea E., Devane D., Murphy K., Cooney A., Casey D., Jordan F. Effectiveness of a structured education reminiscence-based programme for staff on the quality of life of residents with dementia in long-stay units: a study protocol for a cluster randomised trial. Trials. 2011;12:41. doi: 10.1186/1745-6215-12-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamel A.V., Sims T.L., Klassen D., Havey T., Gaugler J.E. Memory matters: a mixed-methods feasibility study of a mobile aid to stimulate reminiscence in individuals with memory loss. J Gerontol Nurs. 2016;42:15–24. doi: 10.3928/00989134-20160201-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Subramaniam P., Woods B. The impact of individual reminiscence therapy for people with dementia: systematic review. Expert Rev Neurother. 2012;12:545–555. doi: 10.1586/ern.12.35. [DOI] [PubMed] [Google Scholar]
- 15.Inel Manav A., Simsek N. The effect of reminiscence therapy with internet-based videos on cognitive status and apathy of older people with mild dementia. J Geriatr Psychiatry Neurol. 2019;32:104–113. doi: 10.1177/0891988718819864. [DOI] [PubMed] [Google Scholar]
- 16.Zhang M.W., Ho R.C. Personalized reminiscence therapy M-health application for patients living with dementia: Innovating using open source code repository. Technol Health Care. 2017;25:153–156. doi: 10.3233/THC-161253. [DOI] [PubMed] [Google Scholar]
- 17.Ryan AA, McCauley CO, Laird EA, Gibson A, Mulvenna MD, Bond R. “There is still so much inside”: The impact of personalised reminiscence, facilitated by a tablet device, on people living with mild to moderate dementia and their family carers. Dementia (London) 2018 doi: 10.1177/1471301218795242. [DOI] [PubMed] [Google Scholar]
- 18.Bejan A., Gundogdu R., Butz K., Muller N., Kunze C., Konig P. Using multimedia information and communication technology (ICT) to provide added value to reminiscence therapy for people with dementia: Lessons learned from three field studies. Z Gerontol Geriatr. 2018;51:9–15. doi: 10.1007/s00391-017-1347-7. [DOI] [PubMed] [Google Scholar]
- 19.Woods R.T., Bruce E., Edwards R.T., Elvish R., Hoare Z., Hounsome B. REMCARE: reminiscence groups for people with dementia and their family caregivers–effectiveness and cost-effectiveness pragmatic multicentre randomised trial. Health Technol Assess. 2012;16(48):v–xv. doi: 10.3310/hta16480. 1-116. [DOI] [PubMed] [Google Scholar]
- 20.Blake M. Group reminiscence therapy for adults with dementia: a review. Br J Community Nurs. 2013;18:228–233. doi: 10.12968/bjcn.2013.18.5.228. [DOI] [PubMed] [Google Scholar]
- 21.Gonzalez J., Mayordomo T., Torres M., Sales A., Melendez J.C. Reminiscence and dementia: a therapeutic intervention. Int Psychogeriatr. 2015;27:1731–1737. doi: 10.1017/S1041610215000344. [DOI] [PubMed] [Google Scholar]
- 22.Keating F., Cole L., Grant R. An evaluation of group reminiscence arts sessions for people with dementia living in care homes. Dementia (London) 2018:1–17. doi: 10.1177/1471301218787655. [DOI] [PubMed] [Google Scholar]
- 23.Rita Chang H., Chien H.W. Effectiveness of group reminiscence therapy for people living with dementia in a day care centers in Taiwan. Dementia (London) 2018;17:924–935. doi: 10.1177/1471301217725185. [DOI] [PubMed] [Google Scholar]
- 24.Cooney A., Hunter A., Murphy K., Casey D., Devane D., Smyth S. ‘Seeing me through my memories’: a grounded theory study on using reminiscence with people with dementia living in long-term care. J Clin Nurs. 2014;23:3564–3574. doi: 10.1111/jocn.12645. [DOI] [PubMed] [Google Scholar]
- 25.Cohen J. A power primer. Psychol Bull. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 26.Tappen R.M., Williams C.L. Development and testing of the Alzheimer's disease and related dementias mood scale. Nurs Res. 2008;57:426–435. doi: 10.1097/NNR.0b013e31818c3dcc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cantril H. Rutgers University Press; NJ: New Brunswick: 1965. The Pattern of Human Concerns. [Google Scholar]
- 28.Logsdon R.G., Teri L. The pleasant events schedule-AD: psychometric properties and relationship to depression and cognition in Alzheimer's disease patients. Gerontologist. 1997;37:40–45. doi: 10.1093/geront/37.1.40. [DOI] [PubMed] [Google Scholar]
- 29.Cummings J.L. The neuropsychiatric inventory: assessing psychopathology in dementia patients. Neurology. 1997;48:S10–S16. doi: 10.1212/wnl.48.5_suppl_6.10s. [DOI] [PubMed] [Google Scholar]
- 30.Park K, Lee S, Yang J, Song T, Hong GS. A systematic review and meta-analysis on the effect of reminiscence therapy for people with dementia. Int Psychogeriatr. 2019:1–17. doi: 10.1017/S1041610218002168. [DOI] [PubMed] [Google Scholar]